Abstract
Emotional state has been shown to influence cognitive performance. However, the influence of mood on auditory processing is not fully understood. The auditory steady state response (ASSR) is the entrainment of neural activities elicited by periodic auditory stimulation, which is commonly used to evaluate the sensory and cognitive functions of brain. It has been shown that ASSR at 40 Hz is impaired at some psychotic disorders, such as schizophrenia and bipolar disorder. The primary goal of this study is to investigate the effect of emotional arousal on ASSR. To this end, we performed simultaneous recordings of local field potential (LFP) in response to 40 Hz click-train stimuli in the primary auditory cortex (A1) and medial prefront cortex (mPFC) of rats. During the electrophysiological recording, a negative mood was induced by means of the foot shocks occurred randomly in the inter-stimulus intervals. We found that both the power and phase-locking of ASSR in A1 were significantly increased under arousal condition, and phase-locking of ASSR in mPFC was also increased. The enhanced ASSRs were accompanied by an increase in coherence between A1 and mPFC. Our results suggest that A1-to-mPFC information transfer is enhanced under arousal state and the functional connectivity between mPFC and A1 may contribute to the emotional modulation of auditory process.
Introduction
The context of sensory stimuli constantly changes in natural environments, and the adaptive behavior of animals depends on their ability to dynamically modulate the processing of sensory stimuli. Affective states, such as moods and emotions are thought to facilitate adaptive responding to situational demands. Several studies have demonstrated that changes in emotional states are associated with changes in perceptual or cognitive processes, including visual perception (Kuhbandner et al., Citation2009; Lee, Baek, Lu, & Mather, Citation2014), spatial attention, and cognitive control (Chigusa, Moroi, & Shoji, Citation2017). Influences of emotional state on auditory processing, however, are still not completely known.
Nevertheless, we can speculate that auditory processing is easily modulated by emotion state, because the auditory system continuously works as an alarm system to detect potentially relevant stimuli, within, as well as outside of our visual field of view (Asutay & Västfjäll, Citation2015). Modulation of the auditory system by affective state may enhance detection of these potentially relevant stimuli and thereby increase chances of survival. Psychophysiological studies of normal human subjects show that affective arousal induced by threatening events changes the threshold of auditory stimuli perception (Resnik, Sobel, & Paz, Citation2011; Bolders, Band, & Stallen, Citation2017) and the loudness perception (Siegel & Stefanucci, Citation2011). The electrophysiological examinations revealed that the auditory-evoked potentials (AEPs) of inferior colliculus activity were augmented by fear of mild electric shock (Eby, Butts, Hoffman, & Sauer, Citation2015; Al-Abduljawad, Baqui, Langley, Bradshaw, & Szabadi, Citation2008). Studies on animals found that painful stimuli alone trigger the induction of immediate early genes, namely, zif268 and cfos, in the auditory cortex (Gruene et al., Citation2016; Peter et al., Citation2012) and lead to a transient modification in spine turnover (Moczulska et al., Citation2013). Electrophysiological evidence from animal studies also indicated that threat conditioning produces specific and long-lasting plasticity of the conditioned stimulus (CS) responses in auditory cortex (Bakin & Weinberger, Citation1990; Edeline & Weinberger, Citation1993; Wigestrand, Schiff, Fyhn, LeDoux, & Sears, Citation2017) However, irreversible lesions in the entire auditory cortex do not affect learned fear to simple auditory CS in rodents (Campeau & Davis, Citation1995; Falls & Davis, Citation1993; Grosso, Cambiaghi, Concina, Sacco, & Sacchetti, Citation2015; Romanski & LeDoux, Citation1992). These lesion studies demonstrate that the auditory cortex is not essential for simple auditory behaviors in mammals. Therefore, the neural mechanism underlying emotional arousal modulation of auditory system, especially the cooperation between auditory cortex and other brain areas, is worthy to be further explored.
The medial prefront cortex (mPFC) is critical for the generation of appropriate behavioral responses to salient sensory stimuli and emotionally arousing events in the external world and plays a key role in the modulation of emotion state (Dejean et al., Citation2016; Do, Quirk, Li, & Penzo, Citation2016; Herry & Johansen, Citation2014; Karalis et al., Citation2016). Indeed, increased activity in the mPFC is associated with elevated level of emotional arousal (Karalis et al., Citation2016; Likhtik, Stujenske, Topiwala, Harris, & Gordon, Citation2014). The mPFC receives dense innervations from arousal system nuclei in the brainstem (i.e. nucleus of solitary tract, locus coeruleus, A1/A2 cell groups) (Siuda, Al-Hasani, McCall, Bhatti, & Bruchas, Citation2016; Zhang, Muller, & McDonald, Citation2013) and is engaged when emotional stimuli elicit an arousal effect to modulate behavior and processes of emotional memory formation (Atsak et al., Citation2015; Siuda et al. Citation2016).
