Abstract
The melanocortin-4 receptor (MC4R) facilitates hypothalamic–pituitary–adrenocortical (HPA) axis responses to acute stress in male rodents and is a well known to regulator of energy balance. Mutations in the MC4R is the most common monogenic cause of obesity in humans and has been associated with sex-specific effects, but whether stress regulation by the MC4R is sex-dependent, and whether the MC4R facilitates HPA responses to chronic stress, is unknown. We hypothesized that MC4R-signaling contributes to HPA axis dysregulation and metabolic pathophysiology following chronic stress exposure. We measured changes in energy balance, HPA axis tone, and vascular remodeling during chronic variable stress (CVS) in male and female rats with MC4R loss-of-function. Rats were placed into three groups (n = 9–18/genotype/sex) and half of each group was subjected to CVS for 30 days or were non-stressed littermate controls. All rats underwent an acute restraint stress challenge on Day 30. Rats were euthanized on Day 31, adrenals collected for weight, and descending aortas fixed for morphological indices of vascular pathophysiology. We observed a marked interaction between Mc4r genotype and sex for basal HPA axis tone and acute stress responsivity. MC4R loss-of-function blunted both endpoints in males but exaggerated them in females. Contrary to our hypothesis, Mc4r genotype had no effect on either HPA axis responses or metabolic responses to chronic stress. Heightened stress reactivity of females with MC4R mutations suggests a possible mechanism for the sex-dependent effects associated with this mutation in humans and highlights how stress may differentially regulate metabolism in males and females.
Lay summary
The hypothalamic melanocortin system is an important regulator of energy balance and stress responses. Here, we report a sex-difference in the stress reactivity of rats with a mutation in this system. Our findings highlight how stress may regulate metabolism differently in males and females and may provide insight into sex-differences associated with this mutation in humans
1. Introduction
In response to an acute threat to homeostasis or well-being, the hypothalamic–pituitary–adrenocortical (HPA) axis is engaged. The resulting increase in circulating glucocorticoids acts to mobilize fuels, thereby facilitating appropriate behavioral and/or physiological responses to the stressor (Ulrich-Lai & Herman, Citation2009). In agreement with this, neuroendocrine circuits underlying stress integration and systemic fuel homeostasis are substantially intertwined (Ulrich-Lai & Ryan, Citation2014). Pathways involved in the regulation of energy balance also modulate stress responses, and vice versa (Asakawa et al., Citation2001; Chuang et al., Citation2011; Goodson et al., Citation2017; Kuo et al., Citation2007; Ryan et al., Citation2012, Citation2018). For example, the hypothalamic melanocortin system plays a canonical role in the neuroendocrine control of energy balance (Haskell-Luevano & Monck, Citation2001; Marsh et al., Citation1999; Rossi et al., Citation2011; Vaisse, Clement, Guy-Grand, & Froguel, Citation1998; van Dijk, Thiele, Seeley, Woods, & Bernstein, Citation1997; Wilson, Ollmann, & Barsh, Citation1999) and it also modulates behavioral and physiological responses to acute stress (Chaki, Ogawa, Toda, Funakoshi, & Okuyama, Citation2003; Chuang et al., Citation2010; Kheirabad et al., Citation2015; Liu, Garza, Li, & Lu, Citation2013; Liu et al., Citation2007; Park et al., Citation2016; Ryan et al., Citation2014; Serova, Laukova, Alaluf, & Sabban, Citation2013). Activation of the melanocortin-4 receptor (MC4R) by its endogenous agonist α-melanin-stimulating hormone (αMSH) as well as its pharmacological agonists reduces caloric intake, increases energy expenditure, and alters peripheral glucose and lipid metabolism (Huszar et al., Citation1997; Nogueiras et al., Citation2007; Obici et al., Citation2001; Ryan, Woods, & Seeley, Citation2012). In addition, MC4R-signaling increases anxiety-like behavior (De Barioglio, Lezcano, & Celis, Citation1991; Gonzalez, Vaziri, & Wilson, Citation1996; Liu et al., Citation2013) and increases circulating corticosterone (Dhillo et al., Citation2002; Lu, Barsh, Akil, & Watson, Citation2003) in male rats and mice. Conversely, pharmacological blockade or genetic loss of MC4R function increases caloric intake, decreases energy expenditure (Fan, Boston, Kesterson, Hruby, & Cone, Citation1997; Rossi et al., Citation1998), buffers anxiety-like behaviors (Chaki et al., Citation2003; Kokare, Dandekar, Singru, Gupta, & Subhedar, Citation2010; Liu et al., Citation2007; Serova et al., Citation2013), and blunts HPA axis activation in response to an acute stress challenge, in male rodents (Liu et al., Citation2013; Ryan et al., Citation2014).
Although the HPA axis response to an acutely stressful challenge may be advantageous in the short term, repeated and/or persistent stress exposure is associated with increased risk of cardiometabolic disease (McEwen & Stellar, Citation1993; Steptoe & Kivimäki, Citation2012). Chronic exposure to elevated glucocorticoids is thought to be an important underlying mechanism (Scholz, Sprague, & Kernohan, Citation1957; Walker, Citation2007), since pharmacological glucocorticoid treatment similarly increases risk (Handa, Kondo, Suzuki, & Saruta, Citation1984; Iijima & Malik, Citation1988; Krakoff, Citation1988; Walker, Citation2007). Therefore, interventions that blunt HPA responses to stress, and/or those that disrupt critical interactions between stress-regulatory and fuel-regulatory pathways, are expected to buffer metabolic pathophysiology associated with chronic stress.
Because activating the hypothalamic melanocortin system is thought to facilitate HPA axis responses to acute stress, we hypothesized that MC4R signaling also contributes to HPA axis dysregulation and metabolic consequences pursuant to chronic stress. Specifically, we predicted that blocking MC4R function during chronic stress would protect against indices of stress-induced HPA axis dysregulation, weight loss, and vascular pathophysiology. We tested this by applying the chronic variable stress paradigm in a well-characterized rat model for genetic MC4R loss-of-function (Almundarij et al., Citation2016; Mul et al., Citation2012; Ryan et al., Citation2014). Notably, previous work regarding the role of MC4R on HPA axis regulation has been performed almost exclusively in male subjects. This is especially problematic in light of prominent sex differences in HPA axis responsivity and the strong female bias toward stress-related psychopathologies including metabolic disease (Critchlow, Liebelt, Bar-Sela, Mountcastle, & Lipscomb, Citation1963; Earls, Citation1987; Handa, Burgess, Kerr, & O’Keefe, 1994; Murphy & Loria, Citation2017; Solomon et al., Citation2015). Understanding sex-specific effects of the MC4R may help inform treatment of both metabolic and stress-related psychological diseases. Therefore, we included both males and females in our experiments.
