Abstract
Evidence implicates the endocannabinoid (eCB) system as a negative modulator of neural and endocrine responses to acute stressors. Recently, eCB signaling was also reported to contribute to habituation of hypothalamo-pituitary-adrenal (HPA) axis responses to repeated homotypic stress. The present studies were initiated to distinguish a potential role of eCB signaling in the expression vs. the acquisition of habituation of the HPA axis response to repeated stress. In each of three experiments, adult male Sprague Dawley rats were exposed to daily, 30-minute sessions of loud white noise (95 dB), which resulted in a progressive decrease in HPA axis response over successive days. Cannabinoid receptor 1 (CB1) antagonist AM251 (0.5, 1.0 or 2.0 mg/kg, i.p.) was used to examine the role of eCB signaling in homotypic stressor habituation and heterotypic (novel) stressor cross-sensitization of neuroendocrine activity. Pretreatment with high dose (2.0 mg/kg) AM251 before each of 7 consecutive, daily loud noise exposures (acquisition of habituation) resulted in potentiation of stress-induced HPA axis activation and disruption of habituation. After an 8th loud noise exposure without AM251 pretreatment, the same group of rats displayed a habituated plasma corticosterone (CORT) level similar to that of controls, indicating that CB1 receptor antagonist pretreatments did not disrupt the acquisition of habituation. In two additional experiments, rats acquired habituation to loud noise drug free, then lower doses of AM251 (0.5 and 1.0 mg.kg) were administered before a final exposure (expression of habituation) to the homotypic stressor and/or a novel heterotypic stressor. CB1 receptor antagonism disrupted the expression of CORT response habituation and some of the c-fos mRNA reduction associated with it and facilitated novel stressor sensitization in doses that did not potentiate acute responses to these stressors. Collectively, these data suggest a progressive intensification of neural eCB signaling at CB1 receptors with repeated stress exposures.
Introduction
Habituation of neuroendocrine responding to repeatedly experienced psychological stressors is an adaptive mechanism that can limit the accumulation of stress-related pathology (Herman, Citation2013). Impaired ability to habituate to stressors has been reported in populations with psychiatric disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD) (Brierley & Jamieson, Citation1974; Chattopadhyay et al., Citation1980; Lader & Wing, Citation1964; Thomson & Craighead, Citation2008), and this impairment may contribute to development and maintenance of these disorders. Strategies designed to correct, protect, or improve habituation to repeatedly experienced stressors may have therapeutic potential, but our knowledge is limited with regards to the neural circuitry and mechanisms responsible for this specific form of stress adaptation.
The endogenous cannabinoid (eCB) system diminishes the amplitude of neural and hormonal responses to acute psychological stress (Hill & McEwen, Citation2010; Newsom et al., Citation2012; Patel et al., Citation2004; Riebe & Wotjak, Citation2011) and may protect against pathology resulting from repeated stress (review: Hill & Patel, Citation2013). Increases in eCB signaling at central CB1 receptors have been postulated to contribute to the habituation of behavioral, neural and endocrine reactions to repeatedly experienced psychological stressors (Hill et al., Citation2010; Kamprath et al., Citation2006; Patel et al., Citation2005; Patel & Hillard, Citation2008). Repeated stress has been reported to result in stress-induced elevations of eCB ligands in stress-reactive limbic structures in patterns different than those reported to occur after acute stress (Hill et al., Citation2010; Patel et al., Citation2004, Citation2005; Patel and Hillard, Citation2008). Additionally, differences in neuroendocrine responses to CB1 receptor antagonists administered before exposure to a familiar stressor compared to the same dose given before an initial experience have supported a view that the eCB system contributes to the expression of habituated responses (Hill et al., Citation2010; Patel et al., Citation2005, Citation2008). In this context, “expression” of habituated responses refers to the observation of reduced stress-evoked responses after repeated exposures to the same (homotypic) stress situation. However, it is unclear from these experiments whether CB1 receptors also contribute to the “acquisition” of habituated responses, that is, whether CB1 receptor activity is necessary during the initial repeated homotypic stress exposures, to reduce stress responses elicited during a later homotypic stress exposure.
Under some conditions, homotypic stress habituation also produces larger or “sensitized” responses to subsequent novel stressors (Grissom et al., Citation2008; Weinberg et al., Citation2009). Antagonism of CB1 receptors as well as CB1 receptor KO both facilitate neural, behavioral, and hormonal responses to acute stress, and limbic eCB signaling is disrupted in some models of chronic stress (heterotypic – repeated exposures to different stress situations), as well as repeated glucocorticoid exposures (Hill & Patel, Citation2013). It is unclear if these instances of eCB signaling disruption directly contribute to the cross-sensitization to novel stressors that co-occurs during some repeated homotypic stress paradigms (Bhatnagar & Dallman, Citation1998; Grissom et al., Citation2008; Weinberg et al., Citation2009).
The current experiments were designed to distinguish the role of eCB signaling at CB1 receptors in the acquisition of habituated neuroendocrine responses to repeated homotypic stress, as compared to their contribution during the expression of habituated neuroendocrine responses (Campeau et al., Citation2002; Citation2008). In addition, we examined the potential contribution of eCB signaling in the cross-sensitization of neuroendocrine stress-reactivity that can co-occur following homotypic stress habituation.
Materials and methods
Subjects
One hundred and ten male Sprague-Dawley rats (Harlan, Indianapolis IN) weighing 300–350 grams were used. Animals were housed in polycarbonate tubs containing wood shavings, with wire lids providing rat chow and water ad libitum. Conditions in the animal colony were controlled to constant humidity and temperature, with a 12:12 hour light/dark cycle (lights on at 7:00 am). Testing was performed between 8:30 am and 12:30 pm during the circadian nadir for the HPA axis. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the United States of America National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
Acclimation
Animals were allowed two weeks of acclimation to the colony before testing. The first week, animals were housed in groups of three to four. During the second week of acclimation, rats were individually housed and handled daily, in the colony room, from days one through four. On each of the last three days before testing, rats were transported in their home cages from the colony to the testing room, handled, returned to their home cages, and placed inside individual acoustic chambers (without noise exposure) for thirty minutes. This pre-exposure was intended to familiarize the rats to all of the testing procedures and minimize novelty related responses on testing days.
Drug treatment
The CB1 receptor antagonist AM251 (Ascent Scientific, Princeton, NJ) was used to assess the involvement of the endogenous cannabinoid system on plasma CORT and limbic c-fos mRNA responses to acute and repeated audiogenic stress. AM251 was dissolved in dimethyl sulfoxide (DMSO), Tween 80, and physiological (0.9%) saline (in a 1:1:8 ratio, respectively). A stir plate was used to maintain suspension of AM251 in vehicle, and syringes were loaded immediately prior to dosing. Rats received acute or repeated intraperitoneal (i.p.) injections in doses of AM251 at 0.5, 1.0, or 2.0 mg/kg, in injection volumes of 1 ml/kg. On drug treatment days, control rats received a similar volume of vehicle (DMSO/Tween 80/0.9% saline) 30 minutes prior to placement in the acoustic chambers. All rats were weighed on testing days involving drug and vehicle injections to ensure specificity of dose and volume administered. During Experiment 1, the general health of rats receiving repeated injections with solutions containing DMSO and Tween 80 was monitored for visible disturbances, including fluctuation in body weight or lesions at the injection site, which were not observed. This study examined the involvement of CB1 receptors in stress habituation and sensitization rather than CB2 receptors given the primary importance of CB1 in neural regulation (Cota, Citation2007).
Experimental design
Experiment 1: Test of the necessity of CB1 receptor activity in the acquisition of neuroendocrine habituation to repeated loud noise
Rats were randomly assigned to one of three groups (n = 8/group) to receive intraperitoneal (i.p.) injections of CB1 receptor antagonist AM251 at high dose (2 mg/kg), low dose (0.5 mg/kg), or vehicle thirty minutes before each of seven consecutive daily 30-minute exposures to loud noise stress (95 dBA), as indicated in . This regimen of loud noise stress exposures consistently induces reliable habituation of HPA axis responses (Masini et al., Citation2008). Rats were not given drug pretreatment on the 8th day of loud noise exposure, to test for potential lasting alterations in habituation caused by the antagonism of CB1 receptor signaling during the previous 7 exposures to the stressor, and distinguish habituation-related alterations from acute effects of AM251 on HPA axis reactivity. Blood samples were collected immediately after stress exposures on days 1, 3, 7, and 8, using heparinized capillary tubes and a small tail nick at the base of the tail. Plasma was assayed to measure corticosterone (CORT) levels. The plasma volumes collected were too small to assay adrenocorticotropic hormone (ACTH) levels, perhaps overlooking a level of neuroendocrine activity that could provide a different functional dynamic assessment of stress reactivity.
Figure 1. Experimental design summaries for Experiments 1-3. CORT: plasma corticosterone measured in blood sample taken via tail nick except for D9 blood sample taken via trunk blood; D: Testing day; Env, (Novel) Environment testing.
Experiment 2: Test of CB1 receptors in the expression of habituated neuroendocrine responses and sensitization to a mild heterotypic stressor
The results of Experiment 1 suggested that CB1 receptor signaling was not necessary for the acquisition of HPA axis habituation to repeated loud noise stress, but that repeated stress experience altered the eCB system such that CB1 receptors increased their inhibitory influence over HPA axis response to an increasingly familiar stressor. Experiment 2 was designed to test the necessity of CB1 receptor signaling in the expression of a previously acquired habituated neuroendocrine response. Additionally, given the general role of the eCB system in stress reactivity, we examined whether alterations in CB1 receptor signaling resulting from repeated homotypic stress experience would sensitize reactivity to a subsequent, mild heterotypic stressor. A moderate dose (1 mg/kg) of AM251 previously shown to have negligible effects on acute neuroendocrine stress responses (Hill et al., Citation2010) was selected for use in Experiment 2 to avoid antagonist-induced potentiation of acute stress reactivity as obtained with 2 mg/kg AM251 in Experiment 1.
