Abstract
Chronic, ongoing stress can impact negatively on health and wellbeing. Indigenous Australians are at an increased risk of experiencing multiple stressors. Hair glucocorticoids have been used as a marker for chronic stress. This study aimed to assess the associations of hair cortisol and cortisone with sociodemographic (age, gender, Indigenous Identification), substance use, emotional wellbeing, and emotional stress, in a cohort at increased risk of stressful events and psychological distress. Cross-sectional data (age 21–28 years) are presented from two Australian longitudinal studies; the Aboriginal Birth Cohort (n = 253) and non-Indigenous Top End Cohort (n = 72). A third of the cohort reported psychological distress, with Indigenous participants reporting higher rates of stressful events compared to non-Indigenous (6 vs. 1; p < .001). Significantly higher levels of cortisone were seen in Indigenous women compared to non-Indigenous women (β 0.21; p = .003). A positive association with age was present in hair cortisol and cortisone in Indigenous young adults (β 0.29 and β 0.41; p < .001, respectively). No association with substance use, emotional wellbeing or emotional stress was seen. Sub-analysis in women suggested a possible curvilinear relationship between hair cortisone and the number of stressful events. In this culturally diverse cohort, hair sampling provides a noninvasive, easily conducted and generally well tolerated mechanism to measure stress markers. The association with age, even in this narrow age range, likely represents the manifold changes in circumstances (financial independence, becoming parents, increased risk of substance use and mental illness) that occur during this transitional period of life, particularly for young Indigenous women.
LAY ABSTRACT
Chronic stress can impact negatively on health and emotional wellbeing. A hair sample is an easy way to measure chronic stress in Indigenous and non-Indigenous young people. The markers of chronic stress, cortisol and cortisone, were different between Indigenous and non-Indigenous, men and women and increased with age in Indigenous young adults.
Introduction
Cumulative exposure to stress over a long period has been shown to impact negatively on an individual’s health and wellbeing. Indigenous Australians are at increased risk of mental health disorders, including psychological distress (Australian Bureau of Statistics, Commonwealth of Australia, Citation2013) and suicide (Australian Bureau of Statistics, Commonwealth of Australia, Citation2016), compared to non-Indigenous Australians. Indigenous Australians often live in a high stress environment, stemming from colonization, socioeconomic and political marginalization, and racial discrimination (Gracey & King, Citation2009; Kelly, Dudgeon, Gee, & Glaskin, Citation2009). They have lower socio-economic status, high rates of unemployment, low education level, and experience a higher number of stressful events, such as death of a family member, alcohol/drug problems or abuse/violent crime (Blair, Zubrick, & Cox, Citation2005; Davison, Nagel, & Singh, Citation2017). Several studies have shown a link between chronic stress and mental health, including depression and anxiety (Michl, McLaughlin, Shepherd, & Nolen-Hoeksema, Citation2013). The occurrence of multiple stressful events has been associated with an increased risk of psychological distress and lower positive wellbeing in Indigenous Australians (Blair et al., Citation2005; Davison et al., Citation2017).
Exposure to stress triggers the activation of the hypothalamic-pituitary-adrenal (HPA) axis, inducing the release of glucocorticoids from the adrenal cortex. Cortisol and cortisone are downstream factor resulting from the activation of the HPA, with cortisol converted into inactive cortisone (Staufenbiel, Penninx, de Rijke, van den Akker, & van Rossum, Citation2015). Cortisol is converted into inactive cortisone by the enzyme 11-beta-hydroxysteroid dehydrogenase (11β-HSD) type-2, with cortisone converted back to active cortisol by the enzyme 11β-HSD type-1. These enzymes of the cortisol-cortisone interconversion have been shown to be influenced by body size, particularly obesity (Valsamakis et al., Citation2004). Whereas transient increases in these glucocorticoids can be protective, excessively high levels can be harmful and increase the risk of physical and mental health issues (Rabkin & Struening, Citation1976). Ongoing exposure to a high stress enviroment, often beginning early in life and occuring across the life course, can result in the stress system physiology being attenuated, resulting in a blunting of the HPA axis and low level of glucocorticoids (Susman, Citation2006). Glucocorticoids (cortisol and cortisone) levels that are at either end of the spectrum, both high and low, may set the stage for the pathogenic process that predisposes individuals to illness (Miller, Chen, & Zhou, Citation2007).
In the past decade, hair cortisol concentration has been proposed as a measure of long-term accumulation of cortisol (Stalder et al., Citation2017). However, the source of the cortisol remains a controversial topic. Although theorized that cortisol found in hair is accumulated in a steady state manner, the exact mechanism remains unclear. Studies have shown an increase in cortisol in response to local stressors suggesting it is also produced by local hair follicles and may be independent of central cortisol (Sharpley, Kauter, & McFarlane, Citation2009). Cortisone concentration in hair has been less studied; however, when used in conjunction with cortisol it can provide comprehensive information on the cumulative amount of active (cortisol) and inactive (cortisone) glucocorticoids in the body (Staufenbiel et al., Citation2015; Zhang, Chen, Chen, Xu, & Deng, Citation2017). Considerable evidence supports the robustness of glucocorticoids concentrations to a range of hair-related external factors such as dyeing, washing and other treatments (Dettenborn, Tietze, Kirschbaum, & Stalder, Citation2012; Kristensen, Larsen, Olsen, Fahrenkrug, & Heitmann, Citation2017).
