Abstract
A recent study reported for the first time, that DNA methylation of the KITLG gene mediates the association between childhood trauma and cortisol stress reactivity. Our study aimed to provide the first independent replication of these findings. ESPRIT is a prospective study of community-dwelling participants (age ≥ 65), randomly selected from the electoral rolls of the Montpellier district, in France. Clinical depression was assessed using the Mini-International Neuropsychiatric Interview (MINI, French version 5.00), and the Centre for Epidemiological Studies Depression Scale (CES-D). Experiences of childhood adversity were ascertained via a 25-item questionnaire. Morning, evening, and diurnal salivary cortisol was measured under basal and stress conditions and determined using direct radioimmunoassay analysis. DNA methylation of the KITLG gene was quantified in whole blood using the SEQUENOM MassARRAY EpiTYPER platform. A significant negative association was observed between KITLG DNA methylation and both morning cortisol (β = −1.846 ± 0.666, p = .007) and diurnal cortisol (area under curve [AUC]) (β = −19.429 ± 8.868, p = .031) under a stress condition. However, only the former association was significant after correcting for multiple testing. Further, this association remained after adjusting for age, sex, and depression status. No significant association was observed between childhood trauma and KITLG DNA methylation in this older population. This study provides support for an association between KITLG methylation and stress cortisol levels, suggesting that DNA methylation of this gene may play a role in the longer term regulation of the stress system.
The significant negative association between KITLG DNA methylation and morning cortisol, measured under a stressful condition, suggests that individuals with higher KITLG methylation will secrete lower levels of cortisol whilst under stress.
Lay summary
Introduction
Severe or repeated stress or trauma has been shown to result in long-term disruptions in stress signaling (Lupien, McEwen, Gunnar, & Heim, Citation2009). This in turn has been associated with an increased risk of mental health problems over the lifetime, including mood and addictive disorders (Gilbert et al., Citation2009). It remains unclear, however, how trauma can have an enduring effect on the risk of disease, which can occur years and even decades after the initial exposure.
The hypothalamic pituitary adrenal (HPA) axis plays an essential role in stress signaling. In response to stress, the HPA axis releases a cascade of hormones that stimulate the secretion of glucocorticoids (i.e. cortisol in humans), which influences a broad range of physiological processes (Gaffey, Bergeman, Clark, & Wirth, Citation2016). Cortisol secretion is thus used as an indicator of HPA axis responsiveness to stress. Disrupted cortisol secretion is a feature of a number of psychiatric disorders (Beluche et al., Citation2009; Morris, Compas, & Garber, Citation2012). Abnormal cortisol secretion and blunted cortisol response have also been reported following early-life stress (Doom, Cicchetti, & Rogosch, Citation2014), and can remain stable persisting into later life (Olff, Guzelcan, de Vries, Assies, & Gersons, Citation2006).
In recent years, interest into the molecular mechanisms which account for the long-lasting and persistent effects of stress has been increasing. Accumulating evidence indicates that stress and trauma can impact the epigenome and these changes can remain stable with the potential for long-term health effects (Gluckman & Hanson, Citation2004). The epigenome, the collection of potentially reversible modifications which regulate gene activity, is responsive to environment. Adult females reporting childhood adversities had an increased risk of developing depression and were also found to have lower methylation levels of the monoamine oxidase A (MAOA) gene, involved in the metabolism of neurotransmitters (Melas et al., Citation2013). Most consistently reported, however, has been the association between early-life stress and increased glucocorticoid receptor DNA methylation (NR3C1), another major component of HPA-axis signaling. For example, retrospective reporting by adults of parental loss, lack of parental care or maltreatment in childhood, has been associated with increased NR3C1 DNA methylation in blood (Tyrka, Price, Marsit, Walters, & Carpenter, Citation2012). Furthermore, differential NR3C1 DNA methylation in adulthood has also been linked to an attenuated cortisol stress response (Tyrka et al., Citation2012). Recently, findings have been published from the first study to investigate DNA methylation patterns associated with cortisol stress reactivity, using an epigenome-wide approach (rather than focusing on specific candidate genes) (Houtepen et al., Citation2016). The investigators identified a locus (KITLG) where DNA methylation was significantly correlated with cortisol stress reactivity in a cohort of 85 healthy adults aged 18–69 years. The KITLG protein, otherwise referred to as stem cell factor (SCF), mast cell growth factor or steel factor, promotes cell survival, proliferation, differentiation, adhesion, and functional activation (Ashman, Citation1999). It is essential for hematopoiesis, melanogenesis, and fertility. It appears to be important for mast cells, which are cells of the immune system that increase their activity in response to cortisol (Ashman, Citation1999; Nautiyal, Ribeiro, Pfaff, & Silver, Citation2008). DNA methylation at this loci was also observed to mediate the association between increased childhood trauma and lower cortisol reactivity in adulthood (Houtepen et al., Citation2016). This finding furthers our understanding of how KITLG methylation may help regulate the stress response, and provides a mechanism that could possibly explain the varying resilience to toxic environments that has been observed within the population. To date, no other study has reported replication of these findings. The primary aim of this study is to determine whether KITLG DNA methylation continues to be associated throughout life with cortisol response by examination of an older cohort. If a significant association is observed, a secondary aim is to determine whether retrospective reporting of childhood trauma is also associated with KITLG DNA methylation.
