Abstract
Previous studies have revealed stress-induced dysregulation of hypothalamic-pituitary-adrenal (HPA) axis in women with premenstrual syndrome (PMS). So far, however, the results about the relationship between HPA axis dysregulation and PMS are mixed. To this end, it is necessary to investigate the basal activity of the HPA axis in women with PMS instead of only assessing a certain stressor. Therefore, this study evaluated the relationship between the cortisol awakening response (CAR) and PMS. Thirty-two women with PMS (mean age 22.47 ± 2.20 years) and 36 healthy controls (mean age 22.28 ± 2.43 years) were included in this study. Saliva samples of our participants were collected successively at 0, 30, 45, and 60 min after awakening to assess CAR during each of two phases of the menstrual cycle (the mid-follicular phase and the late luteal phase). The results showed a significantly attenuated CAR in women with PMS compared with the healthy controls, especially at 45 and 60 min after awakening, regardless of the menstrual cycle phases. Furthermore, there was a significant negative correlation between PMS severity as measured by PMS scale and AUCi (i.e. the Area Under the Curve with respect to increase) in the mid-follicular phase. Our findings suggested that an attenuated CAR activity profile may be an important risk factor for the development of PMS.
1. Introduction
After entering puberty, women’s endometrium is affected by ovarian hormones and periodic uterine bleeding occurs, which is called menstruation (Farage, Osborn, & MacLean, Citation2008). Almost 80% of women report having some symptoms during the one to two weeks prior to menstruation (Biggs & Demuth, Citation2011). These symptoms interfere with normal life and therefore qualify as premenstrual syndrome (PMS) in 20–30% of women. PMS refers to a group of symptoms linked to the menstrual cycle, which is characterized by a constellation of emotional, physical, and behavioral symptoms that appear at a few days before menstruation and remit with the onset of menses (Cunningham, Yonkers, Oʼbrien, & Eriksson, Citation2009). Considering PMS is so highly prevalent in premenopausal women (Klatzkin, Bunevicius, Forneris, & Girdler, Citation2014; Qiao et al., Citation2012), understanding its underlying mechanisms has gradually become a focus of many experimental research over the past decades (Campagne & Campagne, Citation2007).
A wealth of evidence suggests that PMS is associated with increased daily stress and is exacerbated by stressful life events (Perkonigg, Yonkers, Pfister, Lieb, & Wittchen, Citation2004; Sadler et al., Citation2010). Based on this evidence, many researchers have investigated the stress response dysregulation among PMS individuals (Huang, Zhou, Wu, Wang, & Zhao, Citation2015; Klatzkin, Lindgren, Forneris, & Girdler, Citation2010; Klatzkin et al., Citation2014). Furthermore, the stress-related biological basis also attracts people’s attention, especially the hypothalamic-pituitary-adrenal (HPA) axis (Huang et al., Citation2015; Klatzkin et al., Citation2010). So far, however, the results about relationship between HPA axis dysregulation and PMS are controversial. On the one hand, some studies have found that the attenuated stress reactivity profile represents a phenotype of PMS (Girdler, Straneva, Light, Pedersen, & Morrow, Citation2001; Huang et al., Citation2015; Klatzkin et al., Citation2010; Roca et al., Citation2003). On the other hand, others suggest that PMS is related to the hyper-reactive stress responses (Rabin et al., Citation1990). The discrepancies in these results might be partly due to the methodological differences across studies.
For example, these studies used different tasks and stressors, with some studies used only a single stressor (e.g. Girdler et al., Citation2001; Rabin et al., Citation1990; Roca et al., Citation2003), whereas others used multiple stressors (e.g. Klatzkin et al., Citation2010). Furthermore, most prior studies did not consider the specificities of the HPA axis function. The HPA axis has a diurnal rhythm (Young, Carlson, & Brown, Citation2001) and shows a cyclical change with the change of the menstrual cycle (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, Citation1999). However, the experimental time varied across studies and was not effectively controlled between groups in each study.
The cortisol awakening response (CAR) typically peaks at approximately 30–45 min after awakening and has been seen as a distinctive part of the cortisol circadian cycle (Clow, Hucklebridge, Stalder, Evans, & Thorn, Citation2010; Clow, Thorn, Evans, & Hucklebridge, Citation2004; Pruessner et al. Citation1997). Pruessner et al. (Citation1997) first proposed that CAR was characterized by marked intra-individual stability thus could be a reliable biological marker for the individual’s adrenocortical activity. Although later studies have shown that magnitude of the CAR in healthy individuals affected by some state variables such as ambient light in the morning, morning waking times, anticipation of workload, and negative experiences (see Law, Hucklebridge, Thorn, Evans, & Clow, Citation2013), considerable empirical effort has examined the important role of dysfunctional CAR in stress-induced diseases.
For example, attenuated CAR has been linked to burnout (Pruessner, Hellhammer, & Kirschbaum, Citation1999), chronic pain (Geiss, Varadi, Steinbach, Bauer, & Anton, Citation1997), chronic fatigue syndrome (Roberts, Wessely, Chalder, Papadopoulos, & Cleare, Citation2004), posttraumatic stress disorder (Wessa, Rohleder, Kirschbaum, & Flor, Citation2006), depressed postpartum women (de Rezende et al., Citation2016), and women living in chronic material hardship (Ranjit, Young, & Kaplan, Citation2005). These findings suggest that dysfunctional CAR can be seen as an important risk factor for stress-induced diseases (Fries, Dettenborn, & Kirschbaum, Citation2009). However, little is still known about CAR in women with PMS, which might be associated with dysregulation of stress response.
In this study, we aimed at characterizing the basal HPA activity as measured by CAR in women with PMS. More specifically, saliva samples of women with PMS and healthy controls were collected successively at 0, 30, 45, and 60 min after awakening during each of the two phases of the menstrual cycle (i.e. the mid-follicular phase and the late luteal phase). Based on previous findings, we expected that women with PMS would experience dysfunctional CAR compared with the healthy controls.
2 Methods
2.1 Participant screening
The participant screening procedure is similar to the one used in a previous study (Huang et al., Citation2015). Specifically, potential participants were recruited via posters or online ads at universities. 251 interested women were instructed to assess themselves daily according to the Chinese version of the American College of Obstetricians and Gynecologists (ACOG) recommendations (Cirillo, Passos, Bevilaqua, López, & Nardi, Citation2012; Qiao et al., Citation2012) for PMS diagnosis for two consecutive cycles. 227 women completed the two consecutive cycles of the survey (the completion rate was 90.44%).
The Chinese version of ACOG recommendations has been demonstrated to be valid in our previous studies (Huang et al., Citation2015). These recommendations outlined a total of 10 emotional and physical symptoms: depression, angry outbursts, irritability, anxiety, confusion, social withdrawal, breast tenderness, abdominal bloating, headache, and swelling of extremities. Participants were classified as having PMS if they met all of the five diagnostic criteria: (a) at least one symptom occurred during the five days before menses in each of the three previous menstrual cycles and also that (b) it occurred reproducibly during the next two cycles; (c) these symptoms must be relieved or improved during the 4–13 d of the menstrual cycle and (d) be associated with identifiable dysfunction in work, study or life, additionally, (e) these symptoms must occur in the absence of any pharmacologic therapy, hormone ingestion or alcohol abuse (Cirillo et al., Citation2012). A diary form of questionnaires that contained the 10 symptoms and an additional four PMS diagnostic criterion (above-mentioned criteria (a, c, d, and e) was designed in our study to operationalize the ACOG recommendations. Participants were instructed to indicate whether these symptoms and criteria occurred or not for two consecutive cycles (except both the criteria (a) and (c), which were completed once on the first complete day), and also to indicate the days of menses, thus the criteria (b) were prospectively validated. Participants were asked to complete the printed versions of the questionnaires for two consecutive cycles every night before going to bed. Those who met the above-mentioned five criteria were confirmed as women with PMS, whereas others were confirmed as women without PMS. Based on the criteria described above, among the 227 women who completed the ACOG recommendations daily for two consecutive cycles, 77 (33.92%) women met the criteria for PMS, and the other 150 (66.08%) women were confirmed as non-PMS. Such percentage is consistent with that from previous studies (e.g. Klatzkin et al., Citation2014).
Of the 77 women with PMS, 40 women volunteered to participate in the study. Furthermore, 40 healthy controls were selected from the pool of 150 non-PMS women (control group). Then, these participants were further screened for the inclusion criteria: (a) did not have a cardiovascular disorder or endocrine disorder as confirmed by a complete hospital physical examination; (b) were not taking hormones and medications; (c) had regular menstrual cycles; (d) did not have alcohol and substance abuse; did not currently have Axis I psychiatric disorders that were ascertained using the Chinese version of Mini International Neuropsychiatry Interview, based on DSM-IV and ICD-10 (Si et al., Citation2009); and self-reported of no treatment for depression prior to the screening. After screening, 33 women with PMS and 39 healthy controls were included.
The menstrual cycle phases of each participant were calculated with regard to the self-reported date of her last menses, the average length of a single menstrual cycle, and the predicted time of the next menses, which could be found in demographic data that were collected before the experiment (). The test days for each participant were adjusted according to her cycle length and the duration of menstrual flow. The late luteal phase was validated according to the exact time of the onset of the next menses. Four participants were further excluded because the hormone values and the test days did not correspond to either of two menstrual phases (one with PMS and three healthy controls). Consequently, 32 women with PMS and 36 matched controls composed of the sample for this study. All participants provided written informed consent to participate in this study. This study was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning of Beijing Normal University. This study was performed in accordance with the Declaration of Helsinki.
Table 1. Demographic data and PMS scores of PMS and control group.
2.2 PMS severity measurement
The PMS scale, which consisted of 12 items related to emotional and physical symptoms, can provide the degree of severity of PMS (Bancroft, Citation1993). All items were scored on a four-point Likert scale, with 0 “no symptoms”, 1 “mild symptoms”, 2 “symptoms affecting life, study, and work, but tolerable”, and 3 “symptoms seriously affecting life, study, and work, needing treatment”. Total scores of 6–10, 11–20, and >20 points indicated mild, moderate, and severe PMS, respectively. The validated Chinese PMS scale demonstrated good reliability and validity (Wu, Liang, Wang, Zhao, & Zhou, Citation2016). The PMS scores of PMS and healthy controls are listed in .
2.3 Study protocol and saliva collection
Each participant completed the CAR test twice: in the mid-follicular phase (days 6–9 for 28-day menstrual cycles) and in the late luteal phase (days –4 to –1 for 28-day menstrual cycles). The order of the test was counterbalanced across participants. Participants’ cycle phases were adjusted accordingly if the cycle lengths were longer or shorter than 28 days. The two CAR tests sessions extended across one or two consecutive menstrual cycles. Based on self-reported time (time of last menses, the average length of a single menstrual cycle, and predicted time of the next menses), we calculated the testing time for each participant. The late luteal phase was validated according to the exact time of the onset of the next menses. Additionally, salivary estradiol and progesterone levels taken during the baseline period of the CAR (0-min post-awakening) were used to verify self-reported menstrual cycle phases (). Participants were instructed to collect samples of their saliva immediately, 30, 45, and 60 min after awakening using a Salivette sampling device (DRG Inc., Marburg, Germany). All of the participants received oral and written instructions describing the sampling procedures and were requested to use an alarm clock after awakening to ensure accurate timing of their saliva collection. Those samples that exceeded a 5-min deviation from the preset sampling times were excluded from analysis to avoid a potentially confounding effect of sampling time, especially the delay between awakening time and the first saliva collection (Kudielka, Broderick, & Kirschbaum, Citation2003). Additionally, the participants were asked to refrain from eating or drinking and not to brush their teeth before all samples were collected. Furthermore, a paper diary had to be filled out in which participants reported sleep times, awakening times, and exact sampling times.
Table 2. Awakening time, sleep duration, and saliva data of the two groups in the mid-follicular phase and the late luteal phase (M ± SD).
2.4 Biochemical assays
All saliva samples were collected with the Salivette sampling device (DRG Inc., Marburg, Germany) and were stored at –20 °C until assayed. All saliva samples were collected and sent to the biochemical laboratory of Beijing Huada Protein Research and Development Center (Beijing, China) for analysis. Cortisol, estradiol, and progesterone analyses were determined by a competitive enzyme-linked immunosorbent assay. The intra- and inter-assay coefficients of variation were all below 12%. Cortisol, estradiol and progesterone levels of PMS and control group tested in the follicular phase and the late luteal phase are listed in .
2.5 Statistical analyses
All statistics were conducted using SPSS, version 19.0 (Chicago, IL). Where appropriate, the Greenhouse-Geisser correction was applied. Post-hoc Bonferroni tests were used in case of significant ANOVA effects. Partial η2 is presented as a measure of the effect size.
In this study, a significant skewness in the distributions of basal cortisol and sex hormonal data was identified. Accordingly, these raw data were log-transformed so that data could follow a standard normal distribution, as confirmed by Kolmogorov-Smirnov and Shapiro-Wilk tests (p’s > .05). All subsequent statistical analysis on basal cortisol and sex hormonal data were performed on these log-transformation data.
