Abstract
Women’s experience of trauma may cause lifelong alterations in physiological stress regulation, which can be transmitted to offspring in utero. We investigated, in a prospective pregnancy cohort, associations among maternal lifetime interpersonal trauma (IPT) history, prenatal cortisol dysregulation, and children’s memory domains. Sex-specific effects were also explored. Pregnant women were enrolled from Brigham & Women’s Hospital and affiliated clinics near Boston, MA, in 2002–2007. IPT was assessed with the Revised Conflict Tactics Scale, short form. Salivary cortisol was measured at five time points on each of three days in one week at 29.0 ± 5.1 weeks gestation, and morning rise and diurnal slope were calculated. The Wide Range Assessment of Memory & Learning, 2nd Edition was administered at 6.5 ± 1.0 years and scores were generated for general memory and three sub-domains: verbal, visual, and attention/concentration. In total, 258 maternal-child dyads provided memory and IPT and/or cortisol data. IPT was positively associated with verbal memory in boys (β ± SE: 4.6 ± 2.6) and inversely associated with visual memory score in girls (−6.5 ± 3.2). IPT did not predict prenatal cortisol, but prenatal cortisol modified the association between IPT history and child memory in varying coefficient models allowing for non-linear effect modification. The strongest evidence of interaction was for visual memory in boys: IPT history was associated with poorer visual memory only in those with flatter prenatal diurnal slope (interaction p = .005). Maternal lifetime IPT that leads to prenatal HPA dysregulation may have consequences for child memory, more so than either trauma or elevated cortisol alone. Boys may be more vulnerable to effects. Sex- and timing-specific effects require further investigation.
Introduction
Maternal lifetime experience of trauma and its psychological correlates, even during her childhood, may negatively impact fetal neurodevelopment. For example, in several studies maternal childhood abuse or symptoms of post-traumatic stress disorder (PTSD) predicted her child’s poorer emotional regulation in infancy (Bosquet Enlow et al., Citation2011; Bosquet Enlow et al., Citation2009; Lang, Gartstein, Rodgers, & Lebeck, Citation2010). Trauma can chronically impact the mother’s neurobiology, manifesting as sustained or periodic psychological dysfunction, such as symptoms of PTSD, depression or anxiety, and/or impaired hypothalamic-pituitary-adrenal (HPA) axis function (Anda et al., Citation2006; Kaufman & Charney, Citation2001; Rich-Edwards et al., Citation2011; Teicher et al., Citation2003). Clinical and preclinical evidence link prenatal psychological dysfunction and HPA axis dysregulation to offspring brain function (Sanjuan et al., Citation2016) and cognitive performance (Markham, Taylor, Taylor, Bell, & Koenig, Citation2010), with the hippocampus, critical for spatial and working memory, particularly affected (Negrón-Oyarzo, Neira, Espinosa, Fuentealba, & Aboitiz, Citation2015; Son et al., Citation2006).
The effect of maternal lifetime trauma on fetal neurodevelopment is thought to be mediated by the HPA axis (Entringer, Buss, & Wadhwa, Citation2015). Animal studies using exogenously-administered glucocorticoids during gestation support that dysregulated HPA axis could cause neurocognitive outcomes similar to those observed with prenatal stress (Peffer et al., Citation2015; Pryce, Aubert, Maier, Pearce, & Fuchs, Citation2011). Human studies have been less conclusive about the role of the HPA axis, both those linking lifetime trauma to HPA axis dysregulation in pregnancy (Bublitz & Stroud, Citation2013; Miller, Chen, & Zhou, Citation2007; Schreier, Bosquet Enlow, Ritz, Gennings, & Wright, Citation2015; Schreier et al., Citation2016) and those linking prenatal cortisol to child cognitive outcomes (Zijlmans, Riksen-Walraven, & de Weerth, Citation2015). Evidence that trauma may cause hyper- or hypocortisolemia (Luo et al., Citation2012; Miller et al., Citation2007) underscores that the role of the HPA axis in transmitting the effects of trauma on fetal development may be complex. In addition, clinical and preclinical studies of prenatal stress on neurocognition point to sex-specific effects (DiPietro & Voegtline, Citation2017; Luine, Citation2002; Mueller & Bale, Citation2007, Citation2008; Van den Bergh et al., Citation2017; Zuena et al., Citation2008), with most, but not all, reporting greater in utero susceptibility in male offspring. The reason for this is not fully elucidated, but proposed mechanisms include differential endocrine regulation of HPA axis activity (DiPietro, Costigan, Kivlighan, Chen, & Laudenslager, Citation2011; Ellman et al., Citation2008) and placental function (O'Donnell, O'Connor, & Glover, Citation2009).
