Abstract
Social Signal Transduction Theory of Depression hypothesizes that social stress upregulates inflammatory activity, which in turn contributes to depression for some individuals. However, the specific cognitive processes underlying social stress-induced increases in inflammatory activity remain unclear. We addressed this issue by examining two separate relations: (1) between executive control measured following a laboratory-based social stress induction and individuals’ pro-inflammatory cytokine responses to the same stress induction and (2) between pro-inflammatory cytokine responses and participants’ depressive symptom levels. Healthy young participants (Mage = 18.58 years old) were randomly assigned to either a stress condition or control condition. Executive control, and the inflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α, were measured before and after the social stress induction or control task. Regression analyses (stress condition, n = 20; control condition, n = 16) demonstrated that in the stress condition only, greater increases in interleukin-6 were associated with more depressive symptoms. Additional analyses in the stress condition (n = 16) indicated that greater impairment in executive control following the social stress induction was related to greater social stress-induced increases in interleukin-6. These findings are consistent with Social Signal Transduction Theory of Depression and with the hypothesis that impairment in executive control during times of stress may be one process that contributes to stress-induced inflammatory activity, which may in turn increase risk for depression.
Social Signal Transduction Theory of Depression hypothesizes that social stress upregulates inflammatory activity, which in turn contributes to depression, and that cognitive processes play a role in structuring these effects. Consistent with this theory, greater social stress-induced increases in the inflammatory cytokine interleukin-6 were associated with more depressive symptoms. In addition, greater impairment in executive control following the social stress induction was related to greater social stress-induced increases in interleukin-6, highlighting potential links between social stress, cognition, inflammation, and depression.
Lay Summary
Exposure to social stress in humans can lead to a variety of biological changes that affect health. These changes include activation of the hypothalamic-pituitary-adrenal axis, resulting in the release of cortisol, and upregulation of the sympathetic nervous system, resulting in the release of epinephrine and norepinephrine, with all three hormones in turn having the ability to interact with immune cells to influence innate immune system activity (Grebe et al., Citation2010). One consequence of this innate immune system activation is the release of proinflammatory cytokines, which are small proteins that upregulate inflammatory activity and facilitate cell-to-cell communication during times of threat (Irwin & Cole, Citation2011). Although the primary purpose of this response is to accelerate wound healing and recovery following physical threat or injury (Medzhitov, Citation2007), this response may also be triggered by social stressors, such as social evaluation and rejection (Slavish, Graham-Engeland, Smyth, & Engeland, Citation2015).
Social stress-induced increases in inflammatory activity can promote a variety of physical and mental health problems, especially if sustained (Glaser & Kiecolt-Glaser, Citation2005; Slavich & Irwin, Citation2014). According to Social Signal Transduction Theory of Depression, for example, increased inflammatory activity in response to social stress plays a central role in promoting depression (Slavich & Irwin, Citation2014; Slavich & Sacher, Citationin press). This multi-level theory is supported by experimental studies demonstrating that depressed individuals mount greater inflammatory responses to acute social stressors relative to healthy controls (Pace et al., Citation2006; Weinstein et al., Citation2010), and also by research showing that inflammatory challenges induce several depressive symptoms, including sad mood, anhedonia, fatigue, loss of appetite, and suicidal ideation (Capuron et al., Citation2002; Janssen, Brouwer, van der Mast, & Schalm, Citation1994; Lotrich, Rabinovitz, Gironda, & Pollock, Citation2007).
Substantial individual differences exist in the extent to which people mount strong inflammatory responses to social stress. Understanding processes that contribute to such differences in reactivity may thus help explain differences in risk for inflammation-related conditions like depression (Slavich, Citation2015). However, only a few studies have examined this issue. We addressed this topic in the present study by investigating the association between social stress-induced impairment in executive control and individual differences in inflammatory responses to social stress.
Executive control broadly describes cognitive processes that allow individuals to carry out complex tasks such as planning and problem solving (Friedman & Miyake, Citation2017). Individual differences in executive control have been linked to the ability to regulate responses to stressful life events (Joormann & Vanderlind, Citation2014; Schweizer, Grahn, Hampshire, Mobbs, & Dalgleish, Citation2013). One aspect of the stress response that executive control may influence is inflammatory activity. To date, though, only one study has investigated this relation and found that better performance on an executive control task was related to reduced inflammatory reactivity to watching an emotionally stressful video (Shields, Kuchenbecker, Pressman, Sumida, & Slavich, Citation2016). This study highlights the possibility that better executive control may help reduce individuals’ inflammatory responsivity to social stress, perhaps by lessening individuals’ perceived stress severity or improving their emotion regulation capability.