Independent sample t tests were used to compare the demographic variables and PMS scale scores between women with PMS and the healthy controls. Awakening times, sleep durations, and sex hormonal levels were analyzed using two-way mixed analyses of variance (ANOVAs) with group (PMS and control) as a between-subject factor and menstrual cycle (the mid-follicular phase and the late luteal phase) as a within-subject factor. CAR was assessed using three-way mixed ANOVAs with sampling times (0, +30, +45, +60 min post-awakening) and menstrual cycle as within-subject factors and group as a between-subject factor. Furthermore, we calculated Area Under the Curve with respect to ground and increase (AUCg and AUCi) of cortisol levels after awakening, as described in Pruessner, Kirschbaum, Meinlschmid, and Hellhammer, (Citation2003). Then the AUCg and AUCi were analyzed using two-way mixed ANOVAs with group as a between-subject factor and menstrual cycle as a within-subject factor. Finally, the correlation between AUCg/AUCi and PMS score was calculated, respectively.
3 Results
3.1 Demographic information
The demographic data of our participants were reported in . As shown in this table, these two groups were matched. We only found a significant main effect of group on PMS scale scores (t (66) =5.41, p < .001), with significantly higher PMS scores in women with PMS than in women without PMS ().
In addition, for awakening times and sleep durations, no main or interaction effect was significant (all F (1,66) < 3.33, ps > .07) ().
3.2 Salivary hormone levels
shows the salivary estradiol and progesterone levels in women with PMS and healthy controls across the menstrual cycle.
For our analysis on progesterone data, we found a main effect of menstrual cycle (F (1,66) = 34.94, p < .001, partial η2 = 0.35), with progesterone levels during the late luteal phase being significantly higher than that during the mid-follicular phase. No other main or interaction effects were found (all F < 0.30, ps >.59). For estradiol, we did not find any main or interaction effect (all F < 3.24, ps > .08). These results were consistent with finding from previous studies (Soni, Curran, & Kamboj, Citation2013).
3.3 Cortisol awakening response
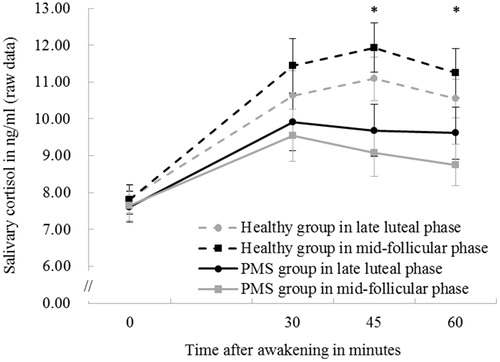
Our analysis revealed a significant main effect of sampling times on CAR (F (3, 198) = 41.69, p < .001, partial η2 = 0.39). There was also a significant main effect of group (F (1, 66) = 4.94, p = .03, partial η2 = 0.07), with a significantly attenuated CAR in women with PMS compared to that of the healthy controls. Furthermore, there was a significant interaction between group and sampling times (F (3, 198) = 3.59, p = .02, partial η2 = 0.05), which was driven by the attenuated CAR in women with PMS at 45 (p = .005, partial η2 = 0.11) and 60 min (p = .03, partial η2 = 0.07) after awakening (). No other main or interaction effect was significant (ps > .13).
Figure 1. CAR at 0, 30, 45, and 60 min after awakening during mid-follicular and the late luteal phases in both healthy controls and women with PMS. Error bars represent standard errors. *p < .05 in women with PMS relative to healthy controls at 45 and 60 min after awakening, regardless of the menstrual cycle phases.
For the AUCg, there was a significant main effect of group (F (1, 66) = 4.25, p = .04, partial η2 = 0.06), with a significantly lower AUCg in women with PMS (542.95 ± 139.46) compared with that of the healthy controls (620.05 ± 165.64). No other main or interaction effect was significant (ps > .19). Furthermore, our analysis also revealed a significant main effect of group on AUCi (F (1, 66) = 5.33, p = .02, partial η2 = 0.08), with a significantly lower AUCi in women with PMS (85.19 ± 126.19) compared with that of the healthy controls (150.61 ± 107.53). No other main or interaction effect was significant (ps > .13), too.
