Abstract
Maternal separation (MS) is an animal model widely used to evaluate the influence of early-life stress exposure on ethanol consumption and dependence. The goal of this study was to evaluate the effects of brief and prolonged MS on the pattern of consumption and ethanol conditioned place preference (CPP) in male and female rats during adolescence and adulthood. Wistar rat pups were separated daily from their dams for 15 or 180 minutes during the 2 to 10 postnatal days (PND). In adolescence, half of the litter from each group was evaluated in the ethanol consumption test using the three-bottle test choice paradigm. In addition, using biased procedure, ethanol-conditioned place preference was also evaluated. In adulthood, the other half of the litter was evaluated on the same tests. Our results showed that there are differences in consumption pattern and in alcohol reinforcement between males and females, adolescents and adults. While prolonged MS had no effect on total ethanol consumption in adolescents of both sexes, it induced CPP in these animals. In turn, in adults, previous exposure to prolonged MS increased ethanol consumption without altering ethanol-CPP.
Giving the importance of the mother-children (dam-pups when talking about rodents) relationship to proper brain development, the separation of pups from their dam is broadly used as an animal model to study the impact of early-life stress exposure. Here, we used a protocol of brief or prolonged maternal separation to study the impact of early-life stress exposure in the alcohol consumption and conditioned place preference in rats, and how age and sex influence it. We showed that, overall, the prolonged maternal separation increased alcohol consumption in both males and females, but only when animals were tested during the adulthood. In the other hand, prolonged maternal separation increased ethanol conditioned place preference in adolescent rats, both male and female.
Lay summary
1. Introduction
Ethanol is one of the most consumed substances worldwide. It is estimated that approximately 2.3 million people consume alcoholic beverages annually, and the trend is that these numbers increase over the next 10 years (World Health Organization [WHO], Citation2018). By 2016, about 3 million deaths were caused entirely or partially by abusive use of alcoholic beverages, which represents a higher mortality than that found in diseases such as AIDS, diabetes and tuberculosis (WHO, Citation2018). An intriguing aspect related to ethanol consumption is that not all individuals who drink alcohol develop addiction problems or alcoholism. Some factors seem to be associated with increased vulnerability in developing these disorders, such as the early onset of drinking, genetic predisposition, and environmental factors (Amodeo, Kneiber, Wills, & Ehlers, Citation2017; Bell et al., Citation2016; Berardo, Fabio, & Pautassi, Citation2016).
Data from the World Health Organization (WHO) warn of an increase in the number of women with diseases related to ethanol abuse, a problem that was previously considered predominantly male (Vatsalya, Liaquat, Ghosh, Mokshagundam, & McClain, Citation2017; WHO, Citation2018). In the United States, between 2000 and 2015, there was a 57% increase in deaths caused by cirrhosis among adult women aged 45 to 64 years, while in men of the same age this percentage was 21% (WHO, Citation2018). When individuals aged 25-44 years were considered, women presented an increase of 18% in the mortality rate due to liver cirrhosis, while in men there was a reduction of 10% (WHO, Citation2018).
Among adolescents, the consumption of ethanol is also increasing. About 27% of all young people in the world, aged 15 to 19, consume ethanol frequently (WHO, Citation2018). Moreover, surveys conducted in schools in several countries show that ethanol consumption begins before age 15, with few differences between men and women (WHO, Citation2018).
The consequences of the early onset of drinking on ethanol consumption have been greatly explored in animal studies (Amodeo et al., Citation2017; McMurray, Amodeo, & Roitman, Citation2016). Adolescent rats, for example, are less sensitive to the stimulating and sedative effects of ethanol (Doremus-Fitzwater, Varlinskaya, & Spear, Citation2010). Concerning environmental factors, preclinical and clinical studies show that exposure to early life stress or adversity, such as neglect, physical or mental abuse, deprivation and maltreatment may contribute to increased consumption of ethanol (Andersen et al., Citation2008; Levine, Worrell, Zimnisky, & Schmauss, Citation2012). Such stressful experiences produce persistent and long-term deleterious effects on the reward system and emotional circuits of the brain (Ganguly & Brenhouse, Citation2015). High levels of glucocorticoids observed in response to the presence of stressors promote disorders in neuronal division, dendrite development, neuronal metabolism, as well as inhibition of brain growth (Meaney et al., Citation1993).
In humans, adverse early-life experiences have been associated with an increased risk for psychiatric diseases, including drug abuse, during the childhood that persists until the adulthood (Kalmakis & Chandler, Citation2015; Kessler et al., Citation2010). Living apart from parents, besides being an adverse life experience, also increase the risk for drug abuse in children whom experience early-life adversities. For example, children looked after by local authorities (i.e., living with foster families, relatives or friends, or in community homes) are more likely to smoke and use illegal drugs than children living with their parents (Williams et al., Citation2001). Street children whom report having no family or being partially disconnected from them reported more substance abuse problems compared to street children who are connected to their parents (Kerfoot et al., Citation2007). Recently, a study showed that parental monitoring and good relationship with mother are protective factors against alcohol-related problems (Clements-Nolle, Oman, Lu, Lensch, & Moser, Citation2019).
Several animal models have been used to evaluate the consequences of exposure to stressful events in early life, among which the paradigms of brief and prolonged maternal separation (MS) stand out (Levine et al., Citation2012; Nylander & Roman, Citation2013).
In the brief MS model, also known as handling, the pups are separated from their dams for intervals of 3 to 15 minutes in the first two weeks of life (Newport, Stowe, & Nemeroff, Citation2002), mimicking the rodents’ natural behavior. In their natural habitat, rodents have the regular habit to leave the nest to forage over a period that can last from 10 minutes to an hour, depending on the age of the pups (Grota & Ader, Citation1969). The brief MS protocol has been associated with a reduction in the responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis in adult life, which constitutes a kind of adaptation to stress (Raineki, Lucion, & Weinberg, Citation2014).
In the model of prolonged MS, the pups are separated from their dams for longer periods than which dams usually leave the nest in nature. In the scientific literature, the length of prolonged MS protocols varies from 60 minutes to 24 hours (Jaime, Venus, Graciela, Tania, & Lucía, Citation2016). There are some reports showing that 180 minutes of maternal separation is enough to induce behavioral alterations in the pups when tested later in life (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, Citation2008; Berardo et al., Citation2016; Daoura, Haaker, & Nylander, Citation2011). While some studies show a reduction in ethanol consumption in rats submitted to brief MS (Roman & Nylander, Citation2005), other studies show that prolonged MS contributes to increased consumption of this substance (Aisa et al., Citation2008).
Thus, exposure to brief and prolonged periods of stress in early life seems to differentially influence the propensity to consume and to exhibit behaviors related to ethanol dependence. In this context, this study investigated the effects of brief and prolonged MS on the positive reinforcing effects of ethanol in two animal models: voluntary consumption model and conditioned place preference (CPP) model. Although other articles have already addressed the influence of the MS model on the reinforcing effects of ethanol on rodents, none of them analyzed in the same study whether there are sex and age differences. It is important to note that the use of MS as a model to assess the impact of early stress exposure on vulnerability to use and seek for ethanol is of great biological relevance. As this model shows similarity to some types of psychosocial stress in humans, it may help in understanding both the mechanisms underlying stress and responses related to the use of ethanol.