Rats (n = 18) were initially exposed to daily, 30-minute loud noise stress sessions (95 dBA) for seven days to habituate HPA axis responses (see ). An additional control group of rats (n = 3) was transported from the colony daily and placed into quiet noise chambers for the same duration to later serve as an acute stress treatment group on day 9. Blood samples were taken via tail nick after the first day of loud noise stress for determination of acute stress CORT release. On day 8, rats were randomly assigned to one of two groups (n = 9/group) to receive pre-injection of AM251 (1.0 mg/kg), or vehicle before a final loud noise stress exposure. Blood samples were taken immediately following stress treatment. On day 9, rats were again given pretreatment of AM251 (1.0 mg/kg) or vehicle 30 minutes before being exposed to a mild heterotypic psychological stressor (15 minutes in a novel environment) to examine a possible sensitization of HPA axis response resulting from the previous 8 days of loud noise stress. To ensure absence of a confounding additive effect of AM251 treatment from day 8 to day 9, group assignments (vehicle/AM251) for roughly half of the repeated stressed rats were randomly selected and switched to the other drug treatment before day 9 testing. Control rats without repeated loud noise exposures were pretreated with AM251 before novel environment exposure. Blood samples were taken from all rats immediately following novel environment exposure on day 9. Plasma CORT was later measured as an index of stress-reactivity.
Experiment 3: Further examination of CB1 receptor signaling in expression of habituation, cross-sensitization to a heterotypic stressor, and acute compared to repeated stress-induced neural activity patterns
Rats (n = 65) were randomly assigned to receive repeated loud noise stress (n = 34) or to be used as acute loud noise stress controls (n = 19) or acute restraint stress controls (n = 12). Rats designated for repeated stress received 7 daily loud noise stress exposures (once/day, 30-minute durations, 95dBA) to induce neuroendocrine stress habituation. Acute stress rats were handled and transported the same way as the repeatedly stressed rats but were placed in quiet noise chambers for 7 daily 30-minute sessions. On day 8, CB1 receptors were antagonized to test for cross-sensitization of reactivity to an acute restraint episode (see ). Repeatedly stressed rats were assigned to 3 groups (Vehicle, n = 11; 0.5 mg/kg AM251, n = 12; 1.0 mg/kg AM251, n = 11). Drugs were administered via i.p. injections 30 minutes prior to the 30-minute session of restraint stress. Blood samples were taken via tail nick immediately after restraint. On day 9, control rats were divided into two groups (Acute stress control/Vehicle, n = 9; Acute stress control/0.5 mg/kg AM251, n = 10), and all rats were exposed to loud noise stress with repeatedly stressed rats receiving the same drug pretreatments as day 8. Immediately following loud noise stress treatment, acute and repeatedly stressed rats were sacrificed. Trunk blood was collected for CORT measurement, and brains were rapidly excised and frozen for later sectioning and immediate early gene (c-fos mRNA) measurements. An independent cohort of rats was randomly divided into two groups (n = 6/group) and given i.p. injections of AM251 (0.5 mg/kg) or vehicle 30 minutes prior to a single restraint stress treatment (lasting 30 minutes). A blood sample was taken via tail nick following restraint. The low 0.5 mg/kg AM251 treatment was not expected to increase stress-induced CORT in rats experiencing loud noise or restraint for the first time, but both doses of the CB1 receptor antagonist were expected to induce higher CORT responses in rats that previously experienced repeated loud noise stress.
Loud noise stress
The acoustic chambers used in this experiment have been described in detail in Day et al. Citation2009 and Nyhuis et al., Citation2016b. In brief, each acoustic chamber consists of ventilated double wooden walls designed to fit the entire home cage of a rat directly underneath a 15.24x22.86 cm speaker that is mounted in the top of the inner chamber. The speaker receives amplified noise produced by a General Radio (#1381) solid-state random-noise generator with the bandwidth set at 2 Hz-50 kHz. The inner wall of the large acoustic chamber is lined with 2.54 cm of Celotex™ insulation to attenuate noise levels, allowing for loud noise stress and “no noise” control treatments to be performed simultaneously in the same testing room. On the testing day, rats were placed in the acoustic chambers in their home cages thirty minutes after vehicle or AM251 injection. Rats were either kept under quiet “no noise” control conditions (background noise of fans approximately 57 dB SPL - A scale) or loud noise (95 dBA) was turned on immediately and remained on for thirty minutes.
Restraint stress
Restrainers were constructed from 0.64 cm wire mesh. The mesh was formed into 7.6 cm diameter cylinders that were 30.5 cm long. A 5.1 cm wide, 0.64 cm thick piece of white painted wood was placed at the bottom of the mesh cylinder to form a platform for the rat to sit on. The mesh was stapled to the wood on the outside of the cylinder. The ends of the cylinders were plugged with 7.6 cm diameter plastic atrium grates. Sections of the grates were removed to allow the rats' tails to protrude from the cylinders. The grates were secured on both sides of the restrainers with small bungee cords. With the grates in place, the internal dimensions of the wire mesh restrainers were similar to those of standard Plexiglas restrainers (17.8 cm length and 6.4 cm diameter) and have shown comparable stress-induced ACTH and CORT hormone release (Masini et al., Citation2012). Restraint stress occurred in the same room as loud noise exposure after appropriate acclimation to testing procedures and location. Rats were removed from their cages immediately before being placed into restraint tubes, which were then placed on a lab bench covered in lab mat. Restraint tubes were cleaned with dish soap, hot water, and dried between uses.
Novel environment stress
Rats were placed in clean, white five-gallon buckets (without bedding) for fifteen minutes as a mild novel stressor (Babb et al., Citation2014). Buckets were cleaned with 50% ethanol and air-dried between uses to minimize odor cross-contamination.
Corticosterone enzyme linked ImmunoSorbent assays (ELISA)
The corticosterone assay was performed according to the manufacturer’s instructions (kit #K014-H5– Arbor Assays, Ann Arbor, MI) using 10 microliters of plasma. Levels were quantified on a BioTek Elx808 microplate reader and calculated against a standard curve generated concurrently.
In situ hybridization
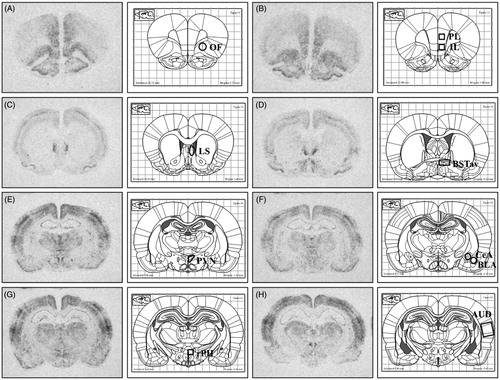
The method for in situ hybridization histochemistry has been previously described (Day & Akil, Citation1996). Briefly, 12 μm sections were cut on a cryostat (Leica model 1850), thaw-mounted on polylysine-coated slides and stored at −80 °C. A [35S]-UTP-labeled riboprobe against c-fos mRNA (680-mer; courtesy of Dr. T. Curran, St Jude Children’s Hospital, Memphis TN) was generated using standard transcription methods. Sections were fixed in 4% paraformaldehyde (1 hour), acetylated in 0.1 M triethanolamine with 0.25% acetic anhydride (10 min.) and dehydrated through graded alcohols. Sections were hybridized overnight at 55 °C with a [35S]-UTP-labeled riboprobe diluted in hybridization buffer containing 50% formamide, 10% dextran sulfate, 2× saline sodium citrate (SSC), 50 mM PBS, pH 7.4, 1× Denhardt’s solution, and 0.1 mg/ml yeast tRNA. The following day, sections were treated with RNase A, 200 ug/ml at 37 °C (1 hour), and washed to a final stringency of 0.1× SSC at 65 °C (1 hour). Dehydrated sections were exposed to X-ray film (BioMax MR; Eastman Kodak, Rochester, NY) for structure-appropriate times (1–3 weeks) and the films analyzed as described below. Structures chosen for c-fos mRNA analysis include regions with high levels of neuronal activity (as indicated by induction of c-fos mRNA) in response to acute loud noise stress (Burow et al., Citation2005) and regions displaying AM251-induced potentiation of noise stress-evoked c-fos mRNA (Newsom et al., Citation2012). The rostral posterior hypothalamus was analyzed due to recent work in our lab implicating involvement of this region in habituation-related plasticity (Nyhuis et al., Citation2016a). The basolateral amygdala (BLA) and central amygdala (CeA) were analyzed due to previous reports of repeated stress-related increases in eCB activity and levels in the amygdala (Hill et al., Citation2010; Patel et al., Citation2005). Frontal cortex regions, including prelimbic (PL), infralimbic (IL), and orbitofrontal (OF) were analyzed due to contribution of these regions to regulation of stress reactivity in some stressor paradigms (Campeau et al., Citation2002; Weinberg et al., Citation2010) and in fear-related learning (Mahan & Ressler, Citation2012; VanElzakker et al., Citation2014). contains atlas images indicating the locations of the neural regions analyzed (Paxinos and Watson, Citation1998).
Semi-quantitative x-ray film analysis
Levels of c-fos mRNA were analyzed by computer-assisted optical densitometry. Anatomical landmarks were based on the white matter distribution of unstained tissue sections, according to a standard rat brain atlas (Paxinos and Watson, Citation1998). Brain sections were captured digitally (CCD camera, model XC-77; Sony, Tokyo, Japan), and the relative optical density of the x-ray film was determined using Scion Image version 4.0 for PC. A macro was written (Dr. S. Campeau) that enabled signal above background to be determined automatically. For each section, a background sample was taken over an area of white matter, and a signal threshold was calculated as mean gray value of background + 3.5 standard deviation. The section was automatically density sliced at this value, so that only pixels with gray values above these criteria were included in the analysis. Quantification templates were created in Scion Image with size and geometric shape specific to each neural region of interest and are indicated, along with representative autoradiographic images of acute stress-induced c-fos mRNA expression, in .
Statistical analyses
Prism (v 6.0, GraphPad Software Inc.) was used for all statistical analyses, which included two-way analyses of variance (ANOVA, repeated measures design when applicable), Bonferroni’s multiple comparisons test for post-hoc analyses, and two-tailed t-tests for pilot testing and planned comparisons, as indicated in the text. Significance for all tests was established at a P = 0.05. All data presented in the figures are listed as mean values +/− 1 standard error. Outlier values were identified as those being greater than 2 standard deviations from the group mean when included in the dataset and were excluded. An exception to this occurred in the repeated measures ANOVA in Experiment 1, in which 3 individual outlier values (one from each drug treatment group) were replaced with group means in the final graph and report of statistical results, which did not modify the overall results of this study. Some variation in degrees of freedom reflects sample loss during processing.
Results
Experiment 1: Test of the necessity of CB1 receptor activity in the acquisition of neuroendocrine habituation to repeated loud noise
Plasma CORT values from blood samples taken after 1, 3, 7, and 8 days of loud noise stress are presented in . CORT values were used to determine the effects of CB1 receptor antagonism on acquisition of HPA axis habituation to repeated loud noise stress. Analysis of plasma CORT was performed with two-way repeated measures (RM) ANOVA with day of stress treatment (1,3,7, and 8) and drug treatment (vehicle, 0.5, and 2.0 mg/kg AM251) as factors. A significant main effect of day (F(3,28) = 58.72, p < 0.001) indicated significant habituation of CORT response to loud noise stress occurred in response to repeated exposures. Post hoc analyses using Bonferroni’s multiple comparisons test confirmed that in all three groups, day 7 values of plasma CORT were significantly lower than day 1 values (p < 0.05) indicating that all treatment groups displayed significant habituation. A significant main effect of drug treatment (F(2,56) = 42.45, p < 0.001) indicated that CB1 receptor antagonism altered plasma CORT values. Bonferroni’s post hoc comparisons confirmed that 2 mg/kg AM251 treatment significantly increased plasma CORT compared to the lower 0.5 mg/kg dose and vehicle treatment groups (p < 0.001), which were not different from each other (p > 0.05). Significant interaction between drug treatment and day of stress treatment (F(6,56) = 4.58, p < 0.001) indicated that daily CB1 receptor antagonism altered the measured habituation rate of CORT response to repeated loud noise stress. Bonferroni post hoc comparisons confirmed that 2 mg/kg AM251 pretreatment resulted in insignificant reduction of HPA axis responses between days 1 and 3 as well as between days 3 and 7 (p > 0.05 for both), compared to significant reductions between these time points in both 0.5 mg/kg AM251 and vehicle treatments (p < 0.05 for all).
Figure 2. Dose-dependent ability of CB1 receptor antagonism disrupts the expression, but not the acquisition, of CORT response habituation to repeated loud noise stress. Rats (n = 8/group) were given systemic pretreatment with CB1 receptor antagonist AM251 (2.0 or 0.5 mg/kg) or vehicle before each of the first 7 days of loud noise stress exposure (30 min/day, 95 dB). Repeated Measures (RM) two-way ANOVA indicated that 2.0 mg/kg AM251 treatment significantly potentiated plasma corticosterone (CORT) responses and resulted in slower rate of habituation (# significant interaction of stress and drug, p < 0.001, confirmed post hoc with Bonferroni mct). Rats were not given drug treatment before the 8th loud noise stress exposure in which all three groups displayed similar level of HPA axis response habituation. The 2.0 mg/kg AM251 treatment group displayed significant reduction from day 7 to day 8, but 0.5 mg/kg AM251 and vehicle treatment groups did not (*p < 0.001).
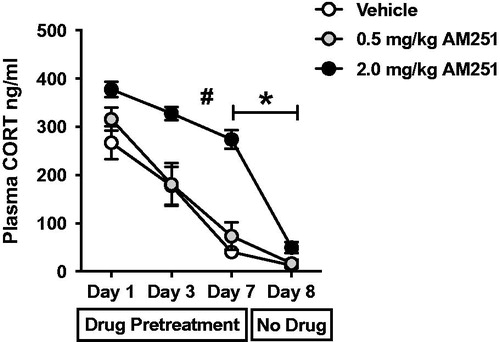
On the 8th day of loud noise exposure, all rats were injection free to test the acquisition of HPA axis habituation without CB1 receptor antagonism. Bonferroni’s post hoc comparisons used to examine repeated measures two-way ANOVA interaction effects indicated that 2 mg/kg AM251-treated rats demonstrated significantly greater reduction of plasma CORT response from day 7 to day 8 (p < 0.001) compared to 0.5 mg/kg AM251- and vehicle-treated rats, which did not display significant reductions between day 7 and day 8 (p > 0.05). Importantly, no differences between the three groups were observed on day 8 CORT values (p > 0.05). These analyses indicate that daily CB1 receptor antagonism has a dose dependent effect on HPA axis response to repeated loud noise stress, such that 2 mg/kg AM251 pretreatments increased CORT responses to noise stress and altered the rate of habituation. This led to an apparent disruption of the habituation of the HPA axis response to repeated loud noise stress by the higher antagonist dose, but when rats were tested drug-free (day 8), significant CORT response habituation was readily observable, and statistically similar to that of the vehicle and low dose treatment groups. These results suggest that CB1 receptor antagonism disrupts the expression of HPA axis habituation to repeated stress exposures, while not preventing the acquisition of the plasticity necessary for normal habituation. Further, these analyses indicate 0.5 mg/kg AM251 to be a dose without overt effect in the acquisition or expression of habituated CORT responses.
Experiment 2: Test of CB1 receptors in the expression of habituated neuroendocrine responses and sensitization to a mild heterotypic stressor
Plasma CORT values from initial (day 1, non-treated) and repeated loud noise stress (day 8, drug or vehicle-treated) are presented with non-stress controls in . Two-way, RM ANOVA using day of stress exposure (day 1 or day 8) and drug treatment (vehicle, 1 mg/kg AM251) as factors indicated significant main effects of day of drug/stress treatment (F(1,34) = 17.52, p < 0.001) and drug treatment (F(1,15) = 10.08, p < 0.01), and a significant interaction between the two factors (F(1,15) = 11.71, p < 0.01). Bonferroni post hoc comparisons confirmed that rats administered vehicle on day 8 of loud noise stress displayed significantly habituated HPA axis responses compared to day 1 values (p < 0.001), but that 1 mg/kg AM251 pretreatment on day 8 resulted in plasma CORT level indistinguishable from day 1 values (p > 0.05). Planned comparison of day 8 CORT values with two tailed t-test indicated that 1 mg/kg AM251 treatment significantly increased HPA axis response compared to the habituated response of vehicle treated controls (T(16) = 4.05, p < 0.001), confirming our hypothesis that CB1 receptor antagonism disrupts the expression of previously acquired habituated responses, as suggested in Experiment 1.
Figure 3. Low dose CB1 receptor antagonist AM251 (1 mg/kg) pretreatment disrupts the expression of HPA axis habituation. Test of CB1 receptor involvement in heterotypic stressor sensitization. (A) Rats (n = 9/group) were exposed to daily loud noise stress without drug treatment (30 min/day, 95 dB). A group of control rats (n = 3) received similar handling but were not exposed to noise stress during the first 8 days, so serve as a control for day 9 testing (n = 3) included for day 9 control group. Repeated measures two-way ANOVA indicates that pretreatment with 1.0 mg/kg AM251 before the 8th loud noise exposure completely prevented expression of a habituated HPA axis response. Vehicle-treated controls display robust habituation of plasma CORT response to the 8th loud noise exposure, compared to the 1st exposure (^p < 0.001, post hoc Bonferroni mct). 1 mg/kg AM251 treatment results in significantly higher plasma CORT response to the 8th loud noise exposure, compared to vehicle controls (*p < 0.001, post hoc Bonferroni mct). (B) Heterotypic stressor sensitization test: After 8 days of loud noise stress, rats were exposed to 15 minutes of novel environment stress on day 9. 1 mg/kg AM251 treatment significantly increased plasma CORT levels compared to vehicle-treated rats with the same stress history (*p < 0.05, post hoc Bonferroni mct). A lack of facilitating effect on plasma CORT response to 1.0 mg/kg AM251 was observed in control rats without recent stress history when compared to their previous non-stress values or to vehicle treated rats with repeated stress history. However, the difference between the two 1.0 mg/kg-treated groups failed to reach significance (p > 0.05, post hoc Bonferroni mct).
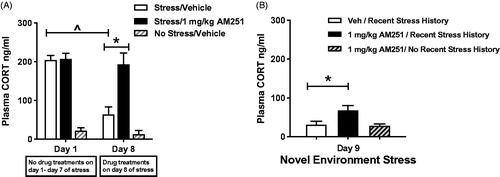
An additional group of non-stressed control rats was placed in quiet noise chambers for 8 days during the repeated stress treatments. This group received the same acclimation and study-related handling as the stress-treated groups, including blood samples being taken on days 1 and 8 and i.p. vehicle injection on day 8. These values are included in the graph for visual comparison but were not analyzed in the repeated measures two-way ANOVA. Mean plasma CORT values for these rats were 22.83 (± 6.74) and 14.04 (± 8.78) ng/ml on day 1 and day 8, respectively. Analysis by two-tailed, paired t-test indicates these values to be statistically similar (p = 0.47).
Plasma CORT response to the mild stress of a novel environment () was used to examine whether repeated noise stress experience would result in a cross-sensitization to a heterotypic stressor that may be dependent on disruption of CB1 receptor signaling. Control rats without repeated noise stress history were included for examination of acute effects of 1 mg/kg AM251 on reactivity to novel environment stress. A one-way ANOVA indicated a significant difference among treatment groups (F(2,17) = 4.43, p < 0.05). Bonferroni post hoc analysis indicated that 1 mg/kg AM251 treatment before novel environment stress resulted in higher plasma CORT levels in rats with repeated noise stress experience compared to vehicle-treated controls with the same stress history (p < 0.05), providing some evidence for stressor cross-sensitization mediated by eCB receptor disruption. However, in the same post hoc analysis, plasma CORT responses in AM251-treated rats without recent stress history were not found to statistically differ from either treatment group (p > 0.05). Comparisons of the two previous day 8 treatments within each group found no effect of previous drug treatment on day 9 CORT level (t- test, p = 0.64, 0.73; data not shown).
Experiment 3: Further examination of CB1 receptor signaling in expression of habituation, cross-sensitization to a heterotypic stressor, and acute compared to repeated stress-induced neural activity patterns
A lower dose (0.5 mg/kg) of AM251 treatment group was added in Experiment 3 to ensure that any stimulatory effects of AM251 observed on HPA axis response to the stressors used for habituation and cross-sensitization testing after repeated stress were independent of acute potentiation from CB1 receptor antagonism. This dose was expected to be without acute stimulatory effect given the results of Experiment 1. Habituation to repeated loud noise stress was analyzed by comparing plasma CORT levels from the 1st and 8th exposures to loud noise stress (. Two-way repeated measures ANOVA with stress treatment (acute vs. repeated) and drug treatment (vehicle, 0.5, and 1.0 mg/kg AM251) indicated significant main effects of stress treatment (F(1,28) = 71.4, p < 0.001), but not drug treatment (F(2,28) = 1.65, p = 0.21), and a significant interaction between the two (F(2,28) = 5.24, p < 0.05). Bonferroni post hoc comparisons confirmed that rats receiving vehicle treatment and 0.5 mg/kg AM251 displayed significantly habituated plasma CORT levels compared to non-treated day 1 values (p < 0.001). However, 1.0 mg/kg treatment before the 8th day of noise exposure resulted in statistically similar values to day 1 (p > 0.05; . Planned comparisons of plasma CORT levels from the 8th day of loud noise stress exposure were performed with independent two-tailed t-tests, and indicated that pretreatment with 1.0 mg/kg of the CB1 receptor antagonist resulted in significantly higher stress-induced plasma CORT compared to vehicle-treated controls (T(19) = 3.78, p < 0.001), but this effect did not reach significance with the 0.5 mg/kg treated group (T(19) = 1.69, p = 0.11). However, when these data were analyzed as the percentage of habituation of initial noise stress-induced plasma CORT level, both doses of AM251 were found to result in significantly less habituation compared to that of vehicle controls (. A one-way ANOVA indicated significant alteration of percent habituation by AM251 treatment (F(2,30) = 4.81, p < 0.5). Planned comparisons with two-tailed t-tests demonstrated that both doses significantly decreased habituation percentage (0.5 mg/kg: T(19) = 2.10, p < 0.05; 1.0 mg/kg: T(19) = 4.22, p < 0.001; ), indicating that the CB1 receptor antagonist treatments partially disrupted the expression of HPA axis habituation in a dose-dependent manner.
Figure 4. Low doses of CB1 receptor antagonism partially disrupt the expression of CORT response habituation to loud noise stress, and result in sensitized response to a heterotypic stressor. (A) Vehicle and 0.5 mg/kg AM251 treatment groups display significant habituation on Day 9 (^p < 0.05, RM two-way ANOVA with Bonferroni post hoc mct). 1.0 mg/kg AM251 significantly reduced the expression of habituation on Day 9 (*p < 0.05, planned t-test comparison). 0.5 mg/kg treatment did not reach significance in this measure (p = 0.1, planned t-test comparison). (B) Plasma CORT response to repeated noise stress graphed as percentage of Day 1 response. Both doses of AM251 significantly reduced the percentage of habituation, indicating a disruption of the expression of habituation (*p < 0.05, planned t-test comparisons). (C) Day 8 cross-sensitization testing. Repeatedly stressed rats displayed significantly increased plasma CORT response to restraint, compared to vehicle-treated group (*p < 0.05). (D) Acute noise and restraint stress controls. 0.5 mg/kg AM 251 did not potentiate plasma CORT response to the initial loud noise exposure in acute stress control rats (p = 0.34, t-test). Tissue was collected from rats after acute noise stress for comparisons of neural activity to repeatedly stressed rats. In a separate cohort of rats without recent repeated stress history, 0.5 mg/kg AM251 did not potentiate plasma CORT response to restraint stress (p = 0.75, t-test).
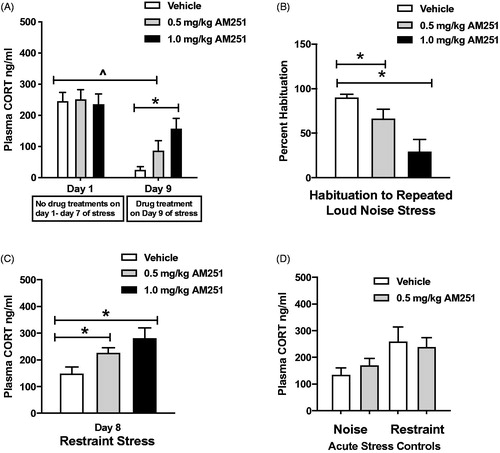
Cross-sensitization to restraint stress was tested on day 8 of experiment 3 (. One-way ANOVA indicated a significant difference in restraint-induced plasma CORT levels (F(2,28) = 5.68, p < 0.01). Planned comparisons between vehicle-treated rats and rats treated with 0.5 or 1.0 mg/kg AM251 with independent two-tailed t-tests confirmed that both doses of AM251 resulted in facilitated HPA axis responses compared to vehicle treatment (0.5 mg/kg: T(18) = 2.58, p < 0.05; 1.0 mg/kg: T(19) = 2.98, p < 0.01). In contrast to the cross-sensitizing effect of 0.5 mg/kg AM251 in rats habituated to repeated loud noise stress, the test of this low dose in a separate cohort of rats without recent repeated stress history (n = 12) demonstrated a lack of HPA axis potentiating effect compared to vehicle treatment in acutely restrained rats without recent repeated stress history (T(10) = 0.32, p = 0.75; . Two additional groups of rats without repeated loud noise stress history were exposed to acute loud noise stress on day 9 of Experiment 3 after pretreatment with 0.5 mg/kg AM251 (n = 10) or vehicle (n = 9). These treatments were found to result in similar plasma CORT values when compared with two-tailed t test (T(17) = 1.03, p = 0.34; . Tissue from these acutely stressed rats without repeated loud noise stress history was used for comparison to tissue from repeatedly stressed rats in neural measures.
Stress-induced c-fos mRNA was measured in tissue from rats administered 0.5 mg/kg AM251 or vehicle before acute or repeated loud noise. contains atlas images indicating the locations of the neural regions analyzed, along with representative autoradiographic images of acute stress-induced c-fos mRNA expression. We hypothesized that 0.5 mg/kg of CB1 receptor antagonist AM251 would not result in increases in c-fos mRNA compared to tissue from vehicle-treated rats after acute loud noise stress but would in tissue collected from rats given repeated loud noise stress. This pattern was measured in several hypothalamic and extra-hypothalamic limbic structures able to modulate stress-reactivity. Data for all regions were analyzed with two-way ANOVA, post hoc Bonferroni multiple comparisons, and independent two-tailed t-test for planned comparison between vehicle and 0.5 mg/kg AM251 treatment in repeatedly stressed rats. Consistent with the majority of HPA axis measures in this experiment, 0.5 mg/kg AM251 was determined by post hoc analyses to not alter c-fos mRNA induction by acute noise stress treatment (p > 0.05). This was consistent in all neural regions examined. Values for all c-fos mRNA analyses are graphed as a percent of the average value for control (acute stress, vehicle-treatment) rats.
Figure 5. Representative autoradiographs of c-fos mRNA with corresponding atlas images (Paxinos and Watson). These representative autoradiographs indicate the regions quantified, as well as the template size, shape, and placement used in analysis. (A) Orbitofrontal cortex (OF). (B) top: Prelimbic cortex (PL), bottom: Infralimbic cortex (IL). (C) Lateral Septum (LS). (D) Antero-ventral bed nucleus of the stria terminalis (BSTav). (E) Paraventricular nucleus of the hypothalamus (PVN). (F) top/medial: Central nucleus of the amygdala (CeA), bottom/lateral: Basolateral nucleus of the amygdala (BLA). (G) Rostral posterior hypothalamus (rPH). (H) Auditory cortex.
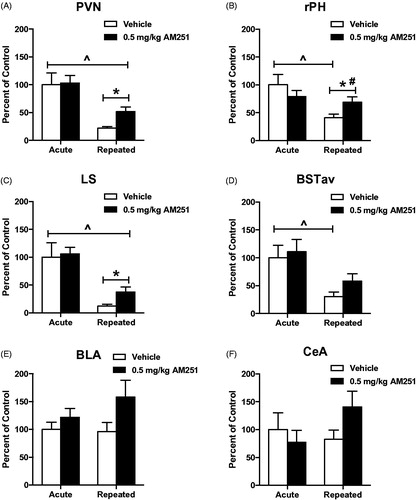
The paraventricular nucleus of the hypothalamus (PVN) was measured to display a pattern similar to plasma CORT values in rats treated with 0.5 mg/kg AM251, such that this dose resulted in significant increase in c-fos mRNA expression in rats with repeated noise stress history, but not in acutely stressed rats (. Two-way ANOVA with stress treatment (acute vs. repeated) and drug treatment (vehicle or 0.5 mg/kg AM251) as factors indicated a significant main effect of stress treatment (F(1,38) = 27.55, p < 0.001), but not overall drug treatment (F(1,38) = 1.76 p = 0.19) or interaction between the two treatments (F(1,38) = 1.19 p = 0.28). Bonferroni post hoc analysis confirmed that c-fos mRNA values were significantly decreased after the 8th day of exposure to loud noise stress compared to values measured in acute rats, in both vehicle (p < 0.001) and AM251 (p < 0.05) treatment groups, and that AM251 treatment was without effect in acutely stressed rats (p > 0.05). Planned comparison indicated that in repeatedly stressed rats, 0.5 mg/kg AM251 treatment increased PVN c-fos mRNA compared to vehicle treatment (T(21) = 3.14, p < 0.01), supporting involvement of a central CB1 receptor mechanism in the disrupted expression of CORT habituation.