3.4 Correlation between AUCg/AUCi and PMS score
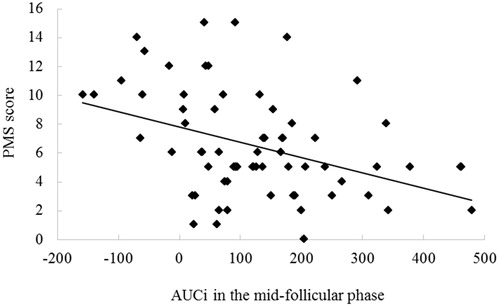
Correlation results indicated that AUCi in the mid-follicular phase was negatively correlated with PMS score (r = –0.398, p < .01). No other significant correlations were found (ps > .11) ().
4 Discussion
The aim of this study was to characterize basal HPA axis activity as measured by the CAR in women with PMS. We found an attenuated CAR in women with PMS as compared to that of the healthy control. In particular, such an attenuated CAR was observed at 45 and 60 min after awakening and was independent of the menstrual cycle. Moreover, the CAR was negatively correlated with PMS severity measured by the PMS scale.
Specifically, the attenuated CAR found in women with PMS was consistent with findings from previous studies that examined the dysregulation of HPA axis using laboratory physiological or psychological stressors (Huang et al., Citation2015; Klatzkin et al., Citation2010). The attenuation of CAR reactivity in women with PMS may be partially accounted by the chronic symptomatology of PMS. Based on previous studies on the relationship between PMS and stress (Deuster, Adera, & South-Paul, Citation1999; Perkonigg et al., Citation2004; Sadler et al., Citation2010), for women with PMS, the cyclic recurrence of negative symptoms in the late luteal phase can be regarded as a cyclical negative event (i.e. stressor). Human and animal studies suggested that chronic or repeated stress exposure may lead to persistent alterations in neurobiological systems, especially the HPA axis (Heim, Newport, Bonsall, Miller, & Nemeroff, Citation2001; Lindley, Carlson, & Benoit, Citation2004). Thus, the long-term effect of the physiological responses to stress in women with PMS may result in the exhaustion of the HPA axis, leading to an attenuated basal HPA axis response reflected by an attenuation of the CAR.
Although the exact functions of the CAR are not well understood, previous studies argued that an attenuated CAR might be due to a lack of regulating effects of cortisol at the metabolic, immune, and central nervous system levels (Heim, Ehlert, & Hellhammer, Citation2000; Meinlschmidt & Heim, Citation2005). Thus, an attenuated CAR might weaken individuals’ ability to respond to the demands of a stressful situation (Fries et al., Citation2009), leading to increased vulnerability for somatic and mental health problems. The observed attenuated CAR in women with PMS in our study seemed to be in line with a popular view that hypoactivity of the HPA axis might be an important risk factor for the development of stress-related disorders (Ehlert, Gaab, & Heinrichs, Citation2001).
Another key finding of our study was that there was no significant of menstrual cycle on the CAR. This result was in concordance with three previous studies showing no differences in CAR during the follicular and the luteal phase in healthy women (Bouma, Riese, Ormel, Verhulst, & Oldehinkel, Citation2009; Kudielka & Kirschbaum, Citation2003; Wolfram, Bellingrath, & Kudielka, Citation2011). Our results extended this phenomenon to women with PMS and thus advanced our understanding on the relationship between CAR and the menstrual cycle. Moreover, the significant correlation between AUCi in the mid-follicular phase and PMS scores was consistent with a previous study (Duchesne & Pruessner, Citation2013). In this study, they found that the negative correlation between subjectively reported stress and stress-induced cortisol reaction only existed in the follicular phase. Thus, future studies should examine whether menstrual cycles can also affect the relationship between stress neuroendocrine response and symptom severity.