Methods
Study participants
The data for this analysis was derived from a large, on-going prospective population study; the ESPRIT study (Enquete de Sante Psychologique – Risques, Incidence et Traitement) (Ritchie et al., Citation2004). Community-dwelling participants aged 65 years and over were randomly selected from the electoral rolls of the Montpellier district, in France. When inclusion criteria were not met, or a participant did not accept the offer to participate, a further random sample was selected from the same electoral roll in order to maintain a representative sample. After providing written informed consent, participants responded to general and health questionnaires, and completed standardized neurological and psychiatric examinations (Ritchie et al., Citation2004). Biological samples, including whole blood for DNA methylation analysis, were also extracted at the time of this examination. The ESPRIT study protocol was approved by the Ethical Committee of the University Hospital of Kremlin-Bicetre, and all procedures were undertaken with verbal and written consent of the participants. The ESPRIT study is ongoing with continual follow-up of participants.
After obtaining written informed consent from all participants, they were administered interviews by trained staff and underwent a number of clinical examinations at baseline and every two years thereafter.
This study is based on a sub-sample of 142 participants who participated in the salivary cortisol study, had complete and valid data for these measures and also provided blood samples for DNA extraction and KITLG DNA methylation analysis (see Supplemental Methods).
Childhood adversity
The Childhood Adversity Questionnaire (Ritchie et al., Citation2009) was administered to the participants at the third wave of assessment (four years after recruitment) by which time a close relationship had been formed between participants and the interviewers, permitting the request for sensitive information. The questionnaire lists 25 adverse childhood events and the participants indicated whether or not had experienced these. For this analysis, we considered two of the major events which were experienced by a number of participants: physical and/or sexual abuse and death (including suicide) of a parent. We also considered the total number of adverse events from the 25-items.
KITLG DNA methylation
The KITLG gene encodes the ligand of the tyrosine-kinase receptor. This gene is pleiotropic. In utero, it is involved in neural cell development, while in adults is required for hematopoiesis.
Using the Epidesigner software (http://www.epidesigner.com/), an assay was designed to amplify a 258 bp region of the gene, just downstream of the CpG island (Ch37hp19; chr12: 88973136-88974846) (). This region was chosen to include the CpG site identified by Houtepen et al. (Citation2016). The measure of DNA methylation is described in the Supplemental Methods.
Statistical analysis
All statistical analyses were performed using Stata 15 (StataCorp, College Station, TX). For each measure of cortisol (i.e. morning, night time, and diurnal) a pairwise correlation determined the strength and direction of the relationship between basal and stress-induced cortisol. A univariate linear regression model was used to determine the association between KITLG methylation (%), at each of the seven CpG units, and cortisol levels (morning, evening (ng/dL), and diurnal (AUC)) (Model 1). A number of a priori covariates were then included in a multivariate regression analysis: initially age and sex (Model 2) as key covariates, and then depression (Model 3) (Ancelin et al., Citation2013), thus replicating covariates modeled by Houtepen et al. (Citation2016). To reduce the risk of false positive findings a more stringent p value of .01 was applied.
The association between participant’s experiences of childhood trauma (e.g. parental death, or childhood abuse) and KITLG methylation (log-transformed to normalize) was first investigated using an independent t-test with unequal variances, an ANOVA (for the three categorical variable of number of adverse events) or a simple linear regression model (when the number of adverse events were treated as a continuous variable). As no significant associations were observed, no further analysis was performed.
