Abstract
Glucocorticoid signaling is fundamental in healthy stress coping and in the pathophysiology of stress-related diseases, such as post-traumatic stress disorder (PTSD). Glucocorticoids are metabolized by cytochrome P450 (CYP) as well as 11-β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and 2 (11βHSD2). Acute stress-induced increase in glucocorticoid concentrations stimulates the expression of several CYP sub-types. CYP is primarily responsible for glucocorticoid metabolism and its increased activity can result in decreased circulating glucocorticoids in response to repeated stress stimuli. In addition, repeated stress-induced glucocorticoid release can promote 11βHSD1 activation and 11βHSD2 inhibition, and the 11βHSD2 suppression can lead to apparent mineralocorticoid excess. The activation of CYP and 11βHSD1 and the suppression of 11βHSD2 may at least partly contribute to development of the blunted glucocorticoid response to stressors characteristic in high trait anxiety, PTSD, and other stress-related disorders. Glucocorticoids and glucocorticoid-metabolizing enzymes interact closely with other biomolecules such as inflammatory cytokines, monoamines, and some monoamine-metabolizing enzymes, namely the monoamine oxidase type A (MAO-A) and B (MAO-B). Glucocorticoids boost MAO activity and this decreases monoamine levels and induces oxidative tissue damage which then activates inflammatory cytokines. The inflammatory cytokines suppress CYP expression and activity. This dynamic cross-talk between glucocorticoids, monoamines, and their metabolizing enzymes could be a critical factor in the pathophysiology of stress-related disorders.
Lay summary
Glucocorticoids, which are produced and released under the control by brain regulatory centers, are fundamental in the stress response. This review emphasizes the importance of glucocorticoid metabolism and particularly the interaction between the brain and the liver as the major metabolic organ in the body. The activity of enzymes involved in glucocorticoid metabolism is proposed to play not only an important role in positive, healthy glucocorticoid effects, but also to contribute to the development and course of stress-related diseases.
Introduction
Stress has a key role in the etiology of numerous central nervous system (CNS) disorders including depression (Checkley, Citation1992; Tafet & Nemeroff, Citation2016), chronic fatigue syndrome (Fenouillet et al., Citation2016), anxiety disorders (Jezova & Hlavacova, Citation2008), and post-traumatic stress disorder (PTSD) (Brooks, Amlot, Rubin, & Greenberg, Citation2018). Similarly, stressful life events influence the development and course of many somatic diseases (Jezova & Herman, Citation2016; Roy, Riley, & Sinha, Citation2018; Simas, Nunes, Crestani, & Speretta, Citation2018).
Stress was originally defined as a nonspecific neuro-endocrine response of the body to the stimuli disturbing its homeostasis (Selye, Citation1973). Later on, the term allostasis has been introduced and defined as the ability to sustain homeostasis in the presence of stressors (Sterling & Eyer, Citation1988). Correspondingly, allostatic load is a burden on the body’s intrinsic systems required to sustain homeostasis under stress (McEwen, Citation2000). When allostatic load overcomes the body’s capabilities, subsequent exposure to stressors leads to the development of stress-related disorders. The vulnerability to stress-related disorders is determined by the nature, intensity, and duration of the stress situation and also by the organism’s intrinsic properties, such as interactions between biological molecules responsible for the allostasis (Jezova, Jurankova, Mosnarova, Kriska, & Skultetyova, Citation1996).
McEwen (McEwen, Citation2000) described four major types of allostatic load characterized by different glucocorticoid response patterns to stressors. The first type (“major hits”) results from repeated exposure to unknown stressful stimuli and it is characterized by recurrent increase in glucocorticoid concentrations. The second type (“lack of adaptation”) is due to an abnormally high glucocorticoid response after repeated exposure to known stressors. The third type (“prolonged response”) demonstrates abnormally long duration of glucocorticoid response to stressors. Finally, the fourth type of allostatic load (“inadequate response”) reflects an abnormally low glucocorticoid response to stressors, and this insufficient response to stressors has been observed in the following patients; those with PTSD (Smith et al., Citation1989; Strohle, Scheel, Modell, & Holsboer, Citation2008; Zaba et al., Citation2015), depression (Lopes et al., Citation2012), allergy (Buske-Kirschbaum, Ebrecht, & Hellhammer, Citation2010; Hlavacova, Solarikova, Marko, Brezina, & Jezova, Citation2017), high trait anxiety (Duncko, Makatsori, Fickova, Selko, & Jezova, Citation2006; Jezova, Makatsori, Duncko, Moncek, & Jakubek, Citation2004), and panic disorder (Jezova, Vigas, Hlavacova, & Kukumberg, Citation2010; Petrowski, Herold, Joraschky, Wittchen, & Kirschbaum, Citation2010).
The development of a blunted glucocorticoid response to stressors might be therefore an important element in the pathophysiology of PTSD and other stress-related disorders. This review is summarizing the regulation of glucocorticoid signaling in relation to psychopathological processes, with a focus on glucocorticoid-metabolizing enzymes.
Factors regulating glucocorticoid signaling
Circulating glucocorticoid concentrations are determined by their biosynthesis in the adrenal cortex and by their metabolism in the local tissues and the liver. Increased glucocorticoid synthesis and/or decreased metabolism leads to hypercortisolism and decreased synthesis and/or increased metabolism produce hypocortisolism (). In addition, glucocorticoid docking by corticosteroid-binding globulin is also an important mechanism regulating circulating glucocorticoid levels (Qian et al., Citation2011).
Figure 1. Role of production and metabolism of glucocorticoids in the regulation of their concentrations in the blood. The circulating levels of cortisol (CORT) are balanced by the production of this enzyme in the zona fasciculate of the adrenal cortex, its metabolism to dihydrorocortisol and tetrahydrocortisol with subsequent glucuronidation to tetrahydrocortisone glucuronide (THCG) in the liver and clearance of THCG via the kidneys. Decrease in cortisol biosynthesis and/or increase in its metabolism lead to hypocortisolism. Increase in cortisol biosynthesis and/or decrease in its metabolism lead to hypercortisolism.
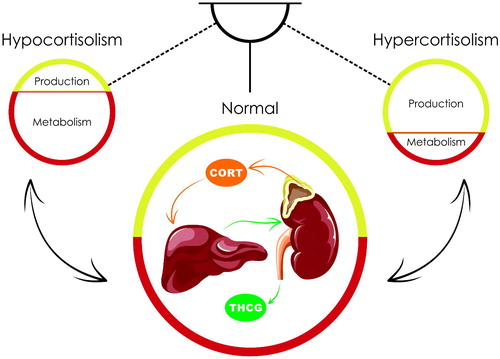
Glucocorticoids penetrate biological cells and their nuclei and directly affect the expression of specific genes via the nuclear glucocorticoid receptors (GRs). Target organ sensitivity to glucocorticoids is therefore regulated by the density and activity of GRs. GR expression is auto-regulated by glucocorticoids so that hypercortisolism usually results in decreased and hypocortisolism in increased GR expression (Leistner & Menke, Citation2018; van Zuiden, Kavelaars, Geuze, Olff, & Heijnen, Citation2013). The first three types of allostatic load have increased glucocorticoid levels associated with decreased sensitivity to these hormones (McEwen, Citation2000). These glucocorticoid signaling changes are similar to those observed in Cushing’s (Granner, Wang, & Yamamoto, Citation2015) and glucocorticoid resistance syndromes (Nicolaides & Chrousos, Citation2018), but to a lesser extent. Finally, the fourth type of allostatic load has decreased glucocorticoid bioavailability and increased target organ sensitivity, and similar alterations have been observed in some stress-related disorders (McEwen, Citation2000).
GR affinity to glucocorticoids is regulated by co-chaperone proteins. These include FK506-binding protein 5 (FKBP5) and its overexpression leads to decreased GR sensitivity and impaired negative feedback regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis (Matosin, Halldorsdottir, & Binder, Citation2018; Tatro, Everall, Kaul, & Achim, Citation2009). It is possible that GR modulation by FKBP5 has a role in allostatic load and in stress-related pathophysiological processes because altered FKBP5 gene expression has been recorded in PTSD patients (Kang, Kim, Choi, So, & Kim, Citation2019) and also in patients with depression (Rao et al., Citation2016) and alcohol-use disorder (Qiu et al., Citation2016).
Although GRs are the primary target of glucocorticoids, these hormones also bind to mineralocorticoid receptors (MRs). Actually, MRs have higher affinity to glucocorticoids than GRs (de Kloet, Reul, & Sutanto, Citation1990). The MR-mediated glucocorticoid signaling boosts attention and memory retrieval; these factors are important for the initiation of the behavioral response to a stressful stimulus (Oitzl & de Kloet, Citation1992). In contrast, GR-mediated signaling regulates memory storage and consolidation (Nikzad, Vafaei, Rashidy-Pour, & Haghighi, Citation2011) and it is critical for termination of the stress response (de Kloet, Meijer, de Nicola, de Rijk, & Joels, Citation2018). The balance between GR- and MR-mediated signaling is therefore important in the stress response, and its disturbance can contribute to stress-related psychopathological processes.
Regulation of glucocorticoid signaling by 11-β-hydroxysteroid dehydrogenase
The balance between GR- and MR-mediated glucocorticoid signaling is controlled by multiple factors. Of particular importance are the enzymes belonging to the of 11-β-hydroxysteroid dehydrogenase (11βHSD) family which act mainly at the local, tissue level (Chapman, Holmes, & Seckl, Citation2013). The 11βHSD has two isoforms: 11βHSD1 and 11βHSD2.
The 11βHSD1 isoform is localized on the luminal surface of the endoplasmic reticulum and it is nicotinamide adenine dinucleotide phosphate (NADPH)-dependent. It is highly expressed in the brain, adrenal glands, liver, and adipocytes and it converts inactive cortisone to cortisol. Its main NADPH source comes from the glucose-6-phosphate-dehydrogenase (H6PDH) reaction, and therefore glucose-6-phosphate deficiency is often accompanied by decreased 11βHSD1 catalytic activity (Tu et al., Citation2008). 11βHSD1 over-expression in white adipose tissue induces hyperphagia (Michailidou et al., Citation2012) and it is also associated with enhanced liver gluconeogenesis (Draper & Stewart, Citation2005). Moreover, the 11βHSD1 isoform contributes up to 30% of extra-adrenal cortisol synthesis in healthy humans (Basu et al., Citation2004). In the brain, 11βHSD1 is expressed in brain neurons and glial cells, and relatively high densities of this enzyme have been recorded in the pituitary, hypothalamus, and hippocampus (Varga et al., Citation2016; Wyrwoll, Holmes, & Seckl, Citation2011).
The 11βHSD2 isoform interacts with MRs and is particularly active as a selective MR protective factor in aldosterone target tissues (Edwards et al., Citation1988; Funder, Pearce, Smith, & Smith, Citation1988) and it provides local conversion of bio-active glucocorticoids into the inactive ketone form. It is localized in the cell endoplasmic reticulum, but also profoundly expressed in the placenta and fetal tissues where it creates a barrier preventing maternal glucocorticoid transfer to the fetus (Wyrwoll et al., Citation2011; Yehuda & Seckl, Citation2011). Varga et al. (Citation2013) also recorded higher 11βHSD2 expression in the rat’s early postnatal period.
The 11βHSD2 isoform was long considered absent in brain tissue until highly sensitive mRNA probes revealed its expression in the nucleus of the solitary tract (NTS) (Evans et al., Citation2016; Gasparini et al., Citation2018; Citation2019; Geerling & Loewy, Citation2007). NTS is involved in the regulation of electrolyte balance, salt appetite, and blood pressure. Since glucocorticoid concentrations in the brain are 100–1000 times higher than those of mineralocorticoids and the affinity of MRs to glucocorticoids and mineralocorticoids is comparable, the MRs located in brain areas not expressing 11βHSD2 are glucocorticoid-preferable (Geerling & Loewy, Citation2008). Co-expression of 11βHSD2 and MRs makes NTS neurons sensitive to mineralocorticoids, via a mechanism involving intracellular inactivation of glucocorticoids (Geerling & Loewy, Citation2007). The 11βHSD2-mediated mineralocorticoid-sensitivity of MRs was considered unique for the NTS. A more recent study however reported, that 11βHSD2, as well as 11βHSD1, are also expressed in the hypothalamus and hippocampus (Varga et al., Citation2016). In the same study, the highest concentration of 11βHSD2 mRNA was detected in the hippocampus of neonatal vasopressin-deficient Brattleboro rats, suggesting that hippocampal MRs might be mineralocorticoid-sensitive as well, particularly in the early postnatal period and/or under specific physiological or pathological conditions, such as low vasopressin concentrations.
The affinity of 11βHSD2 to the substrate is one hundred times higher than that of 11βHSD1 (Chapman et al., Citation2013). Therefore, in the structures in which these enzymes are co-expressed, such as hypothalamus and hippocampus, 11βHSD2 might play a dominant role in glucocorticoid balance. Indeed, the administration of glycyrrhetinic acid, a nonspecific inhibitor of 11βHSD1 and 11βHSD2, decreased CRH concentration in the hypophysial portal blood (Seckl, Dow, Low, Edwards, & Fink, Citation1993), via a mechanism presumably involving hypothalamic 11βHSD2 rather than 11βHSD1.
The inhibition of 11βHSD2 led to the state of apparent mineralocorticoid excess (Ferrari, Citation2010). Moreover, Evans et al. (Citation2016) demonstrated, using NTS-specific knockout mice, that reduced 11βHSD2 activity in the brain did not intrinsically cause hypertension, but stimulated salt intake and the transition from salt resistance to salt sensitivity. The authors then suggested that 11βHSD2-expressing neurons integrate salt appetite and the blood pressure response to dietary sodium through an MR-dependent pathway.
While the suppression of 11βHSD2 activity leads to increased mineralocorticoid signaling on a local level, 11βHSD1 inactivation in glucocorticoid-feedback sites results in stimulation of the entire HPA axis. The 11βHSD1 gene knockout in mice promoted adrenal hypertrophy and augmented ACTH-stimulated circulating corticosterone concentrations (Abrahams et al., Citation2012). It is therefore possible that 11βHSD1 expressed in the hypothalamic paraventricular nucleus (PVN), and perhaps in some other brain areas, affects both local and circulating glucocorticoid levels. Interestingly, patients with type 2 diabetes treated with selective 11βHSD1 inhibitors (Rosenstock et al., Citation2010), and also 11βHSD1 knockout mice, exhibited increased ACTH levels, and adrenal hypertrophy (Harris, Kotelevtsev, Mullins, Seckl, & Holmes, Citation2001). This was summarized in Yehuda & Seckl’s (Citation2011) minireview supporting a metabolic hypothesis for low cortisol activation in stress-related psychiatric disorders.
highlights that 11βHSD1 and 11βHSD2 modulate glucocorticoid signaling on both local tissue and systemic circulation levels, where increased 11βHSD1 tissue activity increases corticosteroid bioavailability, and augmented 11βHSD1 activity suppresses HPA axis and possibly promotes adrenal hypotrophy. In contrast, increased 11βHSD2 activity leads to decreased tissue glucocorticoid availability in aldosterone target cells.
Table 1. Regulation of glucocorticoid signaling by 11-β-hydroxysteroid dehydrogenase types 1 and 2 and by cytochrome P450.
Obut et al. (Citation2009) suggested that repeated, but not acute, stressors activated 11βHSD1 and inhibited 11βHSD2, and other authors consider that the glucocorticoid stimulatory effect on 11βHSD1 is mediated, at least in part, by the GRs (Pal’chikova, Kuznetsova, Selyatitskaya, Cherkasova, & Kuz’mina, Citation2016). Since 11βHSD1 inhibition results in HPA axis activation and adrenal hypertrophy, its activation by repeated stress may have opposite effects. It is therefore possible that low glucocorticoid concentrations observed in some animal models of PTSD (Boero et al., Citation2018; Kondashevskaya et al., Citation2017; Lazuko et al., Citation2018), and the adrenal hypotrophy reported in one of these models (Manukhina et al., Citation2018), were at least partly caused by increased 11βHSD1 activity. While it is not clear if 11βHSD2 inhibition results in altered circulating glucocorticoid levels, it may attenuate GR-mediated glucocorticoid signaling at the tissue level. These hypotheses, however, require clarification.
Cytochrome P450 and control of glucocorticoid concentration
Cytochrome P450 (CYP) is a microsomal and mitochondrial enzyme superfamily which catalyzes oxidation of various endogenous and exogenous biological molecules. These include steroid hormones, catecholamines, and medications including antidepressant drugs (Munro, McLean, Grant, & Makris, Citation2018; Rendic, Citation2002). The CYP3A subfamily comprises several microsomal enzymes involved in glucocorticoid metabolism and it irreversibly metabolizes corticosterone to 6β-corticosterone in rodents (isoforms CYP3A1 and CYP3A2) and cortisol to 6β-cortisol in humans (isoforms CYP3A4 and CYP3A5) (Peng et al., Citation2011).
CYP3A activity is stimulated by glucocorticoids (Dvorak & Pavek, Citation2010), and dexamethasone induces CYP3A mRNA and CYP3A immunoreactive protein expression in cultivated rodent (Kocarek, Schuetz, Strom, Fisher, & Guzelian, Citation1995) and human (Cooper, Cho, Thompson, & Wallace, Citation2008) hepatocytes, and in the rat liver (Kishida et al., Citation2008; Telhada, Pereira, & Lechner, Citation1992) and in the mouse liver (Yanagimoto, Itoh, Muller-Enoch, & Kamataki, Citation1992) and brain (Stamou, Wu, Kania-Korwel, Lehmler, & Lein, Citation2013).
In addition to its regulation by glucocorticoids, CYP3A activity is also modulated by other hormones and neurotransmitters, including monoamines. While α- and β-adrenoceptor agonists stimulated CYP3A1 and CYP3A2 expression in cultivated rat hepatocytes, their in vivo administration showed that the α-adrenergic agonist decreased CYP3A1 and CYP3A2 expression in the liver and in vivo β-adrenergic receptor antagonists increased CYP3A1 and CYP3A2 mRNA levels in that organ. These findings indicate that the complex network of central and peripheral pathways may override the hepatic adrenergic receptor effects (Daskalopoulos, Lang, Marselos, Malliou, & Konstandi, Citation2012). Accordingly, specific brain norepinephrine neuron lesion by 6-hydroxydopamine led to the activation of several hepatic CYPs, including CYP3A, while norepinephrine administration to the cerebral ventricle had the opposite effect.
It has also been suggested that central norepinephrine negatively regulates hepatic microsomal oxidation of glucocorticoids and that this effect is mediated at least in part by growth hormone (Kot et al., Citation2015). In addition to catecholamines, hepatic microsomal oxidation of glucocorticoids is also influenced by central 5-HT pathways, because selective lesion of 5-HT neurons and inhibition of 5-HT synthesis both led to robust activation of multiple CYPs, including CYP1A and CYP3A (Kot & Daniel, Citation2011). Dopamine appears to directly modulate hepatic CYP3A, CYP2C, and CYP2D activities by a D2-receptor-mediated mechanism (Daskalopoulos et al., Citation2012); and more recently, the D2 receptor antagonist sulpiride has been shown to down-regulate CYP1A1, CYP1A12, and CYP1B1 expression in the rat liver (Harkitis et al., Citation2015).
Peripheral catecholamines, including adrenal epinephrine, suppress microsomal oxidation of glucocorticoids, and Daskalopoulos et al. (Citation2012) suggested that this effect may contribute to the increased levels of glucocorticoids in response to acute stressors. However, this is quite unlikely because peripheral catecholamines were not found to participate in HPA axis activation in acute stress (Jezova et al., Citation1987; Makara et al., Citation1986).
Next, microsomal oxidation of glucocorticoids by CYPs in both the brain and liver is also modulated by inflammatory cytokines, and it has long been accepted that the bacterial toxin lipopolysaccharide (LPS) can suppress CYP1A activity in the brain via mechanisms mediated by tumor necrosis factor alpha (TNF-α), interleukin-1α (IL-1α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) (Nicholson & Renton, Citation1999). Finally, the LPS-induced downregulation of CYP3A in the liver also appears to be mediated by TNF-α (Li et al., Citation2008).
Since CYP is fundamental in the modulation of the circulating levels of glucocorticoids, it is possible that abnormal expression or activity of this enzyme is responsible, at least in part, for the blunted glucocorticoid signaling in certain stress-related disorders. In patients with depression showing a blunted response in the dexamethasone suppression test (DST) plasma half-life of dexamethasone is shorter compared to that in depressive patients exhibiting a normal DST response (Holsboer, Wiedemann, & Boll, Citation1986; Stokes, Sikes, Lasley, & Stoll, Citation2002).
Regulation of monoamine oxidize activity by glucocorticoids
As described above, the glucocorticoid metabolism effected by 11βHSD and CYP is controlled by monoamines which are metabolized by monoamine oxidase (MAO). The following three putative mechanisms enable glucocorticoids to modulate MAO activity.
First, MAO is a mitochondrial enzyme, and glucocorticoids control general mitochondrial functions. An example here is glucocorticoid dose-dependent regulation of three key characteristics of mitochondrial function: oxidation level, membrane potential, and calcium holding capacity (Du et al., Citation2009). Second, MAO activity is dependent on the lipid microenvironment, and the ability of glucocorticoids to regulate mitochondrial lipid peroxidation enables them to modulate MAO activity (Ekstedt & Oreland, Citation1976; Singer, Citation1995). Finally, glucocorticoids regulate MAO expression, and it has been established that dexamethasone increased krueppel-like factor 11 (KLF11) mRNA and protein levels in cultured neuronal cells. Over-expression of KLF11 resulted in increases in both the MAO-A mRNA concentration and its enzymatic activity (Grunewald et al., Citation2012; Harris et al., Citation2015). MAO activity correlated with the microsomal oxidation phenotype (Tseilikman et al., Citation2016) and the microsomal oxidation inhibitor proadifen reduced MAO activity in both the brain and liver (Kozochkin et al., Citation2017).
Potential changes in MAO activity in stress-related psychopathologies are not well understood. While one study recorded that increased MAO activity was detected in the platelets from patients with PTSD compared to healthy subjects (Davidson, Lipper, Kilts, Mahorney, & Hammett, Citation1985) a more recent study gave the opposite result (Pivac et al., Citation2007) and GR blockade in an animal model of PTSD diminished the stress-induced increase in MAO activity (Tseilikman et al., Citation2015).
Therefore, the elevation in glucocorticoid concentrations following acute stress exposure could contribute to the increase in MAO activity. This increase in MAO activity may hypothetically result in decreased monoamine transmission which is one of the pathophysiological mechanisms in several mood disorders.
The role of glucocorticoids, CYP, and MAO in oxidative tissue damage
It is well established that stress conditions increase the concentration of free radicals, and that enzymes of the CYP and MAO families generate reactive oxygen species (Benedetti Strolin & Tipton, Citation1999). It is therefore possible that increases in free radicals during stress are mediated by mechanisms involving changes in CYP and MAO. Treatment with both the GR blocker mifepristone (RU486) and the MAO type B (MAO-B) inhibitor selegilin diminished stress-induced production of reactive oxygen species, as measured by increased lipid peroxidation in the rat brain cortex (Tseilikman et al., Citation2015). Earlier studies showed that both MAO-B (Tazik et al., Citation2009) and MAO-A inhibitors reversed dexamethasone-induced apoptosis in cultivated human neuroblastoma cells (Johnson et al., Citation2010).
It is therefore most likely that stress-induced generation of reactive oxygen species by MAO-A and MAO-B is involved in the pathophysiology of certain neurodegenerative disorders, such as Parkinson disease (Goldstein, Jinsmaa, Sullivan, Holmes, Kopin, & Sharabi, Citation2016a, Citation2016b; Goldstein & Lieberman, Citation1992 ). In addition, apoptotic and neurodegenerative processes induced by oxidative tissue damage have also been reported in depression (Leonard & Maes, Citation2012; Leonard, Citation2017) and PTSD (Miller & Sadeh, Citation2014; Schumacher et al., Citation2015). As stated earlier, glucocorticoid-induced stimulation of CYP and MAO expression and activity can lead to increased concentration of reactive oxygen species, and these may contribute to the neurodegenerative and apoptotic processes observed in several stress-related disorders. CYP- and MAO-induced oxidative tissue damage is also likely to activate inflammatory cytokines including TNF-α, IL-1β, and IL-6 (Al Ghamdi et al., Citation2015; Megha et al., Citation2015; Tuorkey, Abdul-Aziz, & Zidan, Citation2013). It is well known that increased inflammatory cytokine levels activate the HPA axis (Serrats, Grigoleit, Alvarez-Salas, & Sawchenko, Citation2017) and down-regulate CYP expression (Morgan, Citation2001). This cytokine-induced inhibition of CYP suppression has a function in stress-related liver injury (Tseilikman et al., Citation2012), and more recently, the cytokine-induced activation of the HPA axis is reported to be blunted in both depression (Niemegeers et al., Citation2016) and panic disorder (Quagliato & Nardi, Citation2018).
Brain-liver axis: mechanisms involved
There are three potential mechanisms that enable monoamines to regulate hepatic glucocorticoid metabolism: (1) monoaminergic innervation of the cell bodies of sympathetic and parasympathetic nerves innervating the liver; (Jensen, Llewellyn-Smith, Pilowsky, Minson, & Chalmers, Citation1995), (2) direct monoaminergic innervation of the liver (Ruddell, Mann, & Ramm, Citation2008), and (3) neuroendocrine pathways connecting the brain and liver (Bromek, Wojcikowski, & Daniel, Citation2013; Rysz, Bromek, & Daniel, Citation2016).
Brain 5-HT neurotransmission and hepatic CYP activity have mutual influence. The selective lesion of 5-HT neurons or inhibition of 5-HT synthesis leads to robust activation of hepatic CYP (Kot & Daniel, Citation2011), and administration of the 5-hydroxytryptophan 5-HT precursor to the lateral cerebral ventricle has the opposite effect (Rysz et al., Citation2016). It can therefore be suggested that the functional deficit in 5-HT neurotransmission characteristic in depression and other stress-related disorders is associated with altered expression of CYP isoforms. This likelihood is supported by brain 5-HT neuron lesions leading to CYP3A over-expression (Kot & Daniel, Citation2011). Accordingly, the Flinder sensitive line (FSL) rats which serve as an animal model of depression are characterized by decreased extracellular monoamine levels (Dremencov, Weizmann, Kinor, Gispan-Herman, & Yadid, Citation2006; Dremencov et al., Citation2004; Citation2005) and also by increased hepatic CYP3A1 activity (Kotsovolou et al., Citation2010). Moreover, high hepatic CYP activity was found to be associated with high brain MAO-A activity and low plasma 5-HT levels (Tseilikman et al., Citation2016). Herein, highlights the neural and endocrine pathways modulating the interaction between brain monoamines and hepatic CYP, 11βHSD1, and MAO.
Figure 2. Regulation of hepatic glucocorticoid-metabolism enzymes by central monoamines. Dopamine (DA) neurons of the ventral tegmental area and serotonin (5-HT) neurons of the raphe nuclei innervate the dorsal nucleus of the vagus nerve. DA inhibits, and 5-HT-stimulates cholinergic (ACH) vagus efferents projecting to the liver, via dopamine-2 (D2) and serotonin-3 (5-HT3) receptors, respectively. Vagal ACH in turn stimulates hepatic 11-β-hydroxysteroid dehydrogenase type 1 (11βHSD1), most likely, via the nicotine acetylcholine receptors (NAChR). Serotonin also positively regulates somatostatin (ST) and growth hormone-releasing hormone (GHRH) secretion from the paraventricular (PVN) and arcuate (ARN) nuclei of hypothalamus, respectively. Norepinephrine (NE), originating from the locus coeruleus, induces opposite effects on the ST and GHRH. ST and GHRH are released into the hypophysial portal blood and these balance growth hormone (GH) secretion from the pituitary. GH is released into the general circulation and arrives in liver where it positively modulates 11βHSD1 and cytochrome P450 (CYP). Serotonin and NE projections incorporated in the parasympathetic and sympathetic efferents may also directly modulate hepatic CYP and monoamine oxidase (MAO). The serotonin-2A (5-HT2A) receptors may be involved in direct 5-HT regulation of CYP and MAO activity.
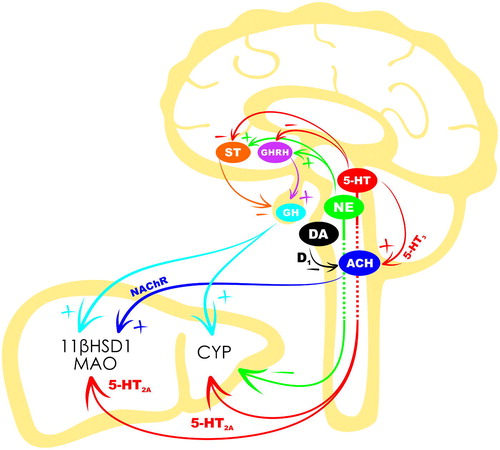
Liver is excessively innervated, and CNS-liver projections may therefore be involved in the central control of hepatic glucocorticoid metabolism. The liver is innervated by post-ganglionic sympathetic nerves originating in the celiac and superior mesenteric ganglia, and the cell bodies of the pre-ganglionic nerves projecting to these ganglia are in the intermediolateral column of segments T7-T12 in the spinal cord. The cell bodies in the spinal cord receive serotonergic innervation, and 5-HT input may stimulate norepinephrine release from the post-ganglionic sympathetic neurons (Jensen et al., Citation1995). The main type of adrenergic receptor expressed in the liver is the β-adrenergic receptor; particularly the β3-adrenergic receptor subtype (Douris et al., Citation2017; Negres et al., Citation2016). The α-adrenergic receptors, mainly the α1-adrenergic subtype, are also present in the liver (Huan et al., Citation2017). In addition, Daskalopoulos, Malliou, et al. (Citation2012) found that activation of the hepatic adrenergic receptors suppressed CYP expression, and it is therefore possible that 5-HT indirectly regulates hepatic CYP activity by activating the visceral adrenergic pathways that suppress CYP expression.