2. Materials and methods
2.1. Rats
Age-matched male and female rats were generated in-house from breeding pairs heterozygous for a loss-of-function mutation in Mc4r (Mul et al., Citation2012). This mutation introduces a stop codon (K314X) in Mc4r, resulting in impaired membrane-binding and subsequent non-functionality of the receptor. We have previously demonstrated functional loss of MC4R signaling with this mutation. For example, loss of in vivo MC4R function was confirmed by intracerebroventricular administration of agouti-related protein (AgRP), an MC4R inverse agonist, or Melanotan-II, an MC4R agonist, which altered feeding behavior in wild-type rats but did not in homozygous mutant rats (Mul et al., Citation2012). Genotypes were identified by PCR with forward primers designed against wildtype or mutant Mc4r and a common reverse primer (WT-forward: GTCAA GAACT GAGGA AAACC TTCA; mutant-forward: GTCAA GAACT GAGGA AAACC TTCT; reverse: CTTTT TCCCC ACGTC AAAAG TC). Rats were homozygous (HOM, male n = 13, female n = 10), heterozygous (HET, male n = 18, female n = 18) or wild-type (WT, male n = 12, female n = 14) for the mutation. All rats were singly housed in an AAALAC-approved, temperature- (20 °C – 22 °C) and humidity-controlled facility with a 12-12 light/dark cycle (lights on at 0700 h and off at 1900 h) and allowed ad libitum access to standard rat chow (Formulab #5008 LabDiet, MO) and water unless otherwise noted. All animal experiments were approved by the IACUC of the University of California, Davis.
2.2. Chronic variable stress
8-week-old male and female rats were divided into weight-matched (within genotype) chronic variable stress (CVS) and non-stressed control (CON) groups. Rats in the CVS group were subjected to twice daily stressors delivered in randomized order for four weeks. Stressors included crowding (6–8 rats in a standard rat cage for 2 h), white noise (∼75 dB for 1 h), shaker platform (100 rpm for 1 h), strobe light (∼250 flashes/min for 1 h), hypoxia (8% O2, 92% N2 for 30 min), no bedding (overnight), and 45° cage tilt (overnight). For the crowding stressor, animals receiving CVS treatment were mixed together within each sex, with particular animals crowded together randomized each time this stressor was used. Sample sizes for each group are detailed in . The CVS protocol is outlined in .
Table 1. Sample sizes of treatment groups.
Table 2. Chronic variable stress protocol.
2.3. HPA axis testing
On day 17 of CVS treatment, we collected a basal ‘unstressed’ tail-clip blood sample at 1500 h, near the peak of the diurnal rhythm. Briefly, the last 0.5 mm of the tail was removed, and blood was collected into chilled, EDTA-coated tubes. Importantly, samples were collected within 3 minutes of first disturbing the rat’s home cage. This timeframe provides a “snapshot” of unstressed hormones, since it is completed prior to any increases in corticosterone that occur in response to handling the animal (Vahl et al., Citation2005). On day 30 of CVS treatment, we collected a second, ‘unstressed’ blood sample at 0900 h, near the nadir of the diurnal rhythm from both CVS and CON rats. Rats were quickly placed into well-ventilated restraint tubes, the tail clipped, and blood collected as above. Rats remained in the restraint tube for 30 minutes and additional samples were collected 15, 30, 60 and 120 minutes after the onset of restraint. In both cases, blood was centrifuged at 900×g for 20 min at 4 °C. Plasma was stored at −80 °C until later analysis. Plasma corticosterone was measured using the Corticosterone Double Antibody RIA Kit (MP Biomedicals, Santa Ana, CA) according to manufacturer’s instructions.
2.4. Adrenal weights
Left and right adrenals from each animal were collected at sacrifice (1300 h, on day 31 of CVS treatment), cleaned, and weighed as an indirect index of chronic HPA activation.
2.5. Aortic histology
Descending aortas were collected and cleaned at sacrifice, fixed in 10% neutral buffered formalin and stored at 4 °C. A 2–3 mm piece of the descending aorta was later collected 15–18 mm from the diaphragm, embedded in paraffin, sectioned (5 µm) and stained with Masson’s trichrome (Research Histology Laboratory, UC Davis). Luminal and medial areas, luminal diameter, and tunica media thickness, were quantified using ImageJ, as in (Goodson et al., Citation2017).
2.6. Statistical analysis
Data were analyzed by the appropriate mixed-model 2-factor or 3-factor ANOVA, as noted, with Tukey’s HSD post hoc analysis. In all cases, α = 0.05.
3. Results
3.1. Basal HPA axis activity
We and others have previously demonstrated that MC4R loss-of-function abrogates acute responses to stress in male rodents (Chaki et al., Citation2003; Liu et al., Citation2013; Ryan et al., Citation2014; Serova et al., Citation2013). Therefore, we predicted that MC4R loss-of-function would also protect against increased basal (non-stress) HPA axis activity typically observed during CVS.
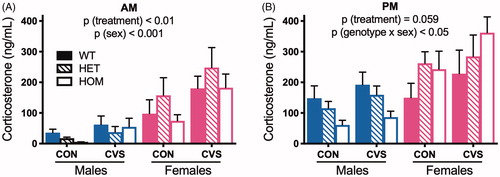
Consistent with the known effect of CVS to increase basal HPA axis tone (Ulrich-Lai & Herman, Citation2009), we observed a main effect of treatment (F(1,61) = 7.26, p < .01), such that CVS-treated rats exhibited greater AM corticosterone compared to CON. Consistent with known sex differences in the HPA axis (Handa et al., Citation1994; Kudielka & Kirschbaum, Citation2004), we observed a main effect of sex (F(1,61) = 26.95, p < .001), such that females exhibited greater AM corticosterone compared to males. However, contrary to expectation, there was no effect of genotype and no significant interactions (3-way ANOVA, ). For the samples collected in the PM (3-way ANOVA, ), near the peak of the diurnal rhythm, we observed a significant genotype x sex interaction (F(2,61) = 3.96, p < .05). Within males, loss of MC4R function decreased plasma corticosterone in a gene dose-dependent manner, though it did not abrogate any increase observed with CVS (i.e. no genotype x treatment interaction). Unexpectedly, loss of MC4R function among females increased plasma corticosterone in a gene dose-dependent manner. Again, this occurred regardless of treatment. Thus, we conclude that loss of MC4R function has opposing effects on basal HPA axis tone in males vs females, and this is independent of exposure to CVS.
Figure 1. MC4R loss-of-function has sex-dependent effects on basal HPA axis tone. Rats exposed to chronic variable stress (CVS) exhibited increased basal plasma corticosterone, compared to unstressed littermate controls (CON), when measured near the nadir of its diurnal rhythm (AM) (A). This was independent of genotype. Near the peak of diurnal rhythm (PM) (B), CVS likewise trended towards increased basal plasma corticosterone. Moreover, we observed a significant genotype x sex interaction, such that MC4R loss-of-function dose-dependently decreased and increased corticosterone in males and females, respectively. At both time points, females had significantly higher basal corticosterone than males. WT: wild-type; HET: heterozygous mutant; HOM: homozygous mutant. Data presented as mean ± S.E.M., 3-way ANOVA, n = 3-9/sex/genotype/treatment.