This study had several limitations. First, although studies showed that CAR exhibited moderate to high stability (Hucklebridge, Hussain, Evans, & Clow, Citation2005; Wust et al., Citation2000), other studies found that the CAR of a single day is largely influenced by environmental factors (Stalder, Evans, Hucklebridge, & Clow, Citation2010; Zoccola, Dickerson, & Yim, Citation2011). Future research should test CAR for more than one day to obtain more reliable results (Hellhammer et al., Citation2007). Second, we did not collect data regarding women’s traumatic history and socioeconomic status, which was related to HPA axis activity in previous studies (Cohen, Doyle, & Baum, Citation2006; Klaassens et al., Citation2009; Kudielka et al., Citation2006). Future research should take into account these factors and eliminate the potential effect of these confounding factors. Third, we did not collect the saliva samples during ovulation. It should be noted, however, that women may show increased CAR during ovulation (Wolfram et al., Citation2011), which may be mediated by peak concentrations of sex steroid levels during the ovulation period. Therefore, whether the regulation of CAR by menstrual cycle is only occurred in the ovulation period remains to be clarified in future studies. Additionally, although we used the estradiol and progesterone levels to confirm the selection of menstrual phase and four participants were excluded due to the abnormal hormone levels, we only used subjective report to determine the menstrual phase during the experiment. Future research should incorporate multiple approaches (e.g. chromatographic ovulation test) to determine the test time more accurately. Finally, although there were statistical differences of CAR between the PMS group and the healthy control group, the differences were relatively small but comparable to those from other studies (e.g. de Rezende et al., Citation2016), caution should be taken in when drawing conclusions.
In summary, our study revealed that women with PMS displayed an attenuation of the CAR compared to women without PMS and such a hypoactivity of HPA axis occurred 45 and 60 min awakening later. These findings supported the notion that an attenuated basal HPA axis activity as indexed by the CAR might be an important risk factor for PMS and may have important implications for our understanding on the role of basal stress responses in the pathological basis of PMS.
Notes on contributor
All authors have made a significant contribution to this work. Y.H. collected the data. L.H., Y.H. and R.Z. analyzed and interpreted the data. L.H. wrote the current version of this article with critical revision of Y.H. and R.Z. All authors have approved the final version of the article.
Acknowledgments
We thanked the volunteers of this study and the funding that supported the project. We also would like to thank Dr Senqi Hu for his assistance in giving comments and suggestions. Moreover, we would like to thank Dr Rachel Han for her assistance in language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bancroft, J. (1993). The premenstrual syndrome–a reappraisal of the concept and the evidence. Psychological Medicine Monograph Supplement, 24, 1–47. doi:10.1017/S0264180100001272
- Biggs, W.S., & Demuth, R.H. (2011). Premenstrual syndrome and premenstrual dysphoric disorder. American Family Physician, 84, 918–924.
- Bouma, E.M., Riese, H., Ormel, J., Verhulst, F.C., & Oldehinkel, A.J. (2009). Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology, 34, 884–893. doi:10.1016/j.psyneuen.2009.01.003
- Campagne, D.M., & Campagne, G. (2007). The premenstrual syndrome revisited. European Journal of Obstetrics & Gynecology and Reproductive Biology, 130, 4–17. doi:10.1016/j.ejogrb.2006.06.020
- Cirillo, P.C., Passos, R.B.F., Bevilaqua, M.C.D.N., López, J.R.R.A., & Nardi, A.E. (2012). Bipolar disorder and premenstrual syndrome or premenstrual dysphoric disorder comorbidity: A systematic review. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil: 1999), 34, 467–479. doi:10.1016/j.rbp.2012.04.010
- Clow, A., Thorn, L., Evans, P., & Hucklebridge, F. (2004). The awakening cortisol response: Methodological issues and significance. Stress, 7, 29–37. doi:10.1080/10253890410001667205
- Clow, A., Hucklebridge, F., Stalder, T., Evans, P., & Thorn, L. (2010). The cortisol awakening response: More than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews, 35, 97–103. doi:10.1016/j.neubiorev.2009.12.011