Figure 6. Low dose CB1 receptor antagonism disrupts the habituation of neural activity in multiple limbic regions. Stress-induced c-fos mRNA significantly habituated in the A. Paraventricular nucleus of the hypothalamus (PVN), B. Rostral posterior hypothalamus (rPH), C. Lateral septum (LS), and in the D. Anteroventral bed nucleus of the stria terminalis (BSTav) after 8 exposures to repeated loud noise stress (^p < 0.05, two-way ANOVA). 0.5 mg/kg AM251 did not significantly increase c-fos mRNA responses to initial, acute loud noise stress, but this dose significantly disrupted the expression of c-fos mRNA habituation in the (A) PVN, (B) rPH, and (C) LS (*p < 0.05 planned t-test comparisons), but this measure did not reach significance in the (D) BSTav (p = 0.1). A significant interaction of drug and stress experience was measured in the PH (#p < 0.05, two-way ANOVA). BLA and CeA c-fos mRNA did not display significant increase by CB1 receptor antagonism (BLA: p = 0.1, CeA: p = 018, planned t-test comparisons).
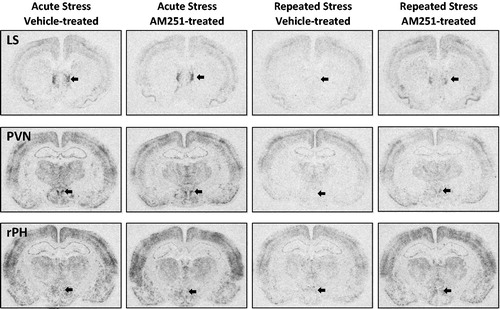
Rostral posterior hypothalamus (rPH) analysis indicated a similar pattern of CB1 receptor antagonist-mediated disruption of habituation in repeatedly stressed rats, with AM251 treatment resulting in complete restoration of c-fos mRNA induction to levels measured in rats treated with AM251 before acute stress (see . Two-way ANOVA indicated a significant main effect of stress treatment (F(1,37) = 9.06, p < 0.01), but not drug treatment (F(1,37) = 0.1, p = 0.76), and a significant interaction between drug and stress treatments (F(1,37) = 4.49, p < 0.05). Post hoc analysis determined that c-fos mRNA induction was significantly habituated on the 8th day of loud noise stress exposure compared to the first exposure in vehicle-treated rats (p < 0.01). AM251 pretreatment on the 8th day of noise stress exposure significantly increased c-fos mRNA values compared to vehicle-treated controls (T(20) = 2.41, p < 0.01). Analysis of lateral septum (LS, ) c-fos mRNA induction with two-way ANOVA revealed significant main effect of stress treatment (F(1,35) = 29.05, p < 0.001), but not drug (F(1,35) = 1.14, p = 0.29), or interaction between the two (F(1,35) = .46, p = 0.50). Bonferroni post hoc comparisons confirmed that both AM251 and vehicle-treated rats displayed significantly habituated c-fos mRNA values after the 8th day of noise stress compared to acute stress controls (p < 0.01). As measured in the PVN and rPH, 0.5 mg/kg AM251 treatment significantly increased c-fos mRNA in the LS on the 8th day of loud noise stress compared to vehicle treatment (T(19) = 2.32, p < 0.05). A similar pattern in the anterior ventral bed nucleus of the stria terminalis (BSTav, ) failed to reach significance in t-test comparison (T(20) = 1.74, p = 0.1). In this region, two-way ANOVA revealed a significant effect of stress treatment (F(1,37) = 13.04, p < 0.001) that was confirmed by post hoc comparisons to indicate that in vehicle-treated rats, repeated noise stress resulted in significantly reduced c-fos mRNA induction compared to acute noise stress (p < 0.05). Interestingly, in the same post hoc comparisons, BSTav c-fos mRNA induction was not found to significantly differ between acute and repeated stress conditions in AM251-treated rats (p > 0.05). contains representative autoradiographs of LS, PVN, and rPH c-fos mRNA expression.
Figure 7. Representative autoradiographs of c-fos mRNA for neural regions with disruption of habituated response by low dose CB1 receptor antagonism. These images demonstrate the autoradiographic pattern observed in the neural regions in which a low dose of CB1 receptor antagonist AM251 (0.5 mg/kg, intraperitoneal injection) was not measured to potentiate expression of the immediate early gene resulting from a single acute loud noise stress exposure, but did significantly disrupt the expression of habituation of this marker of neural activity compared to vehicle-treated controls. LS: Lateral septum, PVN: Paraventricular nucleus of the hypothalamus, rPH: Rostral posterior hypothalamus (regions generally indicated with arrows).
Analysis of basolateral amygdala (BLA) c-fos mRNA with two-way ANOVA indicated no significant differences due to stress (F(1,36) = 0.53, p = 0.47) drug (F(1,36) = 3.44, p = 0.07), or stress x drug interaction (F(1,36) = 1.79, p = 0.38; . Planned comparison of BLA c-fos mRNA in repeatedly stressed rats found a trend of increase due to drug treatment (T(21) = 1.74, p = 0.1). Similarly, central amygdala (CeA) c-fos mRNA values () were not found to differ due to stress (F(1,34) = 0.39, p = 0.53) drug (F(1,34) = 0.15, p = 0.70), or interaction of stress and drug treatments (F(1,34) = 2.02, p = 0.16). Planned comparison of vehicle and AM251 treatments in repeatedly stressed rats did not indicate a difference in CeA c-fos mRNA (T(17) = 1.38, p = 0.18).
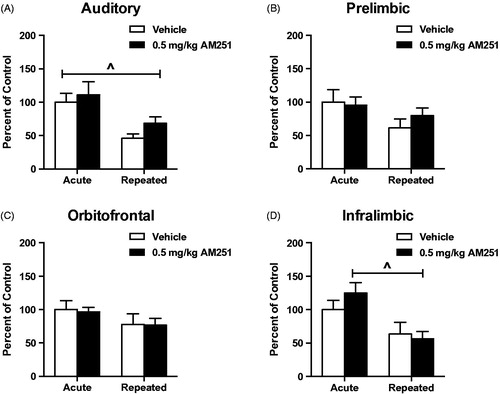
Cortical c-fos mRNA was measured in several stress-related regions as shown in . In general, this marker of neural activity was found to habituate in the auditory (AUD) and infralimbic (IL) regions, but not in the orbitofrontal (OF) or prelimbic (PL) regions. None of the four regions displayed significant effect of CB1 receptor antagonism in acute or repeated stress conditions. For AUD cortex c-fos mRNA, 2-way ANOVA revealed significant effect of stress (F(1,36) = 15.04, p < 0.001), but not drug treatment (F(1,36) = 1.78, p = 0.19), or stress x drug interaction (F(1,36) = 0.21, p = 0.65; . Bonferroni multiple post hoc comparisons indicated significant habituation in repeatedly stressed, vehicle-treated rats compared to acute stress controls (p < 0.05), but this difference was not significant in AM251 treatment groups (p > 0.05). Planned comparison of AM251 to vehicle treatment found a trend toward increase in c-fos mRNA expression from CB1 receptor antagonism on the 8th day of loud noise stress (T(20) = 1.97, p = 0.06). Analysis of PL and OF c-fos mRNA revealed no effects of stress treatment (PL: F(1,35) = 3.83, p = 0.06; OF: F(1,38) = 2.99, p = 0.09), drug treatment (PL: F(1,35) = 0.25, p = 0.62; OF: F(1,38) = 0.04, p = 0.84), or stress x drug interaction (PL: F(1,35) = 0.69, p = 0.41, OF: F(1,38) = 0.02, p = 0.90; )). Planned comparisons in repeated stress groups for both regions did not indicate difference resulting from drug treatment (PL: T(19) = 1.03, p = 0.31; OF: T(21) = 0.04, p = 0.97). Analysis of IL c-fos mRNA with 2-way ANOVA revealed significant effect of stress treatment (F(1,37) = 12.82, p = 0.001) but not drug treatment (F(1,37) = 0.37, p = 0.55) or interaction between the two (F(1,37) = 1.19, p = 0.28; . Planned comparison in repeatedly stressed rats indicated no difference attributable to drug treatment in this region (T(20) = 0.35, p = 0.73).
Figure 8. Low dose CB1 receptor antagonism does not alter responses of cortical neural activity in rats habituated to loud noise stress. (A) Auditory cortex and (D). Infralimbic cortex c-fos mRNA were measured to significantly habituate after 8 loud noise stress exposures (^p < 0.001, two-way ANOVA). (B) Prelimbic and (C). Orbitofrontal (C) cortex c-fos mRNA did not significantly habituate to repeated loud noise stress (p > 0.05, two-way ANOVA). Low dose CB1 receptor antagonism did not alter c-fos mRNA responses in rats habituated to loud noise stress in any of these cortical regions (p > 0.05, planned t-test comparisons).