3.2. Adrenal weights
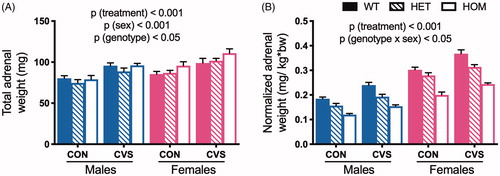
In agreement with the differences in basal HPA axis tone, we observed main effects of sex and treatment on adrenal weight. For raw, uncorrected values, there was a significant main effect of treatment (F(1,73) = 38.5, p < .001) such that CVS induced adrenal hypertrophy relative to CON. As expected (Critchlow et al., Citation1963), female adrenals were heavier than males’ (F(1,73) = 19.67, p(sex)< .001). We also observed a main effect of genotype (F(2,73) = 3.51, p < .05) such that adrenals from HOM rats were heavier than those of HET rats (Tukey’s post hoc, p < .05; 3-way ANOVA, ). Because sex, treatment, and genotype additionally influence body weight (see ), we also analyzed body weight-adjusted values (3-way ANOVA, ). We again observed a significant main effect of treatment (F(1,73) = 59.62, p < .001) such that CVS induced adrenal hypertrophy compared to CON. In this analysis, there was also a significant interaction between genotype and sex (F(2,73) = 4.06, p < .05). Within each genotype, the corrected adrenal weights from females were heavier than males (Tukey’s post hoc, p < .001), and within each sex, there was a gene dose-dependent effect of MC4R loss-of-function to decrease the corrected adrenal weight (Tukey’s post hoc, p < .001) – likely as a simple consequence of MC4R-dependent changes in body weight. Taken together, we conclude that CVS induces adrenal hypertrophy, and this occurs independent of Mc4r genotype.
Figure 2. Chronic variable stress induced adrenal hypertrophy independent of genotype. Adrenals were collected and weighed after 31 days of chronic variable stress (CVS) as an indirect measure of HPA tone. Rats exposed to CVS exhibited increased total adrenal weight compared to unstressed littermate controls (CON) (A), and female adrenals were heavier than males’. When adjusted for body weight (B), MC4R loss-of-function was associated with decreased relative adrenal weight in a dose-dependent manner, likely as a simple consequence of MC4R-dependent changes in body weight (see ). WT: wild type; HET: heterozygous mutant; HOM: homozygous mutant. Data presented as mean ± S.E.M., 3-way ANOVA, n = 4-9/sex/genotype/treatment.
3.3. HPA axis response to acute restraint
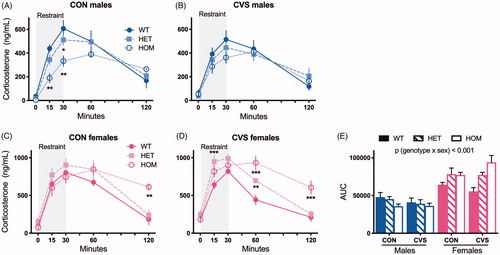
We challenged both CON and CVS-treated male and female rats with a novel, acute restraint stress on day 30 of CVS. In agreement with our previous work (Ryan et al., Citation2014), the acute restraint-induced increase in circulating corticosterone was blunted with MC4R loss-of-function in CON males (, 2-way RM ANOVA, F(8,56) = 2.88, p(genotype x time)<.01, Tukey’s post hoc). Lower corticosterone levels in the first 30 minutes of HOM males suggest a reduced HPA axis activation. However, contrary to our expectation, this was less apparent among CVS males (, 2-way ANOVA, F(4,68) = 64.27, p(time)<.001). Conversely, and contrary to our expectation, loss of MC4R function was associated with an exaggerated stress-evoked increase in circulating corticosterone among both CON females (, 2-way RM ANOVA, F(8,56) = 2.73, p(genotype x time) < .05, Tukey’s post hoc), and CVS females (, 2-way RM ANOVA, F(8,64) = 8.15, p(genotype x time)<.001), suggesting impaired negative feedback on the HPA axis in females. An integrated analysis of the areas under the curve for all rats (AUC, ) revealed an interaction between genotype and sex (3-way ANOVA, F(2,61) = 9.36, p < .001) such that MC4R loss-of-function blunted the corticosterone response among males and exaggerated the corticosterone response among females in a gene dose-dependent manner. Thus, we conclude that MC4R has opposing effects on the restraint-evoked corticosterone response in males vs females, independent of exposure to CVS.
Figure 3. MC4R loss-of-function has sex-dependent effects on the corticosterone response to an acute restraint stress. Rats were placed in a well-ventilated Plexiglas restrainer for 30 minutes and blood was collected from the tip of the tail vein at indicated times. MC4R loss-of-function blunted the corticosterone response to restraint in unstressed, control (CON) males (A, 2-way RM ANOVA, F(8,56)=2.88, p (genotype x time) < .01) but this effect was less apparent among rats exposed to chronic variable stress (CVS) (B). On the contrary, MC4R loss-of-function heightened the corticosterone response in both CON (C, 2-way RM ANOVA, F(8,56)=2.73, p (genotype x time) < .05) and CVS females (D, 2-way RM ANOVA, F(8,64)=8.15, p (genotype x time) < .001). *p < .05, **p < .01, ***p < .001, Tukey’s post hoc. Areas under the curve (E) revealed a significant interaction between genotype and sex (3-way ANOVA). WT: wild type; HET: heterozygous mutant; HOM: homozygous mutant. Data presented as mean ± SEM., n = 3-9/sex/genotype/treatment.
3.4. Energy balance
Because MC4R plays a key role in the regulation of energy balance, and because previous work by ourselves and others suggests MC4R signaling facilitates stress responsiveness, we predicted that MC4R loss-of-function would abrogate the weight loss and decreased food intake typically observed during CVS.
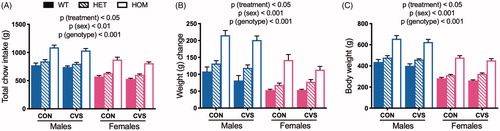
Consistent with previous findings (Goodson et al., Citation2017), we observed a main effect of CVS to reduce both chow intake (, 3-way ANOVA, F(1,73) = 7.51, p(treatment)<.01), weight change (, 3-way ANOVA, F(1,73) = 4.71, p(treatment)<.05), and absolute body weight (, 3-way ANOVA, F(1,73) = 4.53, p(treatment)<.05). As expected, there was a significant main effect of sex such that males were heavier (3-way ANOVA, F(1,73) = 278.35, p(sex)<.001) and ate more (3-way ANOVA, F(1,73) = 126.27, p(sex)<.001) than females. Lastly, as expected, there was also a significant gene dose-dependent effect of Mc4r genotype (HOM > HET > WT) on both body weight (3-way ANOVA, F(2,73) = 156.07, p(genotype)<.001) and chow intake (3-way ANOVA, F(2,73) = 78.20, p(genotype) < .001). Contrary to our prediction, there was no significant interaction between genotype and stress.
Figure 4. Chronic variable stress decreased food intake and body weight independent of genotype. Food intake (A) and body weight change (B) were measured throughout 31 days of chronic variable stress (CVS) treatment. MC4R loss-of-function increased total food intake and body weight change in both sexes in a dose-dependent manner, and males ate more and were heavier than females. This was also reflected in body weight (C) at the conclusion of the study. CVS treatment decreased all three measures, compared to unstressed littermate controls (CON), and this was independent of genotype. WT: wild type; HET: heterozygous mutant; HOM: homozygous mutant. Data presented as mean ± SEM., 3-way ANOVA, n = 5-9/sex/genotype/treatment.
3.5. Vascular pathology
Chronic exposure to either stress or exogenous glucocorticoids increases risk of hypertension and vascular stiffening in both rodents and humans (Goodson et al., Citation2017; Handa et al., Citation1984; Iijima & Malik, Citation1988; McEwen & Stellar, Citation1993; Scholz et al., Citation1957; Steptoe & Kivimäki, Citation2012; Walker, Citation2007). Therefore, we predicted that CVS-treatment would increase related anatomical markers, for example by increasing the intima-medial thickness and/or decreasing the luminal area. Given the findings in 3.1 and 3.3 (above), we further predicted an interacting effect of sex and genotype on vascular morphology such that MC4R loss-of-function protects against indices of vascular remodeling among males and exaggerates this response among females. Because there are no reports of MC4R expression in the vasculature, and because we likewise observed only very low expression of Mc4r mRNA in the aortas (data not shown), we expect any potential effect of genotype occurs indirectly.
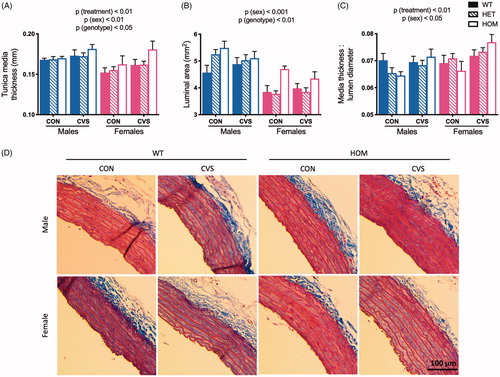
As expected, we observed a main effect of CVS-treatment to increase tunica media thickness (, 3-way ANOVA, F(1,72) = 7.98, p < .01). Moreover, there was a main effect of both sex (F(1,72) = 9.16, p < .01) and genotype (F(2,72) = 3.32, p < .05) such that larger rats, e.g. males and HOMs, exhibited larger tunica media thickness. This is consistent with previous findings that tunica media thickness increases with body weight in both rodents (Ma et al., Citation2010) and human populations (Woo et al., Citation2004; Wunsch, de Sousa, Toschke, & Reinehr, Citation2006). There were no significant interactions. For luminal area (), there was again a main effect of both sex (3-way ANOVA, F(1,72) = 60.12, p < .001) and genotype (F(2,72) = 6.81, p < .01) such that larger rats exhibited a greater luminal area. There was no significant effect of treatment and no significant interactions. Finally, when we analyzed media thickness as a ratio to lumen diameter (Pitol et al., Citation2015; Xiao et al., Citation2016; Zhu et al., Citation2018) (), and in agreement with increased risk of cardiovascular morbidity upon exposure to chronic stress (Black & Garbutt, Citation2002; Chumaeva et al., Citation2009; Grippo & Johnson, Citation2009; Lehman, Taylor, Kiefe, & Seeman, Citation2009; Steptoe & Kivimäki, Citation2012), we observed a main effect of CVS-treatment to increase this ratio (3-way ANOVA, F(1,72) = 9.82, p < .01). Contrary to expectation, however, there was no significant difference of genotype and no significant interactions. Representative images of aortas are shown in .
Figure 5. Chronic variable stress induced markers of vascular remodeling independent of genotype. Descending aortas were collected and fixed in formalin after 31 days of CVS, and a 2-3 mm piece was embedded in paraffin and stained with Masson’s trichrome. Exposure to chronic variable stress (CVS) increased tunica media thickness compared to unstressed littermate controls (CON) (A), with additional effects of sex and genotype such that larger rats exhibited larger thicknesses (also see ). CVS did not significantly affect luminal area (B), but this also increased with larger rats. Lastly, CVS increased the media thickness: lumen diameter ratio (C) independent genotype. Representative images of aortas are shown in (D). WT: wild type; HET: heterozygous mutant; HOM: homozygous mutant. Data presented as mean ± SEM., 3-way ANOVA, n = 3-9/sex/genotype/treatment.
4. Discussion
The neural circuitries controlling brain stress integration and systemic metabolism exhibit significant functional overlap (Ulrich-Lai & Ryan, Citation2014). In agreement with this, metabolic diseases like obesity and diabetes have a high incidence of co-morbidity with stress-associated psychological disorders like anxiety and depression (Anderson, Freedland, Clouse, & Lustman, Citation2001; Faith, Matz, & Jorge, Citation2002; Gariepy, Nitka, & Schmitz, Citation2010; Stunkard, Faith, & Allison, Citation2003; Ulrich-Lai & Ryan, Citation2014). Accordingly, interventions targeting these critical neuroanatomical connections may represent a promising strategy for preventing chronic stress-induced metabolic and cardiovascular consequences. However, with regard to the hypothalamic melanocortin system, the present findings do not support this possibility. Consistent with our previous report (Ryan et al., Citation2014), we found that melanocortin 4 receptor (MC4R) loss-of-function significantly diminished the acute stress-induced rise in plasma corticosterone in naïve male rats (). Nevertheless, Mc4r genotype did not modulate responses to chronic stress. Specifically, MC4R loss-of-function did not protect against chronic stress-associated increases in basal HPA axis tone and adrenal hypertrophy ( and ), nor against chronic stress-induced anorexia and weight loss (). Lastly, loss of MC4R function did not reduce chronic stress-induced vascular remodeling ().
The MC4R is a G protein-coupled receptor expressed widely in the adult central nervous system (Cone, Citation2005; Tao, Citation2010). Its activity is coordinated by opposing actions of its endogenous agonist, αMSH, and its endogenous antagonist, agouti-related protein (AgRP) (Fong et al., Citation1997; Ollmann et al., Citation1997; Shutter et al., Citation1997). In addition, the receptor has intrinsic constitutive activity on which AgRP can act as an inverse agonist (Srinivasan et al., Citation2004). αMSH producing neurons in the arcuate nucleus of the hypothalamus are activated by restraint stress (Liu et al., Citation2007) and provide melanocortinergic input to MC4R-expressing neurons in key stress and feeding-regulatory brain regions including the paraventricular nucleus of the hypothalamus (PVN), the medial amygdala (MeA), and the nucleus accumbens (NAc) (Balthasar, Citation2006; Wang et al., Citation2015). Activation of MC4Rs by αMSH or pharmacological agonists acutely stimulates the HPA axis in male rats and mice (Liu et al., Citation2013) and induces weight loss by reducing caloric intake and increasing energy expenditure in both sexes (Fan et al., Citation1997; Hamilton & Doods, Citation2002). Conversely, loss of MC4R function (Ryan et al., Citation2014) or its pharmacological blockade (Kokare et al., Citation2010; Liu et al., Citation2007; Serova et al., Citation2013) blunts acute restraint stress-induced corticosterone elevation in male rats and mice. MC4R loss-of-function also induces weight gain by increasing caloric intake and decreasing energy expenditure in both sexes (Huszar et al., Citation1997). Our results () are consistent with these reports.
