Abstract
Stress is a powerful moderator of brain plasticity and may affect several physiological functions such as the endocrine and the immune system. The impact of stress can be protective or detrimental according to several factors such as level of the stressor and age of occurrence. Also, the impact may differ in males and females. We aim to analyze the effect of mild levels of early and recent stress on white matter microstructure in healthy volunteers. MRI acquisition of diffusion tensor images with a 3.0 T scanner was performed on 130 healthy subjects (71 males and 59 females). Severity of early and recent stress was rated, respectively, on the Risky Families Questionnaire and on the Schedule of Recent Experiences; subjects were divided into low stress and mild stress groups. Mild early stress associated with lower fractional anisotropy (FA) in the cingulate gyrus compared to low early stress. Females reported reduced FA compared to males in the low-stress group in the internal capsule, posterior corona radiata, posterior thalamic radiation, superior longitudinal fasciculus, and sagittal stratum whereas no difference was observed in the mild stress group. An additive effect of early and recent stress was observed in posterior corona radiata, retrolenticular part of the internal capsule, and superior longitudinal fasciculus. The impact of early stress on WM microstructure in healthy subjects is different in males and females. While males seem to be more sensitive to early stress, an additive effect of early and recent stress manifests itself in females.
Mild levels of early stress associate with lower white matter integrity measured by fractional anisotropy.
Females and males show differences in white matter integrity when exposed to low levels of early stress with females showing lower white matter integrity compared to males.
No difference in white matter integrity was observed for males and females exposed to mild levels of stress.
Mild stress in females is associated with higher white matter integrity.
Males seem to be more sensitive to early stress while females are more affected when early stress is followed by stress in adult life.
Layman summary
1. Introduction
Approximately 38% of the total brain volume is composed of myelinated axons forming the white matter (WM) (Leonard et al., Citation2008). Myelination begins during late stages of fetal development and continues into early adulthood (Dean et al., Citation2014; Lebel et al., Citation2012). Genetic influences and spontaneous electrochemical activity guide the neurodevelopmental processes that shape the neurobiological features of WM such as the number of axons, their diameter, and the thickness of the myelin sheath (Paus, Citation2010). To estimate the bundle coherence and the integrity of WM tracts in vivo diffusion-tensor imaging (DTI) measure the diffusion of water within fiber tracts. A reduced tendency to diffuse along the principal direction of the fiber (axial diffusivity) associates with reduced integrity of axons and myelin sheaths (Boretius et al., Citation2012), while an increased water diffusion perpendicular to axonal walls (radial diffusivity) associates with dysmyelination and demyelination (Song et al., Citation2002). Fractional anisotropy (FA) reflects the dominance of the largest axial component and is the most used DTI index to investigate the degree of organization of WM tracts (i.e. fibers density, axonal diameter, and myelination). FA, together with the regional development and degradation of myelin sheaths, changes over the lifetime (Lebel et al., Citation2012). Indeed, FA increases during adolescence and then decreases from the fifth decade (Bartzokis et al., Citation2012; Lebel et al., Citation2012). Early experiences may then shape the maturation process through plasticity mechanisms that act on synapsis formation or pruning (Ben-Ari & Spitzer, Citation2010). Changes in WM microstructure following early experiences may promote resilience, as suggested by the presence of higher FA in the corpus callosum of resilient adolescent (Galinowski et al., Citation2015), or psychiatric disorders such as mood disorders which are characterized by reduced WM integrity (Benedetti & Bollettini, Citation2014).
It is now well established that early stress may lead to poorer emotional and physical functioning, higher vulnerability to further trauma, and physical and mental health consequences (Anda et al., Citation2006; Edwards, Holden, Felitti, & Anda, Citation2003; Felitti et al., Citation1998; Moffitt, Caspi, & Rutter, Citation2005; Rutter, Citation2002; Teicher, Samson, Sheu, Polcari, & McGreenery, Citation2010; Wermter et al., Citation2010). Early stress in its different forms has been extensively associated with in vivo measures of WM microstructure in adult healthy subjects (Choi, Jeong, Rohan, Polcari, & Teicher, Citation2009; Choi, Jeong, Polcari, Rohan, & Teicher, Citation2012; Eluvathingal et al., Citation2006; Teicher et al., