Abstract
Several studies found that acute stress leads to increased risk taking in humans. However, this effect appears to be time-dependent because the few studies that examined delayed (>40 min after stress onset) stress effects show in fact a decrease in risk taking. In 32 young healthy women, we intra-individually examined whether psychosocial stress decreases risk taking 80 min after stress induction. All participants performed the Balloon Analog Risk Task (BART) twice: once after exposure to the Trier social stress test (TSST) and once after a control condition Placebo-TSST (P-TSST). The experimental order was randomized across participants. The psychophysiological stress response increased after the TSST compared to the P-TSST, indicated by elevated cortisol concentrations, elevated alpha-amylase activity, and elevated blood pressure. We found a significant interaction of stress condition and experimental order. Compared to the control condition psychosocial stress decreased risk taking in novel decision situations but not when participants were already familiar with the BART from the prior condition. Delayed effects of psychosocial stress lead to a decrease in risk taking in unfamiliar but not familiar conditions 80 min after stress exposure.
It has been suggested that stress exerts delayed effects on risk taking propensity. We found that individuals who are exposed to psychosocial stress take less risk when confronted with novel decisions even 80 min after the stressor compared to individuals who are not stressed.
Lay summary
Introduction
Stress has wide-ranging effects on risk taking, as people take more risk when confronted with acute stress (Starcke & Brand, Citation2016). The stress effects on risk taking are modulated by several factors, such as gender (Lighthall, Mather, & Gorlick, Citation2009; Van den Bos, Harteveld, & Stoop, Citation2009) and there is first evidence that the time interval between stressor and decision situation is important (Pabst, Brand, & Wolf, Citation2013). So far, most studies examined risk taking immediately after or up to 30 min after stress. Less is known about possible delayed stress effects more than 1 h after stress exposure.
Two well-established methods to induce stress in humans are primarily used in the field: the cold pressor test (CPT; Hines & Brown, Citation1936) and the Trier social stress test (TSST; Kirschbaum, Pirke, & Hellhammer, Citation1993). While the CPT mainly induces an activation of the sympathetic nervous system (SNS), the TSST additionally activates the hypothalamic pituitary adrenal (HPA) axis leading to increased cortisol concentrations (McRae et al., Citation2006; Schwabe, Haddad, & Schachinger, Citation2008). While studies that used a psychosocial stressor (e.g. TSST) predominantly found that stress increases risk taking propensity, the results of studies based on the CPT are rather heterogeneous (for an overview, see Sokol-Hessner, Raio, Gottesman, Lackovic, & Phelps, Citation2016). Thus, cortisol might be crucial for the effects of stress on risk taking.
Cortisol acts via glucocorticoid (GR) and mineralocorticoid (MR) receptors in the brain. The early stress response is primarily mediated via activation of membrane MR and GR and takes place minutes after stress induction (de Kloet, Citation2014). Approximately, 1 h after corticosteroids reach their peak in response to acute stress, the delayed GR effects occur (de Kloet, Meijer, de Nicola, de Rijk, & Joëls, Citation2018). The early stress response seems to be associated with increased risk taking, since it has been observed early after stress induction (Starcke & Brand, Citation2016) and in studies using a pharmacological stimulation of the MR receptor (Deuter et al., Citation2017; Putman, Antypa, Crysovergi, & van der Does, Citation2010).
To this point, few studies examined delayed stress effects on risk taking using longer time intervals between stressor and the assessment of risk taking. Although there is preliminary evidence that risk taking decreases 45 min (Bendahan et al., Citation2017) and 120 min (Yamakawa, Ohira, Matsunaga, & Isowa, Citation2016) after psychosocial stress compared to a control condition, the observation is based on two studies, of which the latter had a small sample size. Thus, we examined the delayed stress effects on risk taking comparing the performance on the Balloon Analog Risk Task (BART; Lejuez et al., Citation2002) 80 min after the TSST and after a control condition. Because practice with a risk taking task might influence risk taking propensity (Robinson, Bond, & Roiser, Citation2015; Sokol-Hessner et al., Citation2015, Citation2016), we controlled for experimental order. We hypothesized that there is a delayed effect of psychosocial stress on risk taking leading to decreased risk taking propensity.