Despite its clear influence on both acute stress responses and energy balance, the present findings indicate that MC4R-signaling does not play a major role in chronic stress-induced facilitation of basal HPA-axis tone, anorexia or weight loss. Notably, these outcomes are consistent with previous work identifying MC4Rs in the MeA as a critical mediator for the acute HPA axis response to central melanocortinergic tone in male rats (Liu et al., Citation2013), and supporting that lesions of the MeA blunt neuroendocrine responses to acute, but not chronic, stress in male rats (Solomon, Jones, Packard, & Herman, Citation2010). Our findings are inconsistent, however, with another previous study, which concluded that MC4Rs in the NAc are necessary for chronic-stress induced weight loss. Specifically, Lim and colleagues (Lim, Huang, Grueter, Rothwell, & Malenka, Citation2012) found that male mice treated with AAV-GFP locally in the NAc, and later subjected to chronic restraint stress, lost weight compared to untreated, non-stressed controls, whereas mice treated with AAV-MC4R-shRNA to knockdown Mc4r expression in the NAc did not lose weight during chronic restraint stress, again in comparison to the untreated and unstressed wildtype controls. The inconsistent conclusions drawn between this study and ours may result from experimental differences including differences in model species (rat vs mouse), differences in the method of knockdown (whole-body loss-of-function vs virally-directed gene knockdown), differences in the chronic stress model (heterotypic vs homotypic stressors), or from other details of the experimental design (e.g., use of a full-factorial model vs not). Further study will be necessary to resolve the discrepancy.
Our findings are in line with previous reports by ourselves (Goodson et al., Citation2017) and others (Neves et al., Citation2009) that chronic stress induces vascular remodeling in male rodents, and with the many epidemiological reports identifying psychological stress as a significant risk factor for cardiovascular disease in human populations (reviewed by (Rozanski, Blumenthal, & Kaplan, Citation1999; Ryan, Citation2014; Steptoe & Kivimäki, Citation2012)). Specifically, we report that chronic stress increases tunica media thickness, and increases the ratio of tunica media thickness: lumen diameter, in chronically-stressed male and female rats. This is consistent with known effects of stress to induce hypertension and vascular stiffening (Heagerty, Aalkjaer, Bund, Korsgaard, & Mulvany, Citation1993), and was independent of Mc4r genotype. Although vascular remodeling may be induced in part by chronic glucocorticoid exposure (Fishel et al., Citation1995) during prolonged or repeated stress exposure, we found no association between basal or stress-induced corticosterone and vascular morphology. That is, although Mc4r genotype had opposing effects on HPA axis activity in males and females (discussed below), both sexes exhibited stress-induced vascular remodeling, suggesting glucocorticoid exposure was not the primary mechanism underlying vascular remodeling in this rat chronic variable stress model.
Perhaps the most striking outcome we observed was the marked interaction between Mc4r genotype and sex for basal HPA axis tone and acute stress responsivity. First, in line with well-documented sex differences in basal and stress-induced HPA axis activity (Goel, Workman, Lee, Innala, & Viau, Citation2014; Handa et al., Citation1994; Solomon, Jankord, Flak, & Herman, Citation2011), female rats exhibited greater basal corticosterone when measured at both the nadir and peak of its circadian rhythm, greater restraint-induced increases in corticosterone, and greater chronic stress-induced adrenal hypertrophy, compared to male littermates. Importantly, we (unexpectedly) also observed opposing effects of Mc4r genotype to modulate these endpoints depending on sex. In males, MC4R loss-of-function decreased basal corticosterone and blunted the response to acute restraint, in a gene dose-dependent manner. Exactly the opposite effect was observed among female littermates. In female rats, MC4R loss-of-function exhibited greater basal corticosterone and exaggerated responses to acute restraint, also in a dose-dependent manner, resulting in a significant genotype x sex interaction in the 3-way ANOVA.
The opposing effects of MC4R function on stress reactivity in males and females may provide mechanistic insight regarding the sex-dependent associations between Mc4r genotype and eating behavior reported in human populations. In humans, heterozygous Mc4r loss-of-function mutations are the most common genetic cause of obesity (Farooqi & O’Rahilly, Citation2004), and sex has repeatedly been shown to modify the association between Mc4r genotype and feeding behavior together with other markers of obesity (Cauchi et al., Citation2009; Liu et al., Citation2010; Renström et al., Citation2009). Generally, these studies report a greater effect of the mutation in women compared to men. For example, Horstmann and colleagues (Horstmann et al., Citation2013) found that only female carriers of a common genetic variant near the Mc4r gene exhibited a significant increase in “disinhibited” and “emotional” eating, together with increased gray matter volume in the amygdala. “Emotional eating” has been linked to stress (Adam & Epel, Citation2007) and our present study found that females with Mc4r mutations have heightened stress reactivity compared to males, suggesting a possible mechanism for the sex-dependent effects associated with this mutation in humans. However, in contrast to the human literature but consistent with several rodent studies (Huszar et al., Citation1997; Ste Marie, Miura, Marsh, Yagaloff, & Palmiter, Citation2000), we did not find a sex-dependent effect of the Mc4r genotype on food intake or body weight in this study. This discrepancy may be due to difficulty in modeling “emotional eating” in rodents. In the current study, for example, rats were not offered the choice to consume palatable foods. It would be interesting, in future work, to investigate if sex-dependent effects of MC4R signaling on energy balance are revealed using a rodent model of binge eating. Furthermore, the heightened stress reactivity was evident in both CON and CVS females, though more exaggerated in CVS females (). Because CVS can alter the female reproductive cycle via disruption of the hypothalamic-pituitary-gonadal axis (Valsamakis, Chrousos, & Mastorakos, Citation2019), further studies are needed to delineate the role of reproductive hormones to modulate MC4R signaling, specifically with respect to the stress response.
In summary, the present findings support that MC4R-signaling exerts a sex-dependent effect on acute stress-responsivity and basal HPA axis tone. Whereas MC4R loss-of-function facilitated the acute stress-induced rise in plasma corticosterone in a gene dose-dependent manner in male rats, it had a gene dose-dependent opposing effect in female littermates. Future work will focus on identifying genetic, developmental, and/or hormonal mechanisms contributing to this sex difference, and implications for stress-associated psychopathologies. Importantly, and contrary to our overall hypothesis, Mc4r genotype did not modulate responses to chronic stress in either sex. Specifically, MC4R loss-of-function did not protect against chronic stress-associated increases in HPA axis tone, adrenal hypertrophy, anorexia, weight loss, or vascular remodeling. Therefore, with regard to these endpoints, we conclude that the hypothalamic melanocortin system is not a critical communication link between brain metabolic and stress systems during exposure to chronic stress.