At the lower level of the auditory hierarchy, the primary auditory cortex (A1) is the first station of cortical auditory processing, which plays a key role in the sound representation and auditory perception (Dong, Qin, Liu, Zhang & Sato, Citation2011; Dong, Qin, Zhao, Zhong & Sato, Citation2013). The A1 is connected to the mPFC via both direct and indirect anatomical pathways (Van Eden, Lamme, & Uylings, Citation1992). Previous electrophysiological studies on animals have shown that the mPFC and A1 show similar neuronal processes (Rodgers & DeWeese, Citation2014) and dynamically establish a functional connection during auditory discrimination processes (Fritz, David, Radtke-Schuller, Yin, & Shamma, Citation2010). Magnetoencephalographic data from human participants also showed an enhanced feed-forward theta/alpha-band connectivity between auditory-prefrontal networks during auditory mismatch detection (Recasens, Gross, & Uhlhaas, Citation2018). It has been proposed that the auditory-frontal cortical connection may drive the subject’s behavioral choice in response to different sound stimuli (Concina et al. Citation2018).
To date, the impacts of emotional arousal on the neural activities of A1 and mPFC and the interaction between them have not been well investigated. Therefore, in this study, we used foot-shocks to induce emotional arousal and investigate the degree to which activations of the emotion system modulate auditory processing in healthy rats. We used a train of click as stimulus sounds to access the auditory steady state response (ASSR) of A1 and mPFC. ASSR is the entrainment of neural activities elicited by periodic auditory stimulation, which is commonly used to evaluate the sensory and cognitive functions of the central nervous system (Parker & Sweet, Citation2017). The power (magnitude) and phase locking ability (phase consistency across trials) of the ASSR can reflect the functional integrity of the neural circuits that support synchronization across frequencies (Makeig, Debener, Onton, & Delorme, Citation2004). Abnormal ASSR to 40-Hz stimulation has been found to be associated with psychotic disorders, such as schizophrenia (O'Donnell et al., Citation2013), bipolar disorder (Rass et al., Citation2010). We hypothesized that the affective arousal evoked by foot-shock would modulate the 40-Hz ASSRs in A1 and mPFC and affect the coherence between them.
Materials and methods
Animals
A total of 14 male Wistar rats, weighing 350–380 g and aged 12 weeks at the time of surgery, were used in this study. Animals were single-housed under the following laboratory conditions: room temperature 22 °C, relative humidity of 55%, 12 h light cycle beginning at 8 am, food and water ad libitum. Experiments were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996. This study was approved by China Medical University Animal Care and Use Committee.
Surgery of electrode implantation
Following a period of two weeks of handling for at least once a day for 5 min, animals were subjected to a surgery for implantation of chronic single-wire electrodes. Animals were kept under anesthesia during the whole procedure with a gaseous mixture of 2–4% isoflurane in air. Atropine sulfate (0. 1 mg/kg) was used to reduce the viscosity of bronchial secretions. Temperature was monitored rectally and maintained at 37 °C using a feedback-controlled blanket. After placing the animal in a stereotaxic frame (#68001, RWD Life science, Shenzhen, China), the cranium was exposed. Two stainless steel guide tubes (30G) were separately inserted into the left hemisphere of prelimbic region of mPFC (AP= +3.5 mm, ML = 0.6 mm, DV = 3.5 mm) and A1 (AP= −4.0 mm, ML = 7.0 mm, and DV = 5.0 mm) according to the standard rat stereotaxic atlas (Paxinos & Watson, Citation1986). After fixing the tubes using dental acrylic, we lowered a formvar-insulated nichrome microwire (ID 50 µm, OD, #762000, A-M Systems, Hofheim, USA) into each tube and kept the tip of the microwire at the level of 0.5 mm below the opening of the tube. The other end of the microwire was soldered to a pin connector, which was secured onto the cranium of the right hemisphere using dental acrylic resin. A stainless-steel screw electrode over the cerebellum served as ground. Four additional skull screws were implanted serving as anchors. Animals were allowed to recover for 2 weeks.
Auditory stimuli and experiment protocol
After recovery from surgery, animals were familiarized with the sound-attenuated recording room. Briefly, the animals were transported in their home cage to the recording room where they were left alone for 5 min. They were then put in a mesh box (40 × 40 × 60 cm) and tethered to the recording system via a flexible cable headstage and allowed to freely behave in the recording box for 15 min. This procedure was repeated for 4 days. Recording experiments were conducted on the 5th day. The sound stimulus used in our experiments was a train of click sounds to assess ASSR. The waveform of each click was a rectangular pulse of a 0.2 ms duration, which repeated at a rate of 40 cycles/s and continued for 0.5 s duration. The waveforms were generated digitally with a 100 kHz sampling rate using a custom built MATLAB (Mathworks, Natick, MA, USA) program and transferred to a analog signal by a D/A board (PCI-6052E, National Instruments, Austin, Texas, USA), then played through a loudspeaker (K701, AKG, Vienna, Austria) at the top of recording box. The intensity of the sound stimulus was adjusted to be at 70 dB SPL and measured at the center of the recording box (Brüel & Kjær type 2238 sound level meter, Naerum, Danish). In one session, 120 trials of click-train were presented at a random interval between 4 and 8 s. After completed one session of recording under normal condition, the other session of recording was conducted under arousal condition, wherein a mild foot shock (0.7 mA current, 0.5 s duration) was applied through metal bars on the floor of recording box. In one session, 10–15 foot shocks occurred randomly in the inter-stimulus intervals.