Hepatic parasympathetic fibers originate in the vagal dorsal motor nucleus (DMV) in the medulla oblongata and from the enteric ganglia located at the hepatic hilus and hilar spaces (Jensen, Alpini, & Glaser, Citation2013; Lautt, Citation2009 ). The DMV receives serotonergic and dopaminergic innervation (Babic & Browning, Citation2014), and Browning & Travagli (Citation2019) provide supporting evidence of their functional role. One example is the authors’ 1999 in vitro result that 5-HT stimulated the activity of DMV neurons projecting to gastrointestinal organs (Browning & Travagli, Citation1999). This stimulatory effect on the gastrointestinal-projecting vagus neurons is most likely mediated by 5-HT3 receptors (Mussa, Sartor, & Verberne, Citation2008; Mussa & Verberne, Citation2008). In contrast, dopamine may inhibit gastrointestinal-projecting vagal neurons; most likely by a D1-mediated mechanism (Zheng & Travagli, Citation2007).
The post-synaptic parasympathetic neurons release acetylcholine, and both muscarinic and nicotinic cholinergic receptors are expressed in the liver (Koo & Liang, Citation1979; Soeda et al., Citation2012); an example here is that exposure to nicotine during gestation resulted in enhanced 11βHSD1 mRNA expression in the fetal liver (Xu et al., Citation2012). It is, therefore, possible that central 5-HT and dopamine regulate hepatic function, including 11βHSD1 activity, by 5-HT3, D1 and cholinergic nicotine receptors.
In addition to its indirect modulation of liver-projecting sympathetic and parasympathetic fibers, 5-HT has a direct regulatory influence on hepatic functions. The 5-HT fibers are incorporated in both sympathetic and parasympathetic hepatic nerves. Some hepatocytes express 5-HT2A receptors, 5-HT transporters (SERT), and MAO; and the 5-HT2A receptors there could be involved in mitochondrial toxicity and oxidative tissue damage (Ruddell et al., Citation2008).
Brain and liver also communicate through neuroendocrine mechanisms, GH has a major function in brain monoamine and hepatic CYP interaction. GH positively regulates CYP2C11 expression in the liver (Bromek et al., Citation2013; Rysz, Bromek, Haduch, et al., Citation2016), and its secretion from the pituitary is controlled by the hypothalamic inhibitory somatostatin peptide and the stimulatory growth hormone-releasing hormone (GHRH). While norepinephrine can activate the release of hypothalamic regulatory peptides with subsequent modulation of GH and GH-dependent CYP2C11 expression in the liver (Bromek et al., Citation2013), 5-HT in contrast, can either activate or inhibit this cascade (Rysz et al., Citation2016).
GH influence on liver enzymes can be important under stress conditions and its activated release in response to different stress stimuli is well known. There is an adaptation mechanism in the GH response to some types of stressors which involves the central dopamine system (Jezova, Radikova, & Vigas, Citation2007), and although this response to certain stressors was blunted in patients with panic disorder compared to healthy subjects (Jezova et al., Citation2010), direct evidence for GH’s role in the stress-induced down-regulation of CYP expression has only been established in fish species (Saera-Vila, Calduch-Giner, Prunet, & Perez-Sanchez, Citation2009). Further studies are therefore required to confirm its function in stress-induced regulation of CYP in mammals.
Interaction between brain monoamine transmission and liver CYP activity is reciprocal. Complementary to central monoamine regulation of hepatic CYP, the hepatic CYP activity can influence brain monoamine neuron excitability. We have recently found that CYP inhibition by proadifen hydrochloride (SKF525) resulted in inhibition of spontaneous firing of 5-HT neurons in the rat dorsal raphe nucleus (Grinchii, Paliokha, Tseilikman, & Dremencov, Citation2018).
Central mechanisms involved in the modulation of CYP activity by antidepressants
Although antidepressant drugs were primarily designated for treatment of depression, they have also demonstrated efficacy in the treatment of other stress-related disorders, such as generalized anxiety disorder (GAD), social anxiety disorder (SAD), and PTSD (Williams, Stein, & Ipser, Citation2018). The stress-induced increase in glucocorticoid levels activates MAO (Du et al., Citation2009) and this leads to decreased 5-HT levels which may subsequently stimulate CYP (Rysz et al., Citation2016). However, antidepressant drugs which stimulate central monoamine transmission may have opposite brain-mediated effect on hepatic CYP.
Liver CYP is the major metabolizer of antidepressants. As CYP substrates, antidepressants affect the activity of this enzyme, and the direct effects of different antidepressant drugs on the activity of hepatic CYP sub-types have been described in numerous experimental studies summarized in recent reviews by researchers including Ornoy & Koren (Citation2018) and Nassan et al. (Citation2016). However, at least some antidepressant drugs can affect hepatic CYP expression and activity by CNS-mediated mechanisms. Since CYP inhibition attenuates 5-HT neurotransmission (Grinchii et al., Citation2018), the inhibition of this enzyme by antidepressants could interfere with the primary therapeutic effect of these drugs on 5-HT transmission.
To the best of our knowledge, the effect of intracerebral administration of antidepressant drugs on hepatic CYP expression and activity has not yet been directly investigated. However, it has been reported that 5-hydroxytryptophan injection into the lateral cerebral ventricle increased brain 5-HT concentrations and diminished the activity of CYP1A, CYP2A, CYP2B, CYP2C11, and CYP3A and reduced the mRNA levels of CYP1A2, CYP2A2, CYP2C11, CYP3A1, and CYP3A2 in the liver (Rysz et al., Citation2016). It is, therefore, possible that intra-cerebroventricular administration of selective serotonin reuptake inhibitors (SSRIs) has a similar effect on liver CYP. Future direct experimental studies should test this hypothesis.
Other types of antidepressant drugs which do not directly enhance brain monoamine levels may act directly on the hepatic CYP without central mechanism involvement. One example here is Haduch et al.’s. (Citation2006) finding that central effects are not involved in mirtazapine-induced increase in hepatic CYP2D activity.
Conclusions
Glucocorticoid-metabolizing enzymes may be at least partly responsible for development of the blunted glucocorticoid response to stress stimuli in patients with some stress-related psychopathologies. Two lines of evidence support this hypothesis. First, repeated stress-induced elevation in glucocorticoid levels results in activation of 11βHSD1 and inhibition of 11βHSD2, and this latter 11βHSD2 suppression can lead to apparent mineralocorticoid excess. Second, elevated glucocorticoid concentrations induced by stressors stimulates the synthesis and expression of CYP and MAO. herein highlights that increased CYP expression and activity can lead to subsequent decrease in glucocorticoid concentrations due to their increased metabolism.
Acknowledgments
We thank Mr Raymond Marshall for the English language editing and Ms Katsiaryna Shabatsenka for the graphical design of the illustrations.
Disclosure statement
Authors declare no conflict of interest and no financial interest in the publication of this manuscript.
Additional information
Funding
Notes on contributors
Vadim Tseilikman
Vadim Tseilikman, MSc (1982, Chelyabinsk State Pedagogical University, Chelyabinsk Russia), PhD (1992, Siberian State Medical University, Tomsk, Russia), DSc (1999, Institute of General Pathology and Pathological Physiology, Moscow, Russia), was a Head of Biochemical Department of the South Ural State Medical University (2005-2016, Chelyabinsk) and he is currently a Director of School of Medical Biology of the South Ural State University (2017-). His research is focusing in brain-liver interactions in stress;
Eliyahu Dremencov
Eliyahu Dremencov, MMedSc (Hebrew University, Jerusalem, Israel, 2000), PhD (Bar-Ilan University, Israel, 2004) is an Independent Research Fellow in the Institute of Molecular Physiology and Genetics (IMPG), Centre for Biosciences, and Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Science (since 2013), Head of the IMPG Neuropharmacological Laboratory (2016-), Head of the IMPG (2018-), and a Head of the International Neurohepathological Laboratory in the School of Medical Biology, South Ural State University, Chelyabinsk, Russia (2017-);
Olga Tseilikman
Olga Tseilikman, PhD (2001, South Ural State Medical University, Chelyabinsk, Russia), DSc (2005, Omsk State Medical University, Omsk, Russia), is a Professor in the School of Medical Biology, South Ural State University, Chelyabinsk, Russia. Her research is focusing in brain-liver interactions in stress;
Michaela Pavlovicova
Michaela Pavlovicova, PhD (Comenius University in Bratislava, Slovakia, 20013) was a Research Fellow (2003-2015) and an Independent Research Fellow (2015-2016) in the Institute of Molecular Physiology and Genetics (IMPG), Centre for Biosciences, Slovak Academy of Sciences, Bratislava, Slovakia;
Lubica Lacinova
Lubica Lacinova, PhD (1989), DSc (2004), Professor (2018), is a Leading Research Fellow in the Institute of Molecular Physiology and Genetics, Centre for Biosciences, Slovak Academy of Sciences, Bratislava, Slovakia, and Professor of Biophysics at University of Saints Cyril and Methodius, Trnava, Slovakia;
Daniela Jezova
Prof. Daniela Jezova, PharmD is a full professor of Pharmacology at the Faculty of Medicine of Comenius University and the Head of the Laboratory of Pharmacological Neuroendocrinology being a part of the Department of Endocrine Regulations and Psychopharmacology of the Institute of Experimental Endocrinology, Biomedical Research Center, Bratislava, Slovakia. She has been a vice-president of the Slovak Academy of Sciences and vice-president of All European Academies. She is serving as an international expert in European research networks including the panel of European Research Council (ERC) on Endocrinology, Pathophysiology and Physiology. Her research team focused on stress research and psychopharmacology in both humans and animals belongs to the evaluated top research teams in Slovakia.