Acknowledgements
The authors thank James Graham (UC Davis) for expert technical assistance. This work was supported in part by the National Institutes of Health (HL111319) to KKR.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
References
- Adam, T.C., & Epel, E.S. (2007). Stress, eating and the reward system. Physiology & Behavior, 91, 449–458. doi:10.1016/j.physbeh.2007.04.011
- Almundarij, T.I., Smyers, M.E., Spriggs, A., Heemstra, L.A., Beltz, L., Dyne, E., … Novak, C.M. (2016). Physical activity, energy expenditure, and defense of body weight in melanocortin 4 receptor-deficient male rats. Scientific Reports, 6, 37435. doi:10.1038/srep37435
- Anderson, R.J., Freedland, K.E., Clouse, R.E., & Lustman, P.J. (2001). The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care, 24, 1069–1078. doi:10.2337/diacare.24.6.1069
- Asakawa, A., Inui, A., Kaga, T., Yuzuriha, H., Nagata, T., Fujimiya, M., … Kasuga, M. (2001). A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology, 74, 143–147.
- Balthasar, N. (2006). Genetic dissection of neuronal pathways controlling energy homeostasis. Obesity (Silver Spring, Md.), 14, 222S–227S. doi:10.1038/oby.2006.313
- Black, P.H., & Garbutt, L.D. (2002). Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research, 52, 1. doi:10.1016/S0022-3999(01)00302-6
- Cauchi, S., Stutzmann, F., Cavalcanti-Proença, C., Durand, E., Pouta, A., Hartikainen, A.-L., … Froguel, P. (2009). Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. Journal of Molecular Medicine, 87, 537–546. doi:10.1007/s00109-009-0451-6
- Chaki, S., Ogawa, S., Toda, Y., Funakoshi, T., & Okuyama, S. (2003). Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. European Journal of Pharmacology, 474, 95–101. doi:10.1016/S0014-2999(03)02033-8
- Chuang, J. C., Krishnan, V., Yu, H.G., Mason, B., Cui, H., Wilkinson, M.B., … Lutter, M. (2010). A beta3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biological Psychiatry, 67, 1075–1082. doi:10.1016/j.biopsych.2009.12.003
- Chuang, J., Perello, M., Sakata, I., Osborne-Lawrence, S., Savitt, J.M., Lutter, M., & Zigman, J.M. (2011). Ghrelin mediates stress-induced food-reward behavior in mice. 121, 2684–2692. doi:10.1172/JCI57660.2684
- Chumaeva, N., Hintsanen, M., Ravaja, N., Puttonen, S., Heponiemi, T., Pulkki, -Råback, L.,… Rvinen, L. (2009). Interactive effect of long-term mental stress and cardiac stress reactivity on carotid intima-media thickness: the Cardiovascular Risk in Young Finns study. Stress (Amsterdam, Netherlands), 12, 283. doi:10.1080/10253890802372406
- Cone, R.D. (2005). Anatomy and regulation of the central melanocortin system. Nature Neuroscience, 8, 571–578. doi:10.1038/nn1455
- Critchlow, V., Liebelt, R.A., Bar-Sela, M., Mountcastle, W., & Lipscomb, H.S. (1963). Sex difference in resting pituitary-adrenal function in the rat. American Journal of Physiology-Legacy Content, 205, 807–815. doi:10.1152/ajplegacy.1963.205.5.807
- De Barioglio, S.R., Lezcano, N., & Celis, M.E. (1991). Alpha MSH-induced excessive grooming behavior involves a GABAergic mechanism. Peptides, 12, 203–205. doi:10.1016/0196-9781(91)90189-V
- Dhillo, W.S., Small, C.J., Seal, L.J., Kim, M.-S., Stanley, S.A., Murphy, K.G., … Bloom, S.R. (2002). The hypothalamic melanocortin system stimulates the hypothalamo-pituitary-adrenal axis in vitro and in vivo in male rats. Neuroendocrinology, 75, 209–216. https://doi.org/54712 doi:10.1159/000054712
- Earls, F. (1987). Sex differences in psychiatric disorders: origins and developmental influences. Psychiatric Developments, 5, 1–23.
- Faith, M.S., Matz, P.E., & Jorge, M.A. (2002). Obesity–depression associations in the population. Journal of Psychosomatic Research, 53, 935–942. doi:10.1016/S0022-3999(02)00308-2
- Fan, W., Boston, B.A., Kesterson, R.A., Hruby, V.J., & Cone, R.D. (1997). Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature, 385, 165–168. doi:10.1038/385165a0
- Farooqi, I.S., & O’Rahilly, S. (2004). Monogenic human obesity syndromes. Recent Progress in Hormone Research, 59, 409–424. doi:10.1210/rp.59.1.409
- Fishel, R.S., Eisenberg, S., Shai, S.Y., Redden, R.A., Bernstein, K.E., & Berk, B.C. (1995). Glucocorticoids induce angiotensin-converting enzyme expression in vascular smooth muscle. Hypertension (Dallas, Tex.: 1979), 25, 343–349. doi:10.1161/01.HYP.25.3.343
- Fong, T.M., Mao, C., MacNeil, T., Kalyani, R., Smith, T., Weinberg, D., … Van der Ploeg, L.H.T. (1997). ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochemical and Biophysical Research Communications, 237, 629–631. doi:10.1006/bbrc.1997.7200
- Gariepy, G., Nitka, D., & Schmitz, N. (2010). The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. International Journal of Obesity (Obesity), 34, 407–419. doi:10.1038/ijo.2009.252
- Goel, N., Workman, J.L., Lee, T.T., Innala, L., & Viau, V. (2014). Sex differences in the HPA axis. Comprehensive Physiology, 4, 1121–1155. doi:10.1002/cphy.c130054
- Gonzalez, M.I., Vaziri, S., & Wilson, C.A. (1996). Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides, 17, 171–177. doi:10.1016/0196-9781(95)02092-6
- Goodson, M.L., Packard, A.E.B., Buesing, D.R., Maney, M., Myers, B., Fang, Y., … Ryan, K.K. (2017). Chronic stress and rosiglitazone increase indices of vascular stiffness in male rats. Physiology & Behavior, 172, 16–23. doi:10.1016/j.physbeh.2016.03.031
- Grippo, A.J., & Johnson, A.K. (2009). Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress, 12, 1–21. doi:10.1080/10253890802046281
- Hamilton, B.S., & Doods, H.N. (2002). Chronic application of MTII in a rat model of obesity results in sustained weight loss. Obesity Research, 10, 182–187. doi:10.1038/oby.2002.28
- Handa, R.J., Burgess, L.H., Kerr, J.E., & O'Keefe, J.A. (1994). Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior, 28, 464–476. doi:10.1006/hbeh.1994.1044
- Handa, M., Kondo, K., Suzuki, H., & Saruta, T. (1984). Dexamethasone hypertension in rats: role of prostaglandins and pressor sensitivity to norepinephrine. Hypertension, 6, 236–241. doi:10.1161/01.HYP.6.2.236
- Haskell-Luevano, C., & Monck, E.K. (2001). Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regulatory Peptides, 99, 1–7. doi:10.1016/S0167-0115(01)00234-8
- Heagerty, A.M., Aalkjaer, C., Bund, S.J., Korsgaard, N., & Mulvany, M.J. (1993). Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension, 21, 391–397.
