Abstract
The main stage in real-time quantitative PCR is a quantification of gene transcriptomes, in which suitable use of reliable reference genes is critical to normalize accurately. To determine the most stable reference genes in laying hens under heat stress, from a panel of nine typical candidate reference genes, the mRNA transcript of ACTB, HMBS, HPRT1, RPL13, RPL32, 18SrRNA, TBP, TFRC, and YWHAZ was evaluated in the ovary and uterus of both control and heat-stress groups of laying hens. Forty 23-week-old White Leghorn laying hens were housed in two rooms. The control (n = 20) and heat-stress (n = 20) groups were maintained at 21–23 °C and 36–38 °C for 8 weeks respectively. Analysis of this set of genes was done with BestKeeper, geNorm, and NormFinder software programs to find the most stable ones. Candidate reference genes ranked in the uterus of heat-stress and control groups of hens included YWHAZ, HPRT1, HMBS, RPL13, TFRC, ACTB, TBP, RPL32, and 18SrRNA; those in the ovary were YWHAZ, HPRT1, TFRC, HMBS, RPL13, TBP, RPL32, ACTB, and 18SrRNA. The overall results indicated that the most stable genes are YWHAZ, HPRT1, HMBS, RPL13, TFRC, TBP, ACTB, RPL32, and 18SrRNA respectively. In addition, the combination of YWHAZ, HPRT1, and HMBS is suggested as the most stable reference group of genes for more accurate quantitative data normalization in the ovarian and uterine tissues of laying hens under control and heat stress conditions.
Lay summary
Heat stress influences the expression of many genes in the reproductive tissues of birds. Accurate evaluation of these changes via real-time quantitative PCR depends on the determination of reliable reference genes. In this study, nine candidate housekeeping genes were evaluated, and the most stable were YWHAZ, HPRT1, HMBS, RPL13, TFRC, TBP, ACTB, RPL32, and 18SrRNA.
Introduction
Heat stress is one of the main problems in the modern poultry industry, especially in tropical regions. The optimal environmental temperature for birds is in the range of 16–25 °C. Every 1 °C increase in temperature within 21–30 °C, decreases appetite by 1.5%, and every 1 °C increase in temperature within 32–38 °C, reduces feed intake by 4.6% (Allahverdi, Feizi, Takhtfooladi, & Nikpiran, Citation2013; Mashaly et al., Citation2004; Puthpongsiriporn, Scheideler, Sell, & Beck, Citation2001; Sahin & Kucuk, Citation2001; Song, Liu, Sheikhahmadi, Jiao, & Lin, Citation2012). Other adverse effects of heat stress are low feed digestibility, lower eggshell quality, (Lara & Rostagno, Citation2013; St-Pierre, Cobanov, & Schnitkey, Citation2003) and lower absorptive ability of enterocytes for calcium in laying hens, which is an important factor in eggshell mineralization (Allahverdi et al., Citation2013; Mahmoud et al., Citation1996; Quinteiro-Filho et al., Citation2010). Heat stress can impact the expression of many genes. Many studies have been conducted to evaluate the effect of heat stress on the expression of genes (Sonna, Fujita, Gaffin, & Lilly, Citation2002). The failure of poultry reproduction during heat stress, is also due to its effect on the hypothalamic-pituitary-gonad axis in which many gene transcripts are up/down-regulated (Bahadoran, Dehghani Samani, & Hassanpour, Citation2018; Rozenboim, Tako, Gal-Garber, Proudman, & Uni, Citation2007; Sun et al., Citation2015; Wang et al., Citation2013).
Polymerase chain reaction (PCR) techniques, especially real-time quantitative PCR (RT-qPCR), play a principle role in current cellular and molecular biology (Kubista et al., Citation2006). In RT-qPCR, two points must be considered: (1) the quantification process is composed of standardized procedures, assay quality and calculation methods, and (2) normalization of samples to correct for differences in gene inputs (Dorak, Citation2007). In this technique, the accurate relative expression of transcripts and their fold changes are estimated by comparison with a reference gene (housekeeping gene) (Wong & Medrano, Citation2005). In order to function as a reference, the expression of a gene must stay unchanged and constant irrespective of treatment. However, it has been shown that the transcription of these genes is not perfectly unchanged in various types of tissues, cells, phases of disease, and treatment (Gu et al., Citation2011; Vandesompele, Citation2002). Hence, determination of a stable housekeeping gene may be important when it is transcribed differentially across the samples (Kozera & Rapacz, Citation2013; Suzuki, Higgins, & Crawford, Citation2000).
The aim of the present study was to identify the most stable housekeeping genes for application in molecular studies of ovarian and uterine tissues in laying hens under heat stress, and the objective was to compare nine potential housekeeping genes, ACTB, HMBS, HPRT1, RPL13, RPL32, 18SrRNA, TBP, TFRC and YWHAZ, and to find the most stable ones via three software packages, geNorm, BestKeeper, and NormFinder.