References
- Abrahams, L., Semjonous, N. M., Guest, P., Zielinska, A., Hughes, B., Lavery, G. G., & Stewart, P.M. (2012). Biomarkers of hypothalamic-pituitary-adrenal axis activity in mice lacking 11beta-HSD1 and H6PDH. Journal of Endocrinology, 214, 367–372. doi:10.1530/JOE-12-0178
- Al Ghamdi, A. A., Badr, G., Hozzein, W. N., Allam, A., Al-Waili, N. S., Al-Wadaan, M. A., & Garraud, O. (2015). Oral supplementation of diabetic mice with propolis restores the proliferation capacity and chemotaxis of B and T lymphocytes towards CCL21 and CXCL12 by modulating the lipid profile, the pro-inflammatory cytokine levels and oxidative stress. BMC Immunology, 16, 54. doi:10.1186/s12865-015-0117-9
- Babic, T., & Browning, K.N. (2014). The role of vagal neurocircuits in the regulation of nausea and vomiting. European Journal of Pharmacology, 722, 38–47. doi:10.1016/j.ejphar.2013.08.047
- Basu, R., Singh, R. J., Basu, A., Chittilapilly, E. G., Johnson, C. M., Toffolo, G., … Rizza, R.A. (2004). Splanchnic cortisol production occurs in humans: Evidence for conversion of cortisone to cortisol via the 11-beta hydroxysteroid dehydrogenase (11beta-hsd) type 1 pathway. Diabetes, 53, 2051–2059. doi:10.2337/diabetes.53.8.2051
- Benedetti Strolin, M., & Tipton, K.F. (1999). Involvement of monooxygenases and amine oxidases in hydroxyl radical generation in vivo. Neurobiology (Bp), 7, 123–134.
- Boero, G., Pisu, M. G., Biggio, F., Muredda, L., Carta, G., Banni, S., … Serra, M. (2018). Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology, 133, 242–253. doi:10.1016/j.neuropharm.2018.01.045
- Bromek, E., Wojcikowski, J., & Daniel, W.A. (2013). Involvement of the paraventricular (PVN) and arcuate (ARC) nuclei of the hypothalamus in the central noradrenergic regulation of liver cytochrome P450. Biochemical Pharmacology, 86, 1614–1620. doi:10.1016/j.bcp.2013.09.006
- Brooks, S., Amlot, R., Rubin, G. J., & Greenberg, N. (2018). Psychological resilience and post-traumatic growth in disaster-exposed organisations: Overview of the literature. Journal of the Royal Army Medical Corps. doi:10.1136/jramc-2017-000876
- Browning, K. N., & Travagli, R.A. (1999). Characterization of the in vitro effects of 5-hydroxytryptamine (5-HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV). British Journal of Pharmacology, 128, 1307–1315. doi:10.1038/sj.bjp.0702908
- Browning, K. N., & Travagli, R.A. (2019). Central control of gastrointestinal motility. Current Opinion in Journal of Endocrinology, Diabetes & Obesity, 26, 11–16. doi:10.1097/MED.0000000000000449
- Buske-Kirschbaum, A., Ebrecht, M., & Hellhammer, D.H. (2010). Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain, Behavior, and Immunity, 24, 1347–1353. doi:10.1016/j.bbi.2010.06.013
- Chapman, K., Holmes, M., & Seckl, J. (2013). 11beta-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiological Reviews, 93, 1139–1206. doi:10.1152/physrev.00020.2012
- Checkley, S. (1992). Neuroendocrine mechanisms and the precipitation of depression by life events. British Journal of Psychiatry, 160, 7–17. doi:10.1192/S0007125000296633
- Cooper, B. W., Cho, T. M., Thompson, P. M., & Wallace, A.D. (2008). Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicological Sciences, 103, 268–277. doi:10.1093/toxsci/kfn047
- Daskalopoulos, E. P., Lang, M. A., Marselos, M., Malliou, F., & Konstandi, M. (2012). D(2)-dopaminergic receptor-linked pathways: Critical regulators of CYP3A, CYP2C, and CYP2D. Molecular Pharmacology, 82, 668–678. doi:10.1124/mol.112.078709
- Daskalopoulos, E. P., Malliou, F., Rentesi, G., Marselos, M., Lang, M. A., & Konstandi, M. (2012). Stress is a critical player in CYP3A, CYP2C, and CYP2D regulation: Role of adrenergic receptor signaling pathways. American Journal of Physiology-Endocrinology and Metabolism, 303, E40–54. doi:10.1152/ajpendo.00545.2011
- Davidson, J., Lipper, S., Kilts, C. D., Mahorney, S., & Hammett, E. (1985). Platelet MAO activity in posttraumatic stress disorder. The American Journal of Psychiatry, 142, 1341–1343.doi:10.1176/ajp.142.11.1341
- de Kloet, E. R., Meijer, O. C., de Nicola, A. F., de Rijk, R. H., & Joels, M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Frontiers in Neuroendocrinology, 49, 124–145. doi:10.1016/j.yfrne.2018.02.003
- de Kloet, E. R., Reul, J. M., & Sutanto, W. (1990). Corticosteroids and the brain. The Journal of Steroid Biochemistry and Molecular Biology, 37, 387–394. doi:10.1016/0960-0760(90)90489-8
- Douris, N., Desai, B. N., Fisher, F. M., Cisu, T., Fowler, A. J., Zarebidaki, E., … Maratos-Flier, E. (2017). Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Molecular Metabolism, 6, 854–862. doi:10.1016/j.molmet.2017.05.017
- Draper, N., & Stewart, P.M. (2005). 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. Journal of Endocrinology, 186, 251–271. doi:10.1677/joe.1.06019
- Dremencov, E., Gispan-Herman, I., Rosenstein, M., Mendelman, A., Overstreet, D. H., Zohar, J., & Yadid, G. (2004). The serotonin-dopamine interaction is critical for fast-onset action of antidepressant treatment: In vivo studies in an animal model of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 28, 141–147. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14687868 doi:10.1016/j.pnpbp.2003.09.030
- Dremencov, E., Newman, M. E., Kinor, N., Blatman-Jan, G., Schindler, C. J., Overstreet, D. H., & Yadid, G. (2005). Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology, 48, 34–42. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15617725 doi:10.1016/j.neuropharm.2004.09.013
- Dremencov, E., Weizmann, Y., Kinor, N., Gispan-Herman, I., & Yadid, G. (2006). Modulation of dopamine transmission by 5HT2C and 5HT3 receptors: A role in the antidepressant response. Current Drug Targets, 7, 165–175. doi:10.2174/138945006775515491 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16475958
- Du, J., Wang, Y., Hunter, R., Wei, Y., Blumenthal, R., Falke, C., … Manji, H.K. (2009). Dynamic regulation of mitochondrial function by glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America, 106, 3543–3548. doi:10.1073/pnas.0812671106
- Duncko, R., Makatsori, A., Fickova, E., Selko, D., & Jezova, D. (2006). Altered coordination of the neuroendocrine response during psychosocial stress in subjects with high trait anxiety. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 30, 1058–1066. doi:10.1016/j.pnpbp.2006.04.002
- Dvorak, Z., & Pavek, P. (2010). Regulation of drug-metabolizing cytochrome P450 enzymes by glucocorticoids. Drug Metabolism Reviews, 42, 621–635. doi:10.3109/03602532.2010.484462
- Edwards, C. R., Stewart, P. M., Burt, D., Brett, L., McIntyre, M. A., Sutanto, W. S., … Monder, C. (1988). Localisation of 11 beta-hydroxysteroid dehydrogenase-tissue specific protector of the mineralocorticoid receptor. Lancet, 2, 986–989. doi:10.1016/S0140-6736(88)90742-8
- Ekstedt, B., & Oreland, L. (1976). Effect of lipid-depletion on the different forms of monoamine oxidase in rat liver mitochondria. Biochemical Pharmacology, 25, 119–124. doi:10.1016/0006-2952(76)90277-X
- Evans, L. C., Ivy, J. R., Wyrwoll, C., McNairn, J. A., Menzies, R. I., Christensen, T. H., … Bailey, M.A. (2016). Conditional deletion of Hsd11b2 in the brain causes salt appetite and hypertension. Circulation, 133, 1360–1370. doi:10.1161/CIRCULATIONAHA.115.019341
- Fenouillet, E., Vigouroux, A., Steinberg, J. G., Chagvardieff, A., Retornaz, F., Guieu, R., & Jammes, Y. (2016). Association of biomarkers with health-related quality of life and history of stressors in myalgic encephalomyelitis/chronic fatigue syndrome patients. Journal of Translational Medicine, 14, 251. doi:10.1186/s12967-016-1010-x
- Ferrari, P. (2010). The role of 11beta-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochimica et Biophysica Acta, 1802, 1178–1187. doi:10.1016/j.bbadis.2009.10.017
- Funder, J. W., Pearce, P. T., Smith, R., & Smith, A.I. (1988). Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science, 242, 583–585. doi:10.1126/science.2845584
- Gasparini, S., Melo, M. R., Andrade-Franze, G.M.F., Geerling, J. C., Menani, J. V., & Colombari, E. (2018). Aldosterone infusion into the 4th ventricle produces sodium appetite with baroreflex attenuation independent of renal or blood pressure changes. Brain Research, 1698, 70–80. doi:10.1016/j.brainres.2018.06.023
- Gasparini, S., Resch, J. M., Narayan, S. V., Peltekian, L., Iverson, G. N., Karthik, S., & Geerling, J.C. (2019). Aldosterone-sensitive HSD2 neurons in mice. Brain Structure and Function, 224, 387–417. doi:10.1007/s00429-018-1778-y
- Geerling, J. C., & Loewy, A.D. (2007). Sodium depletion activates the aldosterone-sensitive neurons in the NTS independently of thirst. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292, R1338–R1348. doi:10.1152/ajpregu.00391.2006
- Geerling, J. C., & Loewy, A.D. (2008). Central regulation of sodium appetite. Experimental Physiology, 93, 177–209. doi:10.1113/expphysiol.2007.039891
- Goldstein, D. S., Jinsmaa, Y., Sullivan, P., Holmes, C., Kopin, I. J., & Sharabi, Y. (2016a). 3,4-Dihydroxyphenylethanol (hydroxytyrosol) mitigates the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochemical Research, 41, 2173–2178. doi:10.1007/s11064-016-1959-0
- Goldstein, D. S., Jinsmaa, Y., Sullivan, P., Holmes, C., Kopin, I. J., & Sharabi, Y. (2016b). Comparison of monoamine oxidase inhibitors in decreasing production of the autotoxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells. Journal of Pharmacology and Experimental Therapeutics, 356, 483–492. doi:10.1124/jpet.115.230201
- Goldstein, M., & Lieberman, A. (1992). The role of the regulatory enzymes of catecholamine synthesis in Parkinson’s disease. Neurology, 42(4), 8–12.