Methods
Participants
In total, we tested 37 healthy women. The sample derived from a larger research project, in which we tested the effect of psychosocial stress on memory functions in healthy women and women with borderline personality disorder (Duesenberg et al., Citation2019, European Journal of Psychotraumatology). For this study, we asked all healthy women to participate in a decision-making task and those who agreed conducted the BART at the end of the testing day. We had to exclude five participants for several reasons (technical problems, premature termination of participation, and retrospective fulfillment of exclusion criteria). We used the data of 32 participants for statistical analysis. We recruited via email lists, advertisement in universities and via our website. In a telephone interview, we screened for demographic characteristics, psychiatric and somatic illnesses, and intake of medication. We excluded all participants, who had a history of psychiatric disorders or any severe medical condition (especially gastrointestinal illnesses, head injuries, epilepsy or neurological disorders, a body mass index of 30 or higher, illnesses concerning the thyroid glands, Cushing or Addison syndrome, heart or blood illnesses, blood pressure or respiratory problems, liver illnesses, diseases of the immune system or HIV, kidney problems, or anemia), who underwent a psychotherapeutic or psychiatric treatment at present or in the past and all participants who took any medication (except oral contraceptives [OC]) at the time of testing. The study was approved by the ethic commission of the German Psychological Association.
Procedure
All participants were invited for two testing days and were randomized to one of the two experimental orders. One group underwent the TSST on the first, and a control condition (the Placebo-TSST variant called P-TSST) on the second testing day. The other group underwent the P-TSST on the first and the TSST on the second testing day. The experimental procedure was the same for both testing days. Participants arrived at the laboratory at 15:30 h. The TSST/P-TSST begun at approximately 16:00 h. For baseline measures, we assessed blood pressure and heart rate, as well as saliva samples, 15 min before (−15 min) and immediately prior to the TSST or P-TSST (zero point), respectively. Immediately after the stress task or control condition, blood pressure and heart rate were measured and saliva samples were collected (+20 min). The measurements were repeated at time points +30, +45, and +80 min. The BART was conducted after the last measurement. The subjective stress response was measured before the stress induction (−15 min) as well as two times afterwards (+20 and +80 min).
Stress induction
We used the TSST (Kirschbaum et al., Citation1993) to induce psychosocial stress in the laboratory. During this stress task, participants had 5 min time to prepare for a 10 min speech they were asked to hold in front of a committee. The first 5 min consisted of a fictive job interview and the last 5 min consisted of an arithmetical task. Participants were told that the committee was trained in behavioral observation and that the camera and microphone in the room would record the session for the purpose of behavioral analysis. To increase psychosocial stress, the committee consisted of female and male confederate that were instructed to behave cold and distant and to give no positive feedback during the whole task. As control condition, we used the placebo variant of the TSST (Het, Rohleder, Schoofs, Kirschbaum, & Wolf, Citation2009). The P-TSST comprised the same 5 min preparing phase, but the speech participants were asked to hold was about a topic of their choice, the arithmetical task was a simple addition exercise and neither a committee nor a microphone or camera were present at any time. Without inducing stress, the P-TSST is designed to be identical to the TSST, including the effect on orthostatic load.
Psychological and physiological measurement
To measure the psychological stress response, we used the Multidimensional Mood State Questionnaire (MDMQ; Steyer, Schwenkmezger, Notz, & Eid, Citation1997). To measure the physiological stress response, we assessed saliva cortisol concentrations (nmol/l) and alpha-amylase concentrations (u/ml) using Code Blue Salivettes® (Sarstedt, Germany). The Neurobiological Laboratory of the Department of Psychiatry, Charité Universitätsmedizin Berlin analyzed the saliva samples using a homogenous time-resolved fluorescence resonance energy transfer (HTR-FRET) assay. Cortisol sample analysis was based on intra-assay variation coefficients below 8%, inter-assay variation coefficients below 10%, a detection limit of 0.2 nmol/L, and two measurements for all saliva samples and standards (for a detailed description, see Duesenberg et al., Citation2016). The alpha-amylase activity analysis was based on intra-assay and inter-assay variation coefficients below 10% (for a detailed description, see Rombold et al., Citation2016). The measurement of the blood pressure (mmHg) was conducted using the apparatus Boso Medicus Uno (Bosch + Sohn, Jungingen, Germany).
Balloon analog risk task
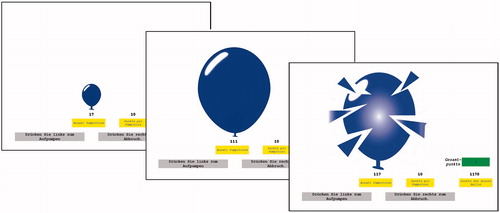
The BART (Lejuez et al., Citation2002) was used to measure risk taking in our study. In the computerized task, participants were asked to pump up a virtual balloon (see ). For every pump, 10 monetary points were earned and the points of every trial were saved on a virtual account that was displayed on the screen. After a random amount of pumps, the balloon exploded and all the points earned in the trial were lost. The number of pumps for the balloon to explode varied between 1 and 128 and was randomized over the 30 trials. Thus, the risk of losing the monetary points due to balloon explosion increased with every pump. However, the more pumps the more monetary points could have been earned in each trial. Since participants underwent several trials, the risk taking task allowed for learning by feedback. With proceeding trials, participants were more and more able to judge the risk of balloon explosion. To measure risk taking, we analyzed the adjusted number of pumps (average number of pumps for trials with no balloon explosion), the amount of monetary points and the number of balloon explosions (see also Deuter et al., Citation2017).
