Abstract
Repeated exposure to stressors, even if mild, may alter the efficiency of optimal stress responses and hinder emotion regulation skills. Mindfulness meditation, by strengthening self-regulation and awareness, may optimize the efficiency of physiological, cognitive, and behavioral reactions to stressful events but typically requires notable commitment to practice, which often leads to disengagement. Recent research suggested that such practices may be made more accessible and that the potential for self-enhancement and stress management of meditation might be improved by supporting mental training with wearable neurofeedback devices able to inform the practicer on ongoing modulation of bodily and brain activity. This study aimed at testing the effect of such novel training approach based on the integration of mental training with brain-sensing wearable devices on physiological (heart rate and variability) and subjective markers of stress (perceived stress, anxiety, and mood states). Participants (N = 55) have been randomly divided into an active control (CONTg) and an experimental group (EXPg). Both groups completed a four-week training constituted by brief daily activities based on mindfulness practices. Experimental participants practiced with the support of dedicated brain-sensing devices. By analyzing pre- and post-training assessments, we observed relevantly decreased stress and anxiety measures in EXPg, as well as relevantly decreased mental fatigue and increased vigor. EXPg also showed improved physiological markers of vagal tone both at rest and during exposure to a cognitive stressor. Reported findings add to the limited available literature on potential effects of technology-supported mental training protocols for promoting subjective well-being and enhancing self-regulation skills.
1. Introduction
Stress has been traditionally defined as an aspecific response of the organism to any kind of exogenous or endogenous stimulus that is able, due to its duration or intensity, to activate adaptation mechanisms to face the stimulus and reestablish homeostasis (Selye, Citation1974). Though such definition has been criticized for being too simplistic and for not explicitly introducing the role of contextual mediators and appraisal mechanisms (Matthews, Lin, & Wohleber, Citation2017), it carries in its core the notion of stress as linked to basic adaptation responses. Primary and secondary stress responses are indeed crucial to activate the organism and to properly face an event or situation, thus supporting a controlled response in which the individual feels to have adequate skills and resources to answer the requests of the context and initiates a positive problem-solving process. Only when the exposure to a stressor agent persists for long periods of time or when the action of the stressor agent becomes too intense such physiological adaptation mechanisms begin to falter, homeostatic regulatory processes become less efficient, and the stress response becomes dysfunctional (Chrousos, Citation2009; Dhabhar, Citation2014).
Indeed, optimal performance levels lay in the middle of the stress curve, while extremely low and extremely high levels of stress associated to the fall of cognitive performance and to the absence of adaptive responses. Consistently, a long research tradition on the effects of acute stress – grounded both on animal studies and studies involving human participants – shaped the relationship between glucocorticoid levels and cognitive performance as an inverted-U curve (Chrousos, Citation2009; Lupien & McEwen, Citation1997).
In contrast, from a neurophysiological point of view prolonged exposure to stress has been associated to a decrease of the production of leukocytes, with a consequent decrease of efficiency of the immune system (Dhabhar, Citation2014). Further, it associates to various clinical consequences such as cardiovascular disorders, hypertension, gastric ulcer, muscular and metabolic dysfunctions, and sleep disturbances (Chrousos, Citation2009). Again, chronic or excessive exposure to stress lead to maladaptive activation of the hypothalamus-pituitary-adrenal (HPA) axis and to consequent alteration of the release of glucocorticoids, which may alter brain plasticity and development, especially within neural structures that are undergoing concurrent structural and functional changes linked to specific stages of lifespan (Lupien, McEwen, Gunnar, & Heim, Citation2009). Beyond known consequences of extreme and chronic exposure to severe stressors, the very same cascade of physiological and psychological events occurs also when people experience common stressors – such as competitions and examinations (Cerin, Szabo, Hunt, & Williams, Citation2000; Saleh, Camart, & Romo, Citation2017) – with relevant consequences on self-perception, performance, and well-being.
In order to promote subjective well-being and to optimize the efficiency of neural, cognitive, and behavioral reactions to stressful events or situations – comprising common environmental stressors that characterize and influence social and working life – it seems then important to train the ability to capitalize on the initial stress-related increase of psychophysiological reactivity while preventing dysfunctional consequences via proper stress management techniques (Subhani et al., Citation2018).
Among stress management techniques, meditation and, in particular, mindfulness practice proved to have positive effects with regard to global physical health, mental health, cognitive and affective efficiency, and social relations (Creswell, Citation2017; Keng, Smoski, & Robins, Citation2011). Further, by practicing mindfulness meditation, individuals may improve the ability to sense and recognize automatic psychological and bodily reactions to eliciting events or situations, thus learning to substitute such uncontrolled reactions with controlled adaptive responses, and may strengthen bodily and self-awareness, thus empowering self- and emotion-regulation skills (Guendelman, Medeiros, & Rampes, Citation2017; Vago & Silbersweig, Citation2012; Wheeler, Arnkoff, & Glass, Citation2017). The potential of mindfulness mental training for improving stress management resources and for containing the impact of dysfunctional emotion-regulation has been further supported by the investigation of training effects in healthy young participants (e.g. Tang et al., Citation2007; Tang, Tang, Jiang, & Posner, Citation2014; Zeidan, Johnson, Diamond, David, & Goolkasian, Citation2010). Overall, available literature highlighted positive training-related effects with regard to the level of mental weariness, energy, and clarity of mind (as measured by the Profile of Mood States (POMS) questionnaire, McNair, Lorr, & Droppleman, Citation1971) and to subjectively reported level of stress (as measured by the Perceived Stress Scale (PSS) Cohen, Kamarck, & Mermelstein, Citation1983).