- Granner, D. K., Wang, J. C., & Yamamoto, K.R. (2015). Regulatory actions of glucocorticoid hormones: From organisms to mechanisms. Advances in Experimental Medicine and Biology, 872, 3–31.doi:10.1007/978-1-4939-2895-8_1
- Grinchii, D., Paliokha, R., Tseilikman, V., & Dremencov, E. (2018). Inhibition of cytochrome P450 with proadifen diminishes the excitability of brain serotonin neurons. General Physiology and Biophysics, 37, 711–713. doi:10.4149/gpb_2018040
- Grunewald, M., Johnson, S., Lu, D., Wang, Z., Lomberk, G., Albert, P. R., … Ou, X.-M. (2012). Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. Journal of Biological Chemistry, 287, 24195–24206. doi:10.1074/jbc.M112.373936
- Haduch, A., Bromek, E., Kot, M., Jemnitz, K., Veres, Z., Vereczkey, L., & Daniel, W.A. (2006). Effect of mirtazapine on the CYP2D activity in the primary culture of rat hepatocytes. Pharmacological Reports: Pr, 58, 979–984.
- Harkitis, P., Daskalopoulos, E. P., Malliou, F., Lang, M. A., Marselos, M., Fotopoulos, A., … Konstandi, M. (2015). Dopamine D2-receptor antagonists down-regulate CYP1A1/2 and CYP1B1 in the rat liver. PLoS One, 10, e0128708. doi:10.1371/journal.pone.0128708
- Harris, S., Johnson, S., Duncan, J. W., Udemgba, C., Meyer, J. H., Albert, P. R., … Wang, J.M. (2015). Evidence revealing deregulation of the KLF11-MAO A pathway in association with chronic stress and depressive disorders. Neuropsychopharmacology, 40, 1373–1382. doi:10.1038/npp.2014.321
- Harris, H. J., Kotelevtsev, Y., Mullins, J. J., Seckl, J. R., & Holmes, M.C. (2001). Intracellular regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase (11beta-HSD)-1 plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: Analysis of 11beta-HSD-1-deficient mice. Endocrinology, 142, 114–120. doi:10.1210/endo.142.1.7887
- Hlavacova, N., Solarikova, P., Marko, M., Brezina, I., & Jezova, D. (2017). Blunted cortisol response to psychosocial stress in atopic patients is associated with decrease in salivary alpha-amylase and aldosterone: Focus on sex and menstrual cycle phase. Psychoneuroendocrinology, 78, 31–38. doi:10.1016/j.psyneuen.2017.01.007
- Holsboer, F., Wiedemann, K., & Boll, E. (1986). Shortened dexamethasone half-life in depressed dexamethasone nonsuppressors. Archives of General Psychiatry, 43, 813–815. doi:10.1001/archpsyc.1986.01800080099014
- Huan, H. B., Wen, X. D., Chen, X. J., Wu, L., Wu, L. L., Zhang, L., … Xia, F. (2017). Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain, Behavior, and Immunity, 59, 118–134. doi:10.1016/j.bbi.2016.08.016
- Jensen, K. J., Alpini, G., & Glaser, S. (2013). Hepatic nervous system and neurobiology of the liver. Comprehensive Physiology, 3, 655–665.doi:10.1002/cphy.c120018
- Jensen, I., Llewellyn-Smith, I. J., Pilowsky, P., Minson, J. B., & Chalmers, J. (1995). Serotonin inputs to rabbit sympathetic preganglionic neurons projecting to the superior cervical ganglion or adrenal medulla. The Journal of Comparative Neurology, 353, 427–438. doi:10.1002/cne.903530310
- Jezova, D., & Herman, J.P. (2016). Lessons from regular gathering of experts in stress research: Focus on pathophysiological consequences of stress exposure. Stress, 19, 339–340. doi:10.1080/10253890.2016.1213515
- Jezova, D., & Hlavacova, N. (2008). Endocrine factors in stress and psychiatric disorders: Focus on anxiety and salivary steroids. Annals of the New York Academy of Sciences, 1148, 495–503. doi:10.1196/annals.1410.050
- Jezova, D., Jurankova, E., Mosnarova, A., Kriska, M., & Skultetyova, I. (1996). Neuroendocrine response during stress with relation to gender differences. Acta Neurobiologiae Experimentalis, 56, 779–785.
- Jezova, D., Kvetnansky, R., Kovacs, K., Oprsalova, Z., Vigas, M., & Makara, G.B. (1987). Insulin-induced hypoglycemia activates the release of adrenocorticotropin predominantly via central and propranolol insensitive mechanisms. Endocrinology, 120, 409–415. doi:10.1210/endo-120-1-409
- Jezova, D., Makatsori, A., Duncko, R., Moncek, F., & Jakubek, M. (2004). High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 28, 1331–1336. doi:10.1016/j.pnpbp.2004.08.005
- Jezova, D., Radikova, Z., & Vigas, M. (2007). Growth hormone response to different consecutive stress stimuli in healthy men: Is there any difference? Stress, 10, 205–211. doi:10.1080/10253890701292168
- Jezova, D., Vigas, M., Hlavacova, N., & Kukumberg, P. (2010). Attenuated neuroendocrine response to hypoglycemic stress in patients with panic disorder. Neuroendocrinology, 92, 112–119. doi:10.1159/000283560
- Johnson, S., Tazik, S., Lu, D., Johnson, C., Youdim, M. B., Wang, J., … Ou, X.M. (2010). The new inhibitor of monoamine oxidase, M30, has a neuroprotective effect against dexamethasone-induced brain cell apoptosis. Frontiers in Neuroscience, 4, 180. doi:10.3389/fnins.2010.00180
- Kang, J. I., Kim, T. Y., Choi, J. H., So, H. S., & Kim, S.J. (2019). Allele-specific DNA methylation level of FKBP5 is associated with post-traumatic stress disorder. Psychoneuroendocrinology, 103, 1–7. doi:10.1016/j.psyneuen.2018.12.226
- Kishida, T., Muto, S., Hayashi, M., Tsutsui, M., Tanaka, S., Murakami, M., & Kuroda, J. (2008). Strain differences in hepatic cytochrome P450 1A and 3A expression between Sprague-Dawley and Wistar rats. The Journal of Toxicological Sciences, 33, 447–457. doi:10.2131/jts.33.447
- Kocarek, T. A., Schuetz, E. G., Strom, S. C., Fisher, R. A., & Guzelian, P.S. (1995). Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metabolism and Disposition, 23, 415–421.
- Kondashevskaya, M. V., Tseilikman, V. E., Manukhina, E. B., Downey, H. F., Komelkova, M. V., Lapshin, M. S., … Tseilikman, O.B. (2017). Disorder in the morphology and function of adrenal glands in experimental post-traumatic stress disorder in rats: Correlation with behavioral markers. Russian Journal of Physiology, 103, 808–818.
- Koo, A., & Liang, I.Y. (1979). Stimulation and blockade of cholinergic receptors in terminal liver microcirculation in rats. American Journal of Physiology, 236, E728–732. doi:10.1152/ajpendo.1979.236.6.E728
- Kot, M., & Daniel, W.A. (2011). Cytochrome P450 is regulated by noradrenergic and serotonergic systems. Pharmacological Research, 64, 371–380. doi:10.1016/j.phrs.2011.06.020
- Kot, M., Sadakierska-Chudy, A., Haduch, A., Rysz, M., Bromek, E., Gołembiowska, K., & Daniel, W.A. (2015). The role of the dorsal noradrenergic pathway of the brain (locus coeruleus) in the regulation of liver cytochrome P450 activity. European Journal of Pharmacology, 751, 34–41. doi:10.1016/j.ejphar.2015.01.014
- Kotsovolou, O., Ingelman-Sundberg, M., Lang, M. A., Marselos, M., Overstreet, D. H., Papadopoulou-Daifoti, Z., … Konstandi, M. (2010). Hepatic drug metabolizing profile of Flinders Sensitive Line rat model of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 34, 1075–1084. doi:10.1016/j.pnpbp.2010.05.029
- Kozochkin, D. A., Manukhina, E. B., Downey, H. F., Tseilikman, O. B., Komelkova, M. V., Vasilyeva, M. V., … Tseilikman, V.E. (2017). The role of microsomal oxidation in the regulation of monoamine oxidase activity in the brain and liver of rats. General Physiology and Biophysics, 36, 455–464. doi:10.4149/gpb_2017012
- Lautt, W.W. (2009). Colloquium series on integrated systems physiology: From molecule to function to disease hepatic circulation: Physiology and pathophysiology. San Rafael (CA): Morgan & Claypool Life Sciences.