LFP data acquisition and analysis
Signals were acquired through a flexible, low noise cable connected to the pin connector implanted on the skull of the rat. The microwire output was delivered to a multi-channel preamplifier (RA16PA; TDT, Alachua, FL, USA), then to a digital signal processing module (RZ-2; TDT). The waveforms of the field potentials were amplified and low-pass filtered with a 300 Hz cutoff frequency. The field potentials were imported into MATLAB for further analysis.
LFPs were analyzed using a wavelet-based analysis algorithm, implemented in custom-written code using eeglab toolbox (https://sccn.ucsd.edu/eeglab/index.php). The trial-based spectrums of LFPs were accessed by the mean trial power (MTP) and phase-locking factor (PLF) (Delorme & Makeig, Citation2004). MTP was computed by averaging the LFP power in the spectral-temporal domain across the 120 trials from one session. PLF measures the synchronization of the LFP phase across individual trials at particular frequencies and time intervals. For the data recorded from one session, the MTP and PLF were calculated to obtain a spectral-temporal function using the multitaper method provided by eeglab. The results presented as a relative estimation of the ratio between the values after stimulus onset and pre-stimulus (stimulus/pre-stimulus).
The coherence between LFP channels was measured by event-related phase coherence (ERCOH), using the function of “newcross” in the eeglab toolbox, which is a phase coupling factor between two input data. The result of ERCOH estimate is a two-dimension array as a spectral-temporal function, which is present by subtraction of coherence values during stimulus presentation and immediately before (stimulus − pre-stimulus).
Heart rate acquisition and analysis
We used the electrocardiogram (EKG) to monitor the heart rate of rats during the experiments. EKG was obtained from an electrode sutured subcutaneously around the thoracic musculature. EKG recordings were obtained from a Teflon-coated stranded stainless steel wire (#793200, A–M Systems) with a patch of insulation removed where the wire would directly oppose the heart. A separate subcutaneous loop of wire was placed over the back to serve as the ground. Both wires were previously soldered into a pin connector, which was then embedded on the skull. EKG signals were amplified, filtered (<300 Hz), and stored (RZ-2; TDT). Heartbeats were threshold edge-detected “R-wave” events (peak voltage of individual heartbeats). Beat times were converted to beats per minute.
Histology
To confirm the position of the electrodes, at the end of the experimental period, all animals were deeply anesthetized with pentobarbital (100 mg/Kg). The animals were then perfused transcardially with fixative (4% paraformaldehyde). The brains were removed and placed in fixative solution. After further fixation the brains were coronally sectioned in 40 µm slices, collected on non-coated glass slides, stained with thinonin. Electrode tip positions were confirmed by microscopic observation of the slides. Representative sections from the A1 and mPFC are presented in .
Statistical analysis
Statistical analysis was performed using SPSS for Windows (18.0, 2010. Chicago: SPSS, Inc.). Paired t-tests were performed on the comparisons between the data of normal and arousal session. Statistical significance was determined at p < .05. Data were presented as mean ± SEM.
Results
Inducing emotional arousal by foot-shock
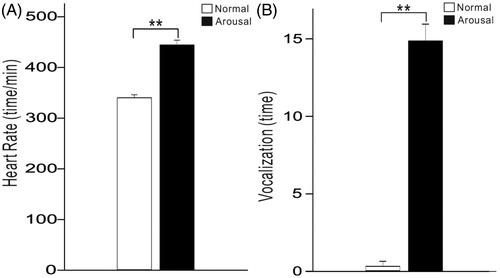
We measured the heart rate of 14 rats using EKG during the session only presenting sound stimuli (normal session) and that presenting both sound stimuli and a mildly painful foot-shock (arousal session). In the arousal session, foot-shocks were randomly presented in some intervals of sound presentation. We adopt this procedure to provoke the arousal level of the rats rather than to establish a solid association between sound and shock. We found that the rat’s heart rate rose from 347 ± 32 beats/min in normal session to 421 ± 43 beats/min in foot-shock session (t13=17.8, p < .001, paired t-test, ). Furthermore, the rats seldom vocalized in normal session, while vocalized 13.5 ± 5.2 times during the 3–4 min period of foot-shock session (t13=28.5, p < .001, paired t-test, ). These results indicate that the foot-shock effectively elevated the arousal level of the rats.