Importantly, other studies indicate that acute stress exposure temporarily impairs executive control in some individuals (Shields, Sazma, & Yonelinas, Citation2016). Therefore, stress-induced impairments in executive control may provide more information about an individual’s ability to regulate responses to stress exposure than executive control per se (Quinn & Joormann, Citation2015). However, no study to date has examined whether executive control under stress is related to stress-induced inflammatory reactivity. To address this issue, we conducted a cross-sectional laboratory-based study in which we assessed participants’ depressive symptoms, as well as their executive control and pro-inflammatory cytokine levels before and after an acute social stressor or control task. Based on the research summarized above, we tested two separate hypotheses: (1) greater social stress-induced inflammatory response will be related to higher levels of depressive symptoms and (2) greater social stress-induced inflammatory response will be related to poorer executive control under acute stress.
Method
Participants
Participants were drawn from a larger sample of 76 healthy young adults who were recruited via advertisements on the Yale University campus. The 39 participants (stress condition, n = 23; control condition, n = 16) included in this report provided saliva samples for assessment of inflammatory activity. The sample (Mage = 18.58 years old, SDage = 0.60) was 69.2% female and was racially and ethnically diverse, with 53.8% self-identifying as White/Caucasian, 33.3% as Asian, 17.9% as Hispanic/Latino, 10.3% as Black/African American, 2.6% as American Indian/Alaska Native, 10.3% as Middle Eastern, and 2.6% as “Other”. The two experimental conditions did not significantly differ on demographic characteristics (ps > .280). To reduce the influence that extraneous factors could have on cytokine levels, all participants were non-smokers, denied any dental concerns or oral disease, and refrained from eating or drinking for at least 30 minutes prior to providing samples.
Three participants were excluded from all analyses: one person refused to complete the social stress induction and two were taking medications that could have influenced the results (i.e., antidepressants and stimulants; Slavish et al., Citation2015). This resulted in 36 participants (stress condition, n = 20; control condition, n = 16) being available for analyses examining associations between the cytokine and depression data. Executive control data were screened for outliers (Snyder, Miyake, & Hankin, Citation2015), and four participants in the stress condition were excluded from executive control analyses due to performance that may reflect poor understanding of, or disengagement from, the task. Specifically, three participants had performance that was not better than chance on one or more blocks (50-57% error rate), and one participant performed slightly better than chance (42% error rate on each n-back task) but pressed the same key for several consecutive trials at a fast rate (reaction times less than 300ms). This resulted in 16 participants in the stress condition who were available for analyses examining associations between the executive control and social stress-induced cytokine reactivity data.
Procedure
Before arriving at the lab, participants were consented and completed an online survey that included a measure of depressive symptoms. All laboratory sessions began between 12:00pm and 4:30pm. Participants were again consented upon arriving for their laboratory visit. Participants were then fitted with electrodes and given five minutes to relax as a part of the larger study (data not reported here). This was followed by the n-back, which is a measure of executive control (see below). A baseline saliva sample was collected approximately 30 minutes after arriving at the lab. After the baseline sample, participants completed additional tasks and questionnaires as part of the larger study (data not reported here). Participants completed either a social stress induction or a non-stressful control task (see below). Participants then completed a second n-back. At the beginning of each n-back administration, participants completed practice trials requiring accuracy ≥90%. After the second n-back, participants were able to relax for the remainder of the session. A second saliva sample was obtained approximately 45 minutes after the start of the social stress induction or control task. All study procedures were approved by an Institutional Review Board.
Social Stress Induction and Control Task
Social Stress Induction. Participants in the stress condition completed a modified version of the Trier Social Stress Test (TSST), which is a 15-minute laboratory induction of social-evaluative threat (Kirschbaum, Pirke, & Hellhammer, Citation1993). Similar to prior studies (Quinn & Joormann, Citation2015), this social stress induction included a speech and arithmetic task completed in front of one experimenter and camera. Participants were told that they would be recorded so their performance could be rated by peers. For the speech, participants spent 3 minutes preparing, and then gave a 5-minute speech on why they are an ideal job candidate. For the arithmetic task, participants counted backwards from 2,083 to 0 in increments of 17. Each time a mistake was made, the experimenter prompted participants to restart at 2,083. After the 5-minute arithmetic task, participants were seated to complete the post-stress n-back. Participants completed two sets of frustrating practice trials. They were informed that they failed the first two sets of practice trials, regardless of actual performance. Participants then completed another set of practice trials that gave accurate feedback. As soon as participants reached 90% accuracy on a set of true practice trials, advancement to the experimental trials was granted.
Control Task. Participants in the control condition completed a 15-minute control version of the TSST (Het, Rohleder, Schoofs, Kirschbaum, & Wolf, Citation2009). The control task was designed to approximate the TSST on physical demands, but did not include social evaluative threat, which has been previously identified as a critical component of stressors that upregulate pro-inflammatory cytokine activity (Slavich, O'Donovan, Epel, & Kemeny, Citation2010). Participants spent 3 minutes thinking about a movie, novel, or recent trip. Participants then stood up and talked about the chosen topic for 5 minutes. Participants then spent 5 minutes counting up from 0 in increments of 15. Participants performed these tasks while alone in a room and were not recorded.
Self-reported Affect. Affect was assessed before and immediately after the social stress induction or control task using a subset of items from the Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, Citation1988). Participants rated how much they experienced five negative emotions: upset, nervous, sad, tense, and irritable. Each emotion was rated using 5-point Likert scales, ranging from 1 (not at all) to 5 (extremely). The average was computed to assess the validity of the social stress induction.
Pro-Inflammatory Cytokines
To assess social stress-induced changes in inflammatory activity, three pro-inflammatory cytokines that are involved in the acute phase response – namely, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α – were assayed from saliva samples obtained before and after the social stress induction or control task. Saliva samples provide a minimally invasive method for measuring inflammatory activity and salivary levels of each of these cytokines are responsive to acute stress inductions (Slavish et al., Citation2015). IL-1β, IL-6, and TNF-α have been implicated in depression (Slavich & Irwin, Citation2014; Slavich & Sacher, Citationin press) and are active early in the immune response cascade (Barton, Citation2008), suggesting that stress-induced activity may be observed within an hour after onset of a stressor (Slavish et al., Citation2015). IL-10 and interferon-γ were also assayed, but not included in this study given our lack of a priori hypotheses regarding relations between these cytokines, executive control, and depression. A baseline sample was collected approximately 30 minutes after arrival at the lab for the study session and a second sample was obtained approximately 45 minutes after the start of the social stress induction or control task. Participants used a straw to expel saliva into vials (SaliCap Set, IBL International). Inhibitors were not added to the samples. All samples were transferred to a −70 °C freezer within 20 minutes of collection and were frozen at −70 °C until shipment to the UNC Cytokine and Biomarker Analysis Facility. Samples were assayed using a Magnetic Luminex Performance Assay for High Sensitivity detection, Cytokine Panel A kit (R&D Systems, Minneapolis, MN). This technique has been previously validated for salivary cytokine assay by comparison to ELISA (Arellano-Garcia et al., Citation2008). In addition, using this same assay, we have shown that salivary cytokines can be reliably assessed (Shields, Slavich, Perlman, Klein, & Kotov, Citation2019) and that they correlate with social stress exposure (Giletta et al., Citation2018), executive control (Shields, Kuchenbecker, et al., Citation2016), and depression severity (Slavich et al., Citation2019). Running the standards in duplicate revealed that the mean intra-assay coefficients of variation were 1.53% for IL-6, 1.12% for IL-1β, and 1.49% for TNF-α, indicating high reliability.
Executive Control Task
Consistent with our prior research (Quinn & Joormann, Citation2015), a two-back, affective version of the n-back task was used to measure executive control. The n-back is often used as a measure of executive control, which requires both the general and updating working memory components of executive control and has demonstrated acceptable construct validity and reliability (Friedman et al., Citation2008; Snyder et al., Citation2015). In the n-back, participants see a series of 120 words, each presented for 500ms, followed by a blank screen for 2500ms. For each word, participants indicate by button press whether it matches the word presented two trials previously. This set of trials lasts 6 minutes. Our measure of executive control includes affective stimuli based on evidence that it may assess processes that are important for the application of executive control in emotional contexts (e.g., emotion regulation; Cohen et al., Citation2015; Schweizer, Hampshire, & Dalgleish, Citation2011). Positive and negative words selected from the Affective Norms of English Words list (Bradley & Lang, Citation1999) were presented in the task. The total number of errors made during the task was the measure of executive control (Snyder et al., Citation2015).
Beck Depression Inventory, Second Edition
The BDI-II (Beck, Steer, & Brown, Citation1996) is a 21-item self-report measure of depressive symptoms. Participants indicated the extent to which they had experienced symptoms of depression during the past two weeks on a scale ranging from 0 to 3, with higher scores representing greater depression severity. The BDI-II has previously demonstrated acceptable reliability and validity (Beck et al., Citation1996; Dozois, Dobson, & Ahnberg, Citation1998), and showed very good reliability in the present sample (α = .88).
Results
Social Stress Induction Manipulation Check
Self-reported negative affect was assessed immediately before and after the social stress induction or control task. A repeated-measures ANOVA was conducted with Time (pre- and post-stress or control task) as the within-subjects factor and Condition (stress, n = 20; control, n = 16) as the between-subjects factor. A significant Time × Condition interaction was detected, Wilk’s λ = 0.81, F(1,34) = 7.83, p = .008, partial η2 = 0.19. Participants in the stress condition reported a significantly greater increase in negative affect (pre-stress: M = 1.30, SD = 0.35; post-stress: M = 1.92, SD = 0.67) relative to those in the control condition (pre-stress: M = 1.16, SD = 0.22; post-stress: M = 1.25, SD = 0.28).