2. Material and methods
2.1. Animals
Male and female Wistar rats from State University of Goiás were used throughout the experiments. Primiparous females (n = 30) were mated with males (n = 15) of the same strain in a 1:1 ratio with the day of finding copulatory plugs and/or sperm defined as Gestation Day (GD) 0. Pregnant rats were kept individually throughout the gestation in polypropylene cages (41 × 34 × 16 cm) containing wood shavings. All animals were kept in temperature-controlled rooms (22 ± 2 °C) and 12 hours light/dark cycle (lights on at 7:00 a.m.). Rodent chow (Presence®) and water ad libitum were available to the animals throughout the study.
Three hundred and sixty-five Wistar rats (females: n = 182, males: n = 183) obtained from the above-mentioned mating were used as experimental animals. The number of animals per group is depicted in . They were kept in groups of 3-5 animals/cage except in the voluntary ethanol consumption experiments when they were individually housed. All animals were kept in the same conditions as mentioned above.
Table 1. Number of animals used in the study and in each experimental group.
All experiments were carried out in accordance with the standards of the National Council for the Control of Animal Experimentation (Concea) and began after approval by the Commission on Ethics in Animal Use (CEUA) of the State University of Goiás on March 13rd, 2017 under the number 008/2016.
2.2. Maternal separation (MS)
Pregnant rats were checked twice a day for newborn litters. The day the pups were born was determined as postnatal day 0 (PND 0). On PND 1, each litter had the maximum number of pups culled to 10 (4 to 5 females and 4 to 5 males). From PND 2 to 10, the pups were randomly assigned to two groups, which were separated from their dams for 15 minutes (MS 15 - 10 litters) or 180 minutes (MS 180 - 10 litters), once a day, from 8:00 to 11:00 a.m. A third group of pups (MS 0 - 10 litters) was kept with the dams until weaning (PND 21) and was only handled during the cleaning of the cages, which occurred in PND 1 and PND 11. During separation, the dams were transferred to cages containing clean wood shavings and kept in a separate room from the one in which the pups were. The pups were maintained in their home cages under red incandescent light to keep the temperature at 32 ± 2 °C. On PND 21, the pups of each group were weaned and housed in number of 3 to 5 per cage.
In order to avoid the bias of the litter effect on the results, two pups of each sex were used from each dam to compose each experimental group. Half of the pups from each group were tested in adolescence, considered between PND 28–42 (females: n = 91, males: n = 92), while the other half were evaluated in adulthood from PND 70 (females: n = 91, males: n = 91). Adolescence and adulthood periods were used as defined previously in the literature based on animals’ behavior and hormonal response to stressors (Spear, Citation2000; Tarazi, Tomasini, & Baldessarini, Citation1998).
2.3. Voluntary ethanol consumption
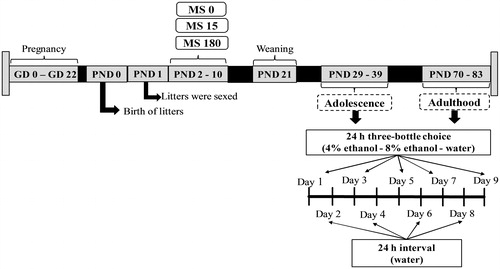
The schematic representation of this experimental design is illustrated in . This procedure was conducted as previously described (Morais-Silva, Fernandes-Santos, Moreira-Silva, & Marin, Citation2016). Adolescent rats (n = 63), males (n = 32) and females (n = 31) of all experimental groups (MS 0, MS 15 and MS 180) were housed individually in cages (41 x 34 x 16 cm) containing wood shavings. Each animal had 24-h free access to three bottles containing 4% ethanol, 8% ethanol and filtered water. Ethanol solutions were prepared from absolute ethanol (Neon Comercial, São Paulo-SP) diluted in filtered water (v/v). After 24 h exposure to the three bottles, each was weighed individually to record the consumption of each solution. Afterwards, only one bottle with water was offered to each animal for another 24 h, without evaluating the consumption. This protocol of intermittent access to ethanol solutions lasted 9 days. In order to minimize spillage of solutions, we used plastic bottles (50 mL) fitted with stainless steel sipper tubes with ball-valve nipples. The positions of the bottles were changed in each session, to avoid position preference. The ethanol and water solutions were changed in each session. The three bottles and the animals were weighed at the end of the light phase of the light/dark cycle between 5:00 and 6:00 p.m. The consumption data of the solutions were corrected by the amount of liquid lost in the drinkers by spillage or evaporation. For this, drinkers containing the three solutions were placed in empty cages at the same time the test solutions were offered to the animals. The values of the consumption were expressed in relation to the weight of the animal (g/kg). The chow was given to the animals throughout the experiment.
Figure 1. Experimental outline used for voluntary consumption of ethanol in adolescent and adult Wistar rats subjected to maternal separation (MS) during postnatal day (PND) 2 to 10 for 0, 15, or 180 minutes. The timeline shows the sequence and duration of the experimental protocol.
Adult rats (n = 62), males (n = 31) and females (n = 31), different from those tested during adolescence, had ethanol consumption assessed according to the protocol described above.
Voluntary ethanol consumption in adolescents was evaluated from PND 29 to 39, and in adults from PND 70 to 83.
2.4. Conditioned place preference (CPP)
The schematic representation of the design of this experiment is illustrated in . The CPP experiments were performed in acrylic, rectangular conditioning boxes (57.2 × 41 × 37.5 cm), divided into 3 compartments, two of them larger and located at the ends, presenting different wall color and texture of the floor (bar or grid). A third compartment, smaller and located in the central part, separated the chambers from the ends by guillotine doors. The time the animals spent in each of the three compartments was recorded by ANY-maze® software (Stoelting Co.).
Figure 2. Experimental protocol used for ethanol conditioned place preference (CPP) in adolescent and adult Wistar rats subjected to maternal separation (MS) during postnatal day (PND) 2 to 10 for 0, 15, or 180 minutes. The timeline shows the sequence and duration of experimental protocol.