Effect of emotional arousal on ASSR of A1 and mPFC
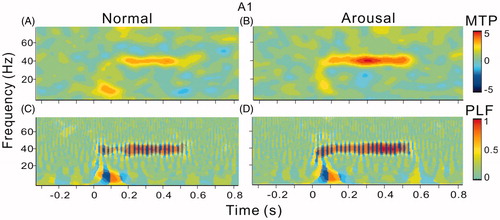
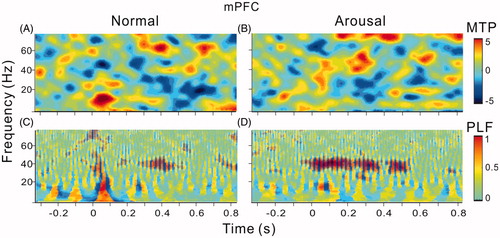
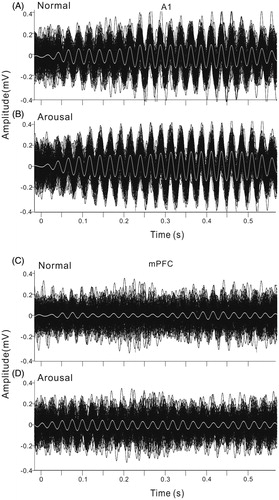
Firstly, we investigated the difference of ASSR between the arousal and normal conditions. For this purpose, we recorded local field potentials (LFPs) from A1 and mPFC (prelimbic area) during both the normal and arousal sessions. shows the LFPs averaged across 120 trials from the A1 and mPFC of an example rat in normal session. LFPs recorded from A1 showed a large deflection at the onset of click-train, then a stable oscillation synchronizing to the click stimuli (ASSR, ), while those recorded from mPFC only showed a weaker response at sound onset, could not tightly synchronize to the on-going clicks (). To compare with the frequency of stimuli, the LFPs were filtered with a bandpass filter of 35–45 Hz (). The 40-Hz ASSR was clear in A1, but obscure in mPFC. In the arousal session, the ASSR in both A1 and mPFC became stronger (). To show the variation of ASSR across different trials, we plot the 120 trials of ASSR of one session together (). The ASSRs of arousal session arranged more neatly comparing to those of normal session, indicating a stronger inter-trial coherence.
Figure 3. Example of averaged LFPs evoked by 40-Hz click-trains in A1 and mPFC under normal and arousal conditions. (A) and (B) unfiltered LFP in A1 and mPFC under normal condition. Vertical lines at the top of the plot represent the stimulus of click-train. (C) and (D) filtered LFP to show the ASSR of 40-Hz. (E) and (F) unfiltered LFP under arousal condition. (G) and (H) filtered ASSR under arousal condition.
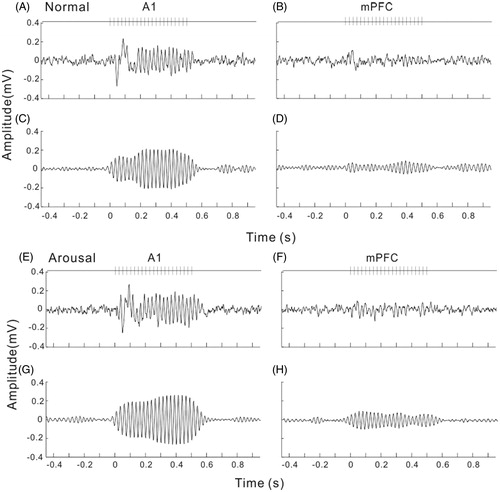
Figure 4. Individual ASSRs of each trial in one session. The same example shown in . (A) and (B) ASSR of A1 under normal and arousal condition. Black lines are the individual ASSRs, white line is the averaged ASSR. (C) and (D) ASSR of mPFC under normal and arousal condition.
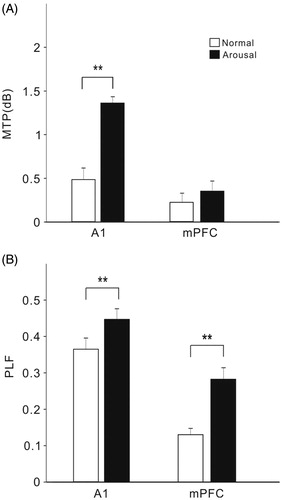
We used MTP and PLF (see Materials and methods) to evaluate the strength and phase-locking of ASSR. The spectral-temporal functions of MTP and PLF are present in and . Consistent with the observation of LFPs in showed a sustained elevation of MTP at 40-Hz during the period of sound presentation (), and the strength of MTP was further increased in the arousal session (). The results of PLF also showed a similar trend (). The MTP at 40 Hz was not prominent in mPFC and was not obviously modulated by arousal (). But the PLF at 40 Hz was notably enhanced by arousal (). To statistically comparing the population results among the 14 recorded rats, we accessed the MTP and PLF of each rat by averaging the values in the spectral-temporal function between 35 and 45 Hz frequency range and during 0–0.5 s post stimulus time window. shows the mean and SE of MTP and PLF in different brain areas and different sessions. In A1, both the MTP and PLF in the arousal session were significantly higher than that in the normal session (t13=6.9, p < .001; t13=3.2, p < .01, paired t-test). In mPFC, only PLF was significantly enhanced by arousal (t13=7.1, p < .001, paired t-test).