Results
The 142 participants included in this study ranged in age from 65 to 80 and nearly half were female (). A small number of individuals (n = 22) had clinically significant depression. Of the 128 participants providing a measure of childhood adversity, a total of 20.3% participants indicated their parents had died during childhood, and 14.8% of individuals reported childhood physical or sexual abuse. The mean levels of cortisol measured in the morning, evening or total cortisol across the day (AUC), under both basal (non-stress), and stress conditions, are also shown in . A pairwise correlation analysis confirmed a strong significant, positive correlation between basal and stress-induced cortisol (morning: r = 0.2961; night time: r = 0.6698, and diurnal: r = 0.6751; p < .01).
Table 1. Participant characteristics (n = 142).
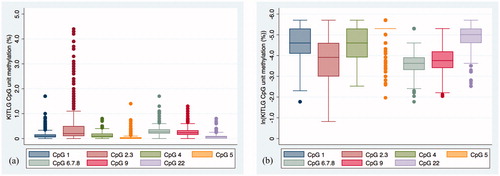
The KITLG assay quantified methylation at seven CpG units located within a 258 bp region of the gene. Overall, mean methylation was low (2.4 ± 3.0%), with CpG unit 2.3 having the highest mean methylation (5.0 ± 7.9%) and CpG unit 5 the lowest (0.6 ± 1.1%) ().
None of the seven CpG units were associated with basal cortisol levels (). On the other hand, CpG 2.3 was significantly negatively associated with morning cortisol levels under the stressful condition (Supplementary Table 2, Figure 2(a)), even after applying a more stringent p value (p < .01). In multivariate models, this association between CpG 2.3 KITLG DNA methylation and morning cortisol levels remained after adjusting for age and sex, as well as further adjustment for clinical depression (). However, when adjusting for multiple testing (p < .01), this association is no longer significant.
Figure 2. Scatterplot indicating the association between KITLG DNA methylation at unit CpG 2.3 and stress reactivity cortisol levels (a) morning cortisol; (b) diurnal cortisol levels (AUC).
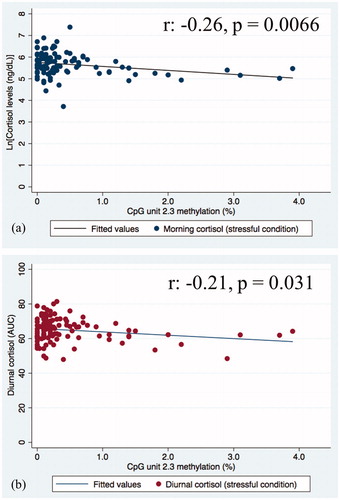
Table 2. Linear regression models for the association between KITLG DNA methylation (%) and cortisol levels under stress.
Similarly, a significant negative association was observed between CpG 2.3 KITLG DNA methylation and total cortisol secretion across the day (AUC) under the stress condition (Supplementary Table 2, Figure 2(b)). Despite remaining significant when controlling for age and sex, adjusting for multiple testing deemed this association no longer significant ().
No association was found between childhood trauma and KITLG DNA methylation, at any of the CpG units (). This includes no significant association between the number of adverse events experienced (in four categories) and KITLG methylation (p between 0.099 and 0.893 for each of the CpG units). These results remained non-significant when adverse events were treated as a continuous variable (p between 0.116 and 0.93 for each of the CpG units).
Table 3. The association between childhood stressful events and KITLG DNA methylation.
Discussion
Severe or chronic stress and trauma can result in long-lasting disruptions in cortisol secretion which negatively impact health outcomes. The molecular mechanism accounting for these persistent effects is unclear, but accumulating evidence suggests a role for epigenetic processes, including DNA methylation, which can result in s changes in gene expression.
As the first study to investigate epigenome-wide DNA methylation patterns correlated with cortisol levels, Houtepen et al. (Citation2016) measured saliva cortisol over a 90 min period in a cohort of 85 healthy Dutch individuals (mean age 33 years), given a stress induction task (Houtepen et al., Citation2016). This task, a version of the Trier social stress test (TSST), involved public speaking and completing an arithmetic task. The researchers identified that a locus within the KITLG gene (cg27512205), was most strongly associated with cortisol stress reactivity, with increased methylation corresponding to lower cortisol levels (AUCi). While this association was not significant when adjusted for multiple testing, they replicated these findings in blood samples from a multiethnic cohort of 45 adults (mean age 28 years), and in buccal cells from 255 Caucasian adolescents (mean age 17 years) (Houtepen et al., Citation2016). The investigators further determined that childhood trauma (a 25 item self-report questionnaire) was positively associated with KITLG DNA methylation, and that DNA methylation-mediated 32% (p = .05) of the association between childhood trauma and cortisol stress reactivity. This latter finding, however, was only identified in one of their cohorts consisting of 85 healthy Dutch individuals, without major depression.