Methods
Birds and treatments
The Institutional Animal Care and Use Committee of the University of Shahrekord approved all procedures throughout this study. Forty 23-week-old White Leghorn laying hens were accommodated in single cages (1600 cm2/bird) under ambient temperatures and were provided 16 h of light (06:00 h. to 23:00 h). Hens were fed ad libitum with commercial layer feed, formulated according to the NRC (Citation1994). Birds were allocated to 2 groups (n = 20) in two separate rooms. Conditions of both rooms were similar (21–23 °C, 35% humidity). This condition was stable for laying hens in the control group during the total days of the experiment (9 weeks) while a high temperature was induced in the room of the treated group (the heat-stress group) after one week. The increase of temperature in this group was 3 °C daily until 36–38 °C was reached according to Mashaly et al. (Citation2004) and Bahadoran et al. (Citation2018). This environmental stress was maintained during the rest of the experiment. Mortalities were recorded in both groups.
Extraction of total RNA and preparation of cDNA
At the end of the experiment, 10 hens from each group were randomly selected, anesthetized (Pentobarbital sodium, 50 mg/mL; dose of 30 mg/kg, IV) and decapitated. Samples of uterine and ovarian tissues were separated. This procedure was performed 10 hours before the expected time of laying when the eggshell was being formed in the uterus. The internal surface of the uterus was scraped, frozen in liquid nitrogen and then stored at −70 °C. The samples were homogenized in a liquid nitrogen bath. Total RNA was extracted from homogenized samples (100 mg) using RNX-Plus (Sinaclon Bioscience, Karaj, Iran) as per the manufacturer’s recommendations and according to Ahmadipour et al. (Citation2015). The yield was treated with DNase (Sinaclon Bioscience, Karaj, Iran) to remove contaminating DNA. To quantify RNA, agarose gel electrophoresis and spectrophotometry were used. The presence of intact 28S and 18S ribosomal bands in the agarose gel and absorbance ratios (A260/280) of 1.8 to 2.0 verified the RNA purity.
The PrimeScript™ RT Reagent Kit (Takara Bio Inc., Japan) was used to make cDNA from 1 µg of RNA in a total volume of 10 µl. Following reverse transcription by this kit, the mix was heated to 85 °C for 5 s to denature the residual RNA and inactivate reverse transcriptase. The cDNA was stored at −20 °C until real-time PCR was performed (Ahmadipour et al., Citation2015).
Real-time PCR
Real-time PCR was performed to analyze expression of the YWHAZ, HPRT1, HMBS, RPL13, TFRC, ACTB, TBP, RPL32, and 18SrRNA genes in the ovarian and uterine tissues of the control and heat-stress groups. Real-time PCR was done using SYBR green Premix Ex Taq II (Takara Bio Inc, Shiga, Japan) and 2 µl cDNA in a total volume of 20 µl, as published in detail previously (Ahmadipour et al., Citation2015; Hassanpour, Khosravi Alekoohi, Madreseh, Bahadoran, & Nasiri, Citation2016). The full name and functions of target genes are presented in . The specific primers of the mentioned genes were designed according to Hassanpour et al. (Citation2018) which their sequences are offered in . The amplification program consists of 40 cycles at 94 °C for 25 seconds, 60 °C to 64 °C for 28 seconds, and 72 °C for 20 seconds. The no-template control and no-reverse-transcriptase control were used for tracing possible contamination in the PCR mix. The melt curve analysis of PCR products was recorded to assess whether single and specific product of genes had been produced (). Threshold cycle numbers were analyzed by LinRegPCR software version 2012.0 (Amsterdam, Netherlands) to calculate mean efficiency values. This parameter is used in geNorm, BestKeeper and NormFinder programs to determine the most stable reference genes.
Figure 1. Specificity of real-time PCR amplification. Melting curves (dissociation curves) of the 9 reference gene amplicons after the real-time PCR reactions, all showing one peak. X-axis (horizontal): temperature (°C); Y-axis (vertical): negative derivative of fluorescence over temperature (-dF/dT).
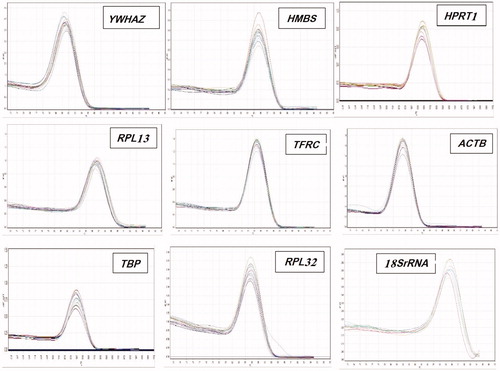
Table 1. Name and function of genes mentioned in the text.