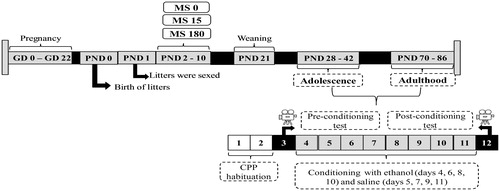
This study was based on Moreira‐Silva, Morais‐Silva, Fernandes‐Santos, Planeta, and Marin (Citation2014) and employed the biased conditioning protocol. The test lasted 12 days and consisted of 4 phases: (i) habituation (days 1 and 2), (ii) pre-conditioning test (day 3), (iii) ethanol conditioning sessions (days 4, 6, 8 and 10) and saline solution (days 5, 7, 9 and 11) and (iv) post-conditioning test (day 12). Each session lasted 20 minutes. In the habituation sessions, the animals were placed in the central compartment with the doors between the compartments open for free exploration of the CPP apparatus. On the day of the pre-conditioning test, the animals were again able to explore all compartments of the CPP box and the time spent in each chamber was recorded to establish the animal’s initial preference for each compartment. In the conditioning sessions, each animal was confined to one of the end compartments shortly after receiving solutions of ethanol (10%) or saline (0.9%). Conditioning sessions were started with animals receiving ethanol solution (0.5 g/kg and 1.0 g/kg, i.p.) and confined to the least preferred compartment of CPP boxes, according to the preconditioning test result. In the next conditioning session, the animals received saline solution (5 mL/kg, i.p.) and were confined to the most preferred environment. On day 12, the conditioning test was conducted, the animals were placed in the central compartment of the apparatus with the doors open to freely explore the three compartments. The place preference was evaluated by comparing time spent in ethanol-paired compartment during post-conditioning session to initial preference in pre-conditioning session. The 10% ethanol solution was prepared from absolute ethanol (Neon Comercial, São Paulo-SP) diluted in 0.9% (v/v) saline solution.
Adolescent rats, males (n = 60) and females (n = 60) of all experimental groups (MS 0, MS 15 and MS 180) were evaluated regarding to ethanol CPP. Adult rats, males (n = 60) and females (n = 60), different from those tested during adolescence, were also evaluated in the CPP test.
CPP in adolescents was evaluated from PND 28 to 42, and in adults from PND 70 to 86.
2.5. Statistical analysis
All results are expressed as mean ± standard error of the mean. Consumption data were analyzed by two-way ANOVA, considering maternal separation (MS 0 vs. MS 15 vs. MS 180) and sex (male vs. female) as factors. To assess the influence of time on ethanol consumption, we performed repeated measures ANOVA considering the factors maternal separation and time (days). Detailed information about these analyses are available in Supplemental material (Supplemental material – Figure S1 and Figure S2). CPP data were analyzed by repeated measures ANOVA, using the factors maternal separation, sex and the test phase (pre-conditioning x post-conditioning). In cases where the ANOVA indicated a significant difference (p ≤ .05), Newman-Keuls post hoc test was applied. The level of significance was set at p ≤ .05.
3.. Results
3.1. Voluntary ethanol consumption
3.1.1. Ethanol consumption in adolescent males and females
In the analysis of the total ethanol (sum of ethanol 4% and 8% bottles) consumption of adolescents (), two-way ANOVA did not show effect of MS (F2,57 = 0.88; p = .42), sex (F1,57 = 1.53; p = .22) and interaction between these two factors (F2,57 = 1.87; p = .16). Then, we performed a separated analysis for 4 and 8% ethanol bottles. Consumption of the 4% ethanol solution was significantly affected by sex (F1,57 = 6.00; p < .05) but not for MS (F2,57 = 1.65; p = .20) factors (. Interaction between sex and MS was not significative (F2,57 = 1.81; p = .17). ANOVA for the ethanol 8% consumption showed significant effect of sex (F1,57 = 7.57; p < .01) but not MS (F2,57 = 0.58; p = .56) or interaction (F2,57 = 0.69; p = .50) (. Then, female adolescent rats consumed more ethanol at low (4%) and less at high (8%) concentration.
Figure 3. Voluntary ethanol consumption in adolescent rats subjected to brief or prolonged maternal separation during the neonatal period (n = 10–12/group). (A) Total ethanol consumption (g/kg); (B) consumption of ethanol solution at 4% (g/kg); (C) consumption of ethanol solution at 8% (g/kg); (D) water consumption (g/kg). Data represent the mean ± standard error of the mean. ANOVA followed by the Newman Keuls post hoc test. $p ≤ .05 compared to the male groups.
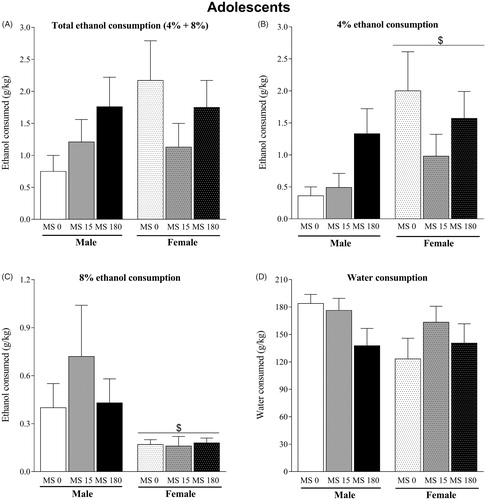
Differences on ethanol consumption were not nonspecific since water consumption was not altered by MS (F2,57 = 1.43; p = .25) or sex (F1,57 = 2.52; p = .12) factors and there was not significant interaction (F2,57 = 1.68; p = .19) ().
3.1.2. Ethanol consumption in adult males and females
illustrates the total (4 and 8%) ethanol consumption of adult rats. Two-way ANOVA indicated effect of the MS (F2,56 = 6.15; p < .01) and sex (F1,56 = 7.61; p < .01) for ethanol consumption. Nevertheless, there was no interaction between factors (F2,56 = 1.67; p = .20). The post hoc test showed that the total ethanol consumption of MS 180 groups was higher compared to control groups (MS 0) (p < .05). Similar effects were observed concerning the consumption of the 4% ethanol solution. There was effect of MS (F2,56 = 3.47; p < .05) and sex (F1,56 = 5.57; p < .05) but not interaction between factors (F2,56 = 0.17, p = .84) (. The Newman-Keuls test indicated that 4% ethanol intake was higher in MS 180 groups compared to MS 0 groups (p < .05). For the consumption of the 8% ethanol solution, there was an effect of the MS (F2,56 = 4.16; p < .05) and interaction between factors (F2,56 = 3.84; p < .05) (. There was no effect of sex (F1,56 = 3.25; p = .08). The post hoc test evidenced that MS 180 males consumed more ethanol 8% solution than MS 15 and MS 0 male groups (p < .05). In addition, MS 15 female consumed more this ethanol solution than MS 15 male group (p < .05).
Figure 4. Voluntary ethanol consumption in adult rats subjected to brief or prolonged maternal separation during the neonatal period (n = 10–11/group). (A) total ethanol consumption (g/kg); (B) consumption of ethanol solution at 4% (g/kg); (C) consumption of ethanol solution at 8% (g/kg); (D) water consumption (g/kg). Data represent the mean ± standard error of the mean. ANOVA followed by the Newman Keuls post hoc test. #p ≤ .05, independent of sex, compared to the MS 0 groups; §p ≤ .05, independent of sex, compared to the MS 15 groups; $p ≤ .05 compared to the male groups; &p ≤ .05 compared to the MS 0 male group; †p ≤ .05 compared to the MS 15 male group.