Research into the relationship between stressful events and hair glucocorticoids has reported mixed results. Several studies have reported positive associations between cortisol and exposure to stressful events in pregnant women (Schreier, Enlow, Ritz, Gennings, & Wright, Citation2015), children (Simmons et al., Citation2016), adults (Kalra, Einarson, Karaskov, Van Uum, & Koren, Citation2007), and young adults (Karlén, Ludvigsson, Frostell, Theodorsson, & Faresjö, Citation2011), while others have not (Dowlati et al., Citation2010; Fischer et al., Citation2017; Gidlow et al., Citation2016). There is a paucity of information on hair cortisone and stressful events; however, in young girls a weak positive association between cortisone and stressful events was observed (Vanaelst et al., Citation2013). This study will provide valuable information on hair cortisol and cortisone levels in a culturally diverse cohort of young adults at an increased risk of psychological distress and multiple stressful events.
Young adulthood can be a vulnerable period with Indigenous young women at an increased risk of psychological distress and increasing rates of suicide seen in Indigenous young men (Australian Bureau of Statistics, Commonwealth of Australia, Citation2013; Leckning, Li, & Cunningham, Citation2016). The 20 s are a period of substantial change and increased stress, as people enter into financial independence, become parents and increased social responsibilities. This increase in stress is particularly pertinent to young Indigenous adults, who are exposed to high rates of unemployment, social change, and cultural conflict (Kelly et al., Citation2009). Indigenous women become mothers at an early age, with traditional parental responsibilities differing between men and women across cultures (Johnstone, Citation2010). The impact these changes in life circumstances have on stress levels in this vulnerable age group, is not fully understood.
Currently there is a paucity of information available on hair cortisol and cortisone in the Australian context, particularly in Indigenous Australians. High urinary cortisol levels have been reported in remote residing Indigenous adults undergoing rapid sociocultural change (Schmitt, Harrison, & Spargo, Citation1998). More recently a blunted cortisol awakening response was seen in Indigenous students associated with increased chronic stress (Berger, Leicht, & Slatcher, Citation2017). To our knowledge there is no published data examining hair glucocorticoids in Indigenous Australians.
In this culturally diverse cohort a comprehensive profile of glucocorticoid concentration including cortisol (active), cortisone (inactive), and total glucocorticoid (cortisol + cortisone) was obtained at an age of increased stress, high rates of psychological distress, and multiple stressful events. The aims of the study were twofold; (i) to assess the cultural acceptability of hair sampling as an objective and noninvasive way of assessing chronic stress level in Indigenous and non-Indigenous young adults; (ii) to investigate whether different health indicators (substance use, emotional wellbeing, and emotional stress) and stress levels measured by hair cortisol and cortisone concentrations were different among comparable young Indigenous and non-Indigenous adults; considering the age of the population, potential gender differences and the increase exposure to multiple stressful events present in Indigenous Australians.
Methods
Recruitment and retention
The Life Course Program is a prospective longitudinal study encompassing two distinct but complementary cohorts; the Aboriginal Birth Cohort (ABC) and the Top End Cohort (TEC).
The recruitment and previous follow-ups of the ABC (Sayers et al., Citation2009) and TEC (Davison, Cunningham, & Singh, Citation2011) studies have been described elsewhere. In brief, between 1987 and 1990, 686 (54% of those eligible) babies born to Indigenous mothers were recruited from the Royal Darwin Hospital, the main referral hospital for the Northern Territory (NT), to the ABC study (Sayers et al., Citation2009). Subsequent follow-ups have occurred at the participant’s residence in over 40 urban and remote communities across the top end of the NT at a mean age of 11 years (86% examined) (Sayers, Mackerras, Halpin, & Singh, Citation2007) and 18 years (71% examined) (Sayers et al., Citation2009). Between 2007 and 2009, 196 non-Indigenous people born in Darwin between 1987 and 1991 were recruited to the TEC study. TEC participants were age matched to participants of the ABC study (Davison et al., Citation2011). Participants of both studies were examined between August 2013 and June 2015 (age 21–28 years) in their community of residence.
Cultural considerations
During the consultation phase concerns were raised by the male Indigenous researcher, members of the Indigenous Reference Group and Expert Reference Group on the cultural acceptance of obtaining hair samples. In some Indigenous cultures/areas, hair has cultural importance with specific traditions to be followed; such as returning any cut hair to a senior member of the family (i.e. grandmother) for protection. Further consultation with key Indigenous stakeholders, both in urban and remote communities, resulted in the conclusion that individual participants should be provided with the opportunity to choose if they wished to provide a hair sample.
Ethics approval
This study was approved by the Human Research Ethics Committee (HREC) of NT Department of Health and Menzies School of Health Research, including the Aboriginal Ethical Sub-committee which has the power of veto (ABC Reference no. 2013-2022 and TEC Reference no. 2013-1986). All participants provided written informed consent.
Hair sample
A lock of hair from the back of the head (posterior vertex position) was cut with fine scissors as close as possible to the scalp. Inclusion criteria include; willingness to provide sample, sufficient hair growth in posterior vertex and sample weight of at least 3 mg and ≥1 cm in length. Hair samples were stored in paper envelope at cooled room temperature until processed (approximately 40 months).
Sample analysis
Hair samples were prepared as previously described (Sharpley et al., Citation2009) with some minor modification of increased extraction preparation for LC/MS analysis. Briefly hair samples were weighted, finely cut with surgical scissors, and placed into glass vials and then extracted for 24 h with 1.5 mL methanol on a platform shaker. The methanol was decanted into 1.5 mL microfuge tubes and the methanol evaporated under vacuum. The samples were extracted twice more and the methanol decanted into the same microfuge tube. The samples were dissolved in 50 µL of 50% methanol:water and then centrifuged before transfer into autosampler vials.