Table 2. Primers used for quantitative real-time PCR analysis of chicken mRNAs.
Data analysis for determining gene expression stability
In this study, three statistical algorithms (BestKeeper, geNorm, and NormFinder) were used to specify the stability rate of gene expression; the most stable genes were identified via Ct values. In the BestKeeper approach, an intrinsic variance of gene expression was calculated to estimate the inter-gene relationship, the Pearson correlation coefficient, the sample integrity, and the expression stability within each candidate gene. The gene data with higher correlation values were unified to calculate the geometric mean of Ct values as a BestKeeper index. Then, the Pearson's correlation coefficient was estimated between each candidate gene and the index, which provides an approximation of the contribution of the gene to that index (Pfaffl, Tichopad, Prgomet, & Neuvians, Citation2004).
In the geNorm program, the calculating procedure is based on normalized Ct values, in which values for an individual gene are normalized to the sample with the least Ct value (the highest expression) for that gene. The normalized Ct values were estimated according to Vandesompele (2002). In this approach, the pairwise variation of a specific gene is determined by all other control genes as the standard deviation of the logarithmically transformed expression ratios. The internal control gene-stability (M) is determined as the mean of the pairwise variation of one gene with other candidate genes. The least M Value means that the expression of the gene is the most stable. To choose the best candidate reference genes, the geNorm method again counts the M stability after elimination of the least stable gene and repeats the analysis until only the two most stable genes remain. The geNorm approach also determines the minimum number of reference genes required for proper data normalization via calculating a pairwise variation (V) using n (number) and n + 1 reference genes. The high V values show a considerable effect of the extra gene on data normalization and affirm the necessity for including this gene among the controls (Vandesompele, Citation2002). NormFinder is an approach that uses normalized Ct values and estimates both the overall variation of the candidate normalization genes and the variation between subgroups of the same samples. This method applies the intra- and inter-group variations to calculate a stability value for each gene, which offers a practical quantity for the systematic error that will be introduced when using the evaluated gene. Candidate reference genes can then be ranked based on the stability value, where the lowest values correspond to the most stable genes (Andersen, Jensen, & Ørntoft, Citation2004; Staines et al., Citation2016).
Results
Mortality rate
The mortality rate in the heat-stress group of hens was 15% while there was no mortality in the control group.
Ranking of candidate reference genes in the uterus
The stability ranking of the nine examined genes in the uterus of laying hens under heat-stress and control groups calculated by NormFinder is as follow (in order of most stable to the least): YWHAZ, HPRT1, HMBS, RPL13, TFRC, TBP, ACTB, RPL32, and 18SrRNA. Likewise, BestKeeper ranked the stability of these genes as YWHAZ, HPRT1, HMBS, TFRC, RPL13, ACTB, 18SrRNA, RPL32, and TBP, while geNorm ranked them in the order of YWHAZ, HPRT1, RPL13, HMBS, TBP, TFRC, ACTB, RPL32, and 18SrRNA. The overall mean result of the three software programs used showed that the most stable reference genes in the uterus of chickens consisted of YWHAZ, HPRT1, HMBS, RPL13, TFRC, ACTB, TBP, RPL32, and 18SrRNA respectively ().
Table 3. Candidate reference genes ranking in the uterus of heat-stress and control hens.
Ranking of candidate reference genes in the ovary
The stability ranking of the examined genes in the ovary of laying hens under heat-stress and control groups by the three software are presented below:
NormFinder- HPRT1, YWHAZ, TFRC, RPL13, HMBS, TBP, ACTB, RPL32 and 18SrRNA.
BestKeeper- YWHAZ, HPRT1, HMBS, TBP, ACTB, RPL13, TFRC, RPL32 and 18SrRNA.
geNorm- YWHAZ, HPRT1, HMBS, TFRC, RPL13, TBP, RPL32, 18SrRNA and ACTB.
The overall mean results of the three programs indicated that the best stable reference genes in the ovary of control and heat-stress chickens consisted of YWHAZ, HPRT1, TFRC, HMBS, RPL13, TBP, RPL32, ACTB, and 18SrRNA respectively ().
Table 4. Candidate reference genes ranking in the ovary of heat-stress and control hens.
In the geNorm program, pairwise variation analysis was carried out between the normalization factors NFn and NFn + 1 to estimate the optimal number of reference genes for normalization. Only a combination of the most stable genes with a pairwise variation value less than the cut off value of 0.15, is proposed as an adequate number of genes (Ghasemi et al., Citation2017). None of yje geNorm results was lower than 0.15 (not shown); thus, it was not possible to determine the optimal number of reference genes by this approach.