Changes in Pro-Inflammatory Cytokines
Each cytokine (pg/ml) was assessed before (M = 33.51 minutes, SD = 3.75, range: 24-44) and after (M = 44.74 minutes, SD = 3.55, range: 38-54) the start of the social stress induction or control task (see for descriptive statistics). Time from the first saliva sample to the beginning of the social stress induction or control task did not significantly differ between conditions, t(33) = 1.30, p = .203. Similarly, time from the beginning of the social stress or control task to collection of the second saliva sample did not significantly differ between conditions, t(33) = 0.85, p = .404. A repeated-measures ANOVA was conducted for each cytokine with Time (pre- and post-stress or control task) as the within-subjects factor and Condition (stress, n = 20; control, n = 16) as the between-subjects factor. Cytokine levels at each time point were positively skewed, resulting in non-normal residuals. To correct for this non-normality, analyses were also conducted on log transformed variables. The pattern of results remained the same. Untransformed data are thus reported to ease interpretation.
Table 1. Means and standard deviations for executive control, cytokine, and depression data by experimental condition.
A significant Time × Condition interaction was not detected for the cytokines (IL-6: Wilk’s λ = 0.99, F(1,34) = 0.18, p = .674, partial η2 = 0.01; IL-1β: Wilk’s λ = 0.99, F(1,33) = 0.17, p = .684, partial η2 = 0.01; TNF-α: Wilk’s λ = 0.91, F(1,34) = 3.24, p = .081, partial η2 = 0.09), indicating that the two conditions did not significantly differ in cytokine response to the social stress induction or control task. Further, within each condition, a significant change in cytokine levels was not observed (ps > .115). Many participants demonstrated a decrease in cytokine levels from pre- to post-stress or control task (stress condition: 50% for IL-6, 55% for IL-1β, 35% for TNF- α; control condition: 56% for IL-6, 33% for IL-1β, 50% for TNF- α), which could be the result of participants habituating to the novel laboratory conditions. As expected, however, many participants in the stress condition exhibited a substantial increase (>20% increase) in cytokine concentrations from pre- to post-stressor (30% for IL-6, 35% for IL-1β, 50% for TNF-α). Also as expected, there was considerable variability in cytokine response to the social stress induction, with percent changes in cytokine levels ranging from −72.5% to 204.1% for IL-6, −82.1% to 669.2% for IL-1β, and −89.8% to 519.5% for TNF-α. Participants thus showed great variability with respect to their cytokine responses, with many individuals exhibiting increased inflammatory activity following the social stress induction.
Pro-Inflammatory Cytokines and Depression
Correlations among the study variables are reported in . To test the hypothesis that social stress-induced inflammatory activity is associated with depression, linear regression analyses were conducted on the sample (stress condition, n = 20; control condition, n = 16) with depressive symptoms as the dependent variable. Change in cytokine levels were used as predictors. In contrast to cytokine levels at individual time points, the change variables were not positively skewed. Regression assumptions were met, including normally distributed residuals. Therefore, untransformed values for change in cytokine levels were used in all primary analyses.
Table 2. Correlations among key study variables by experimental condition (i.e., stress vs. control).
Gender and family income were included as control variables in each model (Slavish et al., Citation2015). In model 1, Condition, Change in IL-6, and Condition × Change in IL-6 were included as predictors. The interaction between Condition and Change in IL-6 significantly improved the model, ΔR2 = .16, F(1,30) = 6.78, p = .014 (see ). As hypothesized, simple slopes analysis demonstrated that greater increases in IL-6 were associated with higher depressive symptoms only for individuals in the stress condition (see ). In model 2, Condition, Change in IL-1β, and Condition × Change in IL-1β were included as predictors. The interaction between Condition and Change in IL-1β did not significantly improve the model, ΔR2 = .03, F(1,30) = 1.09, p = .306 (see ). In model 3, Condition, Change in TNF-α, and Condition × Change in TNF-α were included as predictors. The interaction between Condition and TNF-α did not significantly improve the model, ΔR2 = .04, F(1,30) = 1.29, p = .265 (see ). Overall, these results indicate that greater social stress-induced increases in IL-6 (but not IL-1β or TNF-α) were significantly associated with depressive symptoms.
Figure 1. Changes in interleukin (IL)-6, as a function of experimental condition (stress, n = 20; control, n = 16), predicting depressive symptoms. In the stress (but not control) condition, greater increases in IL-6 were significantly associated with greater depressive symptoms.
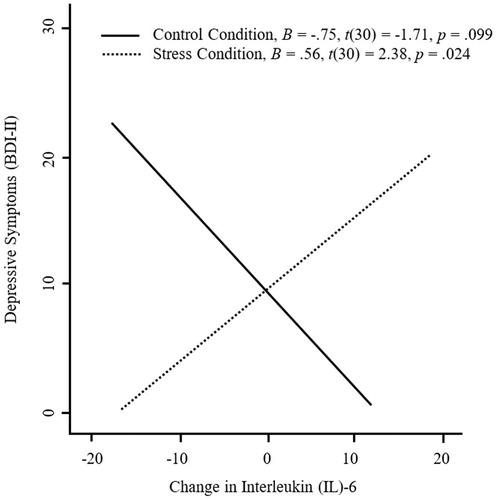
Table 3. Regression models testing the hypothesis that social stress-induced increases in inflammatory activity are associated with participants’ depressive symptom severity.