- Horstmann, A., Kovacs, P., Kabisch, S., Boettcher, Y., Schloegl, H., Tönjes, A., … Villringer, A. (2013). Common genetic variation near MC4R has a sex-specific impact on human brain structure and eating behavior. PLoS One, 8, e74362. doi:10.1371/journal.pone.0074362
- Huszar, D., Lynch, C.A., Fairchild-Huntress, V., Dunmore, J.H., Fang, Q., Berkemeier, L.R., … Lee, F. (1997). Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell, 88, 131–141. doi:10.1016/S0092-8674(00)81865-6
- Iijima, F., & Malik, K.U. (1988). Contribution of vasopressin in dexamethasone-induced hypertension in rats. Hypertension (Dallas, Tex. : 1979), 11, I42–I42. doi:10.1161/01.HYP.11.2_Pt_2.I42
- Kheirabad, M.K., Jahromi, B.N., Tamadon, A., Ramezani, A., Ahmadloo, S., Sarvestani, F.S., … Branch, G. (2015). Expression of melanocortin-4 receptor mRNA in male rat hypothalamus during chronic stress. International Journal of Molecular and Cellular Medicine, 4, 182–187.
- Kokare, D.M., Dandekar, M.P., Singru, P.S., Gupta, G.L., & Subhedar, N.K. (2010). Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology, 58, 1009–1018. doi:10.1016/j.neuropharm.2010.01.006
- Krakoff, L.R. (1988). Glucocorticoid excess syndromes causing hypertension. Cardiology Clinics, 6, 537–545. doi:10.1016/S0733-8651(18)30475-2
- Kudielka, B.M., & Kirschbaum, C. (2004). Sex differences in HPA axis responses to stress: a review. Biological Psychology, 69, 113–132. doi:10.1016/j.biopsycho.2004.11.009
- Kuo, L.E., Kitlinska, J.B., Tilan, J.U., Li, L., Baker, S.B., Johnson, M.D., … Zukowska, Z. (2007). Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nature Medicine, 13, 803–811. doi:10.1038/nm1611
- Lehman, B.J., Taylor, S.E., Kiefe, C.I., & Seeman, T.E. (2009). Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 28, 338–346. doi:10.1037/a0013785
- Lim, B.K., Huang, K.W., Grueter, B. A., Rothwell, P.E., & Malenka, R.C. (2012). Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature, 487, 183–189. doi:10.1038/nature11160
- Liu, J., Garza, J.C., Li, W., & Lu, X.Y. (2013). Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. The International Journal of Neuropsychopharmacology, 16, 105–120. doi:10.1017/S146114571100174X
- Liu, J., Garza, J.C., Truong, H.V., Henschel, J., Zhang, W., & Lu, X.-Y. (2007). The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology, 148, 5531–5540. doi:10.1210/en.2007-0745
- Liu, G., Zhu, H., Lagou, V., Gutin, B., Barbeau, P., Treiber, F.A., … Snieder, H. (2010). Common variants near melanocortin 4 receptor are associated with general and visceral adiposity in European- and African-American youth. The Journal of Pediatrics, 156, 598–605.e1. doi:10.1016/j.jpeds.2009.10.037
- Lu, X.-Y., Barsh, G.S., Akil, H., & Watson, S.J. (2003). Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. The Journal of Neuroscience, 23, 7863–7872. doi:10.1523/JNEUROSCI.23-21-07863.2003
- Ma, L., Ma, S., He, H., Yang, D., Chen, X., Luo, Z., … Zhu, Z. (2010). Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertension Research, 33, 446–453. doi:10.1038/hr.2010.11
- Marsh, D.J., Hollopeter, G., Huszar, D., Laufer, R., Yagaloff, K.A., Fisher, S.L., … Palmiter, R.D. (1999). Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics, 21, 119–122. doi:10.1038/5070
- McEwen, B.S., & Stellar, E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. doi:10.1001/archinte.1993.00410180039004
- Mul, J.D., van Boxtel, R., Bergen, D.J.M., Brans, M.A.D., Brakkee, J.H., Toonen, P.W., … Cuppen, E. (2012). Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring), 20, 612–621. doi:10.1038/oby.2011.81
- Murphy, M.O., & Loria, A.S. (2017). REVIEW sex and gender differences in cardiovascular, renal and metabolic diseases. American Journal of Physiology Regulatory Integrative and Comparative Physiology, 313, 1–9. doi:10.1152/ajpregu.00185.2016.-Car
- Neves, V.J., Moura, M.J.C.S., Tamascia, M.L., Ferreira, R., Silva, N.S., Costa, R., … Marcondes, F.K. (2009). Proatherosclerotic effects of chronic stress in male rats: Altered phenylephrine sensitivity and nitric oxide synthase activity of aorta and circulating lipids. Stress, 12, 320–327. doi: doi:10.1080/10253890802437779
- Nogueiras, R., Wiedmer, P., Perez-Tilve, D., Veyrat-Durebex, C., Keogh, J.M., Sutton, G.M., … Tschöp, M.H. (2007). The central melanocortin system directly controls peripheral lipid metabolism. Journal of Clinical Investigation, 117, 3475–3488. doi:10.1172/JCI31743
- Obici, S., Feng, Z., Tan, J., Liu, L., Karkanias, G., & Rossetti, L. (2001). Central melanocortin receptors regulate insulin action. Journal of Clinical Investigation, 108, 1079–1085. doi:10.1172/JCI12954
- Ollmann, M.M., Wilson, B.D., Yang, Y.K., Kerns, J.A., Chen, Y., Gantz, I., & Barsh, G.S. (1997). Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science, 278, 135–138. doi:10.1126/science.278.5335.135
- Park, S., Daily, J.W., Zhang, X., Jin, H.S., Lee, H.J., & Lee, Y.H. (2016). Interactions with the MC4R rs17782313 variant, mental stress and energy intake and the risk of obesity in Genome Epidemiology Study. Nutrition & Metabolism, 13, 38. doi:10.1186/s12986-016-0096-8
- Pitol, D.L., Siéssere, S., dos Santos, R.G., Rosa, M.L.N.M., Hallak, J.E.C., Scalize, P.H., … Regalo, S.C.H. (2015). Ayahuasca Alters Structural Parameters of the Rat Aorta. Journal of Cardiovascular Pharmacology, 66, 58–62. doi:10.1097/FJC.0000000000000243
- Renström, F., Payne, F., Nordström, A., Brito, E.C., Rolandsson, O., Hallmans, G., … Franks, P.W, GIANT Consortium. (2009). Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Human Molecular Genetics, 18, 1489–1496. doi:10.1093/hmg/ddp041
- Rossi, J., Balthasar, N., Olson, D., Scott, M., Berglund, E., Lee, C.E., … Elmquist, J.K. (2011). Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metabolism, 13, 195–204. doi:10.1016/j.cmet.2011.01.010
- Rossi, M., Kim, M.S., Morgan, D.G.A., Small, C.J., Edwards, C.M.B., Sunter, D., … Bloom, S.R. (1998). A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology, 139, 4428–4431. doi:10.1210/endo.139.10.6332