Overall resultant of analysis
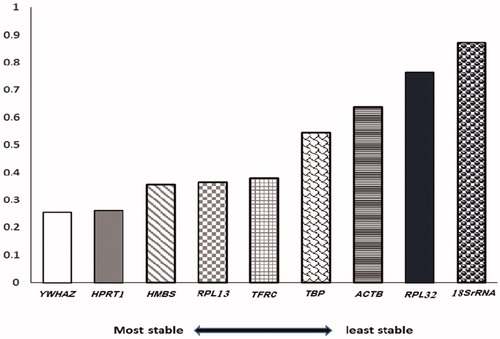
While there were differences in the ranked order presented by all three analysis programs for the uterus and ovary, the overall resultant (mean) of the analysis indicated that candidate reference genes could be ranked as YWHAZ, HPRT1, HMBS, RPL13, TFRC, TBP, ACTB, RPL32, and 18SrRNA (). Commonly, 3 or 4 reference genes are recommended for normalization (Dorak, Citation2007; Pfaffl et al., Citation2004), hence we suggest a combination of YWHAZ, HPRT1, and HMBS genes as the appropriate choice for an accurate normalization strategy in laying hens under heat stress.
Discussion
In order to select the most suitable reference for gene expression normalization in the laying hens under heat stress, we analyzed two critical tissues of the reproductive system i.e. ovary and uterus, and found that the most stable genes are YWHAZ, HPRT1, HMBS, RPL13, TFRC, TBP, ACTB, RPL32, and 18SrRNA respectively. Moreover, selection of the most suitable genes to normalize Ct values is also essential for precise evaluation of the gene expression changes. In addition, the critical character of a suitable reference gene is its stable expression under all pathophysiologic situations in all cells; however, no such ideal reference genes have been found in heat stress (Dheda et al., Citation2004).
BestKeeper, geNorm, and NormFinder programs have commonly been used in previous studies for evaluating reliable housekeeping genes, although some studies have used other approaches such as comparative delta-Ct and RefFinder (Bagés, Estany, Tor, & Pena, Citation2015; Mitra, Bilic, Hess, & Liebhart, Citation2016). It should be noted that the analytic methods used may result in different outputs denoting the most stable genes. Thus, it is more logical to apply the outputs of several analytic approaches for selection of reliable reference genes, as was done in the present study.
We found that the use of the three selected software programs was simple and direct, each estimating an easily available quantification of gene expression stability. It seems that the geNorm program is more amenable to use; those using BestKeeprer can easily consider various stages of calculation and uses equations included in this software. Moreover, BestKeeper facilitates the direct input of cycle threshold values of genes and notes their missing values. It is based on an Excel spreadsheet, without a simple user interface. Conversely, NormFinder is based on normalized Ct values as input identical to geNorm. However, unlike the latter, it does not consider missing values and does not show the equations used in its calculations (Ghasemi, et al., Citation2017).
Many studies have been carried out to pinpoint the reliable reference genes in tissues of birds (Bagés et al., Citation2015; Li, Bang, Handberg, Jorgensen, & Zhang, Citation2005; Olias, Adam, Meyer, Scharff, & Gruber, Citation2014; Zinzow-Kramer, Horton, & Maney, Citation2014). The single most often used non-validated reference gene in chicken tissues employed in many gene expression studies include: ACTB (Hassanpour, Khalaji-Pirbalouty, Nasiri, Mohebbi, & Bahadoran, Citation2015; Hassanpour, Teshfam, Momtaz, Brujeni, & Shahgholian, Citation2010; Zhang et al., Citation2011; Citation2013), HPRT1 (Gomez, Moreno, Iglesias, Coral, & Hernandez, Citation2007; Rojas, Pinzón, & Vásquez, Citation2012), GAPDH (Settle, Falkenstein, & Klandorf, Citation2015; Wang et al., Citation2013) and 28SrRNA (Hamal, Wideman, Anthony, & Erf, Citation2010). In the present study, we ranked three best stable reference genes as YWHAZ, HPRT1, and HMBS that could be appropriate in the gene expression study of the reproductive system in laying hens under heat stress, especially in the ovary and uterine tissues. YWHAZ encodes 14-3-3 protein zeta/delta that is a major regulator of biological processes including metabolism, transcription, apoptosis, protein transport, and cell cycle regulation (Jérôme & Paudel, Citation2014). HPRT1 encodes hypoxanthine-guanine phosphoribosyltransferase that catalyzes the conversion of hypoxanthine to inosine monophosphate and guanine to guanosine monophosphate. This enzyme has a central role in the generation of purine nucleotides through the purine salvage pathway (Sculley, Dawson, Emmerson, & Gordon, Citation1992). HMBS encodes porphobilinogen deaminase that is involved in the heme biosynthetic pathway via catalyzing the condensation of four porphobilinogen molecules into the linear hydroxymethylbilane (Gill et al., Citation2009). These three genes have already been selected as reliable reference genes in previous studies (YWHAZ in the studies of Bagés et al., Citation2015 and Olias et al., Citation2014; RPL13 gene in the studies of Katarzyńska-Banasik, Grzesiak, & Sechman, Citation2017, Mitra et al., Citation2016 and Zhang et al., Citation2017; HMBS in the studies of Nascimento et al., Citation2015, Yin et al., 2017 and Zhang et al., Citation2017). However, unlike our study, their target tissues were not ovary and uterus, or the avian species were not laying hens, except for one study (muscles, liver and abdominal fat of chickens in the study of Bagés et al., Citation2015; the brain and gonad tissues of mallard, chicken, crane, eagle, turkey, cockatiel, penguin, ostrich and zebra finch in the study of Olias et al., Citation2014; the ovarian tissue of chicken in the study of Katarzyńska-Banasik et al., Citation2017; the spleen, liver, cecum and cecal tonsil tissues of turkey and layer/broiler chicken in the study of Mitra et al., Citation2016; the liver and jejunum of yellow-feathered broilers in the study of Zhang et al., Citation2017; the muscle of broiler chickens in the study of Nascimento et al., Citation2015; chicken embryo fibroblasts in the study of Yin et al., 2017). Bahadoran et al. (Citation2018) examined four reference genes, ACTB, TBP, 18SrRNA and RPL32 using the BestKeeper program on the uterus of laying hens, and found that ACTB was the best reference gene in that study.