Again, meditation practice has proved to be able to reduce even physiological markers of stress (Pascoe, Thompson, Jenkins, & Ski, Citation2017). In particular, though different meditation approaches might strictly modulate specific markers, positive effects of practicing meditation have been observed with regard to cortisol levels, concentration of C-reactive protein, level of triglycerides, tumor necrosis factor-alpha, blood pressure, and cardiac activity. Among the measures used to assess physiological stress responsiveness in clinical and research practice, metrics related to heart rate and its variability are probably the most diffused (Bali & Jaggi, Citation2015). The rate of heartbeats is indeed modulated by both sympathetic and parasympathetic influences, and its analysis may then be used to qualify and quantify autonomic nervous system activity (Berntson, Quigley, & Lozano, Citation2007; Mendes, Citation2009). High-frequency components of heart rate variability (HRV), in particular, are thought to mirror parasympathetic influence on the heart, and it has been systematically associated to the intensity of stress response and to the efficiency of coping mechanisms (Appelhans & Luecken, Citation2006; Obrist, Citation1981; Thayer, Hansen, Saus-Rose, & Johnsen, Citation2009).
Recently, it has been proposed that the potential for self-enhancement and stress management of meditation may be improved by supporting practice with noninvasive and highly-usable biofeedback or neurofeedback devices (Balconi, Fronda, Venturella, & Crivelli, Citation2017; Crivelli, Fronda, Venturella, & Balconi, Citation2018; Sliwinski, Katsikitis, & Jones, Citation2017). Such devices, by measuring physiological markers of relaxed vs. aroused or inattentive vs. focused mindsets, are able to keep track of ongoing modulation of bodily and brain activity. By providing the practicers with real-time feedback on the engagement in practice and related physiological underpinnings, those technological devices thus help to make meditation easier, more rewarding, and accessible to a wider audience, especially for beginners.
In order to test whether a mindfulness intervention supported by a brain-sensing neurofeedback device is specifically effective in modulating the subjectively-experienced level of stress and related physiological markers, we devised a controlled longitudinal study. In particular, we hypothesize that: (i) technology-mediated mindfulness practice, compared to an active control intervention, would more relevantly reduce the perceived level of stress as mirrored by subjective reports; (ii) technology-mediated practice would positively modulate physiological markers of stress and, in particular, would improve HRV metrics related to the vagal control of the stress response; and (iii) the technology-supported protocol, by promoting self-regulation skills and awareness of mind-brain states, would also help participants to optimize their adaptive response to environmental demands, with measurable reduction of perceived mental fatigue and increase of perceived vigor.
2. Methods
2.1. Sample
Fifty-five young adults took part in the study (38 ♀, 17 ♂; MAge = 23.212, SDAge = 1.801; MEdu = 16.940, SDEdu = 1.404). Exclusion criteria were: history of psychiatric or neurological diseases; presence of cognitive deficits; ongoing concurrent therapies based on psychoactive drugs that can alter central nervous system functioning; clinically relevant stress, anxiety, or depression levels; occurrence of significant stressful life events during the last 6 months; preceding systematic meditation experience. All of participants had normal or corrected-to-normal hearing and vision.
Participants were randomly divided into an experimental and an active control group. Both groups underwent mental training constituted by brief daily activities based on mindfulness practices. Experimental participants practiced with the support of wearable brain-sensing devices (see Paragraph 2.2.3. “Training intervention”). Statistical comparisons of demographic and psychometric profiles did not highlight relevant across-group differences (see ). Six participants had to interrupt the training sessions before their conclusion due to personal reasons or did not reach the required minimum level of attendance. Consequently, they were not included in subsequent data processing and analysis.
Table 1. Demographic and pre-intervention psychometric data – active control and experimental group – and significance of between-group statistical comparisons.
The study and relative procedures followed the principles of the Declaration of Helsinki and were reviewed and approved by the Ethics Committee of the Department of Psychology of the Catholic University of the Sacred Heart. All participants gave their written consent to take part in the study.
2.2. Procedure
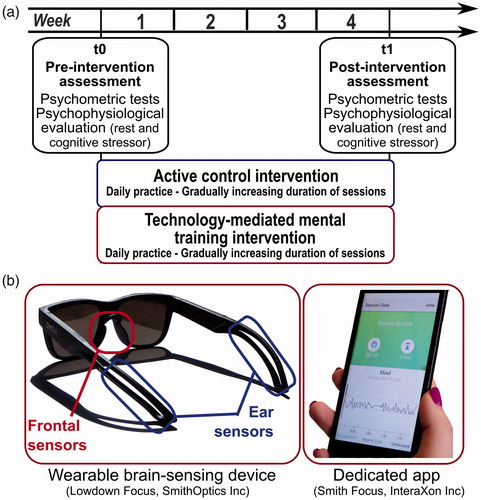
The overall structure of the study included two main assessment steps – i.e. before and at the end of the intervention (see ). During such assessment steps, we collected psychometric measures related to participants’ subjective level of stress and mood states. In addition, we recorded their psychophysiological activity at rest and during a stressor task. Psychophysiological recording sessions were performed in a quiet and moderately darkened room.
Figure 1. (a) Overall structure of the study and experimental steps. (b) An example of wearable brain-sensing device used in the study (Lowdown Focus, SmithOptics Inc.) and of a screenshot of the dedicated app concerning the feedback on session data.
2.2.1. Psychometric assessment
Participants underwent a standardized psychometric assessment in order to exclude the presence of relevant clinical signs of anxiety (State-Trait Anxiety Inventory (STAI); Pedrabissi & Santinello, Citation1989), depression (Beck Depression Inventory (BDI); Ghisi, Flebus, Montano, Sanavio, & Sica, Citation2006), and of a broader set of symptoms of psychological distress (Brief Symptom Inventory (BSI); Leo, Frisoni, Rozzini, & Trabucchii, Citation1993). The short-term effects of tested interventions on the level of anxiety, on perceived stress and on modulation of mood states was respectively assessed via the state subscale of the STAI (Pedrabissi & Santinello, Citation1989), the PSS (Cohen et al., Citation1983), and the POMS (McNair et al., Citation1971), respectively. The 20 items constituting the state scale of the STAI provide a situational measure of the level of anxiety and related negative affectivity. The PSS provides a single score that mirrors the global subjective level of stress related to a given period. The PSS score proved to be positively associated with the high cortisol levels and to physical symptoms of stress (van Eck & Nicolson, Citation1994). The POMS inventory, instead, is constituted by six subscales related to specific affective/mood states; tension, depression, anger, confusion, fatigue, and vigor. Such tool has been consistently used to assess treatment-induced improvement of in state anxiety, mental fatigue and other subjective modulations of mood states, in both pharmacological and non-pharmacological trials (Rossi & Pourtois, Citation2012).
2.2.2. Psychophysiological recording and reduction
In addition to psychometric testing, participants underwent a systematic psychophysiological assessment procedure. Autonomic activity and reactivity were measured at rest and during the exposure to a cognitive stressor. Resting-state recordings included alternated eyes-open and eyes-closed runs (three runs for each condition; duration: 90 s). As a cognitive stressor, we used a challenging computerized Stroop-like task (Stim2 software, Compumedics Neuroscan, Charlotte, NC) tapping on sustained attention and cognitive control skills. During the task, participants were presented with either congruent or incongruent color-word associations (e.g. respectively, the word “RED” written in red or the word “GREEN” written in blue) and had to discriminate between such stimuli by pressing two different buttons. We made the task stressful by manipulating time pressure and by closely presenting the randomized stimuli (stimuli duration: 300 ms; number of trials: 160).
During the psychophysiological assessment, measures of cardiovascular activity were recorded via photoplethysmography by using a Biofeedback2000xpert system (Schuhfried GmbH, Mödling, Austria). The recording sensor was placed in correspondence to the distal phalanx of the second finger of the non-dominant hand. Data were sampled at 40 Hz. Recordings were preceded by an accommodation period to allow sensors to set on baseline levels and participants to habituate to the assessment setting. Measured signals were qualitatively inspected for the presence of artifacts. Inter-beat interval (IBI) metrics were computed starting from raw heart-rate (HR) data. Finally, we extracted the mean HR and the standard deviation of the IBI for each experimental condition. The computation of the standard deviation of IBI mirrors high-frequency components of HRV information corresponding to the vagal influence on cardiovascular activity (Berntson et al., Citation2007; Mendes, Citation2009).
2.2.3. Training intervention
The experimental intervention was based on the mindfulness approach and, in particular, on focused attention meditation practices concerning breathing. Such practices derive from the Vipassanā meditation tradition, which grounds on contemplation and introspection to strengthen concentration, bodily awareness, and awareness of automatic reactions and thoughts. During practice, practicers were asked to intentionally focus their attention on breathing and related bodily sensations and on gently pushing away intrusive thoughts, feelings, and emotions when they enter the space of awareness so to re-orient attention focus on breathing. Mental practice was supported by dedicated brain-sensing wearable devices – namely the Muse™ headband (InteraXon Inc., Ontario, Canada) or the Lowdown Focus glasses (SmithOptics Inc., Clearfield, UT). The devices embed dry electroencephalographic (EEG) electrodes, which allow noninvasive recording of neural activity from frontal and posterior regions of the brain. A dedicated smartphone app then uses such electrophysiological data to immerse the practicer in an interactive sound environment able to provide a real-time feedback on his/her level of focusing vs. distraction, based on related EEG markers. Such wearable neurofeedback system was specifically devised to support meditation practice and inform practicers on their mindset by modulating natural sounds. In particular, the more practicers’ mind got distracted the more weather sounds grew in intensity. In this way, adaptive feedbacks provided them information on mind-wandering and then prompted their nonjudgmental acceptance of such phenomenon and their intentional return to breathing sensations.
The active control intervention was equivalent to the experimental one with regard to overall structure and amount of commitment required to participants. Active control participants were indeed asked to perform daily sessions of breathing awareness practices too. They were also provided with audio clips of natural sounds to be used to recreate a background sound environment similar to the one included in the app, so to keep acoustic stimulation during practice comparable across the groups. Differently from participants included in the experimental group (EXPg), however, they could not benefit from the support of real-time acoustic feedback concerning their focused/inattentive mindset and relative brain activity provided by wearable brain-sensing devices.
In order to grant homogeneity of procedures and comparability of practices, the first session of the protocols took place at the University and, during such session, each participant individually met an expert who introduced and guided them through the breathing awareness practice. Experimental and active control interventions then lasted for four weeks and included daily sessions of practice (gradually incremented duration: from 10 min a day to 20 min a day). To control for potential influence of circadian rhythms on cognitive and physiological processes and reactivity, we asked participants to consistently plan their practices during the day. Further information on the structure and rationale of experimental and active control interventions can be found in Balconi et al. (Citation2017).
2.2.4. Data analysis
Psychometric and psychophysiological data collected before and at the end of the interventions were used to calculate weighted modulation indices by rationalizing post-intervention measures over initial values. Greater-than-1 and lower-than-1 indices respectively mirror a relative increase or decrease of the value that is taken into consideration. Such metrics are robust against potential bias due to inter-individual differences and thus provide a cleaner estimation of intervention effects. Potential computational biases due to pre-intervention between-group differences in psychometric and psychophysiological baseline measures were checked for and excluded via preliminary between-group statistical comparisons (independent-sample t-tests). None of such comparisons revealed statistically-significant difference. Similarly, potential biases related to gender and due to the involvement of sex hormones in stress responses were checked for and excluded. No statistically significant main and interaction effect including gender were observed; then such variable was not included in below-reported analyses.
Weighted indices were analyzed via independent-sample t-tests (PASW Statistics 18, SPSS Inc., Quarry Bay, HK) using Group (Active Control vs. Experimental) as fixed factor. Preliminary Levene’s tests were computed to test homogeneity of variances between the two groups and to adapt the computation of subsequent inferential tests accordingly. Furthermore, we computed Cohen’s d values as a measure of between-group effect size. Effect sizes have been deemed as small when ≥0.2, medium when ≥0.5, and large when ≥0.8, in agreement with Cohen’s (Citation1988) norms.
3. Results
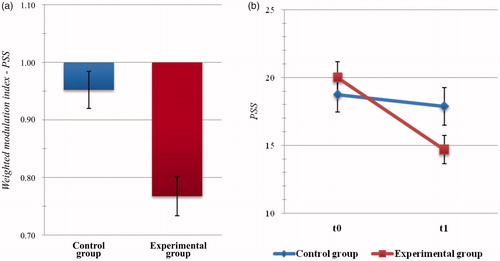
Statistical analysis of weighted indices related to global levels of perceived stress highlighted significant difference between Group scores (t(45) = −3.609, p = .001, d = 1.106). Specifically, perceived stress in the EXPg at the end of the intervention was significantly more reduced than in the Active Control group (MExp = 0.768, SDExp = 0.184; MContr = 0.952, SDContr = 0.124; see ).
Figure 2. Psychometric outcome measures: level of perceived stress. (a) Histogram of post-intervention modulation (weighted modulation indices) and (b) raw data of participants’ scores at the Perceived Stress Scale (PSS). Blue: active control group; dark red: experimental group. Bars represent ± SE.
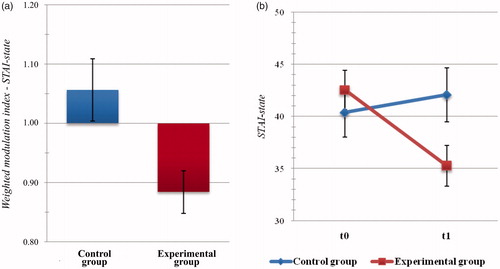
The analysis of weighted change indices for the state subscale of the STAI highlighted significant between-group differences (t(45) = −2.810, p = .007, d = 0.866), with significantly lower levels of anxiety observed in the EXPg with respect to the Active Control one (MExp = 0.884, SDExp = 0.196; MContr = 1.056, SDContr = 0.204; see ).
Figure 3. Psychometric outcome measures: level of anxiety. (a) Histogram of post-intervention modulation (weighted modulation indices) and (b) raw data of participants’ scores at the State-Trait Anxiety Inventory (STAI), state subscale. Blue: active control group; dark red: experimental group. Bars represent ±1 SE.
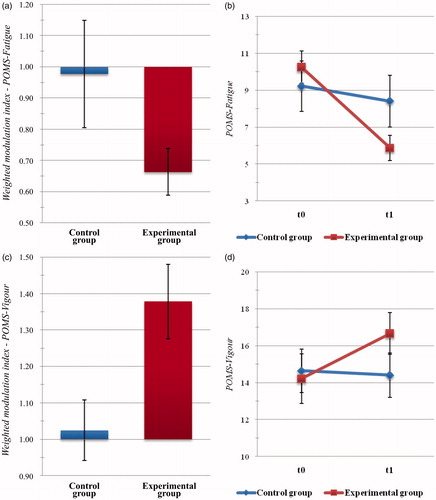
Between-group comparison of weighted indices related to the subscales of the POMS questionnaire highlighted significant differences with regard to the Fatigue (t(44) = −2.019, p = .050, d = 0.646) and the Vigour (t(45) = 2.754, p = .009, d = 0.704) scores. In particular, the EXPg reported significantly reduced levels of fatigue (MExp = 0.663, SDExp = 0.418; MContr = 0.977, SDContr = 0.618; see ) and significantly increased levels of vigor (MExp = 1.378, SDExp = 0.567; MContr = 1.024, SDContr = 0.300; see ) with respect to the Active control group.
Figure 4. Psychometric outcome measures: mood profile. Histogram of post-intervention modulation (weighted modulation indices) and raw data of participants’ scores at the Profile of Mood States questionnaire (POMS), Fatigue (a,b) and Vigor (c,d) subscale. Blue: active control group; dark red: experimental group. Bars represent ±1 SE.
Statistical analyses concerning modulation of HR during resting-state and during the cognitive stressor task did not highlight significant differences between groups (all p > .050).
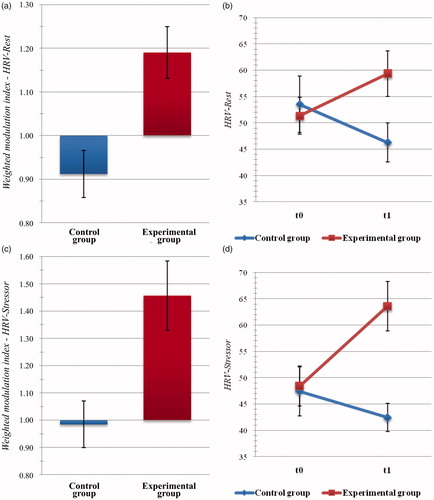
Concerning the analyses of HRV data, the between-group difference of HRV modulation proved to be significant during the eyes-open resting-state (t(47) = 3.146, p = .003, d = 0.942) and during the exposure to the stressor task (t(46) = 3.144, p = .003, d = 0.792). In both cases, HRV modulation indices were significantly greater in the EXPg than in the Active Control one (eyes-open resting-state: MExp = 1.190, SDExp = 0.329; MContr = 0.912, SDContr = 0.215; stressor task: MExp = 1.457, SDExp = 0.694; MContr = 0.985, SDContr = 0.345; see ). In contrast, the analysis of intervention-related HRV modulation indices did not highlight significant differences with regard to eyes-closed resting-state recording.
Figure 5. Psychophysiological outcome measures: heart rate variability (HRV). Histogram of post-intervention modulation (weighted modulation indices) and raw data of participants’ HRV metrics during eyes-open rest (a,b) and during exposure to a cognitive stressor (c,d). Blue: active control group; dark red: experimental group. Bars represent ±1 SE.
4. Discussion
This study aimed at testing the potential of combining traditional mindfulness practice with the support of wearable brain-sensing devices for the reduction of global stress level in healthy people. The analysis of subjective perception of stress and related psychophysiological reactivity highlighted three main consistent sets of findings: (i) the novel technology-mediated mindfulness protocol proved to induce notably greater reduction of perceived stress and anxiety even with respect to an active control condition; (ii) the technology-mediated protocol also lead to the relevant improvement of HRV measures, both at rest and during an activating cognitive stressor task; and (iii) participants who completed the technology-mediated training presented significantly reduced levels of mental fatigue and significantly increased levels of vigor with respect to the active control ones.
The observed reduction in perceived stress and anxiety levels is consistent with available evidence on the effect of mental practices aimed at promoting self-awareness and awareness of automatic affective and stress reactions (Creswell, Citation2017; Guendelman et al., Citation2017; Subhani et al., Citation2018; Vago & Silbersweig, Citation2012). Mindfulness meditation practice, indeed, proved to be useful to empower emotion regulation skills, to build a constructive attitude toward acceptance of negative emotions and thought, and to develop an adaptive detached observational stance with respect to stressful situations and related psychological and physiological responses (Creswell, Citation2017; Guendelman et al., Citation2017; Pascoe et al., Citation2017). Those changes pair with the increase of self-efficacy and sense of control, with the improvement of flexibility of responses to trying situations, and with the reduction of subjectively perceived stress load (Keng et al., Citation2011; Vago & Silbersweig, Citation2012).
Such positive effects of mental practice seemed also systematically enhanced by the concurrent technological support. The effect size of the comparisons between experimental active control groups mirrors a clear reduction of global perceived stress. Those findings are in line with literature on the outcomes of biofeedback and neurofeedback trainings with regard to emotion-regulation and stress reduction (Subhani et al., Citation2018). It has been proposed that the improvement of the outcome of awareness practice due to the integration with brain-sensing wearable devices may depend on the availability of meaningful and easily-accessible information about the modulation of bodily states during practice (Balconi et al., Citation2017; Balconi & Crivelli, Citation2019). By the accurate recording of EEG markers of relaxation vs. arousal and inattention vs. focusing, the devices may act as an external aid and an additional control system that can warn the practicer of the loss of focusing and mind wandering, thus making practice easier for beginners and thus helping to build robust basic meditation skills. Further – and more relevantly – by informing practicers in real time on the status of their mindset and related bodily correlates, the supporting brain-sensing devices may foster the development of self-awareness and, in consequence, self-regulation during stressful situations. Indeed, according to the access theories of consciousness and implicit learning (Cleeremans & Jiménez, Citation2002), awareness of relevant information is the gateway of learning and intentional adaptive changes, and becoming aware of implicit automatic mechanisms (e.g. maladaptive affective responses to trying situations or uncontrolled physiological reactions) is a crucial step to develop the ability to promptly recognize the activation of such automatic mechanisms and intervene to modulate their activation or their effects.