Discussion
The results of these studies support the progressive inhibitory role of endogenous cannabinoid signaling in both the reduction of HPA axis and neural responses to repeated homotypic stress exposures. This progressive stressor-related inhibitory role was also observed in response to acute heterotypic stressors, a mechanism that may be implicated in the sensitization of stress-related responses often reported after multiple types of repeated stress exposures. These two actions of repeated homotypic stress exposures may or may not occur through the same mechanism. Additionally, our results indicate an important distinction of eCB receptor signaling on the expression of habituated neuroendocrine responses to stress, but not as a necessary component of the plasticity mediating the acquisition of this habituated response. Multiple neural regions were found to display increased c-fos mRNA in a pattern indicating a multi-structural consequence of disrupting the increase(s) in, or recruitment of, CB1 receptor signaling that was measured in rats exposed to repeated noise stress, but not in rats without repeated stress history. This pattern is importantly distinguishable from the more widespread potentiation of c-fos mRNA and HPA axis responses to higher dose AM251 administration before an initial acute exposure of loud noise stress (Newsom et al., Citation2012). Together, these results support a multi-structural circuitry involved in the habituation of neuroendocrine responses to familiar, innocuous psychological stressors.
In each of the experiments, stress-induced plasma CORT was quantified and used as an index of reactivity to acute and repeated psychological stressors including: loud noise stress, restraint stress, and novel environment stress. This interpretation is widely accepted in preclinical stress research (Armario et al., Citation1986) and we have previously reported that peak levels of acute stress-induced elevation of plasma CORT correlate well with stressor intensity, and with other measures of HPA axis activation such as plasma adrenocorticotropic hormone (ACTH) levels (Burow et al., Citation2005). Additionally, immediate early gene c-fos mRNA induction was assessed in multiple stress-reactive neural regions in Experiment 3 to characterize regional stress-related neural activity (Senba & Ueyama, Citation1997). Induction patterns of c-fos mRNA resulting from acute psychological stress exposure reliably and predictably correlate with acute stressor intensity in many cortical, limbic, and HPA axis-intrinsic tissues, as well as with the peak amount of stress-induced plasma CORT and ACTH (Burow et al., Citation2005; Campeau & Watson, Citation1997). Also predictable is the general and widespread pattern of a reduction in stress-dependent c-fos mRNA induction that occurs as a mild or moderate stressor is repeatedly experienced and becomes familiar (such as loud noise: Campeau et al., Citation2002, and restraint: Girotti et al., Citation2006), which parallels the pattern of incremental reduction in HPA axis activation that is measurable in both plasma ACTH and CORT (Babb et al., Citation2014) and are characteristic of stress habituation (Spencer and Deak, Citation2017). In the current study, multiple differences were measured in plasma CORT response to repeated stress in AM251-treated rats that are indicative of the progressive CB1 receptor mediation of HPA axis habituation expression. Future research examining CB1 receptor involvement in stress habituation and sensitization may benefit from the use and comparison of multiple CB1 receptor antagonists, as AM251 has been reported to have additional effects at mu opioid receptors (Seely et al., Citation2012). Nevertheless, the congruence of these effects with those measured in the low dose CB1-receptor antagonist dependent alteration of c-fos mRNA habituation in several neural regions suggest the involvement of these regions in the circuitry responsible for stress habituation.
In Experiment 1, daily administration of an acutely potentiating dose (2.0 mg/kg) of CB1 receptor antagonist resulted in increased CORT in blood samples taken immediately after stress treatment on days 1, 3, and 7, and an inhibited habituation rate to repeated loud noise stress exposures compared to both vehicle controls and a lower dose (0.5 mg/kg) of AM251. However, by the 7th loud noise exposure with drug pretreatment, a significant habituation of plasma CORT level was measured compared to the day 1 values for the same group. A final loud noise exposure on day 8 without drug pretreatment revealed that this group displayed a level of habituation that was indistinguishable from the other two groups. This finding indicated that repeated AM251 treatment did not reliably interfere with the acquisition of the neural alterations necessary for the neuroendocrine habituation to this stressor. This dose of AM251 was previously found to robustly potentiate limbic and neuroendocrine reactivity to loud noise stress, as well as to induce significant CORT elevation and activity in various discreet neural regions in the absence of stress (Newsom et al., Citation2012). A potential alternative interpretation for the pattern of plasma CORT measured on days 1, 3, and 7 in rats treated with 2.0 mg/kg AM251 could be that instead of indicating disruption of a central habituation-related mechanism, this pattern simply reflects an additive contribution of direct pharmacological adrenal gland stimulation that can occur with this dose of AM251. However, this interpretation cannot easily account for the altered rate of habituation, and strong evidence from our and other laboratories indicates a widespread, multifaceted ability of central eCB signaling to negatively modulate HPA axis reactivity to acute stress (Evanson et al., Citation2010; Hill and McEwen, Citation2010; Newsom et al., Citation2012; Patel et al., Citation2004, Citation2005; Tasker and Herman, Citation2011). To distinguish the involvement of eCB signaling in habituation and sensitization from the ability of CB1 receptor antagonism to directly stimulate or potentiate neuroendocrine activities, two lower doses of AM251 were employed with limited acute potentiation of CORT release (see also: Hill et al., Citation2010) in the last 2 experiments.
In Experiment 2, 1.0 mg/kg AM251 administration before the 8th day of loud noise exposure resulted in total restoration of plasma CORT values to the levels measured after the first presentation. A control group without recent stress history was given this same dose before novel environment stress on the 9th day. Whereas plasma CORT values for this group were not found to be significantly lower than those of the repeatedly stressed rats receiving the same dose, a comparison indicates them to be similar to the measured non-stressed values of CORT on days 1 and 8 from the same rats, supporting a sub-threshold effect of 1.0 mg/kg AM251 with mild stress such as exposure to a novel environment that would be more easily detectable with larger sample size. A concurrent study has indicated that a dose of 1.0 mg/kg AM251 potentiates some measures of limbic and HPA reactivity to the more intense stress of loud noise exposure (Newsom et al., Citationin review), suggesting that the disruption of HPA axis habituation measured after pretreatment with 1.0 mg/kg AM251 on the 8th day of loud noise stress likely reflects some contribution of the acute potentiating capacity of CB1 receptor antagonism. However, in the same concurrent project, 1.0 mg/kg AM251 did not activate the HPA axis or elevate c-fos mRNA in the absence of stress. Inverse agonist-like effects of AM251 and similar CB1 receptor antagonist SR141716A (Rimonabant) on neuroendocrine activity are not fully understood, but have been demonstrated to be specific to certain neural regions rather than uniformly present in the brain, and to be positively related to dose (Newsom et al., Citation2012; Patel et al., Citation2004). It appears likely that the effects of low doses of AM251 depend on aspects such as stressor intensity and normal or intentional variability in testing environment and paradigm (Dallman et al., Citation1999; Gamble-George et al., Citation2013; Moreira & Wotjak, Citation2010).
In Experiment 3, rats were exposed to 7 days of loud noise stress, then administered 0.5 or 1.0 mg/kg AM251 before exposure to restraint stress on day 8, and before a final exposure to loud noise stress on day 9. Plasma CORT measures indicated both doses to result in sensitized HPA axis response to restraint stress after a repeated stress history, as well as partial disruption of the expression of HPA axis habituation compared to vehicle controls. The patterns of c-fos mRNA expression in multiple stress reactive limbic regions following 0.5 mg/kg AM251 in acute compared to repeated noise stress were very reminiscent of the patterns of plasma CORT resulting from a 1st compared to an 8th loud noise exposure. Stimulation patterns resulting from this low dose most clearly demonstrated the inhibitory involvement of CB1 receptors in (habituated) reactivity to a familiar stressor that is distinct from the participation of the eCB system in tonic inhibition of neuroendocrine activity and in general stress reactivity. Thus, the results suggest that repeated stress alters the eCB system in a manner that increases eCB’s influence on subsequent stress reactivity. The difference between the effects of 0.5 mg/kg AM251 elevating CORT release on the 8th day of loud noise exposure in Experiment 3 but not on days 3 and 7 of Experiment 1 could be explained by the development of a mild tolerance to the repeated administration of the antagonist in Experiment 1. The use of systemic administration of AM251 in this study should be considered in interpretation of the functional importance of CB1 receptors in the expression of stress habituation. Though the lowest dose of AM251 resulted in only partial restoration of c-fos mRNA induction and plasma CORT levels compared to levels measured after an initial exposure to the same stressor, it should be noted that the higher doses of AM251 resulted in more complete disruption of HPA axis habituation. It is possible that eCB signaling at CB1 receptors is a primary and total inhibitory influence within a more specific circuitry involved in stress habituation. However, the use of systemic AM251 administration in this study also allowed us to examine c-fos mRNA activity patterns related to the disruption of HPA axis habituation, which potentially provides novel information about the circuitry of this underexplored learning mechanism.
Experiment 3 was designed to obtain new information about the neural activity patterns associated with AM251-induced impairment of HPA axis habituation expression and the possibility that sensitizing effects of prior repeated homotypic stress to a novel stress situation might be uncovered by CB1R antagonism. By design, the novel stressor test on day 8 might have confounded the final habituation test on day 9. However, the overall level of CORT habituation in the vehicle-treated group observed on day 9 was indistinguishable from the levels obtained in Experiments 1 and 2, in which no intervening sensitization testing took place. Furthermore, antagonist treatment of some of the groups on day 8 of Experiment 3 may also have confounded the results on day 9. The results of Experiment 1 employing repeated higher doses of AM251 (2.0 mg/kg), and Experiment 2 randomly mixing the drug dose conditions without inducing prior drug treatment interaction effects, suggest limited or no confounding effects of day 8 drug or heterotypic stress treatments on the subsequent day 9 CORT habituation levels in Experiment 3.