One of the major factors influencing the selection of a reliable reference gene is a type of challenge used in an experiment. In the previous studies, the best reference genes were determined in birds after different challenge types e.g. silver nanoparticle treatment (Katarzyńska-Banasik et al., Citation2017), different infection models (Mitra et al., Citation2016; Yin et al., Citation2011), lysine supplementation (Nascimento et al., Citation2015), cold-induced pulmonary hypertension (Hassanpour et al., Citation2018), nicarbazin treatment (Samiullah, Roberts, & Wu, Citation2017) and different mitogens (Borowska, Rothwell, Bailey, Watson, & Kaiser, Citation2016). However, in our study, heat stress was the major challenge for ovarian/uterine tissues in laying hens; hence, that the results of this study are not completely similar to previous studies can be considered to be related to the differences in stressors studied. For this reason, reporting of 18SrRNA and RPL32 as the most unstable housekeeping genes in this study was in contrast to Bagés et al. (Citation2015), Olias et al. (Citation2014) and Borowska et al. (Citation2016). In these studies, RPL32 for pectoralis major and abdominal fat of chicken, 18SrRNA for ovary, testis, and brain of zebra finch, and for lymphoid organs of chicken were determined as the most stable reference genes. However, Zinzow-Kramer et al. (Citation2014) reported that 18SrRNA and RPL32 were the most unstable reference genes in testis, ovary, and brain of sparrows. As mentioned, a reference gene could be stable or unstable depending on the type of animal and the tissue.
However, based on the mixed results of previous studies, it can be deduced that a statistically significant difference observed in expression levels of target genes in various studies surely depends on the stability level of the reference gene used for normalization of the target gene expression (Yin et al., Citation2011). As is noted in different studies, critical factors such as animal species, type of cell and tissue, biological conditions and the type of challenge in the cell, can strongly affect the results for the most stable reference genes. Thus, it is important to find the best reference gene for each individual tissue under study with a specific challenge.
In summary, a reference gene assay was designed in this study for reliable normalization of quantitative real-time PCR data, obtained from the ovarian and uterine tissues of control and heat-stress groups of laying hens. The profiling of the gene expression pattern of nine putative reference genes by the BestKeeper, geNorm, and NormFinder programs offered a ranking order of YWHAZ, HPRT1, HMBS, RPL13, TFRC, TBP, ACTB, RPL32, and 18SrRNA. Of these, the combination of YWHAZ, HPRT1, and HMBS can be considered as the most suitable reference genes to facilitate accurate gene expression studies of laying hens under heat stress.
Disclosure statement
There are no conflicts of interest that could have inappropriately influenced the study or the reporting of the findings.
Additional information
Funding
Notes on contributors
Hossein Hassanpour
Hossein Hassanpour is a professor of veterinary physiology.
Zahra Aghajani
Zahra Aghajani and Navid Farhadi are doctoral candidates of faculty of veterinary medicine.
Shahab Bahadoran
Shahab Bahadoran is a doctor of poultry hygiene and diseases.
Navid Farhadi
Zahra Aghajani and Navid Farhadi are doctoral candidates of faculty of veterinary medicine.
Hasan Nazari
Hasan Nazari and Waranyoo Kaewduangta are doctors of biotechnology.
Waranyoo Kaewduangta
Hasan Nazari and Waranyoo Kaewduangta are doctors of biotechnology.