Figure 1. Example of the course of one BART trial, with 17 pumps, followed by 111 pumps, and the balloon explosion after 117 pumps, from the left to the right, from Deuter et al. (Citation2017).
Statistical analysis
For the statistical analysis, we used IBM SPSS Statistics version 23 (IBM SPSS Statistics, Armonk, NY). The demographic data were analyzed with Chi-squared tests (χ2) or t-tests for dichotomous or continuous data, respectively. To analyze risk taking, we conducted mixed design ANOVAs with the within-subject factor stress condition (TSST or P-TSST), the between-subject factor experimental order (TSST first and P-TSST second or P-TSST first and TSST second) and the dependent variables number of pumps, amount of monetary points, and number of balloon explosions.
For the analysis of the psychological and physiological stress response, we used mixed design ANOVAs with the within-subject factors stress condition (TSST or P-TSST) and time (measurement time points −15 min, zero point, +20, +30, +45, and +80 min), the between-subject factor experimental order (TSST first and P-TSST second or P-TSST first and TSST second) and the dependent variables MDMQ subscales, cortisol, alpha-amylase, and blood pressure.
We also controlled for the use of OC) by including the categorical variable “use of oral contraceptives” in the analysis of the cortisol stress response and in the analysis of risk taking. We calculated baseline to peak (BTP) values for cortisol, alpha-amylase, and blood pressure, by subtracting the mean values of both baseline measures from the maximum values of all measurement time points after the TSST or P-TSST, respectively. We also calculated the area under the curve (AUC) for cortisol, alpha-amylase and blood pressure, thereby differentiating between the area under the curve increase (AUCi) and the area under the curve ground (AUCg). In case statistical assumptions were violated, we applied Welch’s F or Greenhouse–Geisser corrections. For post-hoc analysis, we used paired-samples t-tests.
Results
Demographic characteristics
The analysis of the demographic characteristics of our sample revealed no significant differences between the participants of both experimental orders concerning their age, body mass index, years of education, smoking status, intake of oral contraceptives, and menopausal status (all ps > .05; see ).
Table 1. Sample characteristics.
Psychological stress response
For the analysis of the psychological stress response, we used mixed ANOVAs with the within-subject factor stress condition (TSST or P-TSST) and time (measurement time points −15, +20, and +80 min) and the between-subject factor experimental order (TSST first and P-TSST second or P-TSST first and TSST second) for all three subscales of the MDMQ (good-bad mood, wakefulness-fatigue, and calmness-restlessness). We had to exclude data of one participant, because there were missing values for one MDMQ subscale.
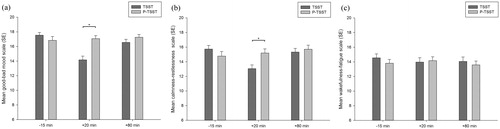
The analysis revealed for the good-bad mood scale a significant main effect of stress condition (F(1,29) = 11.90, p < .01, η2 = 0.29), time (F(2,58) = 18.62, p < .001, η2 = 0.39), but not for experimental order (p > .05). In addition, we found a significant interaction of stress condition × time (F(1.50,43.34) = 19.76, p < .001, η2 = 0.41; see ). For the calmness-restlessness scale, there was no significant main effect of stress condition (p > .05), well for time (F(2,58) = 7.22, p < .01, η2 = 0.20), but not for experimental order (p > .05). In addition, we found a significant interaction of stress condition × time (F(2,58) = 14.54, p < .001, η2 = 0.33; see ). For the wakefulness-fatigue scale, no main effect or interaction reached significance (all ps > .05; see ).
Figure 2. Mean scores of the good-bad mood scale (a), calmness-restlessness scale (b), and wakefulness-fatigue scale (c) for both stress conditions (P-TSST and TSST). Higher scores on the scales indicate (a) better mood, (b) more calmness, and (c) more wakefulness, respectively. Error bars represent standard error and significant differences are marked (*).
Physiological stress response
For the analysis of the cortisol response, alpha-amylase response and blood pressure, we conducted mixed ANOVAs with the within-subject factors stress condition (TSST or P-TSST) and time (measurement time points −15 min, zero point, +20, +30, +45, and +80 min) and the between-subject factor experimental order (TSST first and P-TSST second or P-TSST first and TSST second). For post-hoc analysis, we used paired-samples t-tests and significant differences between stress conditions are marked with an asterisk for every measurement time point in the corresponding figure. The data were log-transformed for statistical analysis, because of a non-normal distribution of the raw data. Figures show raw data. Due to technical difficulties, we had to exclude data of some subjects for the analysis of the psychophysiological stress response (we analyzed for cortisol n = 26, alpha-amylase n = 27, and systolic and diastolic blood pressure n = 31).
Cortisol
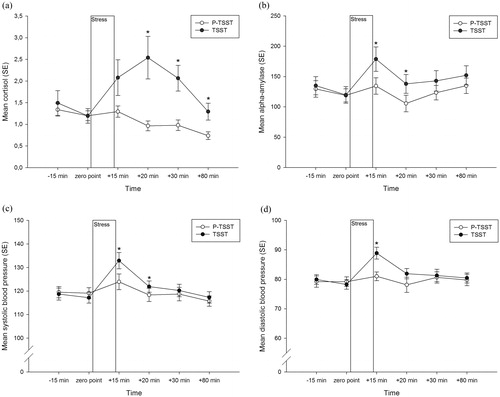
The analysis of the cortisol response revealed a significant main effect of the stress condition (F(1,24) = 4.91, p < .05, η2 = 0.17), a significant main effect of time (F(2.37,56.88) = 6.55, p < .01, η2 = 0.21), but not for experimental order (p = .21). In addition, we found a significant interaction of stress condition × time (F(2.26,54.20) = 11.80, p < .001, η2 = 0.33) but no significant interaction of stress condition × experimental order (p > .05; see ).
Figure 3. Mean (a) cortisol concentration (nmol/l), (b) alpha-amylase activity (u/ml), (c) systolic, and (d) diastolic blood pressure (mmHg) across time for both stress conditions (P-TSST and TSST). Error bars represent standard error and significant differences (p < .05) are marked (*) for every measurement time point.
In a next step, we controlled for the use of OC and found that the main effect of stress condition became non-significant (F(1,22) = 2.75, p = .111, η2 = 0.11). However, the main effect of time (F(2.54,55.93) = 5.07, p < .01, η2 = 0.19) and importantly the interaction of stress condition × time (F(2.15,47.39) = 8.05, p < .01, η2 = 0.27) remained significant. The main effect of experimental order (p > .05) and the interaction of stress condition × experimental order (p > .05) remained non-significant after controlling for OC use.
Alpha-amylase
The analysis of the alpha-amylase response revealed a significant main effect of the stress condition (F(1,25) = 5.23, p < .05, η2 = 0.17), time (F(5,125) = 5.72, p < .001, η2 = 0.19), but not for experimental order (p > .05), and no significant interaction of stress condition × time (p > .05) or stress condition × experimental order (p > .05; see ).
Blood pressure
The analysis of the systolic blood pressure revealed a significant main effect of the stress condition (F(1,29) = 4.77, p < .05, η2 = 0.14), time (F(5,145) = 15.16, p < .001, η2 = 0.34), but not for experimental order (p > .05). In addition, we found a significant interaction of stress condition × time (F(3.00,86.80) = 4.52, p < .01, η2 = 0.14) but no significant interaction of stress condition × experimental order (p > .05; see ).
The analysis of the diastolic blood pressure revealed a significant main effect of the stress condition (F(1,29) = 6.17, p < .05, η2 = 0.18), time (F(1.88,54.59) = 3.89, p < .05, η2 = 0.12), but not for experimental order (p > .05) and no significant interaction of stress condition × time (p > .05) or stress condition × experimental order (p > .05; see ).
Effects of stress on the Balloon Analog Risk Task
We conducted mixed ANOVAs with the within-subject factor stress condition (TSST or P-TSST), the between-subject factor experimental order (TSST first and P-TSST second or P-TSST first and TSST second), and the outcome variables adjusted number of pumps, amount of monetary points, and number of balloon explosions.