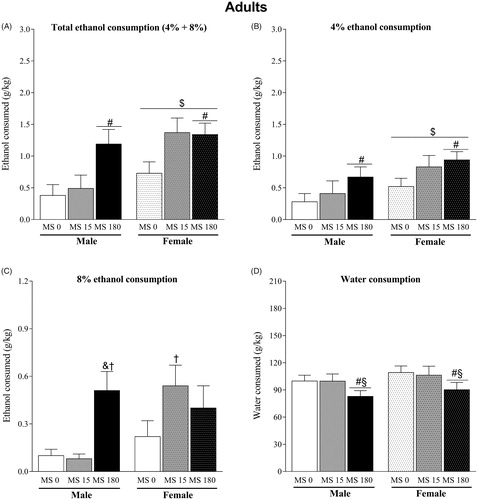
Regarding water consumption, there was only effect of MS (F2,56 = 3.39; p < .05). There was no effect of sex (F1,56 = 1.56; p = .22) and no interaction between factors (F2,56 = 0.02; p = .98) (. The post hoc test showed that water consumption of MS 180 was lower than MS 0 and MS 15 groups (p < .05).
3.2. Conditioned place preference
3.2.1. CPP in adolescent males and females
illustrates the absence of effects of brief and prolonged MS on the induction of ethanol CPP at a dose of 0.5 g/kg in adolescent male and female rats. The repeated measures ANOVA showed no effect of MS (F2,54 = 0.16; p = .85), sex (F1,54 = 0.14; p = .70) and phases (F1,54 = 1.97; p = .17). Additionally, there were any significant interactions (p > .05).
Figure 5. Effects of exposure to neonatal stress on the induction of ethanol-conditioned place preference (CPP) at doses of 0.5 g/kg and 1.0 g/kg. (A) dose of 0.5 g/kg in adolescent rats. (B) dose of 1.0 g/kg in adolescent rats. (C) dose of 0.5 g/kg in adult rats. (D) dose of 1.0 g/kg in adult rats (A). Data represent the mean ± standard error of the mean. Repeated-measures ANOVA followed by the Newman Keuls post hoc test. $p < .05 compared to the male groups; *p < .05 compared to the respective group in the preconditioning test.
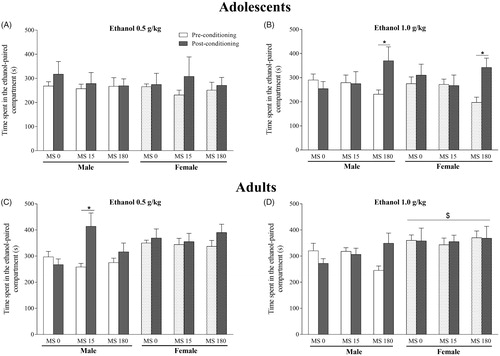
ANOVA of the induction of ethanol CPP at 1.0 g/kg in adolescents showed significative effect of phases (F1,54 = 5.83; p < .05) and interaction between MS x phases (F2,54 = 6.43; p < .01) (. The Newman-Keuls post hoc test evidenced that both male and female MS 180 group presented induction of CPP (post-conditioning time higher than pre-conditioning) for ethanol at a dose of 1.0 g/kg (p < .001).
3.2.2. CPP in adult males and females
In the evaluation of the induction of ethanol CPP at a dose of 0.5 g/kg in adult rats, repeated measures ANOVA evidenced an effect of the sex (F1,54 = 9.59; p < .01), phases (F1,54 = 6.97; p < .01) and interaction between MS × sex × phases (F2,54 = 3.57; p < .05) (. However, there was no significant effect of MS (F2,54 = 0.55; p = .58). The post hoc test showed that only males from MS 15 group presented induction of CPP (p < .05).
Regarding the induction of ethanol CPP at a dose of 1.0 g/kg in adults (), there was only significative effect of sex (F1,54 = 9.90; p < .01) and no interactions.
4. Discussion
Our experiments demonstrated that repeated neonatal intervention on mother-pups interaction caused different effects on ethanol intake or reward depending on pups’ sex and age period of analyses (adolescence or adulthood).
4.1. Voluntary ethanol consumption
The results showed that exposure to brief (15 min) and prolonged (180 min) MS from did not alter the total ethanol consumption in adolescent males and females. On the other hand, prolonged MS increased ethanol consumption in both males and females when tested in adulthood.
Similar results to those found in our study were described by Daoura et al. (Citation2011), who verified that Wistar rats undergoing the protocol of MS for 360 or 15 minutes during PND 1 to 20, did not present difference in the intermittent ethanol consumption pattern in the adolescence period when compared to the control group, while ethanol consumption was increased in the group submitted to 360 minutes of MS and tested in adulthood. In another study that evaluated ethanol consumption in adolescent male and female Wistar rats exposed to the 180 minutes of MS (PND 1-21), it was also observed that the early-life stress protocol did not influence the consumption of ethanol in comparison to the control (Berardo et al., Citation2016). On the other hand, others have found increased ethanol consumption in adolescent rodents after exposure to MS (Daoura et al., Citation2011; García-Gutiérrez et al., Citation2016). It is not clear the reason for such discrepancies, but the differences in animal species and strains used, the period when MS was performed and tests used to evaluate ethanol consumption may have influenced the results obtained in those studies and ours.
One important limitation in our voluntary ethanol consumption protocol is the individual housing necessary for fluid intake measurement. In adolescents, social isolation increases alcohol intake and preference (Butler, Karkhanis, Jones, & Weiner, Citation2016) while it is not effective when animals are isolated during the adulthood (Lopez, Doremus-Fitzwater, & Becker, Citation2011). This could contribute to a ceiling effect in adolescents’ alcohol consumption, which could be a factor that masked the effects of MS in this variable. Future works using advanced technologies to measure ethanol intake could help to confirm or not this hypothesis.
Increased ethanol intake in adults submitted to prolonged MS was described in other studies (Berardo et al., Citation2016; Ploj, Roman, & Nylander, Citation2003). Prolonged separation of the dam in the first days of life may cause hyperresponsiveness of the HPA axis, increasing the concentration of glucocorticoids in the blood (Daskalakis, Bagot, Parker, Vinkers, & De Kloet, Citation2013). The hyperresponsive HPA axis and the high concentration of glucocorticoids can increase the sensitivity of the nucleus accumbens (NAc), promoting neuroadaptations in the dopaminergic system, such as increased dopamine release in this nucleus (Goeders, Citation2002; Graf et al., Citation2013; Walters & Kosten, Citation2019). For example, MS exposure alters epigenetic control in the NAc of adult rats inducing a global hypomethylation of this brain area. This is accompanied by altered response to drug administration (Anier et al., Citation2014). Therefore, these changes result in a greater susceptibility to the development of problems with substances of abuse in humans (Enoch, Citation2011; Kaufman et al., Citation2007), as well as in animals (Champagne, De Kloet, & Joëls, Citation2009; Roman & Nylander, Citation2005). It is interesting that increased ethanol intake in males submitted to prolonged MS is due to an increase in consumption of both high concentrated and low concentrated ethanol solution, while increase ethanol intake in females seems to be related to an increase in consumption of the 4% ethanol solution.
Female rats exposed to brief MS showed an increase in 8% ethanol solution consumption when tested in the adulthood, when compared to males exposed to the same protocol. There is no consensus in the literature on the effects of the brief 15-minute MS model on ethanol consumption in adult rats. Some studies describe this paradigm as being a beneficial intervention for the offspring, being associated with the adaptive changes in neuronal circuits responsible for the responses to stress, making the animals less vulnerable to their consequences throughout the life (Baldini et al., Citation2013; Nylander & Roman, Citation2013). Thus, rodents exposed to the brief MS would present reduced levels of corticotropin releasing hormone (CRH), adrenocorticotropic hormone (ACTH) and corticosterone (O’Donnell, Larocque, Seckl, & Meaney, Citation1994). However, although this model has long-term beneficial and protective effects for offspring, it did not reduce ethanol consumption. It is possible that beneficial effects of brief MS just appear after stress exposure, when it could prevent stress-induced increases in drug intake.
Differences in the consumption profile between males and females have been observed in the literature. Free-bottle choice protocols have shown that female rodents consume more ethanol than males (Barker, Torregrossa, Arnold, & Taylor, Citation2010). On the other hand, most epidemiological studies show that men consume more ethanol than women (Kalaydjian et al., Citation2009; WHO, Citation2018). Nevertheless, recent data released by the World Health Organization (WHO) show an increase in the number of women with alcohol dependence problems (WHO, Citation2018). It seems that the fact that women consume less in relation to men may be related to social and cultural factors. Studies also show that women produce lower concentrations of the enzyme ethanol dehydrogenase, which is responsible for metabolizing ethanol in the liver, slowing down the metabolism, which makes women more susceptible to the toxic effects of ethanol in the body, causing them to consume less (Frezza et al., Citation1990). In our study we showed that ethanol consumption differences between males and females are related to differences in the intake of low concentrated ethanol solutions, when a choice is offered to the animals.
Some studies have revealed that differences in consumption and preference between males and females are not evident in animals younger than 39 days of age, and this difference is more noticeable in adult life (García‐Burgos, González, Manrique, & Gallo, Citation2009). These data show that differences between sexes in preference and pattern of consumption in rodents are not expressed in very young animals but are evident during a brief period of puberty (39 to 41 days) when males drink more than females, and during adulthood with females drinking more than males (Lancaster, Brown, Coker, Elliott, & Wren, Citation1996). The results of the present study reinforce those described in other studies (Peñasco, Mela, López-Moreno, Viveros, & Marco, Citation2015), which pointed out that differences in total ethanol consumption between males and females are not evident during adolescence and seem to be observed in adulthood. On the other hand, we found interesting differences regarding the consumption of different ethanol solutions offered simultaneously. As in adults, female adolescents consumed more the 4% ethanol solution compared to males, while males during the adolescence consumed more the 8% ethanol solution compared to females.
4.2. CPP
The results showed that ethanol at a dose of 0.5 g/kg was not able to induce CPP in both adolescents and adults, except for adult animals exposed to brief MS. On the other hand, 1.0 g/kg ethanol induced CPP in adolescent males and females of the MS 180 group.
There is a large literature that shows the ethanol reinforcing effects on CPP in adult mice (Breit & Chester, Citation2016; Roger-Sánchez, Aguilar, Rodríguez-Arias, Aragon, & Miñarr, Citation2012). On the other hand, CPP is harder to show in outbred rats (Acevedo, Nizhnikov, Spear, Molina, & Pautassi, Citation2013; Philpot, Badanich, & Kirstein, Citation2003; Torres, Walker, Beas, & O'dell, Citation2014), while conditioned place aversion is often described (Cunningham, Fidler, & Hill, Citation2000; Tzschentke, Citation1998). Most of the reports showing CPP in rats involve submitting animals to pretreatments, as stress exposure or drug administration (Fidler, Bakner, & Cunningham, Citation2004). Nevertheless, to date, no studies have been found in the literature investigating the effects of brief and prolonged MS on the induction of ethanol CPP during adulthood in rats and mice.
As well as observed herein, some studies demonstrate that ethanol induces CPP in rats (Torres et al., Citation2014) and mice (Roger-Sánchez et al., Citation2012) at a dose of 1 g/kg during adolescence. Acevedo et al. (Citation2013), for example, found that male and female adolescent (evaluated together) Wistar rats exhibited CPP for ethanol at the 1.0 g/kg dose. Torres et al. (Citation2014), in turn, used male and female adolescents and reported Wistar rats and observed that only females presented ethanol CPP at a dose of 1.0 g/kg. Moreover, a study using male and female adolescent and adult Sprague-Dawley rats showed that the 1.0 g/kg dose was more effective in inducing ethanol CPP in animals aged 45 days. On the other hand, in animals with 25, 35 and 60 days of life this dose generated conditioned place aversion for ethanol (Philpot et al., Citation2003).
Although there is evidence in the literature that ethanol induces CPP in adolescent animals, only one study investigated the effects of brief and prolonged MS stress on ethanol CPP in animals at this age (Portero-Tresserra et al., Citation2018). In this study, adolescent C57BL/6 mice were exposed to prolonged MS (PND 2-5 4 h/day, PND 5-16 8 h/day) and early weaning on the 17th day of life. No induction of ethanol CPP was found at doses of 0.1 and 0.2 g/mL in the animals of the MS group when compared to the control.
Stress has been highlighted as an important factor in the processes of alcohol dependence by increasing sensitivity to the reinforcing effects of ethanol, which can be observed by the increase in the search behavior for this substance in several animal models (Bahi, Citation2013; Breit & Chester, Citation2016; Lucke-Wold, Citation2011). However, exposure to brief MS has been associated with decreased HPA axis responsiveness, which is a kind of adaptation to stress (Benetti, Araujo, Sanvitto, & Lucion, Citation2007; Marco et al., Citation2015; Newport et al., Citation2002). As a result, animals undergoing this procedure present decreased anxiety behaviors, biological responses to stress, and greater ability to cope with stressful events throughout life (Rana, Pugh, Jackson, Clinton, & Kerman, Citation2015; Vetulani, Citation2013). Studies suggest that these adaptive responses are related to increased maternal care that occurs after brief periods of separation of the dam from her litter (Newport et al., Citation2002; Rana et al., Citation2015). Dams of litters which have undergone a brief MS procedure, when return to the nest tend to lick their offspring more than the dams of offspring who have not undergone any maternal deprivation procedure (Rana et al., Citation2015).
In the prolonged MS, there is an increase in the concentration of CRH and the expression of the receptors of this hormone (Aisa et al., Citation2008; Plotsky et al., Citation2005). As a consequence, stress alters glucocorticoid concentration and may increase dopaminergic neurotransmission in reward pathways, thereby modulating behavioral responses to substances of abuse and HPA axis responsiveness in the first days of life (Aisa et al., Citation2008; Blaine & Sinha, Citation2017; Montoya, Bos, Terburg, Rosenberger, & van Honk, Citation2014; Piazza & Le Moal, Citation1997). Thus, the effects triggered by prolonged MS on CPP for ethanol in adolescence may be related to this HPA axis dysfunction.
Concerning our results with adult rats, males exposed to MS 15 and treated with the 0.5 g/kg dose of ethanol were found to have induction of CPP. Further, ethanol at the dose of 1.0 g/kg was also able to induce CPP in the MS 180 group males. As for adult females exposed to MS 15 and MS 180, ethanol at doses of 0.5 and 1.0 g/kg did not induce CPP.