A recent study by our group reported that prenatal cortisol modified rather than mediated the effect of maternal trauma on infant negative affectivity (Bosquet Enlow et al., Citation2017). Trauma history predicted poorer recovery from distress, a dimension of negative affect, only in children of mothers with low prenatal cortisol, and trends toward effect modification by cortisol were observed for trauma and other negative affectivity domains.
In the present study, we extended these prior findings from temperament to cognitive function using memory assessed in mid-childhood, as infant temperament is considered an early precursor to later behavioral and neurocognitive characteristics (Rothbart & Bates, Citation2007), and we explored sex-specific effects. Memory was selected as the outcome of interest because of prior reports of associations between prenatal stress and offspring memory performance described above. We used salivary cortisol, measured throughout the day, as diurnal trends may reveal multiple dimensions of cortisol dysregulation (Adam & Kumari, Citation2009; Vreeburg et al., Citation2009, Citation2010). We aimed to characterize, in a large pregnancy cohort, (1) the association between maternal lifetime trauma and mid-childhood memory function, (2) the effect of maternal lifetime trauma on prenatal diurnal cortisol patterns, (3) modification of the effect of maternal trauma on child memory by prenatal cortisol, and (4) sex differences in these relationships.
Methods
Subjects were from the Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, a pregnancy cohort designed to examine effects of prenatal chemical and non-chemical exposures on childhood asthma risk (Wright et al., Citation2008). Originally, n = 500 English- or Spanish-speaking women receiving prenatal care at two Boston hospitals and affiliated community health centers were consented between August 2002 and January 2007. Seventy-eight percent of women who were approached by research staff on select clinic days were eligible and agreed to enroll. There were no significant differences in race/ethnicity, education, or income between women enrolled and those who declined enrollment. A total of 455 women gave birth to a live-born infant and continued follow-up. Supplemental funding was obtained to assess salivary cortisol rhythms in a subset of mothers in pregnancy and neurocognition in children aged 5–7 years. Procedures were approved by human studies committees at the Brigham and Women’s Hospital and Boston Medical Center; written consent was obtained in the subject’s primary language.
Main exposure and outcome assessments
Maternal interpersonal trauma (IPT) history was assessed with the Revised Conflict Tactics Scale short form (Straus & Douglas, Citation2004), which has demonstrated reliability and validity in English and Spanish (Connelly, Newton, & Aarons, Citation2005). Mothers were asked if anyone physically harmed them during four life stages: childhood (≤11 years old), adolescence (12–17 years old), adulthood prior to this pregnancy and during this pregnancy. For each life stage, exposure was assessed using the same 6 items, asking women whether anyone had ever pushed, grabbed, or shoved them; kicked, bit, or punched them; hit them with something that hurt their body; choked or burned them; forced them to have sexual activities; or physically attacked them in some other way. Women were classified as exposed in each life period if they answered yes to any one of the items.
Prenatal salivary cortisol was measured in saliva samples collected by the participant using the passive drool collection technique at 29.0 ± 5.1 weeks gestation (Suglia et al., Citation2010; Wright et al., Citation2013). Women who reported shift work, steroid use or non-singleton pregnancies were excluded. Five saliva samples per day were collected on three weekdays within the same week: at awakening (T1), 45 minutes after awakening (T2), 4 and 10 hours after awakening (T3 and T4, respectively) and just before going to bed (T5). Cortisol concentrations were measured with a chemiluminescence assay with high sensitivity of 0.16 ng/ml (IBL, Hamburg, Germany); intra- and interassay variabilities were less than 8%.
Child memory function was assessed with the Wide Range Assessment of Memory & Learning, 2nd Edition (WRAML-2) (Sheslow & Adams, Citation2003) at age 6.5 ± 1.0 years (n = 299) by research staff trained in neurodevelopmental testing. The test produces an overall General Memory Index, as well as three sub-scores: Verbal Memory Index, Visual Memory Index, and Attention/Concentration Index, all expressed as age-standardized scores with higher values indicative of better memory performance.
Covariate assessment
Maternal age, race/ethnicity, education completed and self-reported pre-pregnancy weight and height were collected at enrollment. Child sex, date of birth, and gestational age at birth were obtained from medical records. Gestational age at cortisol assessment was calculated based on collection date and maternal-reported date of last menstrual period before pregnancy.
Analytic methods
Data management
Maternal-child dyads with WRAML-2 data plus at least maternal IPT or prenatal cortisol data were included.