- Cohen, S., Doyle, W.J., & Baum, A. (2006). Socioeconomic status is associated with stress hormones. Psychosomatic Medicine, 68, 414–420. doi:10.1097/01.psy.0000221236.37158.b9
- Cunningham, J., Yonkers, K.A., Oʼbrien, S., & Eriksson, E. (2009). Update on research and treatment of premenstrual dysphoric disorder. Harvard Review of Psychiatry, 17, 120–137. doi:10.1080/10673220902891836
- de Rezende, M.G., Garcia-Leal, C., de Figueiredo, F.P., Cavalli, R. D C., Spanghero, M.S., Barbieri, M.A., … Del-Ben, C.M. (2016). Altered functioning of the HPA axis in depressed postpartum women. Journal of Affective Disorders, 193, 249–256. doi:10.1016/j.jad.2015.12.065
- Deuster, P.A., Adera, T., & South-Paul, J. (1999). Biological, social, and behavioral factors associated with premenstrual syndrome. Archives of Family Medicine, 8, 122–128. doi:10.1001/archfami.8.2.122
- Duchesne, A., & Pruessner, J. C. (2013). Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology, 38, 3155–3159. doi:10.1016/j.psyneuen.2013.08.009
- Ehlert, U., Gaab, J., & Heinrichs, M. (2001). Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus–pituitary–adrenal axis. Biological Psychology, 57, 141–152. doi:10.1016/S0301-0511(01)00092-8
- Farage, M.A., Osborn, T.W., & MacLean, A.B. (2008). Cognitive, sensory, and emotional changes associated with the menstrual cycle: A review. Archives of Gynecology and Obstetrics, 278, 299–307. doi:10.1007/s00404-008-0708-2
- Fries, E., Dettenborn, L., & Kirschbaum, C. (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72, 67–73. doi:10.1016/j.ijpsycho.2008.03.014
- Geiss, A., Varadi, E., Steinbach, K., Bauer, H.W., & Anton, F. (1997). Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neuroscience Letters, 237, 65–68. doi:10.1016/S0304-3940(97)00810-0
- Girdler, S.S., Straneva, P.A., Light, K.C., Pedersen, C.A., & Morrow, A.L. (2001). Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biological Psychiatry, 49, 788–797. doi:10.1016/S0006-3223(00)01044-1
- Heim, C., Ehlert, U., & Hellhammer, D.H. (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25, 1–35. doi:10.1016/S0306-4530(99)00035-9
- Heim, C., Newport, D.J., Bonsall, R., Miller, A.H., & Nemeroff, C.B. (2001). Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry, 158, 575–581. doi:10.1176/appi.ajp.158.4.575
- Hellhammer, J., Fries, E., Schweisthal, O.W., Schlotz, W., Stone, A.A., & Hagemann, D. (2007). Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State-and trait components. Psychoneuroendocrinology, 32, 80–86. doi:10.1016/j.psyneuen.2006.10.005
- Huang, Y., Zhou, R., Wu, M., Wang, Q., & Zhao, Y. (2015). Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress (Amsterdam, Netherlands), 18, 160–168. doi:10.3109/10253890.2014.999234
- Hucklebridge, F., Hussain, T., Evans, P., & Clow, A. (2005). The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology, 30, 51–57. doi:10.1016/j.psyneuen.2004.04.007
- Kirschbaum, C., Kudielka, B.M., Gaab, J., Schommer, N.C., & Hellhammer, D.H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162. doi:10.1097/00006842-199903000-00006
- Klaassens, E.R., van Noorden, M.S., Giltay, E.J., van Pelt, J., van Veen, T., & Zitman, F.G. (2009). Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 33, 889–894. doi:10.1016/j.pnpbp.2009.04.011
- Klatzkin, R.R., Bunevicius, A., Forneris, C.A., & Girdler, S. (2014). Menstrual mood disorders are associated with blunted sympathetic reactivity to stress. Journal of Psychosomatic Research, 76, 46–55. doi:10.1016/j.jpsychores.2013.11.002
- Klatzkin, R.R., Lindgren, M.E., Forneris, C.A., & Girdler, S.S. (2010). Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biological Psychology, 84, 235–247. doi:10.1016/j.biopsycho.2010.01.018
- Kudielka, B.M., & Kirschbaum, C. (2003). Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology, 28, 35–47. doi:10.1016/S0306-4530(02)00008-2
- Kudielka, B.M., Broderick, J.E., & Kirschbaum, C. (2003). Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine, 65, 313–319. doi:10.1097/01.PSY.0000058374.50240.BF
- Kudielka, B.M., von Känel, R., Preckel, D., Zgraggen, L., Mischler, K., & Fischer, J.E. (2006). Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biological Psychology, 72, 147–153. doi:10.1016/j.biopsycho.2005.09.001
- Law, R., Hucklebridge, F., Thorn, L., Evans, P., & Clow, A. (2013). State variation in the cortisol awakening response. Stress, 16, 483–492. doi:10.3109/10253890.2013.817552