Additionally, it is worth noting that the reduction of the global subjective level of stress induced by the experimental technology-mediated mindfulness intervention was also paired with a consistent improvement of cardiovascular markers of vagal tone, both at rest and during exposure to a stressing condition. The observation of relevant modulation of the HRV metric beyond the lack of statistically significant modulations of heart rate suggests that the experimental interventions have effectively affected the degree of activation of the parasympathetic branch of participants’ autonomous nervous system. HRV metrics can then be deemed as valuable and informative autonomic measures of the effect of stressors and trying situations, with practical implications both for assessment and intervention (Subhani et al., Citation2018). Consistently, it has been proposed that HRV modulation might mirror the outcome of the mediation of cortical-subcortical appraisal systems over brainstem activity and autonomic responses to the environment, which plays a crucial role in guiding and adaptively controlling behavior and stress coping mechanisms (Thayer, Åhs, Fredrikson, Sollers, & Wager, Citation2012). The sizeable effect size regarding the comparisons between experimental and active control groups hints at the extent of the outcomes of combined mindfulness and neurofeedback practice and at its specific potential for fostering efficient psychophysiological reactivity and homeostatic mechanisms. Further, it hints at its potential for promoting both better subjective experience and objective markers of stress response by strengthening central neural regulatory skills and awareness of EEG signatures of distraction and dysfunctional hyperactivation.
Indeed, the measure of heart-rate variability that we have computed is thought to mirror high-frequency component of HRV, which marks parasympathetic-vagal influence on heart activity (Berntson et al., Citation2007; Mendes, Citation2009). The observed pattern of modulation of the vagal tone is consistent with the neurovisceral integration model of stress response (Thayer et al., Citation2009, Citation2012), which outlines the association between parasympathetic activity and optimal performance on several executive tasks and which, by building on Claude Bernard’s seminal idea on the critical role of the vagus nerve, suggest that HRV measures of vagal tone may mirror the contribution of a medial prefrontal regulatory system in modulating the activity of brainstem nuclei mediating cardiac activity. According to this model, the parasympathetic vagus system acts as a structural and functional link between heart and brain thus creating a loop where visceral data are brought to a central system that integrates perceptual, motor, interoceptive, and memory information into unified representations. In turn, such representations are used to guide adaptation responses and sent back to visceral and muscle effectors. Reduction of high-frequency components of HRV, in particular, has been linked to poor responses to environmental demands – which might follow from dysfunctional stress-related arousal, inefficient orienting responses, and reduced attention and focusing skills – and to poor emotion regulation skills (Appelhans & Luecken, Citation2006; Thayer et al., Citation2009). While – as posed by Obrist (Citation1981) – the gradual increase of the sympathetic influence on heart activity due to the stimulation of beta-adrenergic receptors marks an active coping response when it is proportional to the level of the task demands, parasympathetic control is also crucial to compensate for excessive arousal and avoid overload during challenging tasks, such as the cognitive stressor task that participants had to complete.
It is also worth noting that positive modulations of vagal control were observed both during exposure to a cognitive stressor and at rest. Such findings suggest that bodily awareness and self-regulation skills that are trained thanks to the technology-mediated meditation protocol were adaptively transferred by practicers not only to specific acute stress situations but also to resting non-stressful conditions, thus showing a positive effect on tonic homeostatic balance. This adds to available pieces of evidence concerning the positive correlation between resting HRV, executive control, psychological health, and emotion regulation capabilities (Appelhans & Luecken, Citation2006; Balle et al., Citation2013).
Again, it is interesting to note that, besides the reduction of anxiety and stress levels and the improvement of psychophysiological adaptation to stressors, participants who completed the mental training with the support of dedicated wearable devices also reported significant reduction of mental fatigue and significant increase of vigor at the POMS questionnaire. As underlined by Rossi and Pourtois (Citation2012), the POMS questionnaire has been extensively used to track modulation of mood profile consequent to stress-related interventions in both pharmacological and non-pharmacological trials. Consistent with present reported observations, Zeidan et al. (Citation2010) observed relevant reduction of fatigue even following brief experiences of mindfulness mental training. Moreover, Tang and colleagues reported relevant improvement of vigor and relevant decrease of mental fatigue after brief and intensive meditation practice both in a sample of young adults (Tang et al., Citation2007) and in a sample of adolescents (Tang et al., Citation2014). Taken together, present findings sketch a coherent scenario of subjectively reported and objectively recorded changes that point at global improvement of mental energy, reduction of mental weariness and perceived stress, and improved autonomic reactivity following the experimental training.
5. Conclusions
As underlined by Pascoe et al. (Citation2017) with regard to available literature on the effect of meditation on markers of stress, many studies fail to include proper active control conditions and then prevents from drawing robust conclusions on intervention efficacy. In order to try and overcome such limitation, we implemented a controlled study including an active control condition globally similar to the experimental condition apart from specific target features. And again, the multilevel investigation of stress response is among the strong point of the work. Self-report measures may indeed be affected by social desirability and response biases. The integration of self-report and objective measures of stress response thus allow for containing such evaluation risk.
Therefore, reported findings add to the limited available literature on potential effects of technology-supported mental training protocols for promoting subjective well-being and enhancing self-regulation skills. Given the known psychological and physiological consequences of repeated exposure to stressors, including those related to working and social life, improving self- and bodily awareness might help to break the known vicious cycle linking exposure to stressful events, feeling of inadequacy, and enactment of uncontrolled dysfunctional reactions.