We have conducted the first independent study in an attempt to replicate these findings. In our study of older community-dwelling Caucasians, KITLG DNA methylation was negatively associated with cortisol levels under naturalistic stress conditions. Associations were found with both total cortisol throughout the day (AUC) and morning cortisol levels, but not with evening cortisol levels. Interestingly, the latter appears to be more susceptible to environmental influences (Ancelin et al., Citation2013). Our study thus provides the first independent replication of findings published in the younger cohort (Houtepen et al., Citation2016), however, the exact site of differential methylation varied; CpG 2.3 in our analysis, whereas the locus identified previously corresponds to our CpG 1. The reasons for these differences remain unclear. Overall, KITLG DNA methylation levels in our study were much lower than those in Houtepen et al. (Citation2016); specifically at CpG1 the mean level of methylation in our study was 1.6% compared to 15.0% in the prior study. These could be true differences, reflecting the unique features of the two cohorts, including our sample aged over 65 years, however, it could also be due to the different methodologies used to measure DNA methylation. Methylation was quantified using a probe based array method (Illumina HumanMethylation 450k) in Houtepen et al. (Citation2016) whereas we applied a candidate gene approach with Mass Spectroscopy (Sequenom MassARRAY). Both studies also differ regarding the type of stress (laboratory-type stress versus our naturalistic condition), and protocol for cortisol sampling (afternoon versus our diurnal cortisol sampling). However, despite these differences, as well as populations with different ages (with the HPA-axis becoming more vulnerable to dysregulation in later life and with increased cortisol response to challenge in the elderly (Otte et al., Citation2005), comparable patterns of associations were observed.
Interesting, the association between CpG 2.3 methylation and cortisol was weakened and less significant when clinical depression was included as a covariate. In the study by Houtepen et al. (Citation2016), current major depression was only significantly associated with childhood trauma, although they did not include cases of significantly high depressive symptomatology which did not meet criteria for a major depressive episode, and thus reducing generalizability to the wider population.
We found no association between retrospective reporting of childhood trauma and KITLG DNA methylation, which contrasts with the previous findings (Houtepen et al., Citation2016). In both studies, childhood trauma was measured using a self-report, retrospective questionnaire, although these asked a different set of questions and were considered binary variables (yes/no trauma), whereas Houtpen’s scores were on a continuous scale. Furthermore, their measure of childhood trauma was a total score, the sum of individual questions, whereas we examined specific types of trauma (severe and abuse), as well as the number of traumatic events. However, despite the lack of significance, there was some evidence of a weak association between the experience of severe trauma and DNA methylation at a number of CpGs (i.e. 2.3, 4, 5, 6, 9 and 22), in the direction that those who experienced death of a parent, severe trauma or abuse were more likely to have higher levels of KITLG methylation.
In both our study and the initial report, these retrospective childhood questionnaires are subject to recall bias and under-reporting. The longer time since the occurrence of these events may further reduce accuracy in an older cohort, although death of a parent and physical abuse are highly unlikely to be forgotten. Other possible limitations of our study need to be considered when interpreting the results. Despite being a reasonably sized sample and larger than the discovery cohort of the original publication we were replicating, our sample was still underpowered to detect very small differences in KITLG DNA methylation between groups. Furthermore, only a sample of the original ESPRIT cohort was included in this analysis, as these were the individuals where we had completed KITLG DNA methylation as well as diurnal cortisol levels. The heterogeneity of cell types in whole blood, which was used to measure KITLG DNA methylation, must be kept in mind when interpreting these findings (Bakulski, Halladay, Hu, Mill, & Fallin, Citation2016). However, Houtepen et al. (Citation2016) replicated their findings from whole blood in buccal tissue, which suggests that the observed KITLG methylation results may not be tissue specific. In the context of an older population, both salivary cortisol and whole blood provide a noninvasive, flexible, less stressful approach to measuring cortisol and DNA methylation.