IPT was examined as a single summary variable dichotomized such that an affirmative answer in any of the four queried periods was considered having a history of IPT. Secondary analyses considering IPT experiences within specific life periods did not identify particular vulnerable periods (not shown).
Saliva samples collected outside of pre-specified time windows relative to awakening were omitted (Wright et al., Citation2013). Values for each time point were log-transformed and averaged across the three collection days. Cortisol morning rise (CAR), the difference between the second and first morning values, and diurnal slope, the least squares regression line of cortisol on hours since awakening omitting time 2, were calculated.
Statistical analysis
Main exposure and outcome variables along with maternal and child sociodemographic characteristics were explored for distributions of values and bivariate associations. Summary statistics were calculated overall and by child sex.
Linear regression models were used to evaluate the relationship between maternal IPT (ever/never) or prenatal cortisol (continuous CAR and diurnal slope) and child memory (WRAML-2 index scores), with separate models for each exposure-outcome pair. After log-transformation, cortisol values were standardized prior to modeling so that coefficients reflect the change in WRAML-2 score per SD difference in log-cortisol. Final models were stratified by sex and were adjusted for maternal age, race, education (≥high school vs. not), and child age at cognitive testing. Cortisol models were additionally adjusted for pre-pregnancy BMI and gestational age at cortisol testing.
Associations between IPT history and prenatal cortisol values were also explored in linear regression models with standardized log-transformed cortisol measures by time point and summary measures (CAR and diurnal slope) as the dependent variable and the indicator variable for history of IPT as the independent variable. Separate models were fit for each cortisol measure. Final models were adjusted for maternal age, race, education, pre-pregnancy BMI, and gestational age at cortisol assessment.
Finally, varying coefficient models were developed to examine effect modification of IPT associations with memory scores by maternal prenatal cortisol values using the mgcv package in R (Wood Simon, Citation2011). These models are similar to linear regression models; however, they do not assume a linear effect of cortisol on memory. The rationale for using varying coefficient models was the evidence that a linear assumption was not appropriate for cortisol, as disrupted reactivity of the HPA axis can result in elevated or reduced cortisol levels (Luo et al., Citation2012; Miller et al., Citation2007). In each varying coefficient model, cortisol was entered into the model as an unspecified, potentially nonlinear, smooth function that was estimated from the data in both the main effect of cortisol and the cortisol × IPT interaction. The resulting models allowed the association between IPT and memory scores to vary as a potentially nonlinear function of cortisol. Final models were developed separately by child sex and adjusted for maternal age, race, education, pre-pregnancy BMI, gestational age at cortisol assessment and child age at WRAML-2 testing. Figures were created to present estimates and corresponding confidence intervals for the association of IPT with memory scores, as a function of prenatal cortisol level. α ≤ 0.05 was considered evidence in favor of rejecting the null hypothesis. Statistical significance and confidence interval estimation was also performed adjusting for multiple testing. Specifically, we performed a Bonferroni correction that controls the type 1 error when conducting 8 tests (2 cortisol exposures ×2 memory outcomes ×2 genders). Therefore, for an overall α = 0.05 significance level, we considered an effect significant if the p value was less than .05/8 = .00625 and, in figures, presented 99% confidence intervals.
Analyses were conducted in Stata, version 15 (Stata Corp., College Station, TX) and R, version 3.4.0 (Core Team, Citation2017).
Results
A total of 258 mother-child dyads provided child cognitive data and maternal IPT history, while 99 dyads had both prenatal cortisol and child memory data and 91 had data for all three measures. Women were mean 27.5 ± 5.8 years old at enrollment (). More than half (60%) identified as Hispanic, 28% Black, 9% white and 4% another race or multiple races. Women were approximately evenly split between BMI categories, with 28% normal weight and 36% each overweight and obese. Nearly half of women (46%) reported experiencing IPT during at least one life stage.
Table 1. Descriptive statistics for participating children and their mothers.
Prenatal cortisol was measured at mean gestational age 29.0 ± 5.1 weeks (). Overall, the expected diurnal pattern was observed, with values highest at time 2, soon after waking, and then falling throughout the rest of the day, with evening values lower than the first-morning value.
Slightly more than 50% of children were male. Children completed cognitive testing at mean age 6.5 ± 1.0 years. The participants’ mean memory index scores on the WRAML-2 were at the 38th percentile for general memory, 43th percentile for verbal memory, 35th percentile for visual memory and 47th percentile for attention/concentration.