Pro-Inflammatory Cytokines and Executive Control Under Stress
To test the hypothesis that impairment in executive control under conditions of social stress is associated with stress-induced inflammatory activity, we examined the association between errors made on the post-stress n-back and changes in inflammatory activity in response to the social stress induction. Our goal was to explain variance in inflammatory response to social stress and, although cytokines were assayed in both conditions, participants’ inflammatory response to stress was assessed only in the stress condition; therefore, analyses were limited to the stress condition (n = 16). Gender, family income, and errors made on the n-back task prior to the social stress induction were included as control variables. As hypothesized, errors on the post-stress n-back were significantly associated with changes in IL-6, indicating that impaired executive control under stress, controlling for trait executive control, was associated with greater increase in IL-6 (see ). These same effects were marginally significant for changes in IL-1β and not significant for changes in TNF-α (see ). In contrast, errors made on the pre-stress n-back task, while controlling for gender and family income, were not significantly associated with changes in IL-6 (β = .07, p = .791), changes in IL-1β (β = .40, p = .206), or changes in TNF-α (β = −.17, p = .556).
Table 4. Regression models testing the hypothesis that executive control under stress is associated with participants’ inflammatory reactivity to the laboratory-based social stressor.
Discussion
Social Signal Transduction Theory of Depression posits that social stress can increase inflammatory activity in some individuals, which may in turn promote depressive symptoms and lead to clinical depression for at least some people (Slavich & Irwin, Citation2014; Slavich & Sacher, Citationin press). Examining individual differences that are associated with social stress-induced inflammatory activity may thus improve our understanding of how depression arises following exposure to social stress. To this end, we measured participants’ current depressive symptoms, as well as their pro-inflammatory cytokine levels and executive control before and after an acute laboratory-based social stress induction or control task. Consistent with Social Signal Transduction Theory of Depression, greater increases in IL-6 following the social stress induction were significantly associated with greater depressive symptoms. Separate analyses revealed that greater impairment in executive control measured following the social stress induction was associated with greater social stress-induced increases in IL-6.
Our finding that social stress-induced changes in salivary levels of IL-6 were significantly associated with depressive symptoms is consistent with prior studies that have measured serum or plasma IL-6 levels (Fagundes, Glaser, Hwang, Malarkey, & Kiecolt-Glaser, Citation2013; Pace et al., Citation2006; Weinstein et al., Citation2010). Given the correlational and cross-sectional nature of the present study, we cannot say whether social stress-induced inflammation contributes to depression, although this is one plausible interpretation of these data. Indeed, this interpretation is consistent with Social Signal Transduction Theory of Depression (Slavich & Irwin, Citation2014; Slavich & Sacher, Citationin press) and with numerous studies showing that inflammatory activity promotes depressive symptoms (Capuron et al., Citation2002; Janssen et al., Citation1994; Lotrich et al., Citation2007). The present results add to the growing body of research showing that increases in inflammatory activity are associated with depression.
Given prior evidence showing that increased inflammatory activity can promote depressive symptoms (e.g., Lotrich, Rabinovitz, Gironda, & Pollock, Citation2007), it is important to understand factors that are associated with individual differences in social stress-induced changes in inflammatory activity. To our knowledge, the present study is the first to show that lower levels of executive control under stress, while controlling for baseline executive control, are associated with greater increases in IL-6 following a social stress induction. These results build upon a prior study showing that changes in IL-6 in response to watching an emotionally stressful video were related to performance on a measure of executive control (Shields, Kuchenbecker, et al., Citation2016). The present study and the study by Shields, Kuchenbecker, et al. (Citation2016) suggest that executive control is related to inflammatory responding to social stress. However, the present data are unique in that they suggest that executive control under stress, over and above baseline executive control, is related to changes in inflammatory activity. Given that executive control is thought to influence individuals’ ability to regulate their reactivity to stress (Joormann & Vanderlind, Citation2014), the results of the present study, although correlational, are consistent with the possibility that social stress may impact executive control in a manner that could potentially lead to greater social stress-induced increases in inflammatory activity.
In contrast to IL-6, neither IL-1β nor TNF-α were significantly associated with depressive symptoms. This is similar to a prior study which found that stress-induced increases in TNF-α were not significantly greater in individuals with depression relative to healthy controls (Miller, Rohleder, Stetler, & Kirschbaum, Citation2005). However, other studies have demonstrated that stress-induced increases in serum or plasma measures of IL-1β and TNF-α are associated with depression (Aschbacher et al., Citation2012; Weinstein et al., Citation2010). Additional research is thus needed to clarify how these different cytokines are related to depression.
In the present study, a significant relation was not observed between executive control under stress and either IL-1β or TNF-α, although a marginal (non-significant) relation between executive control under stress and IL-1β was evident. To our knowledge, no prior research has examined the association between executive control under stress and pro-inflammatory cytokines. Given the small sample size in the present study, additional research is needed.