In Experiment 3, c-fos mRNA expression was increased by 0.5 mg/kg AM251 pretreatment in repeatedly stressed rats in multiple neural regions in contrast to a lack of potentiating effect of this dose on acute stress-induced c-fos mRNA, a pattern reminiscent to the regulation of plasma CORT. Many of the regions analyzed provide information either directly (BSTav, rPH) or indirectly (LS, BLA, Prelimbic cortex) to the PVN and are highly interconnected (Radley et al., Citation2015). Of these, the most direct afferents (BSTav, rPH) and stress responsive region (LS) display the highest similarity in c-fos mRNA patterns with the PVN across stress and drug states. Based on these results however, it is still unclear if the CB1R-related expression of habituated HPA axis responses are driven by one or multiple sites, especially from the systemic drug injections employed in this study and the widespread location of CB1 receptors in all regions analyzed. The measured increase in paraventricular nucleus (PVN) c-fos mRNA in repeatedly stressed rats by 0.5 mg/kg AM251 treatment is evidence that the elevation in plasma CORT measured in this treatment group resulted from stimulation of the entire HPA axis rather than pituitary and/or adrenal level antagonist activity (Burow et al., Citation2005; Dallman et al., Citation2006). Similar increases in c-fos mRNA induction by 0.5 mg/kg AM251 administered to rats on the 8th, but not the 1st day of loud noise stress was also measured in the rostral posterior hypothalamus (rPH) and lateral septum (LS). Prior research supports an important regulatory role of the rPH in habituation of stress-induced HPA axis activity (Nyhuis et al., Citation2016a). The LS has been reported to modulate behavioral, neuroendocrine (Singewald et al., Citation2011), and autonomic (Reis et al., Citation2011) responses to stress, and stress-induced Fos protein expression in this region is potentiated by CB1 receptor antagonism, suggesting a regulatory role of eCBs in this structure (Patel et al., Citation2005). Central and basolateral amygdala (CeA, BLA) c-fos mRNA patterns were found to contrast with those of the PVN, rPH, and LS. The amygdala has been the focus of much prior research on eCB regulation of acute and repeated stress (Hill et al., Citation2010; Hill & Patel, Citation2013; Patel et al., Citation2005; Patel & Hillard, Citation2008), and the BLA has been reported to be a primary region of plasticity in stress adaptation (Grissom & Bhatnagar, Citation2011). However, not all studies report c-fos mRNA induction in these amygdala subregions by acute stress exposure (Campeau et al., Citation2002), and c-fos may not be an ideal measure for assessing amygdala activity with an audiogenic paradigm (Day et al., Citation2005). The paraventricular thalamic nucleus is another brain region implicated in the regulation of habituation to stress (Grissom & Bhatnagar, Citation2011), but insufficient tissue was collected in the current study to assess its responsiveness to repeated loud noise and CB1 receptor antagonism. In the current study, the prelimbic (PL) and orbitofrontal (OF) cortex were found to display a notable lack of habituation of stress-induced c-fos mRNA induction. The OF cortex was previously reported to display increased c-fos mRNA after repeated compared to acute loud noise stress (Campeau et al., Citation2002), but there were several procedural differences between these studies. It should also be noted that inhibition of medial prefrontal cortex with muscimol has been demonstrated to interfere with HPA axis habituation to restraint stress as well as habituation of stress-induced LS c-fos mRNA induction (Weinberg et al., Citation2010). The measured habituation of c-fos mRNA in the auditory cortex (AUD) resulting from repeated loud noise stress in the current study is not interpreted to indicate diminished auditory perception of the stressor, as previous research has found maintained c-fos mRNA induction patterns in additional auditory processing structures resulting from acute compared to repeated loud noise stress (Campeau et al., Citation2002). The role of the auditory cortex is also questionable as large ablations of this region do not modify HPA axis habituation to repeated loud noise exposures (Masini et al., Citation2012).
The current study does not include non-stressed comparisons of vehicle treatment to CB1 receptor antagonism with AM251. We have previously reported systemic AM251 administration to have direct stimulatory effect on basal activity in some of the measures utilized in the current study, which are specifically dependent on dose and location of neural/endocrine tissue (Newsom et al., Citation2012) such that some limbic and neuroendocrine tissues are devoid of direct stimulation by CB1 receptor antagonist AM251, and some predictably display the pattern of CB1 receptor antagonist-stimulated basal activity only at 2.0 mg/kg but not at 1.0 mg/kg AM251 (Newsom et al., Citationin review). It is thus unlikely that the potentiation of stress-induced plasma CORT and c-fos mRNA (in LS, PVN, and rPH) measured in repeatedly stressed rats in Experiment 3 resulted from stimulations of basal activity by the lowest dose of AM251 (0.5 mg/kg). Given the widespread expression of CB1 receptors and ligands in the brain and body, careful selection of agonist/antagonist dose and administration strategies will continue to be important for distinction of specific eCB system activities in future research.
The heterotypic stressor sensitization measured in the current study suggests that the repeated experience of loud noise that produces homotypic stressor habituation also contributes to a state of heterotypic stressor sensitization observed only after CB1 receptor antagonism, in the current studies. This sensitization is likely distinct from the general increase in stress-reactivity that results from widespread depletion of limbic eCB levels or multistructural CB1 receptor downregulation that can occur in response to repeated stress (Hill & Patel, Citation2013), and may be experienced as altered emotional reactivity and disrupted ability to habituate to normal stresses as a result of dietary fatty acid deficiency and imbalance (Zamberletti et al., Citation2017). The pattern of habituation to repeated stress resulting in co-development of a sensitized response to subsequent stressors (Grissom et al., Citation2008; Weinberg et al., Citation2009) may be the result of a combination of a neuroplastic mechanism related to the familiarity of the repeatedly experienced stressor (as observed in the current study) and the partial depletion or downregulation of eCB activity that can occur in more disruptive stressor paradigms. The lack of heterotypic stressor sensitization observed in some repeated stress paradigms (Babb et al., Citation2014) might be understood to relate to an absence of limbic eCB system dysfunction resulting from repeated stress exposure that is less disruptive or is conducive to faster and more complete habituation.
Overall, the results of this study suggest a progressive increase in the inhibitory actions of CB1 receptors resulting from repeated stress exposure that contributes to the expression of habituated limbic and HPA axis activity. The use of a CB1 receptor antagonist indicates that the alterations observed are specific to stress-related modifications in receptor activity that facilitate a reduction in reactivity to the familiar stressor. This is consistent with a view that stress habituation does not occur as a general desensitization of stress reactivity (Bhatnagar & Dallman, Citation1998). The pattern of c-fos mRNA induction associated with the disruption of the expression of HPA axis habituation to repeated loud noise stress supports a multistructural involvement of central eCB signaling at CB1 receptors in stress habituation. Further research is necessary to determine if this structural pattern reflects similar CB1 receptor involvement in habituation of additional components of stress reactivity such as autonomic and behavioral responses, and how our results relate to reports of repeated stress-induced increase in eCB levels in limbic structures. Additionally, our results indicate that while alterations in CB1 receptors are involved in the expression of habituated neural and HPA axis responses to stress, normal eCB signaling at CB1 receptors during the repeated stress exposures is not a necessary component of the plasticity responsible for the acquisition of habituated responses. Additional research will be necessary to determine the primary mechanisms and regions involved in this unique and important learning mechanism. Finally, the results of this study suggest that therapies designed to protect or recover general functioning of the eCB system and CB1 receptors may be beneficial in the treatment of psychological and physical disorders which include altered reactivity patterns to repeatedly experienced and mixed stressors such as sensitization and disrupted habituation of neuroendocrine responses.
Disclosures of interest
The authors report no conflicts of interest.
Acknowledgments
We would like to acknowledge the contributions of Drs. Cher Masini, Jessica Babb, Tara Nyhuis, and Heidi Day in various phases of these studies.
Additional information
Funding
References
- Armario, A., Lopez-Calderon, A., Jolin, T., & Balasch, J. (1986). Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neuroscience and Biobehavioral Reviews, 10, 245–250. doi:10.1016/0149-7634(86)90011-4
- Babb, J.A., Masini, C.V., Day, H.E.W., & Campeau, S. (2014). Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress, 17, 224–234. doi:10.3109/10253890.2014.905534
- Bhatnagar, S., & Dallman, M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience, 84, 1025–1039. doi:10.1016/S0306-4522(97)00577-0
- Brierley, H., & Jamieson, R. (1974). Anomalous stress reactions in patients suffering from depression and anxiety. Journal of Neurology, Neurosurgery, and Psychiatry, 37, 455–462. doi:10.1136/jnnp.37.4.455
- Burow, A., Day, H.E.W., & Campeau, S. (2005). A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Research, 1062, 63–73. doi:10.1016/j.brainres.2005.09.031
- Campeau, S., & Watson, S.J. (1997). Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. Journal of Neuroendocrinology, 9, 577–588. doi:10.1046/j.1365-2826.1997.00593.x
- Campeau, S., Dolan, D., Akil, H., & Watson, S.J. (2002). c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress, 5, 121–130. doi:10.1080/10253890290027895
- Campeau, S., Nyhuis, T.J., Sasse, S.K., Day, H.E.W., & Masini, C.V. (2008). Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neuroscience & Biobehavioral Reviews, 32, 1277–1286. doi:10.1016/j.neubiorev.2008.05.014
- Chattopadhyay, P., Cooke, E., Toone, B., & Lader, M. (1980). Habituation of physiological responses in anxiety. Biological Psychiatry, 15, 711–721.