Although some studies have evaluated the influence of other types of stress on ethanol CPP in adult rodents (rats and mice) (Bahi, Citation2013; Breit & Chester, Citation2016; Song et al., Citation2007), there is still no consensus in the literature about the impact of stress on ethanol reinforcing effects in adulthood. For example, a study evaluating ethanol CPP in adult Sprague-Dawley rats exposed to conditioned fear stress using the 0.3 g/kg dose ethanol and a 30-min conditioning period showed that stress induced ethanol-CPP compared to the control group (Matsuzawa, Suzuki, & Misawa, Citation1998). In another study conducted by Bahi (Citation2013) with adult male C57BL/6 mice, it was shown that exposure to chronic psychosocial stress induces ethanol CPP (1.5 g/kg) compared to control animals. On the other hand, a study with adolescent and adult, male and female, mice showed that exposure to footshock stress induced ethanol CPP in adolescents, but not in adults, and that adults only presented ethanol CPP when high doses of ethanol were administered (Song et al., Citation2007). This variability in results may be related to several factors, such as the use of different species and strains in studies involving stress.
Regarding the results of adult females that did not present induction of ethanol CPP at the doses tested, this finding does not seem to be directly related to the hormonal variations that occur in the estrous cycle of these animals. A previous study with female Wistar rats that were tested at different stages of the estrous cycle showed ethanol-aversive effects (2.0 and 2.5 g/kg i.p.) on the CPP test, suggesting that the hormonal variations during the cycle did not influence the effects of this substance (Torres et al., Citation2014).
In our study, although there was no monitoring of the estrous cycle of females, the results showed that there was no difference between the groups (MS 0, MS 15 and MS 180) and only the adolescent females of the MS 180 group showed ethanol CPP (1.0 g/kg dose), suggesting that the estrous cycle is a factor that appears not to be directly related to the induction of ethanol CPP. However, studies show that sex hormones influence development, CNS maturation, and brain system modulation (Moran-Santa Maria, Flanagan, & Brady, Citation2014). Females exhibit greater synaptic remodeling, neuronal pruning, and apoptosis during maturation of the prefrontal cortex during adolescence than males, suggesting that females may be more vulnerable to the effects of drug exposure than males during this period (Willing & Juraska, Citation2015). Furthermore, it is known that prolonged MS promotes lasting CNS changes (Ploj et al., Citation2003), such as morphological and physiological changes in response to stress (De Kloet, Reul, De Ronde, Bloemers, & Ratka, Citation1986; Jahng, Yoo, Kim, Kim, & Lee, Citation2012), which can lead to persistent behavioral changes of pups during adolescence and adulthood (Teicher, Citation2000).
Taken together, our results showed that there are differences in consumption pattern and in alcohol reinforcement between males and females, adolescents and adults. While prolonged MS had no effect on total ethanol consumption in adolescents of both sexes, it induced CPP in these animals. In turn, in adults, previous exposure to prolonged MS increased ethanol consumption without altering ethanol-CPP.
Notes on Contribution
KSL, MC, GMF, MTM and VCSA contributed to the design of the study. KSL and MC performed the experiments. KSL, GMF, MTM and VCSA analyzed and interpreted the data. KSL, GMF, MTM and VCSA drafted and revised the manuscript. All the authors approved the final version of manuscript.
Supplemental_material.docx
Download MS Word (1.5 MB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Acevedo, M.B., Nizhnikov, M.E., Spear, N.E., Molina, J.C., & Pautassi, R.M. (2013). Ethanol‐induced locomotor activity in adolescent rats and the relationship with ethanol‐induced conditioned place preference and conditioned taste aversion. Developmental Psychobiology, 55, 429–442. doi:10.1002/dev.21048
- Aisa, B., Tordera, R., Lasheras, B., Del Rio, J., & Ramirez, M.J. (2008). Effects of maternal separation on hypothalamic–pituitary–adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience, 154, 1218–1226. doi:10.1016/j.neuroscience.2008.05.011
- Amodeo, L.R., Kneiber, D., Wills, D.N., & Ehlers, C.L. (2017). Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol, 59, 43–51. doi:10.1016/j.alcohol.2016.12.002
- Andersen, S.L., Tomada, A., Vincow, E.S., Valente, E., Polcari, A., & Teicher, M.H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences, 20, 292–301. doi:10.1176/jnp.2008.20.3.292
- Anier, K., Malinovskaja, K., Pruus, K., Aonurm-Helm, A., Zharkovsky, A., & Kalda, A. (2014). Maternal separation is associated with DNA methylation and behavioural changes in adult rats. European Neuropsychopharmacology, 24, 459–468. doi:10.1016/j.euroneuro.2013.07.012
- Bahi, A. (2013). Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress (Amsterdam, Netherlands), 16, 441–451. doi:10.3109/10253890.2012.754419
- Baldini, S., Restani, L., Baroncelli, L., Coltelli, M., Franco, R., Cenni, M.C., … Berardi, N. (2013). Enriched early life experiences reduce adult anxiety-like behavior in rats: a role for insulin-like growth factor 1. Journal of Neuroscience, 33, 11715–11723. doi:10.1523/JNEUROSCI.3541-12.2013
- Barker, J.M., Torregrossa, M.M., Arnold, A.P., & Taylor, J.R. (2010). Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. Journal of Neuroscience, 30, 9140–9144. doi:10.1523/JNEUROSCI.0548-10.2010
- Bell, R.L., Hauser, S., Rodd, Z.A., Liang, T., Sari, Y., McClintick, J., … Engleman, E.A. (2016). A genetic animal model of alcoholism for screening medications to treat addiction. International Review of Neurobiology, 126, 179–261. doi:10.1016/bs.irn.2016.02.017
- Benetti, F., Araujo, A.P., Sanvitto, L.G., & Lucion, A.B. (2007). Effects of neonatal novelty exposure on sexual behavior, fear, and stress‐response in adult rats. Developmental Psychobiology, 49, 258–264. doi:10.1002/dev.20181
- Berardo, L.R., Fabio, M.C., & Pautassi, R.M. (2016). Post-weaning environmental enrichment, but not chronic maternal isolation, enhanced ethanol intake during periadolescence and early adulthood. Frontiers in Behavioral Neuroscience, 10, 195. doi:10.3389/fnbeh.2016.00195
- Blaine, S.K., & Sinha, R. (2017). Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 122, 136–147. doi:10.1016/j.neuropharm.2017.01.037
- Breit, K.R., & Chester, J.A. (2016). Effects of chronic stress on alcohol reward‐and anxiety‐related behavior in high‐and low‐alcohol preferring mice. Alcoholism: Clinical and Experimental Research, 40, 482–490. doi:10.1111/acer.12992
- Butler, T.R., Karkhanis, A.N., Jones, S.R., & Weiner, J.L. (2016). Adolescent social isolation as a model of heightened vulnerability to comorbid alcoholism and anxiety disorders. Alcoholism: Clinical and Experimental Research, 40, 1202–1214. doi:10.1111/acer.13075
- Champagne, D.L., De Kloet, E.R., & Joëls, M. (2009). Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Seminars in Fetal and Neonatal Medicine, 14, 136–142. doi:10.1016/j.siny.2008.11.006
- Clements-Nolle, K., Oman, R.F., Lu, M., Lensch, T., & Moser, L. (2019). Youth assets and alcohol-related problems among male and female youth: Results from a longitudinal cohort study. Preventive Medicine, 123, 192–196. doi:10.1016/j.ypmed.2019.03.042
- Cunningham, C.L., Fidler, T.L., & Hill, K.G. (2000). Animal models of alcohol’s motivational effects. Alcohol Research & Health, 24, 85–92.