The KITLG gene encodes the ligand that activates the receptor tyrosine kinase KIT (Mirabello, Kratz, Savage, & Greene, Citation2012), which is responsible for promoting proliferation, migration, survival, and differentiation of hematopoietic progenitors, melanocytes and germ cells (Lennartsson & Ronnstrand, Citation2012). Only a few previous studies have investigated KITLG DNA methylation. Lower methylation has been associated with a specific form of cancer (Mirabello et al., Citation2012), as well as with psychiatric disorders such as schizophrenia and bipolar disorders (Boks et al., Citation2016). It is also unclear what the exact functional consequences of DNA methylation in this region would have on gene expression. However, the location of the KITLG CpG 2.3 sites is within a region enriched for H3K27 acetylation. This is a histone modification more commonly associated with promoters and enhancers (i.e. active regulatory elements), and is considered to play an important role in gene expression (Creyghton et al., Citation2010). This suggests that methylation of this gene region could play a functionally significant role. However, it should be noted that the site analyzed by Houtepen is located within the CpG island shore, a region commonly associated with gene expression, whilst CpG 2.3 was located within the island itself. Nonetheless, the increased expression of the glucorticoticoid receptor (GRα), a functionally important receptor of the stress response system, when erythroid precursors are exposed to the KITLG protein (i.e. full length human KITL) further supports this, and suggests a possible role of KITLG during the stress response (Varricchio et al., Citation2012).
In conclusion, we report a significant association between increased KITLG methylation and decreased cortisol stress response in an older population. This independently replicates the correlation previously measured in a younger cohort, thus suggesting that KITLG gene may continue to regulate the stress response throughout the life-span. On the other hand, we were not able to replicate the correlation between childhood trauma and KITLG methylation. Further studies are required to better understand the relevance of this gene in mediating stress reactivity, and the potential of KITLG methylation to cause a lasting modulation of cortisol secretion.
Supplemental Material
Download MS Word (211.3 KB)Acknowledgments
The authors would like to thank the ESPRIT participants for their involvement in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The datasets analyzed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Ancelin, M.L., Carriere, I., Scali, J., Ritchie, K., Chaudieu, I., & Ryan, J. (2013). Angiotensin-converting enzyme gene variants are associated with both cortisol secretion and late-life depression. Translational Psychiatry, 3, e322. doi: 10.1038/tp.2013.95
- Ashman, L.K. (1999). The biology of stem cell factor and its receptor C-kit. The International Journal of Biochemistry Cell Biology, 31, 1037–1051. doi: 10.1016/S1357-2725(99)00076-X
- Bakulski, K.M., Halladay, A., Hu, V.W., Mill, J., & Fallin, M.D. (2016). Epigenetic research in neuropsychiatric disorders: The “tissue issue”. Current Behavioral Neuroscience Reports, 3, 264–274. doi: 10.1007/s40473-016-0083-4
- Beluche, I., Chaudieu, I., Norton, J., Carriere, I., Boulenger, J.P., Ritchie, K., & Ancelin, M.L. (2009). Persistence of abnormal cortisol levels in elderly persons after recovery from major depression. Journal of Psychiatric Research, 43, 777–783. doi: 10.1016/j.jpsychires.2008.10.011
- Boks, M.P., Rutten, B.P., Geuze, E., Houtepen, L.C., Vermetten, E., Kaminsky, Z., ... Vinkers C.H. (2016). SKA2 Methylation is involved in cortisol stress reactivity and predicts the Development of Post-Traumatic Stress Disorder (PTSD) after military deployment. Neuropsychopharmacology, 41, 1350–1356. doi: 10.1038/npp.2015.286
- Chaudieu, I., Beluche, I., Norton, J., Boulenger, J.P., Ritchie, K., & Ancelin, M.L. (2008). Abnormal reactions to environmental stress in elderly persons with anxiety disorders: Evidence from a population study of diurnal cortisol changes. Journal of Affective Disorders, 106, 307–313. doi: 10.1016/j.jad.2007.07.025