Samples were analyzed in duplicate by LC/MS/MS using the method of Ionita, Fast, and Akhlaghi (Citation2009) with minor modifications as follows. The samples were separated using a Kintetex 2.6 u Evo C18 column (2.1 × 50 mm 2 µm particle size) (Phenomenex). Samples were eluted with a gradient from 10% to 100% methanol with 0.2 mM ammonium fluoride over 6 minutes. Cortisone and cortisol eluted with a retention time of 2.1 and 2.5 min, respectively, and quantitative analysis was performed in multiple reaction monitoring mode (MRM) of the two most abundant product ions (m/z 361 > 163.1 & 121.1 and 363.2 > 121.2 & 309.3). The inter-assay and intra-assay coefficients of variation were determined to be 4.9% and 2.7%, respectively. The results were expressed as pg/mg.
Sociodemographic, body size, and substance use
Sociodemographic factors obtained included gender, age, marital status (married/defacto relationship or single), children, employment (in regular employment or not), and ancestry (Indigenous or non-Indigenous). Body mass index (BMI) was calculated from directly collected measurements (height/weight2 (cm/kg2)). Participants were measured in light clothing and barefoot; height was measured to the nearest millimeter using a calibrated, portable wall mounted stadiometer; and weight was measured to the last complete 0.1 kg with a digital electronic scale (TBF-521, Tanita Corporation, IL). Substance use included alcohol consumption (occurrence and frequency of alcohol consumption), tobacco smoking behavior (current smoker or non-smoker), and marijuana use (current user or non-user).
Emotional wellbeing
Psychological distress in the past 4 weeks was assessed by the Kessler-5, which has been used with Indigenous and non-Indigenous Australians in state-wide and national surveys (Australian Bureau of Statistics, Commonwealth of Australia, Citation2013; McNamara et al., Citation2014). Participants were asked how often they felt: nervous; without hope; restless or jumpy; everything was an effort; and so sad that nothing could cheer them up.
Positive wellbeing in the past 4 weeks was assessed by the Short Warwick-Edinburgh Mental Well-being Scale (Bartram, Sinclair, & Baldwin, Citation2013). Although there is a scarcity of research available from Australia, this scale has been used widely in the United Kingdom and Scotland in teenagers and young adults (Clarke, Friede, & Putz, Citation2011). Participants were asked how often they felt: happy about the future; useful; relaxed; dealt with problems well; thought clearly; close to other people; and been able to make up their own mind.
Emotional stress
Subjective stress in the past 4 weeks was assessed by the Short Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, Citation1983), which has been previously used in Australian Indigenous and non-Indigenous adults (Wiggers et al., Citation2001). Participants were asked how often they were: able to control the important things in their life; handle personal problems; that things were going their way; and that difficulties were piling up so high that they could not overcome them.
The occurrence of stressful events (yes/no) in the past 6 months were adapted from the Negative Life Events Scale (Kowal, Gunthorpe, & Bailie, Citation2007) and included: a close family member has been in an accident; has been in hospital; has been arrested; is in prison; has an alcohol problem; has a drug problem; needs their care most days; has passed away; they didn’t have enough money to buy food or pay bills; them or someone in the house gambles a lot and it gives them money problems; they felt their house doesn’t have enough space for all the people who live there; they were scared by other peoples’ behavior; and physically hurt by someone.
Statistical analysis
All statistical analysis was performed in STATA 15.1. Exploratory analysis was performed to determine whether data were normally distributed and potential outliers. Hair cortisol and cortisone data were log transformed to correct skewness. For descriptive purposes, medians and interquartile range (IQR) in original units (pg/mg) are presented. To further investigate the relevance of hair cortisol and cortisone, total glucocorticoid was calculated as cortisol + cortisone. Categorical demographic data were examined by Indigenous status and gender through Pearson Chi-square tests, with continuous variables through Wilcoxon rank-sum. The linear relationship between hair cortisol and cortisone was assessed by Pearson’s correlation.
Associations with hair cortisol, cortisone, and total glucocorticoid was assessed through linear regression with adjustment for a range of confounding factors such as gender, Indigenous identification, age, and BMI. The explanatory variables, Indigenous identification, gender, and sociodemographic factors, were treated as categorical variables. All other explanatory variables, age, positive wellbeing, perceived stress, stressful events, were treated as continuous. Non-linear relationships were assessed by multiple regression models that included linear and quadratic components. The linearity and multicollinearity of predictor variables were tested. The significance level was set at p ≤ .05.
Results
Cohort characteristics
Of the 576 young adults available for assessment, 493 (86%) consented to providing a hair sample, with no difference seen between Indigenous and non-Indigenous young adults (n = 387; 84% and n = 106; 91% respectively; p = .08). Of the 462 who provided a sample (94% of consented), insufficient hair (<3 mg) was obtained from 45 participants, with a further 4 hair samples excluded as they were >4 standard deviations above the mean weight. As pregnancy can often be a time of increased emotional and physical stress, pregnant women were excluded from this analysis (n = 21). Complete data on emotional variables and hair samples were available on 344 (70%) participants. Sub-analysis was conducted on the 297 of these participants who also provided information on stressful events.
Participants were aged 21–28 years, with a higher proportion of Indigenous (n = 268) compared to non-Indigenous (n = 76), reflective of the available cohort. Women represented a greater portion in both Indigenous (53 vs. 47%) and non-Indigenous (71 vs. 29%) participants. Significant differences in sociodemographic and substance use were seen between Indigenous and non-Indigenous participants. The majority of Indigenous participants were either married or in a defacto relationship and had one or more children. They had lower rates of employment and were significantly more likely to smoke tobacco and use marijuana but less likely to regularly consume alcohol. Indigenous women were more likely to have children, less likely to be employed and less likely to regularly consume alcohol than their male Indigenous counterparts. Indigenous women also had higher psychological distress levels, lower positive wellbeing and higher perceived stress levels than Indigenous men. No sociodemographic differences were seen between women and men in non-Indigenous participants. However, non-Indigenous women were less likely to smoke than their male counterparts. Full details are in .