A1 and mPFC activity are tightly synchronized during emotional arousal
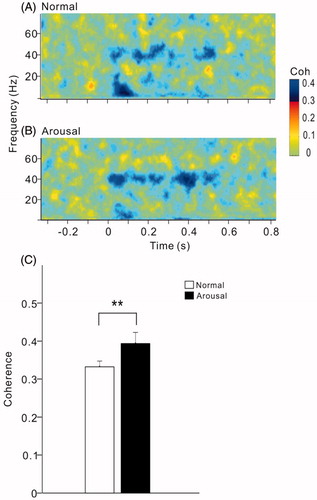
We further investigated whether changes in sound-evoked activity were accompanied by an increase in coherence between A1 and mPFC. show the spectral-temporal functions of coherence between the LFPs of A1 and mPFC in the example present in . The activities of A1 and mPFC showed an obvious coherence at 40-Hz during the period of click-train stimulus (), the 40-Hz coherence was further enhanced under arousal condition (). The 40-Hz coherence was estimated by the value averaged across the frequency range of 35–45 Hz during sound stimulus period. The mean and SE of 40-Hz coherence among the 14 rats are present in . Comparing the coherence during the arousal session with respect to the normal session, the coherence of 40-Hz ASSR between AC and mPFC was significantly increased by arousal (t13=3.0, p < .01, paired t-test).
Figure 8. Spectral-temporal function of coherence between A1 and mPFC under normal (A) and arousal condition (B). The same example shown in . Note an enhancement of coherence at 40-Hz under arousal condition. (C) Bar graph showing the mean and SE of 40-Hz coherence in all the rats under different conditions. **Indicates p < .01, paired t-test.
Discussion
The primary goal of this study is to investigate the effect of emotional arousal on 40-Hz ASSR. To this end, we performed simultaneous recordings of LFP in A1 and mPFC under normal and shock-evoked arousal conditions. We found that the ASSRs of A1 and mPFC were significantly enhanced by arousal. The coherence between the 40-Hz ASSRs of A1 and mPFC was also increased. We concluded that emotional arousal enhanced A1-to-mPFC information transfer and the functional connectivity between mPFC and A1 may contribute to the emotional modulation of auditory process.
Effect of emotional arousal on ASSR
Periodic sounds simultaneously evoke a stable oscillatory response at different levels of the auditory system, which phase-locks to the periodic stimulus—a type of response called the ASSR (Picton, John, Dimitrijevic, & Purcell, Citation2003). Namely, presentation of a train of clicks has been well used to elicit ASSR in the EEG recorded above auditory and frontal cortices (Spencer et al., Citation2004). Previous studies have showed that ASSR can be used to monitor the state of arousal during anesthesia (Picton et al., Citation2003) and 40-Hz ASSR is modulated by attention (Skosnik, Krishnan, & O'Donnell, Citation2007). Here, we found further evidence suggesting that 40-Hz ASSR was modulated by emotion state. Consistent with our previous study (Wang, Ma, Wang, & Qin, Citation2018), the present study shows that under normal condition 40-Hz ASSR is mainly found in A1, and the ASSR in mPFC is not obvious. When the rat was aroused by foot shock, ASSR in A1 was enhanced, and mPFC also showed ASSR.
It has been suggested that the power of the ASSR reflects the excitation of the neurons by afferent synaptic inputs (Pastor et al., Citation2002) and the synchronization of the ASSR reflects the temporal jittering of stimulus inputs (Kalitzin, Parra, Velis, & Lopes, Citation2002). Our results showed that both the MTP and PLF are enhanced by arousal. Especially, the main change of mPFC was the enhancement of PLF. In vitro and in vivo studies suggest that two major cell types, excitatory principal neurons and fast-spiking, parvalbumin (PV) inhibitory interneurons, and two specific receptor types, subtype A of the gamma-amino butyric acid receptor family (GABAA) and the glutamatergic NMDAR, are critical for neural synchronization (Carlén et al., Citation2012) and may be involved in production of the 40-Hz ASSR (Brenner et al., Citation2009;Sivarao, Citation2015; Vierling-Claassen, Siekmeier, Stufflebeam, & Kopell, Citation2008). Optogenetic studies have found that activation of PV projections to mPFC can increase the 40-Hz ASSR (Kim et al., Citation2015). And the activity of PV neuron in mPFC has been shown to be correlated to the level of attention (Kim, Ährlund-Richter, Wang, Deisseroth, & Carlén, Citation2016). NMDAR transmission was also found to be enhanced by acute stress (Yuen et al., Citation2009). Therefore, the questions about which cell type and neural transmission are associated with the arousal-induced enhancement of ASSR are worthy of further investigating.