A significant average increase in inflammatory activity in the stress condition, relative to the control condition, was not observed in the present study. Although increases in salivary IL-1β (Mastrolonardo, Alicino, Zefferino, Pasquini, & Picardi, Citation2007), IL-6 (Izawa et al., Citation2013), and TNF-α (Filaire et al., Citation2011) have been found following stress inductions, findings are mixed (Groer et al., Citation2010; Lester, Brown, Aycock, Grubbs, & Johnson, Citation2010). Similar to the present study, for example, Giletta et al. (Citation2018) found nonsignificant average changes in salivary IL-6 and IL-1β following an acute social stress induction. Despite the lack of average increases in cytokine levels in the present study, however, substantial inter-individual variability in cytokine level changes following the social stress induction was observed. Indeed, this individual variability is central to our hypothesis that individual differences in social stress-induced inflammatory activity are related to depression.
Ultimately, results of the present study should be considered in the context of several limitations. First, the study design was cross-sectional (e.g., depression was measured at only one time point) and the associations among executive control, cytokines, and depressive symptoms are correlational. Therefore, causal effects and temporal precedence cannot be assumed. As a result, several possible explanations exist for the associations observed. Our interpretation of the results, drawing largely from Social Signal Transduction Theory of Depression (Slavich & Irwin, Citation2014; Slavich & Sacher, Citationin press), is that social stress-induced impairment in executive control contributes to increased cytokine activity, which in turn affects depression levels. An alternative explanation, though, is that higher levels of depressive symptoms could contribute to both increased cytokine reactivity and impaired executive control observed under conditions of stress (Dowlati et al., Citation2010; Snyder, Citation2013). It is also possible that other factors could contribute to impaired executive control under stress, increased cytokine reactivity to stress, and depressive symptoms. For example, exposure to early life stress has been associated with increased inflammatory activity (Miller & Chen, Citation2010), impairment in executive control (Quinn et al., Citation2018), and depression (Liu, Citation2017). Moreover, diet, sleep, and exercise levels were not measured in the present study but could play a role (Marsland, Citation2013). Additional research is thus needed to examine other factors and potential causal links among executive control under stress, stress-induced inflammation, and depression.
Second, the present sample consisted of healthy young adults, and it is unclear to what extent the present findings generalize to other populations. In addition, our sample size was small; therefore, there is an elevated risk that some of the reported null results reflect type II errors. These findings should thus be interpreted cautiously, and future research is needed to examine these relations in a larger sample and in other populations. Third, the present study assessed cytokine levels at only two time points. Therefore, cytokine levels at the first sampling timepoint may have been elevated due to arriving at the lab, and it is unclear to what extent cytokine levels at the second timepoint captured the peak reactivity of all participants. As a result, cytokine responses to the stress induction may have been underestimated. In future research, taking additional samples would provide a more nuanced picture of individuals’ cytokine reactivity to social stress. Relatedly, there was variability in the timing of saliva samples and other components of the procedure (e.g., number of practice trials completed to reach 90% accuracy). This variability also may have introduced noise into our analyses. Additionally, the baseline measure of executive control was obtained soon after arriving in the lab and may have been affected by arriving at the lab. In future research, giving participants more time to acclimate to the lab may help to obtain a better baseline measure of executive control.
Finally, salivary measures of inflammatory activity are a relatively new approach for studying the role of inflammation in depression. Although there is some indication that cytokine levels may be higher in saliva samples relative to blood samples, much remains unknown about the pathways leading to increased salivary cytokine levels and how salivary inflammatory levels relate to those obtained from blood (Slavish et al., Citation2015). Nevertheless, the present results combined with those obtained from other studies suggest that more research is warranted to understand how salivary markers of inflammatory activity are relevant for depression.
In summary, the present results indicate that greater social stress-induced increases in IL-6 are associated with higher levels of depression. In addition, we found preliminary evidence that lower levels of executive control under conditions of acute social stress are associated with greater social stress-induced increases in IL-6. These results shed light on a potential cognitive-immunologic link that may have implications for understanding risk for depression. Additional research is needed to examine whether impaired executive control under stress contributes to heightened social stress-induced increases in inflammation, which in turn promote depressive symptoms.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Arellano-Garcia, M.E., Hu, S., Wang, J., Henson, B., Zhou, H., Chia, D., & Wong, D.T. (2008). Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Diseases, 14(8), 705–712. doi:10.1111/j.1601-0825.2008.01488.x
- Aschbacher, K., Epel, E., Wolkowitz, O.M., Prather, A.A., Puterman, E., & Dhabhar, F.S. (2012). Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain, Behavior, and Immunity, 26(2), 346–352. doi:10.1016/j.bbi.2011.10.010
- Barton, G.M. (2008). A calculated response: Control of inflammation by the innate immune system. The Journal of Clinical Investigation, 118(2), 413–420. doi:10.1172/JCI34431
- Beck, A.T., Steer, R.A., & Brown, G.K. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation.