- Cota, D. (2007). CB1 receptors: emerging evidence for central and peripheral mechanism that regulate energy balance, metabolism, and cardiovascular health. Diabetes/Metabolism Research and Reviews, 23, 507–517. doi:10.1002/dmrr.764
- Dallman, M.F., Akana, S.F., Bell, M.E., Bhatnagar, S., Choi, S., Chu, A., … Viau, V. (1999). Warning! Nearby construction can profoundly affect your experiments. Endocrine, 11, 111–113. doi:10.1385/ENDO:11:2:111
- Dallman, M.F., Akana, S.F., Levin, N., Walker, C.D., Bradbury, M.J., Suemaru, S., & Scribner, K.S. (2006). Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Annals of the New York Academy of Sciences, 746, 22–31. discussion 31–2– 64–7. doi:10.1111/j.1749-6632.1994.tb39206.x
- Day, H.E.W., Masini, C.V., & Campeau, S. (2009). Reversible inactivation of the auditory thalamus disrupts HPA axis habituation to repeated loud noise stress exposures. Brain Research, 1276, 123–130. doi:10.1016/j.brainres.2009.04.023
- Day, H.E.W., Nebel, S., Sasse, S., & Campeau, S. (2005). Inhibition of the central extended amygdala by loud noise and restraint stress. European Journal of Neuroscience, 21, 441–454. doi:10.1111/j.1460-9568.2005.03865.x
- Day, H.E., & Akil, H. (1996). Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology, 63, 207–218. doi:10.1159/000126959
- Evanson, N.K., Tasker, J.G., Hill, M.N., Hillard, C.J., & Herman, J.P. (2010). Fast Feedback Inhibition of the HPA axis by Glucocorticoids is Mediated by Endocannabinoid Signaling. Endocrinology, 151, 4811–4819. doi:10.1210/en.2010-0285
- Gamble-George, J.C., Conger, J.R., Hartley, N.D., Gupta, P., Sumislawski, J.J., & Patel, S. (2013). Dissociable effects of CB1 receptor blockade on anxiety-like and consummatory behaviors in the novelty-induced hypophagia test in mice. Psychopharmacology, 228, 401–409. doi:10.1007/s00213-013-3042-8
- Girotti, M., Pace, T.W.W., Gaylord, R.I., Rubin, B.A., Herman, J.P., & Spencer, R.L. (2006). Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience, 138, 1067–1081. doi:10.1016/j.neuroscience.2005.12.002
- Grissom, N.M., & Bhatnagar, S. (2011). The basolateral amygdala regulates adaptation to stress via β-adrenergic receptor-mediated reductions in phosphorylated extracellular signal-regulated kinase. Neuroscience, 178, 108–122. doi:10.1016/j.neuroscience.2010.12.049
- Grissom, N., Kerr, W., & Bhatnagar, S. (2008). Struggling behavior during restraint is regulated by stress experience. Behavioural Brain Research, 191, 219–226. doi:10.1016/j.bbr.2008.03.030
- Herman, J.P. (2013). Neural control of chronic stress adaptation. Frontiers in Behavioral Neuroscience, 7, 61. doi:10.3389/fnbeh.2013.00061
- Hill, M.N., & McEwen, B.S. (2010). Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34, 791–797. doi:10.1016/j.pnpbp.2009.11.001
- Hill, M.N., & Patel, S. (2013). Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biology of Mood & Anxiety Disorders, 3, 19. doi:10.1186/2045-5380-3-19
- Hill, M.N., McLaughlin, R.J., Bingham, B., Shrestha, L., Lee, T.T.Y., Gray, J.M., … Viau, V. (2010). Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences of Sciences, 107, 9406–9411. doi:10.1073/pnas.0914661107
- Kamprath, K., Marsicano, G., Tang, J., Monory, K., Bisogno, T., Marzo, V.D., … Wotjak, C.T. (2006). Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. Journal of Neuroscience, 26, 6677–6686. doi:10.1523/JNEUROSCI.0153-06.2006
- Lader, M.H., & Wing, L. (1964). Habituation of the psycho-galvanic reflex in patients with anxiety states and in normal subjects. Journal of Neurology, Neurosurgery, and Psychiatry, 27, 210–218. doi:10.1136/jnnp.27.3.210
- Mahan, A.L., & Ressler, K.J. (2012). Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neurosciences, 35, 24–35. doi:10.1016/j.tins.2011.06.007
- Masini, C.V., Day, H.E.W., & Campeau, S. (2008). Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behavioral Neuroscience, 122, 210–223. doi:10.1037/0735-7044.122.1.210
- Masini, C.V., Day, H.E.W., Gray, T., Crema, L.M., Nyhuis, T.J., Babb, J.A., & Campeau, S. (2012). Evidence for a lack of phasic inhibitory properties of habituated stressors on HPA axis responses in rats. Physiology & Behavior, 105, 568–575. doi:10.1016/j.physbeh.2011.06.011
- Masini, C.V., Babb, J.A., Nyhuis, T.J., Day, H.E.W., & Campeau, S. (2012). Auditory cortex lesions do not disrupt habituation of HPA axis responses to repeated noise stress. Brain Research, 1443, 18–26. doi:10.1016/j.brainres.2012.01.002
- Moreira, F.A., & Wotjak, C.T. (2010). Cannabinoids and anxiety. Current Topics in Behavioral Neurosciences, 2, 429–450.
- Newsom, R.J., Osterlund, C., Masini, C.V., Day, H.E., Spencer, R.L., & Campeau, S. (2012). Cannabinoid receptor type 1 antagonism significantly modulates basal and loud noise induced neural and hypothalamic-pituitary-adrenal axis responses in male Sprague–Dawley rats. Neuroscience, 204, 64–73. doi:10.1016/j.neuroscience.2011.11.043
- Newsom, R.J., Garcia, R.J., Stafford, J., Osterlund, C., O’Neill, C.E., Day, H.E.W., & Campeau, S. (In review) Remote CB1 receptor antagonist administration reveals multiple sites of tonic and phasic endocannabinoid neuroendocrine regulation. Psychoneuroendocrinology [Under review].
- Nyhuis, T.J., Masini, C.V., Day, H.E.W., & Campeau, S. (2016). Evidence for the Integration of Stress-Related Signals by the Rostral Posterior Hypothalamic Nucleus in the Regulation of Acute and Repeated Stress-Evoked Hypothalamo-Pituitary-Adrenal Response in Rat. The Journal of Neuroscience, 36, 795–805. doi:10.1523/JNEUROSCI.3413-15.2016
- Nyhuis, T.J., Masini, C.V., Taufer, K.L., Day, H.E.W., & Campeau, S. (2016). Reversible inactivation of rostral nucleus raphe pallidus attenuates acute autonomic responses but not their habituation to repeated audiogenic stress in rats. Stress, 19, 248–259. doi:10.3109/10253890.2016.1160281
- Patel, S., Roelke, C.T., Rademacher, D.J., Cullinan, W.E., & Hillard, C.J. (2004). Endocannabinoid Signaling Negatively Modulates Stress-Induced Activation of the Hypothalamic-Pituitary-Adrenal Axis. Endocrinology, 145, 5431–5438. doi:10.1210/en.2004-0638
- Patel, S., & Hillard, C.J. (2008). Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. European Journal of Neuroscience, 27, 2821–2829. doi:10.1111/j.1460-9568.2008.06266.x
- Patel, S., Roelke, C.T., Rademacher, D.J., & Hillard, C.J. (2005). Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. European Journal of Neuroscience, 21, 1057–1069. doi:10.1111/j.1460-9568.2005.03916.x
- Paxinos, G., & Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego. p. 237.
- Radley, J., Morilak, D., Viau, V., & Campeau, S. (2015). Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neuroscience & Biobehavioral Reviews, 58, 79–91. doi:10.1016/j.neubiorev.2015.06.018
- Reis, D.G., Scopinho, A.A., Guimarães, F.S., Corrêa, F.M.A., & Resstel, L.B.M. (2011). Behavioral and autonomic responses to acute restraint stress are segregated within the lateral septal area of rats. PloS One, 6, e23171. doi:10.1371/journal.pone.0023171
- Riebe, C.J., & Wotjak, C.T. (2011). Endocannabinoids and stress. Stress, 14, 384–397. doi:10.3109/10253890.2011.586753
- Seely, K.A., Brents, L.K., Franks, L.N., Rajasekaran, M., Zimmerman, S.M., Fantegrossi, W.E., & Prather, P.L. (2012). AM-251 and Rimonabant Act as Direct Antagonists at Mu-Opioid Receptors: Implications for Opioid/Cannabinoid Interaction Studies. Neuropharmacology, 63, 905–915. doi:10.1016/j.neuropharm.2012.06.046
- Senba, E., & Ueyama, T. (1997). Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neuroscience Research, 29, 183–207. doi:10.1016/S0168-0102(97)00095-3
- Singewald, G.M., Rjabokon, A., Singewald, N., & Ebner, K. (2011). The Modulatory Role of the Lateral Septum on Neuroendocrine and Behavioral Stress Responses. Neuropsychopharmacology, 36, 793–804. doi:10.1038/npp.2010.213
- Spencer, R.L., & Deak, T. (2017). A users guide to HPA axis research. Physiology & Behavior, 178, 43–65. doi:10.1016/j.physbeh.2016.11.014
- Tasker, J.G., & Herman, J.P. (2011). Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress, 14, 398–406. doi:10.3109/10253890.2011.586446
- Thomson, F., & Craighead, M. (2008). Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochemical Research, 33, 691–707. doi:10.1007/s11064-007-9518-3
- VanElzakker, M.B., Dahlgren, M.K., Davis, F.C., Dubois, S., & Shin, L.M. (2014). From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of Learning and Memory, 113, 3–18. doi:10.1016/j.nlm.2013.11.014
- Weinberg, M.S., Bhatt, A.P., Girotti, M., Masini, C.V., Day, H.E.W., Campeau, S., & Spencer, R.L. (2009). Repeated Ferret Odor Exposure Induces Different Temporal Patterns of Same-Stressor Habituation and Novel-Stressor Sensitization in Both Hypothalamic-Pituitary-Adrenal Axis Activity and Forebrain c-fos Expression in the Rat. Endocrinology, 150, 749–761. doi:10.1210/en.2008-0958
- Weinberg, M.S., Johnson, D.C., Bhatt, A.P., & Spencer, R.L. (2010). Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience, 168, 744–756. doi:10.1016/j.neuroscience.2010.04.006
- Zamberletti, E., Piscitelli, F., De Castro, V., Murru, E., Gabaglio, M., Colucci, P., … Parolaro, D. (2017). Lifelong imbalanced LA/ALA intake impairs emotional and cognitive behavior via changes in brain endocannabinoid system. Journal of Lipid Research, 58, 301–316. doi:10.1194/jlr.M068387