Functional connection between A1 and mPFC
In the present study, we showed that the emotional arousal enhanced the coherence between the 40-Hz ASSR of A1 and mPFC. The existence of this long-range interaction between sensory cortex and mPFC has been suggested in the previous studies. Experiments in visual selective attention have revealed that the PFC sends top-down “bias signals” to sensory cortex (Miller & Cohen, Citation2001) to enhance the signal-to-noise ratio of neural representation. Auditory experiments also demonstrated that both the prefrontal cortex and auditory cortex participated in the behavioral task of sound detection and a dynamic connection between the two brain regions can be established during auditory behavior (Fritz et al. Citation2010). A recent study showed that the A1-to-mPFC connection is critical for a rat to successfully differentiate between safe and threatening auditory stimuli (Yang, Jackson, & Huang, Citation2016). Our present study provides further evidence suggesting that the connection between A1 and mPFC is modulated by emotional arousal. Arousal differs from selective attention in the sense that it is not highly selective for specific features of external stimuli, but may be a global activation of cortex (Busse et al., Citation2017). Thus, there is a possibility that lower auditory regions, such as the medial geniculate bodies, project widely to cortical areas, not just the AC (Weinberger, Citation2011; Weinberger, Javid, & Lepan, Citation1995), and could provide the basis for increased coherence between these two cortical regions during emotional arousal. The “high-level” processes like top-down attention may converge with “low-level” process of arousal at some levels of neural circuit. Future work should further address the precise sites of convergence of top-down and bottom-up pathways, to better understand how these different processes interact.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Abduljawad, K.A., Baqui, F., Langley, R.W., Bradshaw, C.M., & Szabadi, E. (2008). Effects of threat of electric shock and diazepam on the N1/P2 auditory-evoked potential elicited by low-intensity auditory stimuli. Journal of Psychopharmacology, 22, 828–835. doi:10.1177/0269881107083843
- Asutay, E., & Västfjäll, D. (2015). Attentional and emotional prioritization of the sounds occurring outside the visual field. Emotion, 15, 281–286. doi:10.1037/emo0000045
- Atsak, P., Hauer, D., Campolongo, P., Schelling, G., Fornari, R.V., & Roozendaal, B. (2015). Endocannabinoid signaling within the basolateral amygdala integrates multiple stress hormone effects on memory consolidation. Neuropsychopharmacology, 40, 1485–1494. doi:10.1038/npp.2014.334
- Bakin, J.S., & Weinberger, N.M. (1990). Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Research, 536, 271–286. doi:10.1016/0006-8993(90)90035-A
- Bolders, A.C., Band, G.P.H., & Stallen, P.J.M. (2017). Inconsistent effect of arousal on early auditory perception. Frontiers in Psychology, 8, 447. doi:10.3389/fpsyg.2017.00447
- Brenner, C.A., Krishnan, G.P., Vohs, J.L., Ahn, W.Y., Hetrick, W.P., Morzorati, S.L., & O'Donnell, B.F. (2009). Steady state responses: Electrophysiological assessment of sensory function in schizophrenia. Schizophrenia Bulletin, 35, 1065–1077. doi:10.1093/schbul/sbp091
- Busse, L., Cardin, J.A., Chiappe, M.E., Halassa, M.M., McGinley, M.J., Yamashita, T., & Saleem, A.B. (2017). Sensation during active behaviors. The Journal of Neuroscience, 37, 10826–10834. doi:10.1523/JNEUROSCI.1828-17.2017
- Campeau, S., & Davis, M. (1995). Prepulse inhibition of the acoustic startle reflex using visual and auditory prepulses: Disruption by apomorphine. Psychopharmacology, 117, 267–274. doi:10.1007/BF02246101
- Carlén, M., Meletis, K., Siegle, J.H., Cardin, J.A., Futai, K., Vierling-Claassen, D., … Tsai, L.-H. (2012). A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular Psychiatry, 17, 537–548. doi:10.1038/mp.2011.31
- Chigusa, S., Moroi, T., & Shoji, Y. (2017). State-of-the-art calculation of the decay rate of electroweak vacuum in the standard model. Physical Review Letters, 119, 211801. doi:10.1103/PhysRevLett.119.211801
- Concina, G., Cambiaghi, M., Renna, A., & Sacchetti, B. (2018). Coherent activity between the prelimbic and auditory cortex in the slow-gamma band underlies fear discrimination. The Journal of Neuroscience, 38, 8313–8328.