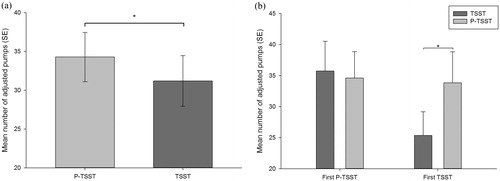
The analysis of the adjusted number of pumps revealed a significant main effect of the stress condition (F(1,30) = 5.38, p < .05, η2 = 0.15), indicating that participants pumped less in reaction to the TSST as compared to the P-TSST, independent of the experimental order (see ). The main effect of experimental order was not significant (p > .05), but we found a significant interaction of stress condition × experimental order (F(1,30) = 9.12, p < .01, η2 = 0.23). Post-hoc analysis revealed, that only participants who were exposed to stress (TSST) on the first day, when they performed the BART for the first time, and the control condition on the second (P-TSST), pumped less in reaction to the TSST as compared to the P-TSST (t(13) = −3.36, p < .01). For participants who underwent the experiment in the other order, we found no significant difference between the stress and the non-stress condition (p > .05; see ).
Figure 4. Mean number of adjusted pumps (a) for both stress conditions (P-TSST and TSST) and (b) for both stress conditions and both experimental orders (TSST first and P-TSST second or P-TSST first and TSST second). Error bars represent standard error and significant differences are marked (*).
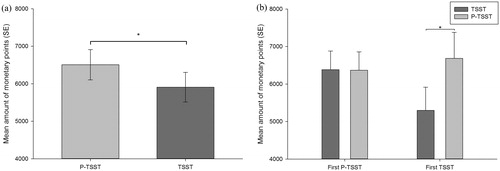
The analysis of the amount of monetary points revealed a significant main effect of stress condition (F(1,30) = 6.23, p < .05, η2 = 0.17), indicating that participants earned less monetary points in reaction to the TSST as compared to the P-TSST, independent of the experimental order (see ). The main effect of experimental order was not significant (p > .05), but we found a significant interaction of stress condition × experimental order (F(1,30) = 6.51, p < .05, η2 = 0.18). Post-hoc analysis revealed that only participants who were exposed to stress (TSST) on the first day, when they performed the BART for the first time, and the control condition on the second (P-TSST), earned less monetary points in reaction to the TSST as compared to the P-TSST (t(13) = −3.33, p < .01). For participants who underwent the experiment in the other order, we found no significant difference (p > .05; see ).
Figure 5. Mean amount of monetary points (a) for both stress conditions (P-TSST and TSST) and (b) for both stress conditions and both experimental orders (TSST first and P-TSST second or P-TSST first and TSST second). Error bars represent standard error and significant differences are marked (*).
The analysis of the number of balloon explosions revealed no significant main effect of stress condition (p > .05), indicating that there was no difference in the number of balloon explosions in reaction to the TSST (M = 8.25, SD = 5.25) compared to the P-TSST (M = 8.75, SD = 4.69). The main effect of experimental order and the interaction of stress condition × experimental order was also not significant (all ps > .05).
In a next step, we controlled for the use of OC and found for pumps that the main effect of stress condition became non-significant (F(1,28) = 3.69, p = .07, η2 = 0.12). However, the interaction of stress condition × experimental order remained significant (F(1,28) = 8.01, p < .01, η2 = 0.23). For monetary points, the main effect of stress condition became non-significant (F(1,28) = 3.27, p = .08, η2 = 0.10) but the interaction of stress condition × experimental order remained significant (F(1,28) = 7.64, p < .05, η2 = 0.21). For balloon explosions, all main effects and interactions remained non-significant as before (all ps > .05).
Risk taking and the psychological stress response
We calculated correlations between all three measures of risk taking (adjusted number of pumps, amount of monetary points and number of balloon explosions) and the subscales of the MDMQ (good-bad mood, wakefulness-fatigue, and calmness-restlessness) for both stress conditions separately. For the P-TSST, we found significant correlations between the good-bad mood scores measured at −15 min and pumps (r = 0.41, p < .05), monetary points (r = 0.36, p < .05) and balloon explosions (r = 0.36, p < .05) and for good–bad mood scores measured at + 80 min and pumps (r = 0.36, p < .05), and monetary points (r = 0.39, p < .05).
Risk taking and the physiological stress response
We calculated correlations between all three measures of risk taking (adjusted number of pumps, amount of monetary points, and number of balloon explosions) and the cortisol and alpha-amylase stress response measurements for the TSST and P-TSST, respectively. No correlation reached significance (all ps > .05).
Subsequently, we calculated correlations between BTP values (cortisol, alpha-amylase, and blood pressure) and all three measures of risk taking (adjusted number of pumps, amount of monetary points, and number of balloon explosions). We found significant correlations between diastolic blood pressure BTP values in response to the TSST and the adjusted number of pumps (r = −0.41, p < .05) as well as the amount of monetary points (r = −0.35, p < .05) in response to the TSST. We also calculated correlations between AUC values (cortisol, alpha-amylase, and blood pressure) and all three measures of risk taking (adjusted number of pumps, amount of monetary points, and number of balloon explosions). No correlation reached significance (all ps > .05).