Maternal history of IPT was associated with lower verbal and visual memory scores in girls, but the effect on visual memory was stronger (). In adjusted models, girls of mothers with a history of IPT had, on average, visual memory scores that were lower by 6.5 points (SE: 3.2, t= −2.05, p value=.043) relative to girls born to mothers without IPT. In boys, on the other hand, IPT was not associated with visual memory but was weakly positively associated with verbal memory scores (β (SE): 4.6 (2.6), t = 1.77, p value=.08). No relationship between IPT history and general memory or attention/concentration indices was observed.
Table 2. Associations between maternal trauma history and prenatal cortisol values and child memory scores stratified by child sex.
Some associations were also observed between prenatal salivary cortisol values and child memory scores (). CAR was not consistently associated with memory outcomes in boys or girls. In adjusted models, diurnal slope was positively associated with all memory scores in boys, with strongest effects observed for general memory and attention/concentration score (6.1 (2.7), t = 2.30, p value=.03 and 5.9 (2.5), t = 2.41, p value=.02, respectively), while in girls associations were weaker. Maternal history of IPT was not associated with prenatal salivary cortisol summary measures or levels at most time points, aside from a positive association observed for time 4 cortisol (7.5–11.5 hours after waking) (Supplemental Table 1).
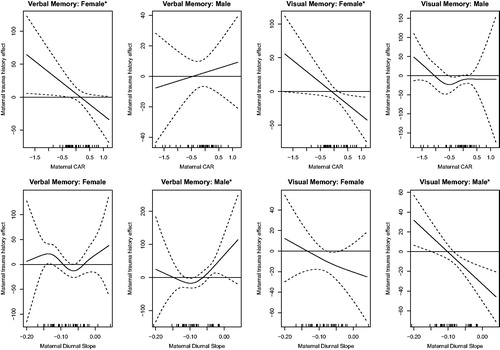
In adjusted sex-stratified varying coefficient models allowing for a smoothed effect of prenatal cortisol to modify the relationship between IPT history and child memory ability, effect modification was observed at some cortisol values (). The strongest effect was observed for IPT and visual memory in boys, where at higher (flatter) prenatal salivary cortisol diurnal slope values (> −0.08), maternal history of IPT was associated with lower visual memory scores (F = 9.524, estimated degrees of freedom (edf)=1, global p value for interaction=.005). Evidence for other effect modification by prenatal cortisol was weaker. In girls, there was a suggestion of a positive association between IPT and verbal memory at low CAR values (F = 4.417, edf =1.000, global p value for interaction=.047), and of a negative association between IPT and visual memory with positive (more typical) prenatal CAR values (F = 4.730, edf =1.000, global p value for interaction=.043). The effect of diurnal slope in girls on the relationship between IPT and either verbal or visual memory was not discernable from null. Associations between IPT and verbal and visual memory in boys appeared to vary somewhat across CAR values, but there was not strong evidence for a statistical interaction. Similarly, diurnal slope appeared to modestly modify the relationship between IPT history and verbal memory function (F = 4.240, edf =1.935, global p value for interaction=.032), such that the positive association observed without accounting for prenatal cortisol persisted only in boys of mothers with higher (flatter) diurnal slope in the range of −0.044 to 0.006, while there was a slight negative association between IPT and verbal memory in those within a band of lower slope values. After adjusting for multiple comparisons (α ≤ .05/8 = .00625 for 2 memory scores ×2 cortisol measures ×2 genders), only the IPT by diurnal slope by visual memory association in boys remained statistically significant (see Supplemental Materials).
Figure 1. Effect modification of maternal IPT history on memory scores by prenatal cortisol values. Panels show the association between maternal trauma history and memory domain as a smooth function of prenatal cortisol morning rise (CAR) or diurnal slope estimated with a varying coefficient model (solid line) with dashed lines showing 95% confidence bounds. Upper and lower dotted bands falling above or below the zero line indicate intervals of cortisol values for which a significant association between maternal IPT history and the memory score was observed. Models were adjusted for mother’s age, race, education and BMI, gestational age at cortisol measurement and child age at cognitive testing. Overall effect modification by prenatal CAR was significant at α ≤ .05 for Visual and Verbal Memory in girls; effect modification by prenatal diurnal slope was significant for Verbal and Visual Memory in boys. (*in the figure title indicates p value ≤.05 for global test of effect modification).