Table 1. Demographics and hair glucocorticoid levels for Indigenous and non-Indigenous Young adults.
Hair cortisol and cortisone concentrations
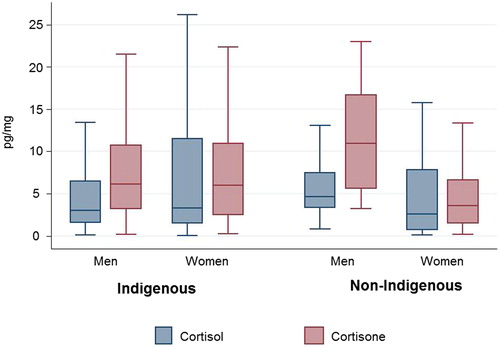
The median hair cortisol of this study was 3.35 (1.45, 7.96) pg/mg, whereas the median cortisone was 5.54 (2.73, 10.56) pg/mg. Log transformed cortisol and cortisone levels showed a weak but significant correlation with each other (r 0.37, p < .001). shows median cortisol and cortisone levels for Indigenous and non-Indigenous, men and women.
Associations with hair concentrations
shows results of the linear and multivariable regression. Initial analysis showed no difference in hair cortisol, cortisone or total glucocorticoid levels between Indigenous and non-Indigenous participants (p = .87, .06, and .15, respectively). On stratifying by gender, this remained for cortisol (see ). However, in women significantly higher levels of cortisone were seen in Indigenous women compared to non-Indigenous women (β 0.21; p = .003). With lower levels of cortisone in Indigenous men compared to their non-Indigenous counterparts (β − 0.17; p = .037). Total glucocorticoid displayed similar results to cortisone with higher levels in Indigenous women compared to non-Indigenous women (β 0.19; p = .009) and lower levels in Indigenous men compared non-Indigenous men (β − 0.16; p = .056).
Table 2. Regression analyses results for hair cortisol, cortisone, and total glucocorticoid.
No difference in cortisol and total glucocorticoid was evident between genders. However, significantly higher cortisone levels were evident in men compared to women, remaining on adjusting for Indigenous identification, age and BMI. On stratification, no association with sex was evident in Indigenous young adults; however, non-Indigenous men had significantly higher levels compared to their female counterparts (see ).
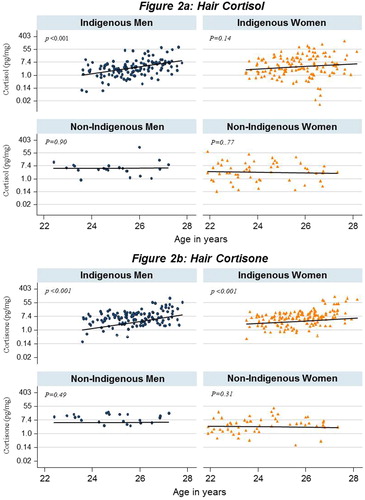
In this relatively small age range, a significant increase in hair cortisol, cortisone, and total glucocorticoid levels was evident with age both in linear and multivariable regression (see ). However on stratification, this significance was only seen in Indigenous participants. The significant increase with age was present for men in both cortisol and cortisone (β 0.44; p < .001 and β 0.37; p < .001, respectively), and women for cortisone (β 0.44; p < .001). depicts the increase levels of cortisol and cortisone seen in Indigenous men and women with increasing age, providing comparison with their non-Indigenous counterparts.
Figure 2. Association of hair cortisol and cortisone concentration with age by Indigenous identification and gender.
Cortisol, cortisone and total glucocorticoid showed no association with emotional status or perceived stress level in linear and multivariable regression (see ). Similarly, no association was evident with tobacco smoking, alcohol or marijuana use.
Hair glucocorticoids and stressful events
Rates of individual stressful events were higher in Indigenous participant, with 30–70% of young adults reporting the occurrence of each event compared to ≤15% of non-Indigenous for the majority. Rates for each stressful events for Indigenous versus non-Indigenous participants were as follows: Family member in an accident (43% vs. 15%); Family member in hospital (58% vs. 38%); Family member arrested (45% vs. 3%); Family member in prison (50% vs. 1%); Family member has alcohol problem (51% vs. 18%); Family member has drug problem; (45% vs. 11%); Family member needs their care most days (48% vs. 6%); Family member has passed away (67% vs. 14%); No money to buy food or pay bills (41% vs. 6%); Them or family member has a gambling problem (31% vs. 1%); House is overcrowded (36% vs. 8%); They were scared by other peoples’ behavior; (31% vs. 4%); They were physically hurt by someone (17% vs. 0%).
Initial analysis showed no association between the accumulative number of stressful events reported and hair cortisol, cortisone or total glucocorticoids supply (β −0.06; p .28, β 0.01; p .86 and β −0.01; p .90, respectively). However, examination of individual events showed significant association with hair cortisone in women. After adjustment for all other individual events, women who reported being scared by someone had significantly higher hair cortisone (β 0.20; p .044). This significance remained after adjustment for age and Indigenous identification (β 0.19; p .044).
Possible curvilinear associations in women
In this cohort who reported high levels of multiple stressful events we further explored the nature of the relationships between stressful events and cortisol, cortisone and total glucocorticoid. The curvilinear associations were tested in a series of three regression models for each of the three biomarkers (see ). The first model includes only the linear component, the second model includes the quadratic component to test whether there is a significant non-linear relation, and the third model being the quadratic component adjusted for the covariates age, gender and Indigenous identification.
Table 3. Quadratic regression analyses on stressful events with hair cortisol, cortisone, and total glucocorticoid by gender.