- Dejean, C., Courtin, J., Karalis, N., Chaudun, F., Wurtz, H., Bienvenu, T.C., & Herry, C. (2016). Prefrontal neuronal assemblies temporally control fear behaviour. Nature, 535, 420–424. doi:10.1038/nature18630
- Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. doi:10.1016/j.jneumeth.2003.10.009
- Do, M.F.H., Quirk, G.J., Li, B., Penzo, M.A. (2016). Retrieving fear memories, as time goes by…. Molecular Psychiatry, 21, 1027–1036. doi:10.1038/mp.2016.78
- Dong, C., Qin, L., Liu, Y., Zhang, X., & Sato, Y. (2011). Neural responses in the primary auditory cortex of freely behaving cats while discriminating fast and slow click-trains. PloS One, 6, e25895. doi:10.1371/journal.pone.0025895
- Dong, C., Qin, L., Zhao, Z., Zhong, R., & Sato, Y. (2013). Behavioral modulation of neural encoding of click-trains in the primary and nonprimary auditory cortex of cats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33, 13126–13137. doi:10.1523/JNEUROSCI.1724-13.2013
- Eby, L.T., Butts, M.M., Hoffman, B.J., & Sauer, J.B. (2015). Cross-lagged relations between mentoring received from supervisors and employee OCBs: Disentangling causal direction and identifying boundary conditions. The Journal of Applied Psychology, 100, 1275–1285. doi:10.1037/a0038628
- Edeline, J.M., & Weinberger, N.M. (1993). Receptive field plasticity in the auditory cortex during frequency discrimination training: Selective retuning independent of task difficulty. Behavioral Neuroscience, 107, 82–103. doi:10.1037/0735-7044.107.1.82
- Falls, W.A., & Davis, M. (1993). Visual cortex ablations do not prevent extinction of fear-potentiated startle using a visual conditioned stimulus. Behavioral and Neural Biology, 60, 259–270. doi:10.1016/0163-1047(93)90504-B
- Fritz, J.B., David, S.V., Radtke-Schuller, S., Yin, P., & Shamma, S.A. (2010). Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nature Neuroscience, 13, 1011–1019. doi:10.1038/nn.2598
- Grosso, A., Cambiaghi, M., Concina, G., Sacco, T., & Sacchetti, B. (2015). Auditory cortex involvement in emotional learning and memory. Neuroscience, 299, 45–55. doi:10.1016/j.neuroscience.2015.04.068
- Gruene, T., Flick, K., Rendall, S., Cho, J.H., Gray, J., & Shansky, R. (2016). Activity-dependent structural plasticity after aversive experiences in amygdala and auditory cortex pyramidal neurons. Neuroscience, 328, 157–164. doi:10.1016/j.neuroscience.2016.04.045
- Herry, C., & Johansen, J.P. (2014). Encoding of fear learning and memory in distributed neuronal circuits. Nature Neuroscience, 17, 1644–1654. doi:10.1038/nn.3869
- Kalitzin, S., Parra, J., Velis, D.N., & Lopes, D. S. F. H. (2002). Enhancement of phase clustering in the EEG/MEG gamma frequency band anticipates transitions to paroxysmal epileptiform activity in epileptic patients with known visual sensitivity. IEEE Transactions on Biomedical Engineering, 49, 1279–1286. doi:10.1109/TBME.2002.804593
- Karalis, N., Dejean, C., Chaudun, F., Khoder, S., Rozeske, R.R., Wurtz, H., … Herry, C. (2016). 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nature Neuroscience, 19, 605–612. doi:10.1038/nn.4251
- Kim, H., Ährlund-Richter, S., Wang, X., Deisseroth, K., & Carlén, M. (2016). Prefrontal parvalbumin neurons in control of attention. Cell, 164, 208–218. doi:10.1016/j.cell.2015.11.038
- Kim, T., Thankachan, S., McKenna, J.T., McNally, J.M., Yang, C., Choi, J.H., … McCarley, R.W. (2015). Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proceedings of the National Academy of Sciences, 112, 3535–3540. doi:10.1073/pnas.1413625112
- Kuhbandner, C., Hanslmayr, S., Maier, M.A., Pekrun, R., Spitzer, B., Pastötter, B., & Bäuml, K.H. (2009). Effects of mood on the speed of conscious perception: Behavioural and electrophysiological evidence. Social Cognitive and Affective Neuroscience, 4, 286–293. doi:10.1093/scan/nsp010
- Lee, T.H., Baek, J., Lu, Z.L., & Mather, M. (2014). How arousal modulates the visual contrast sensitivity function. Emotion, 14, 978–984. doi:10.1037/a0037047
- Likhtik, E., Stujenske, J.M., Topiwala, M.A., Harris, A.Z., & Gordon, J.A. (2014). Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nature Neuroscience, 17, 106–113. doi:10.1038/nn.3582
- Makeig, S., Debener, S., Onton, J., & Delorme, A. (2004). Mining event-related brain dynamics. Trends in Cognitive Sciences, 8, 204–210. doi:10.1016/j.tics.2004.03.008
- Miller, E.K., & Cohen, J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
- Moczulska, K.E., Tinter-Thiede, J., Peter, M., Ushakova, L., Wernle, T., Bathellier, B., & Rumpel, S. (2013). Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proceedings of the National Academy of Sciences of the United States of America, 110, 18315–18320. doi:10.1073/pnas.1312508110
- O'Donnell, B.F., Vohs, J.L., Krishnan, G.P., Rass, O., Hetrick, W.P., & Morzorati, S.L. (2013). The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. Supplements to Clinical Neurophysiology, 2013, 101–112.
- Parker, E.M., & Sweet, R.A. (2017). Stereological assessments of neuronal pathology in auditory cortex in schizophrenia. Frontiers in Neuroanatomy, 2017, 131.
- Pastor, M.A., Artieda, J., Arbizu, J., Marti-Climent, J.M., Peñuelas, I., & Masdeu, J.C. (2002). Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. The Journal of Neuroscience, 22, 10501–10506. doi:10.1523/JNEUROSCI.22-23-10501.2002
- Paxinos, G., & Watson, C. (1986). The rat brain in stereotaxic coordinates. New York: Academic Press.