- Bradley, M.M., & Lang, P.J. (1999). Affective norms for English words (ANEW): Instruction manual and affective ratings. Technical Report C-1, The Center for Research in Psychophysiology. Gainesville, FL: University of Florida.
- Capuron, L., Gumnick, J.F., Musselman, D.L., Lawson, D.H., Reemsnyder, A., Nemeroff, C.B., & Miller, A.H. (2002). Neurobehavioral effects of interferon-α in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology, 26(5), 643–652. doi:10.1016/S0893-133X(01)00407-9
- Cohen, N., Mor, N., & Henik, A. (2015). Linking executive control and emotional response a training procedure to reduce rumination. Clinical Psychological Science, 3(1), 15–25. doi:10.1177/2167702614530114
- Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E.K., & Lanctot, K.L. (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. doi:10.1016/j.biopsych.2009.09.033
- Dozois, D.J.A., Dobson, K.S., & Ahnberg, J.L. (1998). A psychometric evaluation of the Beck Depression Inventory–II. Psychological Assessment, 10(2), 83–89. doi:10.1037/1040-3590.10.2.83
- Fagundes, C.P., Glaser, R., Hwang, B.S., Malarkey, W.B., & Kiecolt-Glaser, J.K. (2013). Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behavior, and Immunity, 31, 172–176. doi:10.1016/j.bbi.2012.05.006
- Filaire, E., Larue, J., Portier, H., Abed, A., Graziella, P.D., Teixeira, A., & Anne, P. (2011). Lecturing to 200 students and its effects on cytokine concentration and salivary markers of adrenal activation. Stress and Health, 27(2), e25–e35. doi:10.1002/smi.1332
- Friedman, N.P., & Miyake, A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. doi:10.1016/j.cortex.2016.04.023
- Friedman, N.P., Miyake, A., Young, S.E., DeFries, J.C., Corley, R.P., & Hewitt, J.K. (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General, 137(2), 201–225. doi:10.1037/0096-3445.137.2.201
- Giletta, M., Slavich, G.M., Rudolph, K.D., Hastings, P.D., Nock, M.K., & Prinstein, M.J. (2018). Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. Journal of Child Psychology and Psychiatry, 59(2), 129–139. doi:10.1111/jcpp.12804
- Glaser, R., & Kiecolt-Glaser, J.K. (2005). Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology, 5, 243–251. doi:10.1038/nri1571
- Grebe, K.M., Takeda, K., Hickman, H.D., Bailey, A.L., Embry, A.C., Bennink, J.R., & Yewdell, J.W. (2010). Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. Journal of Immunology, 184(2), 540–544. doi:10.4049/jimmunol.0903395
- Groer, M., Murphy, R., Bunnell, W., Salomon, K., Van Eepoel, J., Rankin, B., … Bykowski, C. (2010). Salivary measures of stress and immunity in police officers engaged in simulated critical incident scenarios. Journal of Occupational and Environmental Medicine, 52(6), 595–602. doi:10.1097/JOM.0b013e3181e129da
- Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O.T. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the 'Trier Social Stress Test'. Psychoneuroendocrinology, 34(7), 1075–1086. doi:10.1016/j.psyneuen.2009.02.008
- Irwin, M.R., & Cole, S.W. (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625–632. doi:10.1038/nri3042
- Izawa, S., Sugaya, N., Kimura, K., Ogawa, N., Yamada, K.C., Shirotsuki, K., … Nomura, S. (2013). An increase in salivary interleukin-6 level following acute psychosocial stress and its biological correlates in healthy young adults. Biological Psychology, 94(2), 249–254. doi:10.1016/j.biopsycho.2013.06.006
- Janssen, H.L., Brouwer, J.T., van der Mast, R.C., & Schalm, S.W. (1994). Suicide associated with alfa-interferon therapy for chronic viral hepatitis. Journal of Hepatology, 21(2), 241–243. doi:10.1016/S0168-8278(05)80402-7
- Joormann, J., & Vanderlind, W.M. (2014). Emotion regulation in depression: The role of biased cognition and reduced cognitive control. Clinical Psychological Science, 2(4), 402–421. doi:10.1177/2167702614536163
- Kirschbaum, C., Pirke, K.-M., & Hellhammer, D.H. (1993). The 'Trier Social Stress Test': A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. doi:10.1159/000119004
- Lester, S.R., Brown, J.R., Aycock, J.E., Grubbs, S.L., & Johnson, R.B. (2010). Use of saliva for assessment of stress and its effect on the immune system prior to gross anatomy practical examinations. Anatomical Sciences Education, 3(4), 160–167. doi:10.1002/ase.161
- Liu, R.T. (2017). Childhood adversities and depression in adulthood: Current findings and future directions. Clinical Psychology: Science and Practice, 24(2), 140–153. doi:10.1111/cpsp.12190