Discussion
We investigated the delayed effects of psychosocial stress on risk taking in young healthy women. In line with our hypothesis, we observed less risk taking 80 min after psychosocial stress compared to a control condition. The delayed stress effect was moderated by the experience participants already had with the risk taking task. Only when the risk taking task was novel to the participants, their risk taking decreased in reaction to psychosocial stress. Participants who were already familiar with the risk taking task, showed no alterations in risk taking after psychosocial stress. Our study shows that psychosocial stress has delayed effects on risk taking, leading to less risky decisions in unfamiliar situations.
Our finding of decreased risk taking 80 min after psychosocial stress exposure complements the results of previous studies that found decreased risk taking 45 min (Bendahan et al., Citation2017) and 120 min (Yamakawa et al., Citation2016) after psychosocial stress induction compared to a control condition. This is in contrast to most studies that have found an increase in risk taking propensity short after exposure to psychosocial stress (Starcke & Brand, Citation2016). Together, the results indicate that exposure to psychosocial stress leads to an increase in risk taking early after stress and a decrease in risk taking late after stress induction.
Differences in the early and delayed stress response in humans might be responsible for the differences between risk taking early and late after stress. Early after stress exposure catecholamines (adrenaline and noradrenaline) increase and reach their peak within the first minutes (Hermans, Henckens, Joëls, & Fernández, Citation2014). This is followed by an increase of corticosteroids (e.g. cortisol), which reach their peak approximately 20–40 min after stress exposure (Dickerson & Kemeny, Citation2004). Corticosteroids first exert non-genomic effects that take place minutes up to approximately 1 h after stress exposure and then exert genomic effects that last for several hours (for a review see, Joëls, Karst, & Sarabdjitsingh, Citation2018).
The release of catecholamines and corticosteroids exert distinctive effects on decision-making processes. Margittai et al. (Citation2018) found that combined pharmaceutical activation of the GR and noradrenergic stress system decreased loss aversion relative to the activation of either system alone. In our study design, we can rule out the influence of the early catecholamine stress response because we measured risk taking at a time when the catecholamine stress response is already terminated (Hermans et al., Citation2014). In support of this assumption, we only found differences between stress conditions for cortisol 80 min after stress induction, but not for alpha-amylase, a marker of the adrenergic stress response (van Stegeren, Rohleder, Everaerd, & Wolf, Citation2006), and not for blood pressure.
Several studies provide evidence for a distinctive role of early and delayed stress effects on decision-making processes. For instance, decisions are more generous early (20 min) compared to late (90 min) after psychosocial stress (Margittai et al., Citation2015) and people reject unfair offers more often directly compared to long after (75 min) psychosocial stress exposure (Vinkers et al., Citation2013). The corticosteroid stress response seems to be important in this context. One study showed that the administration of hydrocortisone influences intertemporal choices 15 min but not 195 min after drug intake (Cornelisse, Van Ast, Haushofer, Seinstra, & Joels, Citation2013).
The relationship between the behavioral decision-making response to stress and the physiological response to stress is complex. We found that only participants who underwent the TSST on the first and the P-TSST on the second day showed alterations in risk taking after stress, although we found no such effect of experimental order for the physiological stress response. In response to the TSST, we found that a higher response of diastolic blood pressure was related to less risk taking propensity, which fits our observation of decreased risk taking after psychosocial stress. However, all other physiological stress parameters were unrelated to risk taking. Thus, it remains open whether the physiological stress response in our study relates to the alterations in risk taking that we observed 80 min after psychosocial stress.
We interpreted the results as showing that stress only affects risk taking when the task is novel. Such effects of repeated participation on decision-making have been reported earlier (Sokol-Hessner et al., Citation2016). Prior exposure to the experimental setting might have diminished the effect of psychosocial stress on risk taking due to response habituation of our participants. The familiarity with the decision-making task on the second testing day might have led the participants to habitually conduct the BART, so that stress had no more influence on risk taking of our participants.
The use of oral contraceptives influences the human stress response (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, Citation1999). Consequently, we controlled for the variable in further analyses and found that the main effect of stress condition became non-significant for the cortisol response and risk taking. However, the relevant interactions remained significant, showing that psychosocial stress evoked a cortisol stress reaction and changed risk taking in novel decision situations even after controlling for the use of oral contraceptives.
One limitation of our study is the use of a single measurement time point 80 min after stress. Future studies should use several measurement time points to disentangle the early and delayed stress effects on risk taking. For measuring early non-genomic corticosteroid effects, risk taking should be assessed approximately 30 min after stress and for detecting the delayed genomic effects another measurement should be conducted around 120 min post stress.