- Lazuko, S. S., Kuzhel, O. P., Belyaeva, L. E., Manukhina, E. B., Fred Downey, H., Tseilikman, O. B., … Tseilikman, V.E. (2018). Posttraumatic stress disorder disturbs coronary tone and its regulatory mechanisms. Cellular and Molecular Neurobiology, 38, 209–217. doi:10.1007/s10571-017-0517-x
- Leistner, C., & Menke, A. (2018). How to measure glucocorticoid receptor’s sensitivity in patients with stress-related psychiatric disorders. Psychoneuroendocrinology, 91, 235. doi:10.1016/j.psyneuen.2018.01.023
- Leonard, B.E. (2017). Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatrica, 30, 1–16. doi:10.1017/neu.2016.69
- Leonard, B., & Maes, M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience & Biobehavioral Reviews, 36, 764–785. doi:10.1016/j.neubiorev.2011.12.005
- Li, X. Y., Zhang, C., Wang, H., Ji, Y. L., Wang, S. F., Zhao, L., … Xu, D.X. (2008). Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced downregulation of CYP3A in fetal liver: Its repression by a low dose LPS pretreatment. Toxicology Letters, 179, 71–77. doi:10.1016/j.toxlet.2008.04.005
- Lopes, R. P., Grassi-Oliveira, R., de Almeida, L. R., Stein, L. M., Luz, C., Teixeira, A. L., & Bauer, M.E. (2012). Neuroimmunoendocrine interactions in patients with recurrent major depression, increased early life stress and long-standing posttraumatic stress disorder symptoms. Neuroimmunomodulation, 19, 33–42. doi:10.1159/000327352
- Makara, G. B., Kvetnansky, R., Jezova, D., Jindra, A., Kakucska, I., & Oprsalova, Z. (1986). Plasma catecholamines do not participate in pituitary-adrenal activation by immobilization stress in rats with transection of nerve fibers to the median eminence. Endocrinology, 119, 1757–1762. doi:10.1210/endo-119-4-1757
- Manukhina, E. B., Tseilikman, V. E., Tseilikman, O. B., Komelkova, M. V., Kondashevskaya, M. V., Goryacheva, A. V., … Downey, H.F. (2018). Intermittent hypoxia improves behavioral and adrenal gland dysfunction induced by post-traumatic stress disorder in rats. Journal of Applied Physiology (1985), 125, 931–937. doi:10.1152/japplphysiol.01123.2017
- Matosin, N., Halldorsdottir, T., & Binder, E.B. (2018). Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: The FKBP5 model. Biological Psychiatry, 83, 821–830. doi:10.1016/j.biopsych.2018.01.021
- McEwen, B.S. (2000). Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology, 22, 108–124. doi:10.1016/S0893-133X(99)00129-3
- Megha, K., Deshmukh, P. S., Banerjee, B. D., Tripathi, A. K., Ahmed, R., & Abegaonkar, M.P. (2015). Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. Neurotoxicology, 51, 158–165. doi:10.1016/j.neuro.2015.10.009
- Michailidou, Z., Turban, S., Miller, E., Zou, X., Schrader, J., Ratcliffe, P. J., … Seckl, J.R. (2012). Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11beta-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. Journal of Biological Chemistry, 287, 4188–4197. doi:10.1074/jbc.M111.259325
- Miller, M. W., & Sadeh, N. (2014). Traumatic stress, oxidative stress and post-traumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Molecular Psychiatry, 19, 1156–1162. doi:10.1038/mp.2014.111
- Morgan, E.T. (2001). Regulation of cytochrome p450 by inflammatory mediators: Why and how? Drug Metabolism and Disposition: The Biological Fate of Chemicals, 29, 207–212.
- Munro, A. W., McLean, K. J., Grant, J. L., & Makris, T.M. (2018). Structure and function of the cytochrome P450 peroxygenase enzymes. Biochemical Society Transactions, 46, 183–196. doi:10.1042/BST20170218
- Mussa, B. M., Sartor, D. M., & Verberne, A.J. (2008). Activation of cholecystokinin (CCK 1) and serotonin (5-HT 3) receptors increases the discharge of pancreatic vagal afferents. European Journal of Pharmacology, 601, 198–206. doi:10.1016/j.ejphar.2008.11.007
- Mussa, B. M., & Verberne, A.J. (2008). Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neuroscience Letters, 433, 71–76. doi:10.1016/j.neulet.2007.12.048
- Nassan, M., Nicholson, W. T., Elliott, M. A., Rohrer Vitek, C. R., Black, J. L., & Frye, M.A. (2016). Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: A template for psychiatric precision medicine. Mayo Clinic Proceedings, 91, 897–907. doi:10.1016/j.mayocp.2016.02.023
- Negres, S., Zanfirescu, A., Ionica, F. E., Morosan, E., Velescu, B. S., Seremet, O. C., … ChiriTa, C. (2016). Pharmacotoxicological screening on new derivatives of beta-phenylethylamine, potential agonists of beta3-adrenergic receptors. Romanian Journal of Morphology and Embryology, 57, 969–978.
- Nicholson, T. E., & Renton, K.W. (1999). Modulation of cytochrome P450 by inflammation in astrocytes. Brain Research, 827, 12–18. doi:10.1016/s0006-8993(99)01261-5
- Nicolaides, N., & Chrousos, G. (2018). Bilateral adrenal hyperplasia and NR3C1 mutations causing glucocorticoid resistance: Is there an association? European Journal of Endocrinology, 179, C1–C4. doi:10.1530/eje-18-0471
- Niemegeers, P., De Boer, P., Dumont, G.J.H., Van Den Eede, F., Fransen, E., Claes, S. J., … Sabbe, B.G.C. (2016). Differential effects of inflammatory and psychosocial stress on mood, hypothalamic-pituitary-adrenal axis, and inflammation in remitted depression. Neuropsychobiology, 74, 150–158. doi:10.1159/000466698
- Nikzad, S., Vafaei, A. A., Rashidy-Pour, A., & Haghighi, S. (2011). Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress, 14, 459–464. doi:10.3109/10253890.2010.548171
- Obut, T. A., Ovsyukova, M. V., Dement’eva, T. Y., Cherkasova, O. P., Saryg, S. K., & Grigor’eva, T.A. (2009). Effects of dehydroepiandrosterone sulfate on the conversion of corticosterone into 11-dehydrocorticosterone in stress: A regulatory scheme. Neuroscience and Behavioral Physiology, 39, 695–699. doi:10.1007/s11055-009-9179-6
- Oitzl, M. S., & de Kloet, E.R. (1992). Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience, 106, 62–71. doi:10.1037//0735-7044.106.1.62
- Ornoy, A., & Koren, G. (2018). Selective serotonin reuptake inhibitor use in pregnant women; pharmacogenetics, drug-drug interactions and adverse effects. Expert Opinion on Drug Metabolism & Toxicology, 14, 247–259. doi:10.1080/17425255.2018.1430139
- Pal'chikova, N. A., Kuznetsova, N. V., Selyatitskaya, V. G., Cherkasova, O. P., & Kuz’mina, O.I. (2016). Effects of intraperitoneal administration of mifepristone on glucocorticoid status of experimental animals. Bulletin of Experimental Biology and Medicine, 161, 257–260. doi:10.1007/s10517-016-3390-6
- Peng, C. C., Templeton, I., Thummel, K. E., Davis, C., Kunze, K. L., & Isoherranen, N. (2011). Evaluation of 6beta-hydroxycortisol, 6beta-hydroxycortisone, and a combination of the two as endogenous probes for inhibition of CYP3A4 in vivo. Clinical Pharmacology & Therapeutics, 89, 888–895. doi:10.1038/clpt.2011.53
- Petrowski, K., Herold, U., Joraschky, P., Wittchen, H. U., & Kirschbaum, C. (2010). A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology, 35, 414–421. doi:10.1016/j.psyneuen.2009.08.003
- Pivac, N., Knezevic, J., Kozaric-Kovacic, D., Dezeljin, M., Mustapic, M., Rak, D., … Muck-Seler, D. (2007). Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity in combat-related posttraumatic stress disorder. Journal of Affective Disorders, 103, 131–138. doi:10.1016/j.jad.2007.01.017
- Qian, X., Droste, S. K., Gutièrrez-Mecinas, M., Collins, A., Kersanté, F., Reul, J.M.H.M., & Linthorst, A.C.E. (2011). A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology, 152, 3738–3748. doi:10.1210/en.2011-1008
- Qiu, B., Luczak, S. E., Wall, T. L., Kirchhoff, A. M., Xu, Y., Eng, M. Y., … Liang, T. (2016). The FKBP5 gene affects alcohol drinking in knockout mice and is implicated in alcohol drinking in humans. International Journal of Molecular Sciences, 17, 1271. doi:10.3390/ijms17081271
- Quagliato, L. A., & Nardi, A.E. (2018). Cytokine alterations in panic disorder: A systematic review. Journal of Affective Disorders, 228, 91–96. doi:10.1016/j.jad.2017.11.094
- Rao, S., Yao, Y., Ryan, J., Li, T., Wang, D., Zheng, C., … Xu, Q. (2016). Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: A comprehensive meta-analysis. Scientific Reports, 6, 32687. doi:10.1038/srep32687
- Rendic, S. (2002). Summary of information on human CYP enzymes: Human P450 metabolism data. Drug Metabolism Reviews, 34, 83–448. doi:10.1081/DMR-120001392
- Rosenstock, J., Banarer, S., Fonseca, V. A., Inzucchi, S. E., Sun, W., Yao, W., … Huber, R. (2010). The 11-beta-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care, 33, 1516–1522. doi:10.2337/dc09-2315
- Roy, B., Riley, C., Sinha, R. (2018). Emotion regulation moderates the association between chronic stress and cardiovascular disease risk in humans: A cross-sectional study. Stress, 21, 1548–555. doi:10.1080/10253890.2018.1490724
- Ruddell, R. G., Mann, D. A., & Ramm, G.A. (2008). The function of serotonin within the liver. Journal of Hepatology, 48, 666–675. doi:10.1016/j.jhep.2008.01.006
- Rysz, M., Bromek, E., & Daniel, W.A. (2016). Activation of brain serotonergic system by repeated intracerebral administration of 5-hydroxytryptophan (5-HTP) decreases the expression and activity of liver cytochrome P450. Biochemical Pharmacology, 99, 113–122. doi:10.1016/j.bcp.2015.11.014
- Rysz, M., Bromek, E., Haduch, A., Liskova, B., Wojcikowski, J., & Daniel, W.A. (2016). The reverse role of the hypothalamic paraventricular (PVN) and arcuate (ARC) nuclei in the central serotonergic regulation of the liver cytochrome P450 isoform CYP2C11. Biochemical Pharmacology, 112, 82–89. doi:10.1016/j.bcp.2016.04.017
- Saera-Vila, A., Calduch-Giner, J. A., Prunet, P., & Perez-Sanchez, J. (2009). Dynamics of liver GH/IGF axis and selected stress markers in juvenile gilthead sea bream (Sparus aurata) exposed to acute confinement: Differential stress response of growth hormone receptors. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 154, 197–203. doi:10.1016/j.cbpa.2009.06.004
- Schumacher, M., Guennoun, R., Mattern, C., Oudinet, J. P., Labombarda, F., De Nicola, A. F., & Liere, P. (2015). Analytical challenges for measuring steroid responses to stress, neurodegeneration and injury in the central nervous system. Steroids, 103, 42–57. doi:10.1016/j.steroids.2015.08.013
- Seckl, J. R., Dow, R. C., Low, S. C., Edwards, C. R., & Fink, G. (1993). The 11 beta-hydroxysteroid dehydrogenase inhibitor glycyrrhetinic acid affects corticosteroid feedback regulation of hypothalamic corticotrophin-releasing peptides in rats. Journal of Endocrinology, 136, 471–477. doi:10.1677/joe.0.1360471
- Selye, H. (1973). The evolution of the stress concept. American Scientist, 61, 692–699.