Nonetheless, we acknowledge that the investigation of the outcomes of the technology-mediated mindfulness intervention would benefit from additional critical comparisons with further stress management techniques and with different meditation practices, such as open monitoring meditation and compassion meditation, which are thought to exert different effects on practicers’ psychology and behavior (Lippelt, Hommel, & Colzato, Citation2014). In addition, future investigation may benefit from the inclusion of a waiting-list group. Such additional form of methodological control could indeed help to better define the relative advantage of integrating a traditional meditation protocol with dedicated brain-sensing devices and a supporting app fostering practicers during mental training and providing them with easily-accessible information on their mindset. Again, though the computation of weighted indices allowed to directly compare intervention-related modulations across outcome measures and to obtain a quite robust measure of change against inter-individual differences, such computation depends on initial individual values. We have checked and excluded between-group differences that might have relevantly biased computations and following analysis. Nonetheless, we acknowledge that present observations and related remarks would be strengthened by additional investigations that more directly explore differences between pre- and post-intervention data and, relevantly, follow-up data. Moreover, the present investigation highlighted the effect of technology-mediated mental practice on cardiovascular stress-related measures. Though valuable, they are just one of the various aspects of autonomic functioning influenced by stress. The interpretation of the effects of the protocol might be further specified by testing the potential variation of other arousal metrics (e.g. tonic electrodermal activity or number of skin conductance responses). Again, in this work, we opted for a Stroop-like task to explore arousal and stress responses related to an effortful cognitive performance. Still, available data would be enriched by additional complementary investigation of the modulation of stress responses to subjective psychosocial stressors (e.g. by using Trier Social Stress Test). Furthermore, additional studies on the potential role of mediation variables linked to practicers – such as personality traits – or to practice – such as frequency and duration of the intervention protocol – would enrich actual implications for practice and would help to optimize the structure of the intervention protocol. Again, it is worth noting that, against the quite consistent set of intervention outcomes observed in the EXPg, participants in the active control group presented, on average, only limited modulation of psychometric and psychophysiological measures. Such observation would need further replication to be corroborated and properly discussed. Nonetheless, it might be explained by taking into account the duration of the protocol we used. Indeed the vast majority of available evidence on the influence of traditional meditation practices over subjective and, in particular, objective markers of stress grounds on longer intervention protocols that also include longer daily practice sessions (e.g. the standardized mindfulness-based stress reduction protocol lasts eight weeks and requires approximately 45–60 min of individual daily practice). Finally, we also acknowledge that present findings have been observed by focusing on mildly stressed participants. Future studies should try to overcome such limitation and explore the potential of the technology-mediated protocol in case of clinically relevant stress profiles.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
Authors kindly thank Alessandra Coniglio and Marina Ballerio for their support in data collection.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Appelhans, B.M., & Luecken, L.J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10, 229–240. doi: 10.1037/1089-2680.10.3.229
- Balconi, M., & Crivelli, D. (2019). Wearable devices for self-enhancement and improvement of plasticity: Effects on neurocognitive efficiency. In A. Esposito, M. Faundez-Zanuy, F. C. Morabito, & E. Pasero (Eds.), Quantifying and processing biomedical and behavioral signals. Smart innovation, systems and technologies. Heidelberg: Springer. doi: 10.1007/978-3-319-95095-2
- Balconi, M., Fronda, G., Venturella, I., & Crivelli, D. (2017). Conscious, pre-conscious and unconscious mechanisms in emotional behaviour. Some applications to the mindfulness approach with wearable devices. Applied Sciences, 7, 1280. doi: 10.3390/app7121280
- Bali, A., & Jaggi, A.S. (2015). Clinical experimental stress studies: Methods and assessment. Reviews in the Neurosciences, 26, 555–579. doi: 10.1515/revneuro-2015-0004
- Balle, M., Bornas, X., Tortella-Feliu, M., Llabrés, J., Morillas-Romero, A., Aguayo-Siquier, B., & Gelabert, J.M. (2013). Resting parietal EEG asymmetry and cardiac vagal tone predict attentional control. Biological Psychology, 93, 257–261. doi: 10.1016/j.biopsycho.2013.02.012
- Berntson, G.G., Quigley, K.S., & Lozano, D. (2007). Cardiovascular psychophysiology. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (3rd ed., pp. 182–210). New York, NY: Cambridge University Press.
- Cerin, E., Szabo, A., Hunt, N., & Williams, C. (2000). Temporal patterning of competitive emotions: A critical review. Journal of Sports Sciences, 18, 605–626. doi: 10.1080/02640410050082314
- Chrousos, G.P. (2009). Stress and disorders of the stress system. Nature Reviews Endocrinology, 5, 374–381. doi: 10.1038/nrendo.2009.106
- Cleeremans, A., & Jiménez, L. (2002). Implicit learning and consciousness: A graded, dynamic perspective. In R. M. French & A. Cleeremans (Eds.), Implicit learning and consciousness: An empirical, philosophical and computational consensus in the making (pp. 1–40). Hove, England: Psychology Press.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (II). Hillsdale, NJ: Lawrence Erlbaum Associates.
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. doi: 10.2307/2136404
- Creswell, J.D. (2017). Mindfulness interventions. Annual Review of Psychology, 68, 491–516. doi: 10.1146/annurev-psych-042716-051139
- Crivelli, D., Fronda, G., Venturella, I., & Balconi, M. (2018). Supporting mindfulness practices with brain-sensing devices. Cognitive and electrophysiological evidences. Mindfulness, Advance Online Publication. doi: 10.1007/s12671-018-0975-3
- Dhabhar, F.S. (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunologic Research, 58, 193–210. doi: 10.1007/s12026-014-8517-0
- Ghisi, M., Flebus, G. B., Montano, A., Sanavio, E., & Sica, C. (Eds.). (2006). Beck depression inventory – II. Firenze: Giunti OS.