References
- Ahmadipour, B., Hassanpour, H., Asadi, E., Khajali, F., Rafiei, F., & Khajali, F. (2015). Kelussia odoratissima Mozzaf–A promising medicinal herb to prevent pulmonary hypertension in broiler chickens reared at high altitude. Journal of ethnopharmacology, 159, 49–54. doi:10.1016/j.jep.2014.10.043
- Allahverdi, A., Feizi, A., Takhtfooladi, H.A., & Nikpiran, H. (2013). Effects of heat stress on acid-base imbalance, plasma calcium concentration, egg production and egg quality in commercial layers. Global veterinaria, 10, 203–207.
- Andersen, C.L., Jensen, J.L., & Ørntoft, T.F. (2004). Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer research, 64, 5245–5250. doi:10.1158/0008-5472.CAN-04-0496
- Bagés, S., Estany, J., Tor, M., & Pena, R. (2015). Investigating reference genes for quantitative real-time PCR analysis across four chicken tissues. Gene, 561, 82–87. doi:10.1016/j.gene.2015.02.016
- Bahadoran, S., Dehghani Samani, A., & Hassanpour, H. (2018). Effect of heat stress on the gene expression of ion transporters/channels in the uterus of laying hens during eggshell formation. Stress, 21, 51–58. doi:10.1080/10253890.2017.1394291
- Borowska, D., Rothwell, L., Bailey, R., Watson, K., & Kaiser, P.J. (2016). Identification of stable reference genes for quantitative PCR in cells derived from chicken lymphoid organs. Veterinary immunology and immunopathology, 170, 20–24. doi:10.1016/j.vetimm.2016.01.001
- Dheda, K., Huggett, J.F., Bustin, S.A., Johnson, M.A., Rook, G., & Zumla, A. (2004). Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques, 37, 112–119. doi:10.2144/04371RR03
- Dorak, M. T. (2007). Real-time PCR. Taylor & Francis, New York.
- Ghasemi, S., Mirshokraei, P., Hassanpour, H., & Sardari, K. (2017). Identification of reliable reference genes for quantitative real-time PCR in equine fibroblast-like synoviocytes treated by doxycycline. Journal of Equine Veterinary Sciences, 50, 44–51. doi:10.1016/j.jevs.2016.11.008
- Gill, R., Kolstoe, S.E., Mohammed, F., Al D-Bass, A., Mosely, J.E., Sarwar, M., … Shoolingin-Jordan, P.M. (2009). Structure of human porphobilinogen deaminase at 2.8 Å: The molecular basis of acute intermittent porphyria. Biochemical journal, 420, 17–25. doi:10.1042/BJ20082077
- Gomez, A., Moreno, M., Iglesias, A., Coral, P., & Hernandez, A. (2007). Endothelin 1, its endothelin type A receptor, connective tissue growth factor, platelet-derived growth factor, and adrenomedullin expression in lungs of pulmonary hypertensive and nonhypertensive chickens. Poultry science, 86, 909–916. doi:10.1093/ps/86.5.909
- Gu, Y., Li, M., Zhang, K., Chen, L., Jiang, A., Wang, J., & Li, X. (2011). Evaluation of endogenous control genes for gene expression studies across multiple tissues and in the specific sets of fat‐and muscle‐type samples of the pig. Journal of animal breeding and genetics, 128, 319–325. doi:10.1111/j.1439-0388.2011.00920.x
- Hamal, K.R., Wideman, R.F., Anthony, N.B., & Erf, G.F. (2010). Differential expression of vasoactive mediators in microparticle-challenged lungs of chickens that differ in susceptibility to pulmonary arterial hypertension. American journal of physiology. Regulatory, integrative and comparative physiology, 298, R235–R242. doi:10.1152/ajpregu.00451.2009
- Hassanpour, H., Bahadoran, S., Farhadfar, F., Chamali, Z.F., Nazari, H., & Kaewduangta, W. (2018). Identification of reliable reference genes for quantitative real-time PCR in lung and heart of pulmonary hypertensive chickens. Poultry science, 97, 4048–4056. doi:10.3382/ps/pey258
- Hassanpour, H., Khalaji-Pirbalouty, V., Nasiri, L., Mohebbi, A., & Bahadoran, S. (2015). Oxidant and enzymatic antioxidant status (gene expression and activity) in the brain of chickens with cold-induced pulmonary hypertension. International journal of biometeorology, 59, 1615–1621. doi:10.1007/s00484-015-0968-z
- Hassanpour, H., Khosravi Alekoohi, Z., Madreseh, S., Bahadoran, S., & Nasiri, L. (2016). Variation of heat shock protein gene expression in the brain of cold-induced pulmonary hypertensive chickens. British poultry science, 57, 636–642. doi:10.1080/00071668.2016.1196340
- Hassanpour, H., Teshfam, M., Momtaz, H., Brujeni, G.N., & Shahgholian, L. (2010). Up-regulation of Endothelin-1 and Endothelin type A receptor genes expression in the heart of broiler chickens versus layer chickens. Research in veterinary science, 89, 352–357. doi:10.1016/j.rvsc.2010.04.005