- Peter, M., Scheuch, H., Burkard, T.R., Tinter, J., Wernle, T., & Rumpel, S. (2012). Induction of immediate early genes in the mouse auditory cortex after auditory cued fear conditioning to complex sounds. Genes, Brain and Behavior, 11, 314–324. doi:10.1111/j.1601-183X.2011.00761.x
- Picton, T.W., John, M.S., Dimitrijevic, A., & Purcell, D. (2003). Human auditory steady-state responses. International Journal of Audiology, 42, 177–219. doi:10.3109/14992020309101316
- Rass, O., Krishnan, G., Brenner, C.A., Hetrick, W.P., Merrill, C.C., Shekhar, A., & O’Donnell, B.F. (2010). Auditory steady state response in bipolar disorder: Relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disorders, 12, 793–803. doi:10.1111/j.1399-5618.2010.00871.x
- Recasens, M., Gross, J., & Uhlhaas, P.J. (2018). Low-frequency oscillatory correlates of auditory predictive processing in cortical-subcortical networks: A MEG-Study. Scientific Reports, 2018, 8, 14007.
- Resnik, J., Sobel, N., & Paz, R. (2011). Auditory aversive learning increases discrimination thresholds. Nature Neuroscience, 14, 791–796. doi:10.1038/nn.2802
- Rodgers, C.C., & DeWeese, M.R. (2014). Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron, 82, 1157–1170.
- Romanski, L.M., & LeDoux, J.E. (1992). Bilateral destruction of neocortical and perirhinal projection targets of the acoustic thalamus does not disrupt auditory fear conditioning. Neuroscience Letters, 142, 228–232. doi:10.1016/0304-3940(92)90379-L
- Romanski, L.M., & LeDoux, J.E. (1992). Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. The Journal of Neuroscience, 12, 4501–4509. doi:10.1523/JNEUROSCI.12-11-04501.1992
- Siegel, E.H., & Stefanucci, J.K. (2011). A little bit louder now: Negative affect increases perceived loudness. Emotion, 11, 1006–1011. doi:10.1037/a0024590
- Siuda, E.R., Al-Hasani, R., McCall, J.G., Bhatti, D.L., & Bruchas, M.R. (2016). Chemogenetic and optogenetic activation of Gαs signaling in the basolateral amygdala induces acute and social anxiety-like states. Neuropsychopharmacology, 41, 2011–2023. doi:10.1038/npp.2015.371
- Sivarao, D.V. (2015). The 40-Hz auditory steady-state response: A selective biomarker for cortical NMDA function. Annals of the New York Academy of Sciences, 1344, 27–36. doi:10.1111/nyas.12739
- Skosnik, P.D., Krishnan, G.P., & O’Donnell, B.F. (2007). The effect of selective attention on the gamma-band auditory steady-state response. Neuroscience Letters, 420, 223–228. doi:10.1016/j.neulet.2007.04.072
- Spencer, K.M., Nestor, P.G., Perlmutter, R., Niznikiewicz, M.A., Klump, M.C., Frumin, M., … McCarley, R.W. (2004). Neural synchrony indexes disordered perception and cognition in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 101, 17288–17293. doi:10.1073/pnas.0406074101
- Van Eden, C.G., Lamme, V.A.F., & Uylings, H.B.M. (1992). Heterotopic cortical afferents to the medial prefrontal cortex in the rat. A combined retrograde and anterograde tracer study. European Journal of Neuroscience, 4, 77–97. doi:10.1111/j.1460-9568.1992.tb00111.x
- Vierling-Claassen, D., Siekmeier, P., Stufflebeam, S., & Kopell, N. (2008). Modeling GABA alterations in schizophrenia: A link between impaired inhibition and altered gamma and beta range auditory entrainment. Journal of Neurophysiology, 99, 2656–2671. doi:10.1152/jn.00870.2007
- Wang, Y., Ma, L., Wang, X., & Qin, L. (2018). Differential modulation of the auditory steady state response and inhibitory gating by chloral hydrate anesthesia. Scientific Reports, 8, 3683. doi:10.1038/s41598-018-21920-x
- Weinberger, N.M. (2011). The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hearing Research, 274, 61–74. doi:10.1016/j.heares.2010.03.093
- Weinberger, N.M., Javid, R., & Lepan, B. (1995). Heterosynaptic long-term facilitation of sensory-evoked responses in the auditory cortex by stimulation of the magnocellular medial geniculate body in guinea pigs. Behavioral Neuroscience, 109, 10–17. doi:10.1037/0735-7044.109.1.10
- Wigestrand, M.B., Schiff, H.C., Fyhn, M., LeDoux, J.E., & Sears, R.M. (2017). Primary auditory cortex regulates threat memory specificity. Learning & Memory, 24, 55–58. doi:10.1101/lm.044362.116
- Yang, Z., Jackson, T., & Huang, C. (2016). Neural activation during anticipation of near pain-threshold stimulation among the pain-fearful. Frontiers in Neuroscience, 10, 342.
- Yuen, E.Y., Liu, W., Karatsoreos, I.N., Feng, J., McEwen, B.S., & Yan, Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America, 106, 14075–14079. doi:10.1073/pnas.0906791106
- Zhang, J., Muller, J.F., & McDonald, A.J. (2013). Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience, 228, 395–408. doi:10.1016/j.neuroscience.2012.10.035