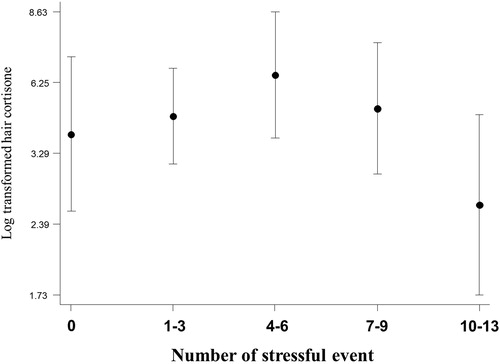
As can be seen in , analyses for stressful events showed no significant linear association with any of the three glucocorticoids in model 1 (linear regression), but a significant negative quadratic association in model 2 for cortisone was seen in women only, indicating a possible inverse U relationship. The significance seen in the quadratic component negated when the covariates were included in the third model.
To further investigate the possible curvilinear association noted above in women, log transformed cortisone was plotted by number of stressful events. The number of stressful events were first broadly categorized as; 0, 1–3, 4–6, 7–9, and 10–13. suggest an inverse U relationship between cortisone and an increasing number of events in women. This curve was also present in women for total glucocorticoid supply but did not reach significance.
Of those women who reported ≥7 stressful events, the majority were Indigenous (48 vs. 1). In these women multivariable regression, adjusting for sociodemographic (age, relationship, children, and employment), psychological distress, positive wellbeing, and perceived stress, showed a significant positive association between cortisone and lower levels of positive wellbeing (β 0.36 p .021) with a negative trend seen with higher rates of psychological distress (β − 0.31 p .066). No associations with hair cortisol levels were evident.
Discussion
In this study, we were able to assess the associations of glucocorticoids in a culturally diverse cohort at increased risk of stressful events and psychological distress. The high consent rates seen in both Indigenous (84%) and non-Indigenous (91%) young adults, highlights the cultural acceptance of hair sampling in this cohort. Cortisone was present at a higher level than cortisol, comparable to that reported by other studies (Raul, Cirimele, Ludes, & Kintz, Citation2004; Staufenbiel et al., Citation2015). When added together to provide an indicator of a general glucocorticoid level (cortisol as an active cortisol and cortisone as an inactivated cortisol) comparable associations were present to those seen with the individual biomarkers.
Despite no initial difference being evident between Indigenous and non-Indigenous young adult, differences were seen between genders. In women, Indigenous women had higher cortisone levels than their non-Indigenous counterparts, while in men the opposite was seen (lower levels in Indigenous compared to non-Indigenous men). Higher cortisone levels were seen in non-Indigenous men compared to non-Indigenous women; however, no difference was evident between Indigenous men and women. This variance in results is consistent with previous hair and salivary cortisol research, which has produced inconsistent results regarding sex differences (Dettenborn et al., Citation2012; Raul et al., Citation2004). The level of stress exposure experienced by Indigenous and non-Indigenous young adults may be influenced by their family, community and cultural role which varies across age groups and genders (Dudgeon, Wright, Paradies, Garvey, & Walker, Citation2014). The variance seen across groups in this cohort supports the need for future studies to consider participant sex and ancestry as possible confounders (Dettenborn et al., Citation2012).
In this the relative small age range (21–28), age was significantly associated with cortisol and cortisone individually and in accumulation in Indigenous men and women in this cohort. The 20 s are often a period of substantial change, as people enter into financial independence, commence employment and become parents. Young Indigenous adults are also exposed to high rates of unemployment, social change and cultural conflict (Hunter & Milroy, Citation2006). Rates of injury, mental illness and substance use peaks in this age group and are higher in Indigenous compared to non-Indigenous young adults (Australian Bureau of Statistics, Commonwealth of Australia, Citation2015; Park, Mulye, Adams, Brindis, & Irwin, Citation2006). Indigenous women become mothers at an early age (mean age 19.2 ± 2.7 years for Indigenous women vs. 23.3 ± 2.6 years for non-Indigenous women in this cohort) and this is particularly evident in in remote parts of the NT (Johnstone, Citation2010). The increase in stress biomarkers seen with increasing age in Indigenous young adults may reflect the degree of influence these changes in life circumstances have on stress levels in this vulnerable age group.
Women in our study had increased rates of psychological distress, lower positive wellbeing, and slightly higher perceived stress. Indigenous women also reported high rates of stressful events, low levels of employment and education attainment. The increase in all hair glucocorticoid markers in women who report being scared by someone may reflect the negative impact this can have on health and wellbeing, with the association strongest in Indigenous women who had children. Indigenous women, particularly those of child-bearing age, are at a greater risk of family violence, with injury and homicide rates much higher than non-Indigenous women (Gracey & King, Citation2009). The increase in glucocorticoid markers may reflect the increase level of stress experienced by these women and the impact caring for and protecting their children has on their stress level.
Prolonged or a high stress environment has been associated with a decreased cortisol secretion, or hypocortisolism (Faresjo et al., Citation2013; Susman, Citation2006). Similar to other Australian Indigenous cohorts (Blair et al., Citation2005), significantly higher numbers of stressful events were reported in Indigenous participants compared to non-Indigenous (6 vs. 1) (Davison et al., Citation2017). Additional analysis in women suggested a possible curvilinear relationship between hair cortisone and the number of stressful events experienced; hair cortisone increased in line with the number of stressful events but then decreased in those experiencing ≥7 events. In response to acute stress the HPA axis normal response is one of hyperactivity and increased glucocorticoid release. However, if the stress exposure is ongoing, or over a long period, and the person is unable to cope with this exposure, a state of exhaustion is reached and the system turns to hypoactive functioning.