- Jérôme, M., & Paudel, H.K. (2014). 14-3-3ζ regulates nuclear trafficking of protein phosphatase 1α (PP1α) in HEK-293 cells. Archives of biochemistry and biophysics, 558, 28–35. doi:10.1016/j.abb.2014.06.012
- Katarzyńska-Banasik, D., Grzesiak, M., & Sechman, A. (2017). Selection of reference genes for quantitative real-time PCR analysis in chicken ovary following silver nanoparticle treatment. Environmental toxicology and pharmacology, 56, 186–190. doi:10.1016/j.etap.2017.09.011
- Kozera, B., & Rapacz, M. (2013). Reference genes in real-time PCR. Journal of applied genetics, 54, 391–406. doi:10.1007/s13353-013-0173-x
- Kubista, M., Andrade, J.M., Bengtsson, M., Forootan, A., Jonák, J., Lind, K., … Strömbom, L. (2006). The real-time polymerase chain reaction. Molecular aspects of medicine, 27, 95–125. doi:10.1016/j.mam.2005.12.007
- Lara, L.J., & Rostagno, M.H. (2013). Impact of heat stress on poultry production. Animals: an open access journal from Mdpi, 3, 356–369.
- Li, Y.P., Bang, D.D., Handberg, K.J., Jorgensen, P.H., & Zhang, M.F. (2005). Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus. Veterinary microbiology, 110, 155–165. doi:10.1016/j.vetmic.2005.06.014
- Mahmoud, K.Z., Beck, M.M., Scheideler, S.E., Forman, M.F., Anderson, K.P., & Kachman, S.D. (1996). Acute high environmental temperature and calcium-estrogen relationships in the hen. Poultry science, 75, 1555–1562. doi:10.3382/ps.0751555
- Mashaly, M.M., Hendricks, G.L., Kalama, M.A., Gehad, A.E., Abbas, A.O., & Patterson, P.H. (2004). Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry science, 83, 889–894. doi:10.1093/ps/83.6.889
- Mitra, T., Bilic, I., Hess, M., & Liebhart, D. (2016). The 60S ribosomal protein L13 is the most preferable reference gene to investigate gene expression in selected organs from turkeys and chickens, in context of different infection models. Veterinary research, 47, 105. doi:10.1186/s13567-016-0388-z
- Nascimento, C.S., Barbosa, L.T., Brito, C., Fernandes, R.P., Mann, R.S., Pinto, A.P.G., … Duarte, M.S. (2015). Identification of suitable reference genes for real time quantitative polymerase chain reaction assays on Pectoralis major muscle in chicken (Gallus gallus). PloS one, 10, e0127935. doi:10.1371/journal.pone.0127935
- NRC. (1994). Nutrient requirements of poultry. Washington D.C.: National Academies Press,
- Olias, P., Adam, I., Meyer, A., Scharff, C., & Gruber, A.D. (2014). Reference genes for quantitative gene expression studies in multiple avian species. PloS one, 9, e99678. doi:10.1371/journal.pone.0099678
- Pfaffl, M.W., Tichopad, A., Prgomet, C., & Neuvians, T.P. (2004). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Best keeper–excel-based tool using pair-wise correlations. Biotechnology letters, 26, 509–515. doi:10.1023/B:BILE.0000019559.84305.47
- Puthpongsiriporn, U., Scheideler, S.E., Sell, J.L., & Beck, M.M. (2001). Effects of vitamin E and C supplementation on performance, in vitro lymphocyte proliferation, and antioxidant status of laying hens during heat stress. Poultry science, 80, 1190–1200. doi:10.1093/ps/80.8.1190
- Quinteiro-Filho, W.M., Ribeiro, A., Ferraz-de-Paula, V., Pinheiro, M.L., Sakai, M., Sá, L.R.M., … Palermo-Neto, J. (2010). Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry science, 89, 1905–1914. doi:10.3382/ps.2010-00812
- Rojas, R.A.A., Pinzón, J.E.C., & Vásquez, A.H. (2012). Diminished pulmonary expression of hypoxia-inducible factor 2-α, vascular endothelial growth factor and hepatocyte growth factor in chickens exposed to chronic hypobaric hypoxia. The journal of poultry science, 49, 205–211. doi:10.2141/jpsa.011036
- Rozenboim, I., Tako, E., Gal-Garber, O., Proudman, J.A., & Uni, Z. (2007). The effect of heat stress on ovarian function of laying hens. Poultry science, 86, 1760–1765. doi:10.1093/ps/86.8.1760
- Sahin, K., & Kucuk, O. (2001). Effects of vitamin C and vitamin E on performance, digestion of nutrients and carcass characteristics of Japanese quails reared under chronic heat stress (34 °C). Journal of animal physiology and animal nutrition, 85, 335–341. doi:10.1046/j.1439-0396.2001.00339.x
- Samiullah, S., Roberts, J., & Wu, S.-B.J. (2017). Reference gene selection for the shell gland of laying hens in response to time-points of eggshell formation and nicarbazin. PloS one, 12, e0180432. doi:10.1371/journal.pone.0180432
- Sculley, D.G., Dawson, P.A., Emmerson, B.T., & Gordon, R.B. (1992). A review of the molecular basis of hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Human genetics, 90, 195–207.