Discussion
In a racially and ethnically diverse urban pregnancy cohort, we investigated associations of maternal lifetime trauma and prenatal HPA axis dysregulation with memory function in mid-childhood. Based on prior evidence (Bosquet Enlow et al., Citation2017), we hypothesized that prenatal cortisol would modify rather than mediate the relationship between trauma and child memory. History of IPT, reported by nearly half of participating mothers, was associated with poorer verbal and visual memory in girls but with better verbal memory in boys. IPT history did not predict prenatal cortisol patterns directly, nor was prenatal cortisol associated with many memory domains, but prenatal cortisol modified the relationship between IPT and memory domains in a sex-specific manner. In boys only, IPT was associated with poorer visual memory in those whose mothers had higher (flatter) salivary cortisol diurnal slopes in mid-pregnancy. Trauma history alone may not elevate the risk of impaired neurocognitive development, but trauma that is followed by persistent or recurrent dysregulated HPA axis during pregnancy may put children at increased risk of impaired neurocognitive development. Boys may be particularly vulnerable.
In this cohort enrolled prenatally and followed through childhood assessing cognitive and other health outcomes, boys of mothers with a history of interpersonal trauma and dysregulated cortisol in the form of flatter diurnal slope had lower visual memory scores than boys of mothers without prior IPT. The same pattern was not observed for boys born to women with more negative diurnal slopes. These findings are consistent with prior research showing that elevated afternoon and evening cortisol levels, captured in flatter, less negative diurnal slope values, tend to occur with chronic stress of various types (Miller et al., Citation2007), and that similar patterns in pregnancy have been associated with adverse child health outcomes (Beijers, Jansen, Riksen-Walraven, & de Weerth, Citation2010). Cortisol is known to be inconsistently associated with prior trauma (Miller et al., Citation2007), and our finding that maternal-reported trauma does not predict cortisol levels in the sample as a whole is not inconsistent with prior literature (Baibazarova et al., Citation2013; Bleker, Roseboom, Vrijkotte, Reynolds, & de Rooij, Citation2017; Davis & Sandman, Citation2010; Shelton, Schminkey, & Groer, Citation2015). Further, elevated prenatal cortisol levels are harmful to fetal development in some cases and beneficial in others (Davis, Head, Buss, & Sandman, Citation2017; Davis & Sandman, Citation2010; Glynn & Sandman, Citation2012), possibly related to timing within gestation, severity of cortisol dysregulation, or developmental outcome being examined. This may explain, in part, why we observed that higher diurnal slope values alone predicted better attention/concentration and general memory scores in boys, but in combination with trauma history, they were associated with poorer visual memory.
When allowing for cortisol to modify the relationship between trauma and memory, we saw that trauma that led to HPA axis dysregulation during pregnancy was associated with poorer neurodevelopment, while trauma that didn’t cause a persistent or recurrent effect on the HPA axis was not associated with impaired fetal development. This corroborates and extends findings of effect modification by prenatal cortisol of the relationship between IPT history and infant temperament reported by our group in another sample (Bosquet Enlow et al., Citation2017). These observations may help to elucidate prior reports that prenatal stress and elevated prenatal cortisol may each either help or harm the developing fetus (Van den Bergh et al., Citation2017); characterizing both stress and cortisol levels during pregnancy may better predict the risk of impaired neurodevelopment.
Our finding that visual memory function, in particular, was affected by maternal IPT plus cortisol dysregulation is consistent with the prenatal stress animal literature, which makes extensive use of tests of spatial learning and memory (Weinstock, Citation2017). Visual memory scores more so than other memory domains tended to be lower in this cohort compared to normative values, which is consistent with that aspect of memory being relatively more susceptible to prenatal insults such a lifetime trauma that are prevalent in the mothers in our sample.
The sex-specific effect that we observed with greater susceptibility in boys is consistent with the prenatal stress literature. Sex-specific effects of prenatal stressors on offspring neurocognitive development are commonly reported in animal models and human studies, with males typically characterized as more susceptible (Bale et al., Citation2010). In an extensive review of sex-specific fetal programing, Sandman et al. presented evidence of sex-specific adaptations and survival tradeoffs in the face of prenatal stressors (Sandman, Glynn, & Davis, Citation2013), suggesting both that sex-specific effects of the type we report are to be expected and that examination of single behavioral or cognitive outcomes may not capture the full effect of an exposure on male and female offspring.
Evidence of interactions between IPT history, prenatal cortisol and child memory was relatively weaker for the other memory outcomes, for CAR and for all cortisol-memory combinations in girls relative to boys. These findings suggest a need for additional studies in different cohorts since the small sample size of dyads with trauma, cortisol and memory data could have limited our ability to detect true, weaker associations in interaction models. In particular, suggestions of protective effects of certain prenatal stress and cortisol exposures on cognitive development are intriguing, especially considering similar findings reported in the literature (Davis & Sandman, Citation2010; DiPietro, Novak, Costigan, Atella, & Reusing, Citation2006). A future study with cumulative cortisol levels in hair or salivary cortisol assessment within a narrower gestational age range or with a larger sample size may reveal stronger associations overall or within certain sociodemographic or trauma/cortisol exposure strata.