Early research measuring cortisol levels indicated that chronic stress (e.g. being a soldier in combat or having a child with cancer) was associated with reduced daily output of cortisol (Bourne, Rose, & Mason, Citation1968; Wolff, Friedman, Hofer, & Mason, Citation1964). Yehuda et al. (Citation1995) examined the influence of chronic stress across generations, showing a decrease in urine cortisol levels in Holocaust survivors and their children in adulthood (Yehuda et al., Citation1995, Citation2000). More recently in hair glucocorticoids lower levels were reported in young Greek adults living in an environment with increased level of economic and social pressure (Faresjo et al., Citation2013). Recent research has shown both high and low levels of cortisol are associated with chronic stress (Miller et al., Citation2007). Chronic exposure to a high stress environment combined with a decrease in glucocorticoid levels may be contributing to the higher rates of morbidity and mortality present in Indigenous Australians. On the other hand, though less likely, the lower level in those experiencing a high number of stressful events may represent an adaptive response. In this study, we do not have sufficient resilience/self-efficacy or quality of life measures to fully explore this theory.
The lack of significant relationships between glucocorticoids measured in hair and self-reported stress and emotional wellbeing questionnaires is consistent with other studies that reported null findings (Dettenborn, Tietze, Bruckner, & Kirschbaum, Citation2010; Dowlati et al., Citation2010). While this may indicate that there is no relationship, methodological explanations also need to be considered. One consideration is the differences in time frames covered. Hair samples >1 cm long are thought to be reflective of ≥1 months whereas the scales used enquired about the past month. Further research into the mechanism of glucocorticoid incorporation in hair is also warranted in order to fully understand the time frame represented in hair analysis.
Several limitations need to be acknowledged. This was a convenience sample taken as part of a large physical and emotional assessment study, with no information obtained on hair details and treatments. However, research has shown that these variables have little impact on the level of cortisol available (Dettenborn et al., Citation2012; Kristensen et al., Citation2017). We did not divide the segment into fragments or gain exact measurements, which would have allowed us to examine the influence length had on concentration levels, but this has not been consistently proven to be a consideration (Sharpley, Kauter, & McFarlane, Citation2010).
As mentioned above limited information was obtained on resilience/self-efficacy or quality of life it is therefore not possible to develop a complete picture of the impact chronic stress has on emotional wellbeing. However, a major strength of this study is that it is part of an ongoing longitudinal study, of which a further follow-up is currently in planning. Information obtained from this experience will allow for refining of procedure and additional information to be obtained on hair characteristics and emotional status (resilience/self-efficacy and quality of life). It will also then have the capacity to provide valuable information on the longitudinal nature of hair glucocorticoids in this population.
In this study, we were able to assess the associations of glucocorticoids (hair cortisol and cortisone) in a cohort at increased risk of stressful events and psychological distress. Hair sampling provided a noninvasive, easily conducted and generally well tolerated mechanism to measure stress markers. The positive association of glucocorticoids with age seen in Indigenous participants may be indicative of the influence stressors such as stressful events, unemployment, cultural conflict, and parental obligations, has on health and wellbeing in young adulthood. This association with age, even in this narrow age range, likely represents the manifold changes in circumstances that occur during this transitional period of life, particularly for young Indigenous women.
Disclosure of interest
No potential conflicts of interest was reported by the author(s).
Acknowledgements
We wish to acknowledge past and present study team members, in particular the late Susan Sayers (AO), founder of the ABC study. We would like to thank Victor Oguoma for providing statistical advice. We especially thank the young adults belonging to the Aboriginal Birth Cohort and Top End Cohort and their families and communities for their co-operation and support and all the individuals who helped in the urban and rural locations.
References
- Australian Bureau of Statistics, Commonwealth of Australia. (2013). Australian aboriginal and Torres Strait Islander health survey: First results, Australia, 2012-13 (Catalog No. 4727.0.55.001). Retrieved from http://www.abs.gov.au/ausstats/[email protected]/Lookup/9F3C9BDE98B3C5F1CA257C2F00145721?opendocument