- Daoura, L., Haaker, J., & Nylander, I. (2011). Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcoholism: Clinical and Experimental Research, 35, 506–515. doi:10.1111/j.1530-0277.2010.01367.x
- Daskalakis, N.P., Bagot, R.C., Parker, K.J., Vinkers, C.H., & De Kloet, E.R. (2013). The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38, 1858–1873. doi:10.1016/j.psyneuen.2013.06.008
- De Kloet, E.R., Reul, J.M.H.M., De Ronde, F.S.W., Bloemers, M., & Ratka, A. (1986). Function and plasticity of brain corticosteroid receptor systems: action of neuropeptides. Journal of Steroid Biochemistry, 25, 723–731. doi:10.1016/0022-4731(86)90301-8
- Doremus-Fitzwater, T.L., Varlinskaya, E.I., & Spear, L.P. (2010). Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition, 72, 114–123. doi:10.1016/j.bandc.2009.08.008
- Enoch, M.A. (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology, 214, 17–31. doi:10.1007/s00213-010-1916-6
- Fidler, T.L., Bakner, L., & Cunningham, C.L. (2004). Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacology Biochemistry and Behavior, 77, 731–743. doi:10.1016/j.pbb.2004.01.010
- Frezza, M., di Padova, C., Pozzato, G., Terpin, M., Baraona, E., & Lieber, C.S. (1990). High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first‐pass metabolism. New England Journal of Medicine, 322, 95–99. doi:10.1056/NEJM199001113220205
- Ganguly, P., & Brenhouse, H.C. (2015). Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental Cognitive Neuroscience, 11, 18–30. doi:10.1016/j.dcn.2014.07.001
- García‐Burgos, D., González, F., Manrique, T., & Gallo, M. (2009). Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse‐like conditions. Alcoholism: Clinical and Experimental Research, 33, 722–728. doi:10.1111/j.1530-0277.2008.00889.x
- García-Gutiérrez, M.S., Navarrete, F., Aracil, A., Bartoll, A., Martínez-Gras, I., Lanciego, J.L., … Manzanares, J. (2016). Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addiction Biology, 21, 847–858. doi:10.1111/adb.12266
- Goeders, N.E. (2002). The HPA axis and cocaine reinforcement. Psychoneuroendocrinology, 27, 13–33. doi:10.1016/S0306-4530(01)00034-8
- Graf, E.N., Wheeler, R.A., Baker, D.A., Ebben, A.L., Hill, J.E., McReynolds, J.R., … Gasser, P.J. (2013). Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. Journal of Neuroscience, 33, 11800–11810. doi:10.1523/JNEUROSCI.1969-13.2013
- Grota, L.J., & Ader, R. (1969). Continuous recording of maternal behaviour in Rattus norvegicus. Animal Behaviour, 17, 722–729. doi:10.1016/S0003-3472(69)80019-9
- Jahng, J.W., Yoo, S.B., Kim, J.Y., Kim, B.T., & Lee, J.H. (2012). Increased mesohippocampal dopaminergic activity and improved depression-like behaviors in maternally separated rats following repeated fasting/refeeding cycles. Journal of Obesity, 2012, 1. doi:10.1155/2012/497101
- Jaime, J.H.P., Venus, B.C., Graciela, J.R., Tania, H.H.O., & Lucía, M.M. (2016). Young-adult male rats’ vulnerability to chronic mild stress is reflected by anxious-like instead of depressive-like behaviors. Neuroscience Journal, 2016, 5317242. doi:10.1155/2016/5317242
- Kalaydjian, A., Swendsen, J., Chiu, W.T., Dierker, L., Degenhardt, L., Glantz, M., … Kessler, R. (2009). Sociodemographic predictors of transitions across stages of alcohol use, disorders, and remission in the National Comorbidity Survey Replication. Comprehensive Psychiatry, 50, 299–306. doi:10.1016/j.comppsych.2008.09.012
- Kalmakis, K.A., & Chandler, G.E. (2015). Health consequences of adverse childhood experiences: A systematic review. Journal of the American Association of Nurse Practitioners, 27, 457–465. doi:10.1002/2327-6924.12215
- Kaufman, J., Yang, B.Z., Douglas-Palumberi, H., Crouse-Artus, M., Lipschitz, D., Krystal, J.H., & Gelernter, J. (2007). Genetic and environmental predictors of early alcohol use. Biological Psychiatry, 61, 1228–1234. doi:10.1016/j.biopsych.2006.06.039
- Kerfoot, M., Koshyl, V., Roganov, O., Mikhailichenko, K., Gorbova, I., & Pottage, D. (2007). The health and well-being of neglected, abused and exploited children: The Kyiv Street Children Project. Child Abuse & Neglect, 31, 27–37. doi:10.1016/j.chiabu.2006.07.003
- Kessler, R.C., McLaughlin, K.A., Green, J.G., Gruber, M.J., Sampson, N.A., Zaslavsky, A.M., … Williams, D.R. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. British Journal of Psychiatry, 197, 378–385. doi:10.1192/bjp.bp.110.080499
- Lancaster, F.E., Brown, T.D., Coker, K.L., Elliott, J.A., & Wren, S.B. (1996). Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague‐Dawley rats. Alcoholism: Clinical and Experimental Research, 20, 1043–1049. doi:10.1111/j.1530-0277.1996.tb01945.x
- Levine, A., Worrell, T.R., Zimnisky, R., & Schmauss, C. (2012). Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiology of Disease, 45, 488–498. doi:10.1016/j.nbd.2011.09.005
- Lopez, M.F., Doremus-Fitzwater, T.L., & Becker, H.C. (2011). Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol, 45, 355–364. doi:10.1016/j.alcohol.2010.08.017
- Lucke-Wold, B. (2011). The varied uses of conditioned place preference in behavioral neuroscience research: an investigation of alcohol administration in model organisms. Impulse (Columbia, SC), 2011. Retrieved from http://impulse.appstate.edu/sites/impulse.appstate.edu/files/Lucke_Wold.pdf
- Marco, E.M., Llorente, R., López-Gallardo, M., Mela, V., Llorente-Berzal, Á., Prada, C., & Viveros, M.P. (2015). The maternal deprivation animal model revisited. Neuroscience & Biobehavioral Reviews, 51, 151–163. doi:10.1016/j.neubiorev.2015.01.015
- Matsuzawa, S., Suzuki, T., & Misawa, M. (1998). Conditioned fear stress induces ethanol-associated place preference in rats. European Journal of Pharmacology, 341, 127–130. doi:10.1016/S0014-2999(97)01456-8
- McMurray, M.S., Amodeo, L.R., & Roitman, J.D. (2016). Consequences of adolescent ethanol consumption on risk preference and orbitofrontal cortex encoding of reward. Neuropsychopharmacology, 41, 1366–1375. doi:10.1038/npp.2015.288