Strengths of the present study include the prospective cohort design and assessment of trauma history, prenatal stress and child memory function with widely-used tools validated in diverse populations. Cortisol assessment was state-of-the-art (Harville et al., Citation2007; Zijlmans et al., Citation2015). Still, cortisol was only measured over a single one-week period in gestation, and saliva collection times were self-reported. Further, the small sample size of maternal-child dyads with both prenatal cortisol and child cognition data limited the complexity of models we could develop, especially with respect to multiple potential effect modifiers. A future study in a larger sample could confirm the present findings, determine whether non-significant trends are real, and explore effect modification by additional maternal characteristics, such as BMI.
The present study finds that maternal history of lifetime trauma may affect child memory function in the presence of certain prenatal HPA axis patterns. Replication in a larger sample would be beneficial to confirm and further explore the role of prenatal HPA axis regulation in modifying effects of maternal trauma history on child cognitive function and to clarify sex-specific effects, critical exposure windows, and maternal and pregnancy characteristics that modify the links between trauma, prenatal cortisol, and child cognition.
Campbell_et_all_Supplemental_materials.pdf
Download PDF (150.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adam, E.K., & Kumari, M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. doi:10.1016/j.psyneuen.2009.06.011
- Anda, R.F., Felitti, V.J., Bremner, J.D., Walker, J.D., Whitfield, C., Perry, B.D., … Giles, W.H. (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. doi:10.1007/s00406-005-0624-4
- Baibazarova, E., van de Beek, C., Cohen-Kettenis, P.T., Buitelaar, J., Shelton, K.H., & van Goozen, S.H. (2013). Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology, 38, 907–915. doi:10.1016/j.psyneuen.2012.09.015
- Bale, T.L., Baram, T.Z., Brown, A.S., Goldstein, J.M., Insel, T.R., McCarthy, M.M., … Nestler, E.J. (2010). Early life programming and neurodevelopmental disorders. Biological Psychiatry, 68, 314–319. doi:10.1016/j.biopsych.2010.05.028
- Beijers, R., Jansen, J., Riksen-Walraven, M., & de Weerth, C. (2010). Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics, 126, e401–e409. doi:10.1542/peds.2009-3226
- Bleker, L.S., Roseboom, T.J., Vrijkotte, T.G., Reynolds, R.M., & de Rooij, S.R. (2017). Determinants of cortisol during pregnancy – The ABCD cohort. Psychoneuroendocrinology, 83, 172–181. doi:10.1016/j.psyneuen.2017.05.026
- Bosquet Enlow, M., Devick, K.L., Brunst, K.J., Lipton, L.R., Coull, B.A., & Wright, R.J. (2017). Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy, 22, 492–513. doi:10.1111/infa.12176
- Bosquet Enlow, M., Kitts, R.L., Blood, E., Bizarro, A., Hofmeister, M., & Wright, R.J. (2011). Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation. Infant Behaviour and Development, 34, 487–503. doi:10.1016/j.infbeh.2011.07.007
- Bosquet Enlow, M., Kullowatz, A., Staudenmayer, J., Spasojevic, J., Ritz, T., & Wright, R.J. (2009). Associations of maternal lifetime trauma and perinatal traumatic stress symptoms with infant cardiorespiratory reactivity to psychological challenge. Psychosomatic Medicine, 71, 607–614. doi:10.1097/PSY.0b013e3181ad1c8b
- Bublitz, M.H., & Stroud, L.R. (2013). Maternal history of child abuse moderates the association between daily stress and diurnal cortisol in pregnancy: A pilot study. Stress (Amsterdam, Netherlands), 16, 706–710. doi:10.3109/10253890.2013.825768
- Connelly, C.D., Newton, R.R., & Aarons, G.A. (2005). A psychometric examination of English and Spanish versions of the Revised Conflict Tactics Scales. Journal of Interpersonal Violence, 20, 1560–1579. doi:10.1177/0886260505280341
- Davis, E.P., Head, K., Buss, C., & Sandman, C.A. (2017). Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology, 75, 56–63. doi:10.1016/j.psyneuen.2016.10.005
- Davis, E.P., & Sandman, C.A. (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81, 131–148. doi:10.1111/j.1467-8624.2009.01385.x