- Lindley, S.E., Carlson, E.B., & Benoit, M. (2004). Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biological Psychiatry, 55, 940–945. doi:10.1016/j.biopsych.2003.12.021
- Meinlschmidt, G., & Heim, C. (2005). Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology, 30, 568–576. doi:10.1016/j.psyneuen.2005.01.006
- Perkonigg, A., Yonkers, K.A., Pfister, H., Lieb, R., & Wittchen, H.U. (2004). Risk factors for premenstrual dysphoric disorder in a community sample of young women: The role of traumatic events and posttraumatic stress disorder. The Journal of Clinical Psychiatry, 65, 1314–1322. doi:10.4088/JCP.v65n1004
- Pruessner, J.C., Gaab, J., Hellhammer, D.H., Lintz, D., Schommer, N., & Kirschbaum, C. (1997). Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology, 22, 615–625. doi:10.1016/S0306-4530(97)00072-3
- Pruessner, J.C., Hellhammer, D.H., & Kirschbaum, C. (1999). Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine, 61, 197–204. doi:10.1097/00006842-199903000-00012
- Pruessner, J.C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D.H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. doi:10.1016/S0306-4530(02)00108-7
- Qiao, M., Zhang, H., Liu, H., Luo, S., Wang, T., Zhang, J., & Ji, L. (2012). Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample in China. European Journal of Obstetrics and Gynecology and Reproductive Biology, 162, 83–86. doi:10.1016/j.ejogrb.2012.01.017
- Rabin, D.S., Schmidt, P.J., Campbell, G., Gold, P.W., Jensvold, M., Rubinow, D.R., & Chrousos, G.P. (1990). Hypothalamic-pituitary-adrenal function in patients with the premenstrual syndrome. The Journal of Clinical Endocrinology and Metabolism, 71, 1158–1162. doi:10.1210/jcem-71-5-1158
- Ranjit, N., Young, E.A., & Kaplan, G.A. (2005). Material hardship alters the diurnal rhythm of salivary cortisol. International Journal of Epidemiology, 34, 1138–1143. doi:10.1093/ije/dyi120
- Roberts, A.D., Wessely, S., Chalder, T., Papadopoulos, A., & Cleare, A.J. (2004). Salivary cortisol response to awakening in chronic fatigue syndrome. British Journal of Psychiatry, 184, 136–141. doi:10.1192/bjp.184.2.136
- Roca, C.A., Schmidt, P.J., Altemus, M., Deuster, P., Danaceau, M.A., Putnam, K., & Rubinow, D.R. (2003). Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. The Journal of Clinical Endocrinology and Metabolism, 88, 3057–3063. doi:10.1210/jc.2002-021570
- Sadler, C., Smith, H., Hammond, J., Bayly, R., Borland, S., Panay, N., … Inskip, H. (2010). Lifestyle factors, hormonal contraception, and premenstrual symptoms: The United Kingdom Southampton Women’s Survey. Journal of Women’s Health, 19, 391–396. doi:10.1089/jwh.2008.1210
- Si, T.M., Shu, L., Dang, W.M., Su, Y.A., Chen, J.X., Dong, W.T., … Zhang, W.H. (2009). Evaluation of the reliability and validity of Chinese version of the Mini-International Neuropsychiatric Interview in patients with mental disorders. Chinese Mental Health Journal, 23, 493–497.
- Soni, M., Curran, V.H., & Kamboj, S.K. (2013). Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiology of Learning and Memory, 104, 32–38. doi:10.1016/j.nlm.2013.04.003
- Stalder, T., Evans, P., Hucklebridge, F., & Clow, A. (2010). State associations with the cortisol awakening response in healthy females. Psychoneuroendocrinology, 35, 1245–1252. doi:10.1016/j.psyneuen.2010.02.014
- Wessa, M., Rohleder, N., Kirschbaum, C., & Flor, H. (2006). Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology, 31, 209–215. doi:10.1016/j.psyneuen.2005.06.010
- Wolfram, M., Bellingrath, S., & Kudielka, B.M. (2011). The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology, 36, 905–912. doi:10.1016/j.psyneuen.2010.12.006
- Wu, M., Liang, Y., Wang, Q., Zhao, Y., & Zhou, R. (2016). Emotion dysregulation of women with premenstrual syndrome. Scientific Reports, 6, 38501. doi:10.1038/srep38501
- Wust, S., Wolf, J., Hellhammer, D.H., Federenko, I., Schommer, N., & Kirschbaum, C. (2000). The cortisol awakening response-normal values and confounds. Noise and Health, 2, 79.
- Young, E.A., Carlson, N.E., & Brown, M.B. (2001). Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology, 25, 267–276. doi:10.1016/S0893-133X(00)00236-0
- Zoccola, P.M., Dickerson, S.S., & Yim, I.S. (2011). Trait and state perseverative cognition and the cortisol awakening response. Psychoneuroendocrinology, 36, 592–595. doi:10.1016/j.psyneuen.2010.10.004