- Meaney, M.J., Bhatnagar, S., Diorio, J., Larocque, S., Francis, D., O'Donnell, D., … Viau, V. (1993). Molecular basis for the development of individual differences in the hypothalamic-pituitary-adrenal stress response. Cellular and Molecular Neurobiology, 13, 321–347. doi:10.1007/BF00711576
- Montoya, E.R., Bos, P.A., Terburg, D., Rosenberger, L.A., & van Honk, J. (2014). Cortisol administration induces global down-regulation of the brain's reward circuitry. Psychoneuroendocrinology, 47, 31–42. doi:10.1016/j.psyneuen.2014.04.022
- Morais-Silva, G., Fernandes-Santos, J., Moreira-Silva, D., & Marin, M.T. (2016). Concomitant stress potentiates the preference for, and consumption of, ethanol induced by chronic pre-exposure to ethanol. Brazilian Journal of Medical and Biological Research, 49, e5009. doi:10.1590/1414-431x20155009
- Moran-Santa Maria, M.M., Flanagan, J., & Brady, K. (2014). Ovarian hormones and drug abuse. Current Psychiatry Reports, 16, 511. doi:10.1007/s11920-014-0511-7
- Moreira‐Silva, D., Morais‐Silva, G., Fernandes‐Santos, J., Planeta, C.S., & Marin, M.T. (2014). Stress abolishes the effect of previous chronic ethanol consumption on drug place preference and on the mesocorticolimbic brain pathway. Alcoholism: Clinical and Experimental Research, 38, 1227–1236. doi:10.1111/acer.12388
- Newport, D.J., Stowe, Z.N., & Nemeroff, C.B. (2002). Parental depression: animal models of an adverse live event. American Journal of Psychiatry, 159, 1265–1283. doi:10.1176/appi.ajp.159.8.1265
- Nylander, I., & Roman, E. (2013). Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology, 229, 555–569. doi:10.1007/s00213-013-3217-3
- O’Donnell, D., Larocque, S., Seckl, J.R., & Meaney, M.J. (1994). Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Molecular Brain Research, 26, 242–248. doi:10.1016/0169-328X(94)90096-5
- Peñasco, S., Mela, V., López-Moreno, J.A., Viveros, M.P., & Marco, E.M. (2015). Early maternal deprivation enhances voluntary alcohol intake induced by exposure to stressful events later in life. Neural Plasticity, 2015, 1–10. doi:10.1155/2015/342761
- Philpot, R.M., Badanich, K.A., & Kirstein, C.L. (2003). Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcoholism: Clinical & Experimental Research, 27, 593–599. doi:10.1097/01.ALC.0000060530.71596.D1
- Piazza, P.V., & Le Moal, M. (1997). Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Research Reviews, 25, 359–372. doi:10.1016/S0165-0173(97)00025-8
- Ploj, K., Roman, E., & Nylander, I. (2003). Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience, 121, 787–799. doi:10.1016/S0306-4522(03)00499-8
- Plotsky, P.M., Thrivikraman, K.V., Nemeroff, C.B., Caldji, C., Sharma, S., & Meaney, M.J. (2005). Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology, 30, 2192–2204. doi:10.1038/sj.npp.1300769
- Portero-Tresserra, M., Gracia-Rubio, I., Cantacorps, L., Pozo, O.J., Gómez-Gómez, A., Pastor, A., … Valverde, O. (2018). Maternal separation increases alcohol-drinking behaviour and reduces endocannabinoid levels in the mouse striatum and prefrontal cortex. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 28, 499–512. doi:10.1016/j.euroneuro.2018.02.003
- Raineki, C., Lucion, A.B., & Weinberg, J. (2014). Neonatal handling: an overview of the positive and negative effects. Developmental Psychobiology, 56, 1613–1625. doi:10.1002/dev.21241
- Rana, S., Pugh, P.C., Jackson, N., Clinton, S.M., & Kerman, I.A. (2015). Inborn stress reactivity shapes adult behavioral consequences of early-life maternal separation stress. Neuroscience Letters, 584, 146–150. doi:10.1016/j.neulet.2014.10.011
- Roger-Sánchez, C., Aguilar, M.A., Rodríguez-Arias, M., Aragon, C.M., & Miñarr, J. (2012). Age-and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicology and Teratology, 34, 108–115. doi:10.1016/j.ntt.2011.07.011
- Roman, E., & Nylander, I. (2005). The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress, 8, 157–174. doi:10.1080/10253890500188666
- Song, M., Wang, X.Y., Zhao, M., Wang, X.Y., Zhai, H.F., & Lu, L. (2007). Role of stress in acquisition of alcohol‐conditioned place preference in adolescent and adult mice. Alcoholism: Clinical and Experimental Research, 31, 2001–2005. doi:10.1111/j.1530-0277.2007.00522.x
- Spear, L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 24, 417–463. doi:10.1016/S0149-7634(00)00014-2
- Tarazi, F.I., Tomasini, E.C., & Baldessarini, R.J. (1998). Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Developmental Brain Research, 110, 227–233. doi:10.1016/S0165-3806(98)00111-4
- Teicher, M.H. (2000). Wounds that time won’t heal: The neurobiology of child abuse. Cerebrum, 2, 50–67.
- Torres, O.V., Walker, E.M., Beas, B.S., & O'dell, L.E. (2014). Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism: Clinical and Experimental Research, 38, 108–115. doi:10.1111/acer.12213
- Tzschentke, T.M. (1998). Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology, 56, 613–672. doi:10.1016/S0301-0082(98)00060-4
- Vatsalya, V., Liaquat, H.B., Ghosh, K., Mokshagundam, S.P., & McClain, C.J. (2017). A review on the sex differences in organ and system pathology with alcohol drinking. Current Drug Abuse Reviews, 9, 87–92. doi:10.2174/1874473710666170125151410
- Vetulani, J. (2013). Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacological Reports, 65, 1451–1461. doi:10.1016/S1734-1140(13)71505-6
- Walters, H., & Kosten, T.A. (2019). Early life stress and the propensity to develop addictive behaviors. International Journal of Developmental Neuroscience, pii: S0736-5748(19)30069-3. doi:10.1016/j.ijdevneu.2019.06.004
- Williams, J., Jackson, S., Maddocks, A., Cheung, W., Love, A., & Hutchings, H. (2001). Case–control study of the health of those looked after by local authorities. Archives of Disease in Childhood, 85, 280–285. doi:10.1136/adc.85.4.280
- Willing, J., & Juraska, J.M. (2015). The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience, 301, 268–275. doi:10.1016/j.neuroscience.2015.05.073
- World Health Organization. (2018). Global status report on alcohol and health 2018. Geneva: World Health Organization.