- DiPietro, J.A., Costigan, K.A., Kivlighan, K.T., Chen, P., & Laudenslager, M.L. (2011). Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology, 36, 588–591. doi:10.1016/j.psyneuen.2010.09.005
- DiPietro, J.A., Novak, M.F., Costigan, K.A., Atella, L.D., & Reusing, S.P. (2006). Maternal psychological distress during pregnancy in relation to child development at age two. Child Development. 77, 573–587. doi:10.1111/j.1467-8624.2006.00891.x
- DiPietro, J.A., & Voegtline, K.M. (2017). The gestational foundation of sex differences in development and vulnerability. Neuroscience, 342, 4–20. doi:10.1016/j.neuroscience.2015.07.068
- Ellman, L.M., Schetter, C.D., Hobel, C.J., Chicz-Demet, A., Glynn, L.M., & Sandman, C.A. (2008). Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology, 50, 232–241. doi:10.1002/dev.20293
- Entringer, S., Buss, C., & Wadhwa, P.D. (2015). Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology, 62, 366–375. doi:10.1016/j.psyneuen.2015.08.019
- Glynn, L.M., & Sandman, C.A. (2012). Sex moderates associations between prenatal glucocorticoid exposure and human fetal neurological development. Developmental Science, 15, 601–610. doi:10.1111/j.1467-7687.2012.01159.x
- Harville, E.W., Savitz, D.A., Dole, N., Herring, A.H., Thorp, J.M., & Light, K.C. (2007). Patterns of salivary cortisol secretion in pregnancy and implications for assessment protocols. Biological Psychology, 74, 85–91. doi:10.1016/j.biopsycho.2006.07.005
- Kaufman, J., & Charney, D. (2001). Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology, 13, 451–471. doi:10.1017/S0954579401003030
- Lang, A.J., Gartstein, M.A., Rodgers, C.S., & Lebeck, M.M. (2010). The impact of maternal childhood abuse on parenting and infant temperament. Journal of Child and Adolescent Psychiatric Nursing, 23, 100–110. doi:10.1111/j.1744-6171.2010.00229.x
- Luine, V. (2002). Sex differences in chronic stress effects on memory in rats. Stress (Amsterdam, Netherlands), 5, 205–216. doi:10.1080/1025389021000010549
- Luo, H., Hu, X., Liu, X., Ma, X., Guo, W., Qiu, C., … Li, T. (2012). Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biological Psychiatry, 72, 65–69. doi:10.1016/j.biopsych.2011.12.020
- Markham, J., Taylor, A., Taylor, S., Bell, D., & Koenig, J. (2010). Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Frontiers in Behavioral Neuroscience, 4, 173. doi:10.3389/fnbeh.2010.00173
- Miller, G.E., Chen, E., & Zhou, E.S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi:10.1037/0033-2909.133.1.25
- Mueller, B.R., & Bale, T.L. (2007). Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiology and Behavior, 91, 55–65. doi:10.1016/j.physbeh.2007.01.017
- Mueller, B.R., & Bale, T.L. (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience, 28, 9055–9065. doi:10.1523/JNEUROSCI.1424-08.2008
- Negrón-Oyarzo, I., Neira, D., Espinosa, N., Fuentealba, P., & Aboitiz, F. (2015). Prenatal stress produces persistence of remote memory and disrupts functional connectivity in the hippocampal–prefrontal cortex axis. Cerebral Cortex, 25, 3132–3143. doi:10.1093/cercor/bhu108
- O'Donnell, K., O'Connor, T.G., & Glover, V. (2009). Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Developmental Neuroscience, 31, 285–292. doi:10.1159/000216539
- Peffer, M.E., Zhang, J.Y., Umfrey, L., Rudine, A.C., Monaghan, A.P., & DeFranco, D.B. (2015). Minireview: The impact of antenatal therapeutic synthetic glucocorticoids on the developing fetal brain. Molecular Endocrinology, 29, 658–666. doi:10.1210/me.2015-1042
- Pryce, C.R., Aubert, Y., Maier, C., Pearce, P.C., & Fuchs, E. (2011). The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: Studies in rhesus macaque and common marmoset. Psychopharmacology, 214, 33–53. doi:10.1007/s00213-010-1989-2
- R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/
- Rich-Edwards, J.W., James-Todd, T., Mohllajee, A., Kleinman, K., Burke, A., Gillman, M.W., & Wright, R.J. (2011). Lifetime maternal experiences of abuse and risk of pre-natal depression in two demographically distinct populations in Boston. International Journal of Epidemiology, 40, 375–384. doi:10.1093/ije/dyq247
- Rothbart, M., E., & Bates, J. (2007). Temperament. In: Handbook of child psychology.