- Serrats, J., Grigoleit, J. S., Alvarez-Salas, E., & Sawchenko, P.E. (2017). Pro-inflammatory immune-to-brain signaling is involved in neuroendocrine responses to acute emotional stress. Brain, Behavior, and Immunity, 62, 53–63. doi:10.1016/j.bbi.2017.02.003
- Simas, B. B., Nunes, E. A., Crestani, C. C., & Speretta, G.F. (2018). Cardiovascular and metabolic consequences of the association between chronic stress and high-fat diet in rats. Stress, 21, 247–256. doi:10.1080/10253890.2018.1437413
- Singer, T.P. (1995). The colorful past and bright future of monoamine oxidase research. Progress in Brain Research, 106, 1–22.
- Smith, M. A., Davidson, J., Ritchie, J. C., Kudler, H., Lipper, S., Chappell, P., & Nemeroff, C.B. (1989). The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biological Psychiatry, 26, 349–355. doi:10.1016/0006-3223(89)90050-4
- Soeda, J., Morgan, M., McKee, C., Mouralidarane, A., Lin, C., Roskams, T., & Oben, J.A. (2012). Nicotine induces fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on hepatic stellate cells. Biochemical and Biophysical Research Communications, 417, 17–22. doi:10.1016/j.bbrc.2011.10.151
- Stamou, M., Wu, X., Kania-Korwel, I., Lehmler, H. J., & Lein, P.J. (2013). Cytochrome p450 mRNA expression in the rodent brain: Species-, sex-, and region-dependent differences. Drug Metabolism and Disposition, 42, 239–244. doi:10.1124/dmd.113.054239
- Sterling, P., & Eyer, J. (1988). Allostasis: A new paradigm to explain arousal pathology. In S. Fisher & J. Reason (Eds.), Handbook of life stress, cognition and health (pp. 629–640). New York, NY: John Wiley & Sons, Inc.
- Stokes, P. E., Sikes, C., Lasley, B., & Stoll, P. (2002). HPA hyperactivity with increased plasma cortisol affects dexamethasone metabolism and DST outcome. Journal of Psychiatric Research, 36, 417–421. doi:10.1016/S0022-3956(02)00059-6
- Strohle, A., Scheel, M., Modell, S., & Holsboer, F. (2008). Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. Journal of Psychiatric Research, 42, 1185–1188. doi:10.1016/j.jpsychires.2008.01.015
- Tafet, G. E., & Nemeroff, C.B. (2016). The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. The Journal of Neuropsychiatry and Clinical Neurosciences, 28, 77–88. doi:10.1176/appi.neuropsych.15030053
- Tatro, E. T., Everall, I. P., Kaul, M., & Achim, C.L. (2009). Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Research, 1286, 1–12. doi:10.1016/j.brainres.2009.06.036
- Tazik, S., Johnson, S., Lu, D., Johnson, C., Youdim, M. B., Stockmeier, C. A., & Ou, X.M. (2009). Comparative neuroprotective effects of rasagiline and aminoindan with selegiline on dexamethasone-induced brain cell apoptosis. Neurotoxicity Research, 15, 284–290. doi:10.1007/s12640-009-9030-4
- Telhada, M. B., Pereira, T. M., & Lechner, M.C. (1992). Effect of dexamethasone and phenobarbital on run-on transcription rate and CYP3A mRNA concentration in rat liver: Changes during development. Archives of Biochemistry and Biophysics, 298, 715–725. doi:10.1016/0003-9861(92)90471-8
- Tseilikman, V., Kozochkin, D., Synitsky, A., Sibiriak, S., Tseilikman, O., Katashinsky, E., … Simbirtsev, A. (2012). Does stress-induced release of interleukin-1 cause liver injury? Cellular and Molecular Neurobiology, 32, 1069–1078. doi:10.1007/s10571-012-9866-7
- Tseilikman, V. E., Manukhina, E. B., Downey, H. F., Tseilikman, O. B., Misharina, M. E., Nikitina, A. A., … N. Sahabutdinov, M. (2016). Duration of hexobarbital-induced sleep and monoamine oxidase activities in rat brain: Focus on the behavioral activity and on the free-radical oxidation. General Physiology and Biophysics, 35, 175–183. doi:10.4149/gpb_2015039
- Tseilikman, V. E., Sinitskii, A. I., Tseilikman, O. B., Deev, R. V., Lapshin, M. S., & Kozochkin, D.A. (2015). Glucocorticoid-related regulation of LPO in brain cortex during anxiogenic stress. Bulletin of Experimental Biology and Medicine, 159, 729–731. doi:10.1007/s10517-015-3060-0
- Tu, H., Powers, J. P., Liu, J., Ursu, S., Sudom, A., Yan, X., … Wang, Z. (2008). Distinctive molecular inhibition mechanisms for selective inhibitors of human 11beta-hydroxysteroid dehydrogenase type 1. Bioorganic & Medicinal Chemistry, 16, 8922–8931. doi:10.1016/j.bmc.2008.08.065
- Tuorkey, M. J., Abdul-Aziz, K. K., & Zidan, A.A. (2013). Active immunization against tumor necrosis factor-alpha decreases proinflammatory cytokines, oxidative stress mediators and adhesion molecules risk factors in streptozotocin-induced diabetic rats. Endocrine, Metabolic & Immune Disorders-Drug Targets, 13, 269–274. doi:10.2174/18715303113139990039
- van Zuiden, M., Kavelaars, A., Geuze, E., Olff, M., & Heijnen, C.J. (2013). Predicting PTSD: Pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain, Behavior, and Immunity, 30, 12–21. doi:10.1016/j.bbi.2012.08.015
- Varga, J., Ferenczi, S., Kovacs, K. J., Csano, A., Prokopova, B., Jezova, D., & Zelena, D. (2016). Dissociation of adrenocorticotropin and corticosterone as well as aldosterone secretion during stress of hypoglycemia in vasopressin-deficient rats. Life Science, 166, 66–74. doi:10.1016/j.lfs.2016.10.011
- Varga, J., Ferenczi, S., Kovacs, K. J., Garafova, A., Jezova, D., & Zelena, D. (2013). Comparison of stress-induced changes in adults and pups: Is aldosterone the main adrenocortical stress hormone during the perinatal period in rats? PLoS One, 8, e72313. doi:10.1371/journal.pone.0072313
- Williams, T., Stein, D. J., & Ipser, J. (2018). A systematic review of network meta-analyses for pharmacological treatment of common mental disorders. Evidence Based Mental Health, 21, 7–11. doi:10.1136/eb-2017-102718
- Wyrwoll, C. S., Holmes, M. C., & Seckl, J.R. (2011). 11beta-hydroxysteroid dehydrogenases and the brain: From zero to hero, a decade of progress. Frontiers in Neuroendocrinology, 32, 265–286. doi:10.1016/j.yfrne.2010.12.001
- Xu, D., Liang, G., Yan, Y. E., He, W. W., Liu, Y. S., Chen, L. B., … Wang, H. (2012). Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic alterations in fetal rats. Toxicology Letters, 209, 282–290. doi:10.1016/j.toxlet.2012.01.006
- Yanagimoto, T., Itoh, S., Muller-Enoch, D., & Kamataki, T. (1992). Mouse liver cytochrome P-450 (P-450IIIAM1): Its cDNA cloning and inducibility by dexamethasone. Biochimica et Biophysica Acta, 1130, 329–332. doi:10.1016/0167-4781(92)90447-8
- Yehuda, R., & Seckl, J. (2011). Minireview: Stress-related psychiatric disorders with low cortisol levels: A metabolic hypothesis. Endocrinology, 152, 4496–4503. doi:10.1210/en.2011-1218
- Zaba, M., Kirmeier, T., Ionescu, I. A., Wollweber, B., Buell, D. R., Gall-Kleebach, D. J., … Schmidt, U. (2015). Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology, 55, 102–115. doi:10.1016/j.psyneuen.2015.02.005
- Zheng, Z., & Travagli, R.A. (2007). Dopamine effects on identified rat vagal motoneurons. American Journal of Physiology-Gastrointestinal and Liver Physiology, 292, G1002–1008. doi:10.1152/ajpgi.00527.2006