- Lotrich, F.E., Rabinovitz, M., Gironda, P., & Pollock, B.G. (2007). Depression following pegylated interferon-alpha: Characteristics and vulnerability. Journal of Psychosomatic Research, 63(2), 131–135. doi:10.1016/j.jpsychores.2007.05.013
- Marsland, A.L. (2013). Adversity and inflammation among adolescents. Psychosomatic Medicine, 75(5), 438–441. doi:10.1097/PSY.0b013e3182983ea6
- Mastrolonardo, M., Alicino, D., Zefferino, R., Pasquini, P., & Picardi, A. (2007). Effect of psychological stress on salivary interleukin-1β in psoriasis. Archives of Medical Research, 38(2), 206–211. doi:10.1016/j.arcmed.2006.09.009
- Medzhitov, R. (2007). Recognition of microorganisms and activation of the immune response. Nature, 449(7164), 819–826. doi:10.1038/nature06246
- Miller, G.E., & Chen, E. (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21(6), 848–856. doi:10.1177/0956797610370161
- Miller, G.E., Rohleder, N., Stetler, C., & Kirschbaum, C. (2005). Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine, 67(5), 679–687. doi:10.1097/01.psy.0000174172.82428.ce
- Pace, T.W.W., Mletzko, T.C., Alagbe, O., Musselman, D.L., Nemeroff, C.B., Miller, A.H., & Heim, C.M. (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry, 163(9), 1630–1633. doi:10.1176/ajp.2006.163.9.1630
- Quinn, M.E., & Joormann, J. (2015). Control when it counts: Change in executive control under stress predicts depression symptoms. Emotion, 15(4), 522–530. doi:10.1037/emo0000089
- Quinn, M.E., Stange, J.P., Jenkins, L.M., Corwin, S., DelDonno, S.R., Bessette, K.L., … Langenecker, S.A. (2018). Cognitive control and network disruption in remitted depression: A correlate of childhood adversity. Social Cognitive and Affective Neuroscience, 13(10), 1081–1090. doi:10.1093/scan/nsy077
- Schweizer, S., Grahn, J., Hampshire, A., Mobbs, D., & Dalgleish, T. (2013). Training the emotional brain: Improving affective control through emotional working memory training. The Journal of Neuroscience, 33(12), 5301–5311. doi:10.1523/JNEUROSCI.2593-12.2013
- Schweizer, S., Hampshire, A., & Dalgleish, T. (2011). Extending brain-training to the affective domain: Increasing cognitive and affective executive control through emotional working memory training. PLoS One, 6(9), e24372. doi:10.1371/journal.pone.0024372
- Shields, G.S., Kuchenbecker, S.Y., Pressman, S.D., Sumida, K.D., & Slavich, G.M. (2016). Better cognitive control of emotional information is associated with reduced pro-inflammatory cytokine reactivity to emotional stress. Stress, 19(1), 63–68. doi:10.3109/10253890.2015.1121983
- Shields, G.S., Sazma, M.A., & Yonelinas, A.P. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience & Biobehavioral Reviews, 68, 651–668. doi:10.1016/j.neubiorev.2016.06.038
- Shields, G.S., Slavich, G.M., Perlman, G., Klein, D.N., & Kotov, R. (2019). The short-term reliability and long-term stability of salivary immune markers. Brain, Behavior, and Immunity. doi:10.1016/j.bbi.2019.06.007
- Slavich, G.M. (2015). Understanding inflammation, its regulation, and relevance for health: A top scientific and public priority. Brain, Behavior, and Immunity, 45, 13–14. doi:10.1016/j.bbi.2014.10.012
- Slavich, G.M., Giletta, M., Helms, S.W., Hastings, P.D., Rudolph, K.D., Nock, M.K., & Prinstein, M.J. (2019). Interpersonal life stress, inflammation, and depression in adolescence: Testing social signal transduction theory of depression. Under review.
- Slavich, G.M., & Irwin, M.R. (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. doi:10.1037/a0035302
- Slavich, G.M., O'Donovan, A., Epel, E.S., & Kemeny, M.E. (2010). Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews, 35(1), 39–45. doi:10.1016/j.neubiorev.2010.01.003
- Slavich, G.M., & Sacher, J. (in press). Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology. doi:10.1007/s00213-019-05326-9
- Slavish, D.C., Graham-Engeland, J.E., Smyth, J.M., & Engeland, C.G. (2015). Salivary markers of inflammation in response to acute stress. Brain, Behavior, and Immunity, 44, 253–269. doi:10.1016/j.bbi.2014.08.008
- Snyder, H.R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. doi:10.1037/a0028727
- Snyder, H.R., Miyake, A., & Hankin, B.L. (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328. doi:10.3389/fpsyg.2015.00328
- Watson, D., Clark, L.A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. doi:10.1037/0022-3514.54.6.1063
- Weinstein, A.A., Deuster, P.A., Francis, J.L., Bonsall, R.W., Tracy, R.P., & Kop, W.J. (2010). Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biological Psychology, 84(2), 228–234. doi:10.1016/j.biopsycho.2010.01.016