Another limitation of our study refers to the all-female sample, which restricts generalizability to men. The results of our study and the two studies available emphasize comparable delayed stress effects for both sexes. We found decreased risk taking in an all-female sample, Yamakawa et al. (Citation2016) found decreased risk taking in an all-male sample and Bendahan et al. (Citation2017) found in their mixed-sex sample no sex effects of stress on risk taking.
An additional limitation refers to the risk taking task. While it is not disadvantageous to take risk in the BART, in other risk taking tasks it is disadvantageous. Since there is evidence that the stress effect on risk taking depends on the advantageousness of taking risk (Starcke & Brand, Citation2016), the generalizability of our results to other decision situations in which risk taking is disadvantageous is limited.
In summary, we show that stress influences risk taking 80 min after stress exposure. Our results complement prior risk taking research, showing that in addition to the early stress response also delayed stress effects affect risk taking in women who are confronted with novel risk taking tasks. The finding has important implications for everyday life. Even when the immediate psychophysiological stress response is over and no longer perceivable for the individual, the cognitive processes can still be affected by stress experienced hours before. Future research should further examine the delayed stress response, taking into account the novelty of the decision situation as well as possible influences of sex.
Disclosure statement
All authors declared no conflicts of interest with respect to the authorship or the publication of this article.
Additional information
Notes on contributors
Jan Nowacki
Christian Deuter and Jan Nowacki: literature research, data analyses
Jan Nowacki: manuscript preparation
Moritz Duesenberg
Moritz Duesenberg: recruitment of participants, data management
Christian Eric Deuter
Christian Deuter and Jan Nowacki: literature research, data analyses
Christian Otte
Katja Wingenfeld & Christian Otte: PI of the study, study design, statistics, and manuscript revision
Katja Wingenfeld
Katja Wingenfeld & Christian Otte: PI of the study, study design, statistics, and manuscript revision
References
- Bendahan, S., Goette, L., Thoresen, J., Loued-Khenissi, L., Hollis, F., & Sandi, C. (2017). Acute stress alters individual risk taking in a time‐dependent manner and leads to anti‐social risk. European Journal of Neuroscience, 45, 877–885. doi:10.1111/ejn.13395
- Cornelisse, S., Van Ast, V., Haushofer, J., Seinstra, M., & Joels, M. (2013). Time-dependent effect of hydrocortisone administration on intertemporal choice.
- de Kloet, E. (2014). From receptor balance to rational glucocorticoid therapy. Endocrinology, 155, 2754–2769. doi:10.1210/en.2014-1048
- de Kloet, E., Meijer, O., de Nicola, A., de Rijk, R., & Joëls, M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Frontiers in Neuroendocrinology, 49, 124–145. doi:10.1016/j.yfrne.2018.02.003
- Deuter, C.E., Wingenfeld, K., Schultebraucks, K., Hellmann-Regen, J., Piber, D., & Otte, C. (2017). Effects of mineralocorticoid-receptor stimulation on risk taking behavior in young healthy men and women. Psychoneuroendocrinology, 75, 132–140. doi:10.1016/j.psyneuen.2016.10.018
- Dickerson, S.S., & Kemeny, M.E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355. doi:10.1037/0033-2909.130.3.355
- Duesenberg, M., Weber, J., Schulze, L., Schaeuffele, C., Roepke, S., Hellmann-Regen, J., … Wingenfeld, K. (2016). Does cortisol modulate emotion recognition and empathy? Psychoneuroendocrinology, 66, 221–227. doi:10.1016/j.psyneuen.2016.01.011
- Duesenberg, M., Wolf, O.T., Metz, S., Roepke, S, Fleischer, J, Elias, V., … Wingenfeld, K. (2019). Psychophysiological stress response and memory in borderline personality disorder. Eur J Psychotraumatol, 10, p1568134.