- Guendelman, S., Medeiros, S., & Rampes, H. (2017). Mindfulness and emotion regulation: Insights from neurobiological, psychological, and clinical studies. Frontiers in Psychology, 8, 220. doi: 10.3389/fpsyg.2017.00220
- Keng, S.L., Smoski, M.J., & Robins, C.J. (2011). Effects of mindfulness on psychological health: A review of empirical studies. Clinical Psychology Review, 31, 1041–1056. doi: 10.1016/j.cpr.2011.04.006
- Leo, D., Frisoni, G.B., Rozzini, R., & Trabucchi, M. (1993). Italian community norms for the brief symptom inventory in the elderly. British Journal of Clinical Psychology, 32, 209–213. doi: 10.1111/j.2044-8260.1993.tb01045.x
- Lippelt, D.P., Hommel, B., & Colzato, L.S. (2014). Focused attention, open monitoring and loving kindness meditation: Effects on attention, conflict monitoring, and creativity – A review. Frontiers in Psychology, 5, 1083. doi: 10.3389/fpsyg.2014.01083
- Lupien, S.J., & McEwen, B.S. (1997). The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research Reviews, 24, 1–27. doi: 10.1016/S0165-0173(97)00004-0
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. doi: 10.1038/nrn2639
- Matthews, G., Lin, J., & Wohleber, R. (2017). Personality, stress and resilience a multifactorial cognitive science perspective. Psychological Topics, 26, 139–162.
- McNair, D.M., Lorr, M., & Droppleman, L.F. (1971). Manual for the profile of mood states. San Diego: Educational and Industrial Testing Services.
- Mendes, W.B. (2009). Assessing autonomic nervous system activity. In E. Harmon-Jones & J. S. Beer (Eds.), Methods in social neuroscience (pp. 118–147). New York, NY: Guilford Press.
- Obrist, P.A. (1981). Cardiovascular psychophysiology: A perspective. Boston, MA: Springer. doi: 10.1007/978-1-4684-8491-5
- Pascoe, M.C., Thompson, D.R., Jenkins, Z.M., & Ski, C.F. (2017). Mindfulness mediates the physiological markers of stress: Systematic review and meta-analysis. Journal of Psychiatric Research, 95, 156–178. doi: 10.1016/j.jpsychires.2017.08.004
- Pedrabissi, L., & Santinello, M. (Eds.). (1989). State-trait anxiety inventory – forma Y. Firenze, Italy: Giunti OS.
- Rossi, V., & Pourtois, G. (2012). Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: A comparative review. Anxiety, Stress and Coping, 25, 603–645. doi: 10.1080/10615806.2011.582948
- Saleh, D., Camart, N., & Romo, L. (2017). Predictors of stress in college students. Frontiers in Psychology, 8, 19 doi: 10.3389/fpsyg.2017.00019
- Selye, H. (1974). Stress without distress. New York, NY: Harper & Row.
- Sliwinski, J., Katsikitis, M., & Jones, C.M. (2017). A review of interactive technologies as support tools for the cultivation of mindfulness. Mindfulness, 8, 1150–1159. doi: 10.1007/s12671-017-0698-x
- Subhani, A.R., Kamel, N., Mohamad Saad, M.N., Nandagopal, N., Kang, K., & Malik, A.S. (2018). Mitigation of stress: New treatment alternatives. Cognitive Neurodynamics, 12, 1–20. doi: 10.1007/s11571-017-9460-2
- Tang, Y.Y., Ma, Y., Wang, J., Fan, Y., Feng, S., Lu, Q., … Posner, M.I. (2007). Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences, 104, 17152–17156. doi: 10.1073/pnas.0707678104
- Tang, Y.Y., Tang, R., Jiang, C., & Posner, M.I. (2014). Short-term meditation intervention improves self-regulation and academic performance. Journal of Child and Adolescent Behaviour, 2, 154. doi: 10.4172/2375-4494.1000154
- Thayer, J.F., Åhs, F., Fredrikson, M., Sollers, J.J., & Wager, T.D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009
- Thayer, J.F., Hansen, A.L., Saus-Rose, E., & Johnsen, B.H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37, 141–153. doi: 10.1007/s12160-009-9101-z
- Vago, D.R., & Silbersweig, D.A. (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience, 6, 296. doi: 10.3389/fnhum.2012.00296
- van Eck, M.M., & Nicolson, N.A. (1994). Perceived stress and salivary cortisol in daily life. Annals of Behavioral Medicine, 16, 221–227. doi: 10.1093/abm/16.3.221
- Wheeler, M.S., Arnkoff, D.B., & Glass, C.R. (2017). The neuroscience of mindfulness: How mindfulness alters the brain and facilitates emotion regulation. Mindfulness, 8, 1471–1487. doi: 10.1007/s12671-017-0742-x
- Zeidan, F., Johnson, S.K., Diamond, B.J., David, Z., & Goolkasian, P. (2010). Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and Cognition, 19, 597–605. doi: 10.1016/j.concog.2010.03.014