- Settle, T., Falkenstein, E., & Klandorf, H. (2015). The effect of allopurinol administration on mitochondrial respiration and gene expression of xanthine oxidoreductase, inducible nitric oxide synthase, and inflammatory cytokines in selected tissues of broiler chickens. Poultry science, 94, 2555–2565. doi:10.3382/ps/pev193
- Song, Z., Liu, L., Sheikhahmadi, A., Jiao, H., & Lin, H. (2012). Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. BioMed research international, 2012, 1–8. doi:10.1155/2012/484869
- Sonna, L.A., Fujita, J., Gaffin, S.L., & Lilly, C.M. (2002). Invited review: Effects of heat and cold stress on mammalian gene expression. Journal of Applied Physiology (Bethesda, Md.: 1985), 92, 1725–1742. doi:10.1152/japplphysiol.01143.2001
- St-Pierre, N.R., Cobanov, B., & Schnitkey, G. (2003). Economic losses from heat stress by US livestock industries. Journal of dairy science, 86, E52–E77. doi:10.3168/jds.S0022-0302(03)74040-5
- Staines, K., Batra, A., Mwangi, W., Maier, H.J., Van Borm, S., Young, J.R., … Butter, C. (2016). A versatile panel of reference gene assays for the measurement of chicken mRNA by quantitative PCR. PloS one, 11, e0160173. doi:10.1371/journal.pone.0160173
- Sun, H., Jiang, R., Xu, S., Zhang, Z., Xu, G., Zheng, J., & Qu, L. (2015). Transcriptome responses to heat stress in hypothalamus of a meat-type chicken. Journal of animal science and biotechnology, 6, 6. doi:10.1186/s40104-015-0003-6
- Suzuki, T., Higgins, P.J., & Crawford, D.R. (2000). Control selection for RNA quantitation. Biotechniques, 29, 332–337. doi:10.2144/00292rv02
- Vandesompele, J. (2002). Accurate normalization of real-time RT-PCR data by geometric averaging of multiple internal control genes. Genome biology, 3, 4.1–11.
- Wang, Y., Guo, Y., Ning, D., Peng, Y., Yang, Y., & Liu, D. (2013). Analysis of Liver Transcriptome in Broilers with Ascites and Regulation by L-Carnitine. The journal of poultry science, 50, 126–137. doi:10.2141/jpsa.0120124
- Wong, M.L., & Medrano, J.F. (2005). Real-time PCR for mRNA quantitation. Biotechniques, 39, 75–85. doi:10.2144/05391RV01
- Yin, R., Liu, X., Liu, C., Ding, Z., Zhang, X., Tian, F., … de Angelis, M.H. (2011). Systematic selection of housekeeping genes for gene expression normalization in chicken embryo fibroblasts infected with Newcastle disease virus. Biochemical and biophysical research communications, 413, 537–540. doi:10.1016/j.bbrc.2011.08.131
- Zhang, J., Feng, X., Zhao, L., Wang, W., Gao, M., Wu, B., & Qiao, J. (2013). Expression of hypoxia-inducible factor 1α mRNA in hearts and lungs of broiler chickens with ascites syndrome induced by excess salt in drinking water. Poultry science, 92, 2044–2052. doi:10.3382/ps.2013-03097
- Zhang, J., Gao, Y.Y., Huang, Y.Q., Fan, Q., Lu, X.T., & Wang, C.K. (2017). Selection of housekeeping genes for quantitative gene expression analysis in yellow-feathered broilers. Italian Journal of Animal Science, 17, 540–546. doi:10.1080/1828051x.2017.1365633
- Zhang, Z., Lv, Z., Li, J., Li, S., Xu, S., & Wang, X. (2011). Effects of cold stress on nitric oxide in duodenum of chicks. Poultry science, 90, 1555–1561. doi:10.3382/ps.2010-01333
- Zinzow-Kramer, W.M., Horton, B.M., & Maney, D.L. (2014). Evaluation of reference genes for quantitative real-time PCR in the brain, pituitary, and gonads of songbirds. Hormones and behavior, 66, 267–275. doi:10.1016/j.yhbeh.2014.04.011