- Hermans, E.J., Henckens, M.J., Joëls, M., & Fernández, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37, 304–314. doi:10.1016/j.tins.2014.03.006
- Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O.T. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the “Trier Social Stress Test”. Psychoneuroendocrinology, 34, 1075–1086. doi:10.1016/j.psyneuen.2009.02.008
- Hines, E.A., & Brown, G.E. (1936). The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. American Heart Journal, 11, 1–9. doi:10.1016/S0002-8703(36)90370-8
- Joëls, M., Karst, H., & Sarabdjitsingh, R.A. (2018). The stressed brain of humans and rodents. Acta Physiologica, 223, e13066. doi:10.1111/apha.13066
- Kirschbaum, C., Kudielka, B.M., Gaab, J., Schommer, N.C., & Hellhammer, D.H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162. doi:10.1097/00006842-199903000-00006
- Kirschbaum, C., Pirke, K.M., & Hellhammer, D.H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. doi:10.1159/000119004
- Lejuez, C.W., Read, J.P., Kahler, C.W., Richards, J.B., Ramsey, S.E., Stuart, G.L., … Brown, R.A. (2002). Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART). Journal of Experimental Psychology: Applied, 8, 75. doi:10.1037//1076-898X.8.2.75
- Lighthall, N.R., Mather, M., & Gorlick, M.A. (2009). Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS One, 4, e6002. doi:10.1371/journal.pone.0006002
- Margittai, Z., Strombach, T., Van Wingerden, M., Joels, M., Schwabe, L., & Kalenscher, T. (2015). A friend in need: time-dependent effects of stress on social discounting in men. Hormones and Behavior, 73, 75–82.
- Margittai, Z., Nave, G., Van Wingerden, M., Schnitzler, A., Schwabe, L., & Kalenscher, T. (2018). Combined effects of glucocorticoid and noradrenergic activity on loss aversion. Neuropsychopharmacology, 43, 334. doi:10.1038/npp.2017.75
- McRae, A.L., Saladin, M.E., Brady, K.T., Upadhyaya, H., Back, S.E., & Timmerman, M.A. (2006). Stress reactivity: Biological and subjective responses to the cold pressor and Trier Social stressors. Human Psychopharmacology: Clinical and Experimental, 21, 377–385. doi:10.1002/hup.778
- Pabst, S., Brand, M., & Wolf, O.T. (2013). Stress and decision making: A few minutes make all the difference. Behavioural Brain Research, 250, 39–45. doi:10.1016/j.bbr.2013.04.046
- Putman, P., Antypa, N., Crysovergi, P., & van der Does, W.A. (2010). Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology, 208, 257–263. doi:10.1007/s00213-009-1725-y
- Robinson, O.J., Bond, R.L., & Roiser, J.P. (2015). The impact of stress on financial decision-making varies as a function of depression and anxiety symptoms. Peer-reviewed Journal, 3, e770. doi:10.7717/peerj.770
- Rombold, F., Wingenfeld, K., Renneberg, B., Hellmann-Regen, J., Otte, C., & Roepke, S. (2016). Influence of the noradrenergic system on the formation of intrusive memories in women: An experimental approach with a trauma film paradigm. Psychological Medicine, 46, 2523–2534. doi:10.1017/S0033291716001379
- Schwabe, L., Haddad, L., & Schachinger, H. (2008). HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology, 33, 890–895. doi:10.1016/j.psyneuen.2008.03.001
- Sokol-Hessner, P., Lackovic, S.F., Tobe, R.H., Camerer, C.F., Leventhal, B.L., & Phelps, E.A. (2015). Determinants of propranolol’s selective effect on loss aversion. Psychological Science, 26, 1123–1130. doi:10.1177/0956797615582026
- Sokol-Hessner, P., Raio, C.M., Gottesman, S.P., Lackovic, S.F., & Phelps, E.A. (2016). Acute stress does not affect risky monetary decision-making. Neurobiology of Stress, 5, 19–25. doi:10.1016/j.ynstr.2016.10.003
- Starcke, K., & Brand, M. (2016). Effects of stress on decisions under uncertainty: A meta-analysis. Psychological Bulletin, 142, 909–933. doi:10.1037/bul0000060
- Steyer, R., Schwenkmezger, P., Notz, P., & Eid, M. (1997). Der mehrdimensionale Befindlichkeitsfragebogen (MDBF). Handanweisung [The multidimensional affect rating scale (MDBF). Manual]. Göttingen, Germany: Hogrefe.
- Van den Bos, R., Harteveld, M., & Stoop, H. (2009). Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology, 34, 1449–1458. doi:10.1016/j.psyneuen.2009.04.016
- van Stegeren, A., Rohleder, N., Everaerd, W., & Wolf, O.T. (2006). Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology, 31, 137–141. doi:10.1016/j.psyneuen.2005.05.012
- Vinkers, C. H., Zorn, J. V., Cornelisse, S., Koot, S., Houtepen, L. C., Olivier, B., … Joëls, M. (2013). Time-dependent changes in altruistic punishment following stress. Psychoneuroendocrinology, 38, 1467–1475.
- Yamakawa, K., Ohira, H., Matsunaga, M., & Isowa, T. (2016). Prolonged effects of acute stress on decision-making under risk: A human psychophysiological study. Frontiers in Human Neuroscience, 10, 444. doi:10.3389/fnhum.2016.00444