- Australian Bureau of Statistics, Commonwealth of Australia. (2015). Commonwealth of Australia Australian health survey: First results, 2011-12 (Catalog No. 4364.0.55.001). Retrieved from http://www.abs.gov.au/ausstats/[email protected]/Lookup/26DB864DADDA4011CA257AA30014BAA7?opendocument
- Australian Bureau of Statistics, Commonwealth of Australia. (2016). Causes of Death, Australia, 2016 (Catalog No. 3303.0). Retrieved from http://www.abs.gov.au/ausstats/[email protected]/Lookup/by%20Subject/3303.0∼2016∼Main%20Features∼Intentional%20self-harm%20in%20Aboriginal%20and%20Torres%20Strait%20Islander%20people∼8
- Bartram, D., Sinclair, J., & Baldwin, D. (2013). Further validation of the Warwick-Edinburgh Mental Well-being Scale (WEMWBS) in the UK veterinary profession: Rasch analysis. Quality of Life Research, 22, 379–391. doi:10.1007/s11136-012-0144-4
- Berger, M., Leicht, A., & Slatcher, A. (2017). Cortisol awakening response and acute stress reactivity in first nations people. Scientific Reports, 7, 41760. doi:10.1038/srep41760
- Blair, E., Zubrick, S., & Cox, A. (2005). The Western Australian Aboriginal Child Health Survey: Findings to date on adolescents. The Medical Journal of Australia, 183, 433–435. Available at https://www-mja-com-au.ezproxy.cdu.edu.au/system/files/issues/183_08_171005/bla10515_fm.pdf
- Bourne, P.G., Rose, R.M., & Mason, J.W. (1968). 17-OHCS Levels in combat. Special forces “A” team under threat of attack. Archives of General Psychiatry, 19, 135–140. doi:10.1001/archpsyc.1968.01740080007002
- Clarke, A., Friede, T., & Putz, R. (2011). Warwick-Edinburgh Mental Well-being Scale (WEMWBS): Validated for teenage school students in England and Scotland. A mixed methods assessment. BMC Public Health, 11, 487. doi:10.1186/1471-2458-11-487
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. doi:10.2307/2136404
- Davison, B., Cunningham, T., & Singh, G. (2011). Engaging adolescents and young adults in a longitudinal health study: Experience from the Top End cohort. Australian and New Zealand Journal of Public Health, 35, 86–87. doi:10.1111/j.1753-6405.2010.00666.x
- Davison, B., Nagel, T., & Singh, G. (2017). Life, lifestyle and location: Examining the complexities of psychological distress in young adult Indigenous and non-Indigenous Australians. Journal of Developmental Origins of Health and Disease, 8(5), 541–549. doi:10.1017/S2040174417000162
- Dettenborn, L., Tietze, A., Bruckner, F., & Kirschbaum, C. (2010). Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology, 35, 1404–1409. doi:10.1016/j.psyneuen.2010.04.006
- Dettenborn, L., Tietze, A., Kirschbaum, C., & Stalder, T. (2012). The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress, 15, 578–588. doi:10.3109/10253890.2012.654479
- Dowlati, Y., Herrmann, N., Swardfager, W., Thomson, S., Oh, P., Van Uum, S., …., Lanctot, K. (2010). Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatric Disease and Treatment, 6, 393–400. doi:10.2147/NDT.S10353
- Dudgeon, W., Wright, M., Paradies, Y., Garvey, D., & Walker, I. (2014). Aboriginal social, cultural and historical contexts. In P. Dudgeon, H. Milroy, & R. Walke (Eds.), Working together: Aboriginal and Torres Strait Islander mental health and wellbeing principles and practice (pp. 3–24). Canberra: Commonwealth Department of Health.
- Faresjo, A., Theodorsson, E., Chatziarzenis, M., Sapouna, V., Claesson, H.P., Koppner, J., & Faresjo, T. (2013). Higher perceived stress but lower cortisol levels found among young Greek adults living in a stressful social environment in comparison with Swedish young adults. PLoS One, 8, e73828. doi:10.1371/journal.pone.0073828
- Fischer, S., Duncko, R., Hatch, S. L., Papadopoulos, A., Goodwin, L., Frissa, S., … & Cleare, A. J. (2017). Sociodemographic, lifestyle, and psychosocial determinants of hair cortisol in a South London community sample. Psychoneuroendocrinology, 76, 144–153. doi: 10.1111/acps.12970
- Gidlow, C. J., Randall, J., Gillman, J., Silk, S., & Jones, M. V. (2016). Hair cortisol and self-reported stress in healthy, working adults. Psychoneuroendocrinology, 63, 163–169. doi: 10.1016/j.psyneuen.2015.09.0220306-4530d
- Gracey, M., & King, M. (2009). Indigenous health. Part 1: Determinants and disease patterns. Lancet (London, England), 374, 65–75. doi:10.1016/S0140-6736(09)60914-4
- Hunter, E., & Milroy, H. (2006). Aboriginal and Torres Strait Islander suicide in context. Archives of Suicide Research, 10, 141–157. doi:10.1080/13811110600556889
- Ionita, I.A., Fast, D.M., & Akhlaghi, F. (2009). Development of a sensitive and selective method for the quantitative analysis of cortisol, cortisone, prednisolone and prednisone in human plasma. Journal of Chromatography B, 877, 765–772. doi:10.1016/j.jchromb.2009.02.019
- Johnstone, K. (2010). Indigenous fertility in the Northern Territory of Australia: What do we know? (and what can we know?). Journal of Population Research, 27, 169–192. doi:10.1007/s12546-011-9048-3
- Kelly, K., Dudgeon, P., Gee, G., & Glaskin, B. (2009). Living on the edge: Social and emotional wellbeing and risk and protective factors for serious psychological distress among Aboriginal and Torres Strait Islander people. Casuarina, NT: Cooperative Research Centre for Aboriginal Health.
- Kalra, S., Einarson, A., Karaskov, T., Van Uum, S., & Koren, G. (2007). The relationship between stress and hair cortisol in healthy pregnant women. Clinical & Investigative Medicine, 30, 103–107. doi:10.25011/cim.v30i2.986
- Karlén, J., Ludvigsson, J., Frostell, A., Theodorsson, E., & Faresjö, T. (2011). Cortisol in hair measured in young adults – A biomarker of major life stressors? BMC Clinical Pathology, 11, 12. doi:10.1186/1472-6890-11-12
- Kowal, E., Gunthorpe, W., & Bailie, R.S. (2007). Measuring emotional and social wellbeing in Aboriginal and Torres Strait Islander populations: An analysis of a Negative Life Events Scale. International Journal for Equity in Health, 6, 18. doi:10.1186/1475-9276-6-18
- Kristensen, S., Larsen, S., Olsen, N., Fahrenkrug, J., & Heitmann, B. (2017). Hair dyeing, hair washing and hair cortisol concentrations among women from the healthy start study. Psychoneuroendocrinology, 77, 182–185. doi:10.1016/j.psyneuen.2016.12.016
- Leckning, B.A., Li, S.Q., & Cunningham, T. (2016). Trends in hospital admissions involving suicidal behaviour in the Northern Territory, 2001–2013. Australasian Psychiatry, 24(3), 300–304. doi:10.1177/1039856216629838
- McNamara, B., Banks, E., Gubhaju, L., Williamson, A., Joshy, G., Raphael, B., & Eades, S. (2014). Measuring psychological distress in older Aboriginal and Torres Strait Islanders Australians: A comparison of the K-10 and K-5. Australian and New Zealand Journal of Public Health, 38, 567–573. doi:10.1111/1753-6405.12271
- Michl, L., McLaughlin, K., Shepherd, K., & Nolen-Hoeksema, S. (2013). Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology, 122, 339. doi:10.1037/a0031994
- Miller, G.E., Chen, E., & Zhou, E.S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25. doi:10.1037/0033-2909.133.1.25
- Park, M., Mulye, T., Adams, S. H., Brindis, C., & Irwin, C. (2006). The health status of young adults in the United States. Journal of Adolescent Health, 39, 305–317. doi:10.1016/j.jadohealth.2006.04.017
- Rabkin, J.G., & Struening, E.L. (1976). Live events, stress, and illness. Science (New York, N.Y.), 194, 1013–1020. doi:10.1126/science.790570
- Raul, J., Cirimele, V., Ludes, B., & Kintz, P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry, 37, 1105–1111. doi:10.1016/j.clinbiochem.2004.02.010
- Sayers, S., Mackerras, D., Halpin, S., & Singh, G. (2007). Growth outcomes for Australian Aboriginal children aged 11 years who were born with intrauterine growth retardation at term gestation. Paediatric and Perinatal Epidemiology, 21, 411–417. doi:10.1111/j.1365-3016.2007.00852.x
- Sayers, S., Singh, G., Mackerras, D., Lawrance, M., Gunthorpe, W., Jamieson, L., …., Fitz, J. (2009). Australian Aboriginal Birth Cohort study: Follow-up processes at 20 years. BMC International Health and Human Rights, 9, 23. doi:10.1186/1472-698X-9-23
- Schreier, H., Enlow, M., Ritz, T., Gennings, C., & Wright, R. (2015). Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. Journal of Epidemiology and Community Health, 69, 1169–1174. doi:10.1136/jech-2015-205541
- Schmitt, L.H., Harrison, G.A., & Spargo, R.M. (1998). Variation in epinephrine and cortisol excretion rates associated with behavior in an Australian Aboriginal community. American Journal of Physical Anthropology, 106, 249–253. doi:10.1002/(SICI)1096-8644(199806)106:2<249::AID-AJPA10>3.0.CO;2-0
- Sharpley, C., Kauter, K., & McFarlane, J. (2009). An initial exploration of in vivo hair cortisol responses to a brief pain stressor: Latency, localization and independence effects. Physiological Research, 58, 757–761.Available at http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.490.579&rep=rep1&type=pdf
- Sharpley, C., Kauter, K., & McFarlane, J. (2010). An investigation of hair cortisol concentration across body sites and within hair shaft. Clinical Medicine Insights: Endocrinology and Diabetes, 3, 17–23. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3411542/
- Simmons, J.G., Badcock, P.B., Whittle, S.L., Byrne, M.L., Mundy, L., Patton, G.C., … Allen, N.B. (2016). The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology, 63, 276–281. doi:10.1016/j.psyneuen.2015.10.004
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., …., Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. doi:10.1016/j.psyneuen.2016.12.017
- Staufenbiel, S., Penninx, B., de Rijke, Y., van den Akker, E., & van Rossum, E. (2015). Determinants of hair cortisol and hair cortisone concentrations in adults. Psychoneuroendocrinology, 60, 182–194. doi:10.1016/j.psyneuen.2015.06.011
- Susman, E.J. (2006). Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience & Biobehavioral Reviews, 30, 376–389. doi:10.1016/j.neubiorev.2005.08.002
- Yehuda, R., Kahana, B., Binder-Brynes, K., Southwick, S.M., Mason, J.W., & Giller, E.L. (1995). Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. The American Journal of Psychiatry, 152, 982–986. doi:10.1176/ajp.152.7.982
- Yehuda, R., Bierer, L.M., Schmeidler, J., Aferiat, D.H., Breslau, I., & Dolan, S. (2000). Low cortisol and risk for PTSD in adult offspring of holocaust survivors. The American Journal of Psychiatry, 157, 1252–1259. doi:10.1176/appi.ajp.157.8.1252
- Valsamakis, G., Anwar, A., Tomlinson, J.W., Shackleton, C.H.L., McTernan, P.G., Chetty, R., … Kumar, S. (2004). 11beta-hydroxysteroid dehydrogenase type 1 activity in lean and obese males with type 2 diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism, 89, 4755–4761. doi:10.1210/jc.2003-032240
- Vanaelst, B., Michels, N., De Vriendt, T., Huybrechts, I., Vyncke, K., Sioen, I., … De Henauw, S. (2013). Cortisone in hair of elementary school girls and its relationship with childhood stress. European Journal of Pediatrics, 172, 843–846. doi:10.1007/s00431-013-1955-1
- Wiggers, J., Radvan, D., Clover, K., Hazell, T., Alexander, J., & Considine, R. (2001). Public housing, public health: Health needs of public housing tenants. Australian and New Zealand Journal of Public Health, 25, 111–114. doi:10.1111/j.1753-6405.2001.tb01830.x
- Wolff, C.T., Friedman, S.B., Hofer, M.A., & Mason, J.W. (1964). Relationship between psychological defenses and mean urinary 17-hydroxycorticosteroid excretion rates. I: A predictive study of parents of fatally ill children. Psychosomatic Medicine, 26, 576–591. doi:10.1097/00006842-196409000-00002
- Zhang, Q., Chen, Z., Chen, S., Xu, Y., & Deng, H. (2017). Intraindividual stability of cortisol and cortisone and the ratio of cortisol to cortisone in saliva, urine and hair. Steroids, 118, 61–67. doi:10.1016/j.steroids.2016.12.008