- Creyghton, M.P., Cheng, A.W., Welstead, G.G., Kooistra, T., Carey, B.W., Steine, E.J., … Jaenisch, R. (2010). Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America, 107, 21931–21936. doi: 10.1073/pnas.1016071107
- Doom, J.R., Cicchetti, D., & Rogosch, F.A. (2014). Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 1206–1215. doi: 10.1016/j.jaac.2014.08.006
- Gaffey, A.E., Bergeman, C.S., Clark, L.A., & Wirth, M.M. (2016). Aging and the HPA axis: Stress and resilience in older adults. Neuroscience and Biobehavioral Reviews, 68, 928–945. doi: 10.1016/j.neubiorev.2016.05.036
- Gilbert, R., Widom, C.S., Browne, K., Fergusson, D., Webb, E., & Janson, S. (2009). Burden and consequences of child maltreatment in high-income countries. Lancet, 373, 68–81. doi: 10.1016/S0140-6736(08)61706-7
- Gluckman, P.D., & Hanson, M.A. (2004). Living with the past: Evolution, development, and patterns of disease. Science, 305, 1733–1736. doi: 10.1126/science.1095292
- Houtepen, L.C., Vinkers, C.H., Carrillo-Roa, T., Hiemstra, M., van Lier, P.A., Meeus, W., … Boks, M.P.M. (2016). Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans. Nature Communications, 7, 10967. doi: 10.1038/ncomms10967
- Lennartsson, J., & Ronnstrand, L. (2012). Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiological Reviews, 92, 1619–1649. doi: 10.1152/physrev.00046.2011
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. doi: 10.1038/nrn2639
- Melas, P.A., Wei, Y., Wong, C.C.Y., Sjöholm, L.K., Åberg, E., Mill, J., … Lavebratt, C. (2013). Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. The International Journal of Neuropsychopharmacology, 16, 1513–1528. doi: 10.1017/S1461145713000102
- Mirabello, L., Kratz, C.P., Savage, S.A., & Greene, M.H. (2012). Promoter methylation of candidate genes associated with familial testicular cancer. International Journal of Molecular Epidemiology and Genetics, 3, 213–227.
- Morris, M.C., Compas, B.E., & Garber, J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, 32, 301–315. doi: 10.1016/j.cpr.2012.02.002
- Nautiyal, K.M., Ribeiro, A.C., Pfaff, D.W., & Silver, R. (2008). Brain mast cells link the immune system to anxiety-like behavior. Proceedings of the National Academy of Sciences of the United States of America, 105, 18053–18057. doi: 10.1073/pnas.0809479105
- Olff, M., Guzelcan, Y., de Vries, G.J., Assies, J., & Gersons, B.P. (2006). HPA- and HPT-axis alterations in chronic posttraumatic stress disorder. Psychoneuroendocrinology, 31, 1220–1230. doi: 10.1016/j.psyneuen.2006.09.003
- Otte, C., Hart, S., Neylan, T.C., Marmar, C.R., Yaffe, K., & Mohr, D.C. (2005). A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology, 30, 80–91. doi: 10.1016/j.psyneuen.2004.06.002
- Radloff, L.S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi: 10.1177/014662167700100306
- Ritchie, K., Artero, S., Beluche, I., Ancelin, M.L., Mann, A., Dupuy, A.M., … Boulenger, J.P. (2004). Prevalence of DSM-IV psychiatric disorder in the French elderly population. British Journal of Psychiatry, 184, 147–152. doi: 10.1192/bjp.184.2.147
- Ritchie, K., Jaussent, I., Stewart, R., Dupuy, A.M., Courtet, P., Ancelin, M.L., & Malafosse, A. (2009). Association of adverse childhood environment and 5-HTTLPR Genotype with late-life depression. The Journal of Clinical Psychiatry, 70, 1281–1288. doi: 10.4088/JCP.08m04510
- Sheehan, D.V., Lecrubier, Y., Sheehan, K.H., Amorim, P., Janavs, J., Weiller, E., … Dunbar, G.C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59, 22–33. quiz 34–57.
- Tyrka, A.R., Price, L.H., Marsit, C., Walters, O.C., & Carpenter, L.L. (2012). Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS One, 7, e30148. doi: 10.1371/journal.pone.0030148
- Varricchio, L., Tirelli, V., Masselli, E., Ghinassi, B., Saha, N., Besmer, P., & Migliaccio, A.R. (2012). The expression of the glucocorticoid receptor in human erythroblasts is uniquely regulated by KIT ligand: Implications for stress erythropoiesis. Stem Cells & Development, 21, 2852–2865. doi: 10.1089/scd.2011.0676