- Sandman, C.A., Glynn, L.M., & Davis, E.P. (2013). Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. Journal of Psychosomatic Research, 75, 327–335. doi:10.1016/j.jpsychores.2013.07.009
- Sanjuan, P.M., Poremba, C., Flynn, L.R., Savich, R., Annett, R.D., & Stephen, J. (2016). Association between theta power in 6-month old infants at rest and maternal PTSD severity: A pilot study. Neuroscience Letters, 630, 120–126. doi:10.1016/j.neulet.2016.07.048
- Schreier, H.M., Bosquet Enlow, M., Ritz, T., Coull, B.A., Gennings, C., Wright, R.O., & Wright, R.J. (2016). Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress, 19, 45–52. doi:10.3109/10253890.2015.1117447
- Schreier, H.M., Bosquet Enlow, M., Ritz, T., Gennings, C., & Wright, R.J. (2015). Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. Journal of Epidemiology and Community Health, 69, 1169–1174. doi:10.1136/jech-2015-205541
- Shelton, M.M., Schminkey, D.L., & Groer, M.W. (2015). Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biological Research for Nursing, 17, 295–302. doi:10.1177/1099800414543821
- Sheslow, D., & Adams, W. (2003). Wide range assessment of memory and learning. Administration and technical manual (2nd ed.). Lutz, FL: Psychological Assessment Resources, Inc.
- Son, G.H., Geum, D., Chung, S., Kim, E.J., Jo, J.-H., Kim, C.-M., … Kim, K. (2006). Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. The Journal of Neuroscience, 26, 3309. [10.1523/JNEUROSCI.3850-05.2006]. p doi:10.1523/JNEUROSCI.3850-05.2006
- Straus, M.A., & Douglas, E.M. (2004). A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence and Victims, 19,507–520. doi:10.1891/088667004780927800
- Suglia, S.F., Staudenmayer, J., Cohen, S., Bosquet Enlow, M., Rich-Edwards, J.W., & Wright, R.J. (2010). Cumulative stress and cortisol disruption among black and hispanic pregnant women in an urban cohort. Psychological Trauma: Theory, Research, Practice, and Policy, 2, 326–334. doi:10.1037/a0018953
- Teicher, M.H., Andersen, S.L., Polcari, A., Anderson, C.M., Navalta, C.P., & Kim, D.M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews, 27, 33–44. doi:10.1016/S0149-7634(03)00007-1
- Van den Bergh, B.R.H., van den Heuvel, M.I., Lahti, M., Braeken, M., de Rooij, S.R., Entringer, S., …., Schwab, M. (2017). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and Biobehavioral Reviews. doi:10.1016/j.neubiorev.2017.07.003
- Vreeburg, S.A., Hoogendijk, W.J.G., van Pelt, J., DeRijk, R.H., Verhagen, J.C.M., van Dyck, R., … Penninx, B.W.J.H. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from. Archives of General Psychiatry, 66, 617–626. doi:10.1001/archgenpsychiatry.2009.50
- Vreeburg, S.A., Zitman, F.G., van Pelt, J., DeRijk, R.H., Verhagen, J.C.M., van Dyck, R., … Penninx, B.W.J.H. (2010). Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic Medicine, 72, 340–347. doi:10.1097/PSY.0b013e3181d2f0c8
- Weinstock, M. (2017). Prenatal stressors in rodents: Effects on behavior. Neurobiology of Stress, 6,3–13. doi:10.1016/j.ynstr.2016.08.004
- Wood Simon, N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 73, 3–36. doi:10.1111/j.1467-9868.2010.00749.x
- Wright, R.J., Fisher, K., Chiu, Y.H., Wright, R.O., Fein, R., Cohen, S., & Coull, B.A. (2013). Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. American Journal of Respiratory and Critical Care Medicine, 187, 1186–1193. doi:10.1164/rccm.201208-1530OC
- Wright, R.J., Suglia, S.F., Levy, J., Fortun, K., Shields, A., Subramanian, S., & Wright, R. (2008). Transdisciplinary research strategies for understanding socially patterned disease: The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Ciência & Saúde Coletiva, 13, 1729–1742. doi:10.1590/S1413-81232008000600008
- Zijlmans, M.A.C., Riksen-Walraven, J.M., & de Weerth, C. (2015). Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience and Biobehavioral Reviews, 53, 1–24. doi:10.1016/j.neubiorev.2015.02.015
- Zuena, A.R., Mairesse, J., Casolini, P., Cinque, C., Alemà, G.S., Morley-Fletcher, S., … Maccari, S. (2008). Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE, 3, e2170. doi:10.1371/journal.pone.0002170