- Rozanski, A., Blumenthal, J.A., & Kaplan, J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation, 99, 2192–2217. doi:10.1161/01.CIR.99.16.2192
- Ryan, K.K. (2014). Stress and metabolic disease. In Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers. Chapter 11, 247–388. doi:10.17226/18822
- Ryan, K.K., Grayson, B.E., Jones, K.R., Schneider, A.L., Woods, S.C., Seeley, R.J., … Ulrich-Lai, Y.M. (2012). Physiological responses to acute psychological stress are reduced by the PPARγ agonist rosiglitazone. Endocrinology, 153, 1279–1287. doi:10.1210/en.2011-1689
- Ryan, K.K., Woods, S.C., & Seeley, R.J. (2012). Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell Metabolism, 15, 137–149. doi:10.1016/j.cmet.2011.12.013
- Ryan, K.K., Mul, J.D., Clemmensen, C., Egan, A.E., Begg, D.P., Halcomb, K., … Ulrich-Lai, Y.M. (2014). Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology, 42, 98–105. doi:10.1016/j.psyneuen.2014.01.010
- Ryan, K.K., Packard, A.E.B., Larson, K.R., Stout, J., Fourman, S.M., Thompson, A.M.K., … Ulrich-Lai, Y.M. (2018). Dietary manipulations that induce ketosis activate the HPA axis in male rats and mice: A potential role for fibroblast growth factor-21. Endocrinology, 159, 400–413. doi:10.1210/en.2017-00486
- Scholz, D.A., Sprague, R.G., & Kernohan, J.W. (1957). Cardiovascular and renal complications of Cushing’s syndrome. New England Journal of Medicine, 256, 833–837. doi:10.1056/NEJM195705022561804
- Serova, L.I., Laukova, M., Alaluf, L.G., & Sabban, E.L. (2013). Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behavioural Brain Research, 250, 139–147. doi:10.1016/j.bbr.2013.05.006
- Shutter, J.R., Graham, M., Kinsey, A.C., Scully, S., Luthy, R., & Stark, K.L. (1997). Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes and Development, 11, 593–602. doi:10.1101/gad.11.5.593
- Solomon, M.B., Jankord, R., Flak, J.N., & Herman, J.P. (2011). Chronic stress, energy balance and adiposity in female rats. Physiology & Behavior, 102, 84–90. doi:10.1016/j.physbeh.2010.09.024
- Solomon, M.B., Jones, K., Packard, B.A., & Herman, J.P. (2010). The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. Journal of Neuroendocrinology, 22, 13–23. doi:10.1111/j.1365-2826.2009.01933.x
- Solomon, M.B., Loftspring, M., de Kloet, A.D., Ghosal, S., Jankord, R., Flak, J.N., … Herman, J.P. (2015). Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology, 156, 2843–2853. doi:10.1210/en.2015-1276
- Srinivasan, S., Lubrano-Berthelier, C., Govaerts, C., Picard, F., Santiago, P., Conklin, B.R., & Vaisse, C. (2004). Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. Journal of Clinical Investigation, 114, 1158–1164. doi:10.1172/JCI21927
- Ste Marie, L., Miura, G.I., Marsh, D.J., Yagaloff, K., & Palmiter, R.D. (2000). A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors., 97 Proceedings of the National Academy of Sciences of the United States of America. 97, 12339–12344.
- Steptoe, A., & Kivimäki, M. (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9, 360–370. doi:10.1038/nrcardio.2012.45
- Stunkard, A.J., Faith, M.S., & Allison, K.C. (2003). Depression and obesity. Biological Psychiatry, 54, 330–337. doi:10.1016/S0006-3223(03)00608-5
- Tao, Y.-X. (2010). The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine Reviews, 31, 506–543. doi:10.1210/er.2009-0037
- Ulrich-Lai, Y.M., & Herman, J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10, 397–409. doi:10.1038/nrn2647
- Ulrich-Lai, Y.M., & Ryan, K.K. (2014). Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapuetic implications. Cell Metabolism, 19, 910–925. doi:10.1016/j.cmet.2014.01.020
- Vahl, T.P., Ulrich-Lai, Y.M., Ostrander, M.M., Dolgas, C.M., Elfers, E.E., Seeley, R.J., … Herman, J.P. (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. American Journal of Physiology. Endocrinology and Metabolism, 289, E823–E828. doi:10.1152/ajpendo.00122.2005
- Vaisse, C., Clement, K., Guy-Grand, B., & Froguel, P. (1998). A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genetics, 20, 113–114. doi:10.1038/2407
- Valsamakis, G., Chrousos, G., & Mastorakos, G. (2019). Stress, female reproduction and pregnancy. Psychoneuroendocrinology, 100, 48–57. doi:10.1016/j.psyneuen.2018.09.031
- van Dijk, G., Thiele, T.E., Seeley, R.J., Woods, S.C., & Bernstein, I.L. (1997). Glucagon-like peptide-1 and satiety? Nature, 385, 214. doi:10.1038/385214a0
- Walker, B.R. (2007). Glucocorticoids and cardiovascular disease. European Journal of Endocrinology, 157, 545–559. doi:10.1530/EJE-07-0455
- Wang, D., He, X., Zhao, Z., Feng, Q., Lin, R., Sun, Y., … Zhan, C. (2015). Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy, 9, 40. doi:10.3389/fnana.2015.00040
- Wilson, B.D., Ollmann, M.M., & Barsh, G.S. (1999). The role of agouti-related protein in regulating body weight. Molecular Medicine Today, 5, 250–256. doi:10.1016/S1357-4310(99)01471-9
- Woo, K.S., Chook, P., Yu, C.W., Sung, R.Y.T., Qiao, M., Leung, S.S.F., … Celermajer, D.S. (2004). Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. International Journal of Obesity, 28, 852–857. doi:10.1038/sj.ijo.0802539
- Wunsch, R., de Sousa, G., Toschke, A.M., & Reinehr, T. (2006). Intima-media thickness in obese children before and after weight loss. Pediatrics, 118, 2334–2340. doi:10.1542/peds.2006-0302
- Xiao, F., He, F., Chen, H., Lin, S., Shen, A., Chen, Y., … Peng, J. (2016). Qingxuan Jiangya decoction reverses vascular remodeling by inducing vascular smooth muscle cell apoptosis in spontaneously hypertensive rats. Molecules (Basel, Switzerland), 21, 956. doi:10.3390/molecules21070956
- Zhu, X., Zhou, Z., Zhang, Q., Cai, W., Zhou, Y., Sun, H., & Qiu, L. (2018). Vaccarin administration ameliorates hypertension and cardiovascular remodeling in renovascular hypertensive rats. Journal of Cellular Biochemistry, 119, 926–937. doi:10.1002/jcb.26258
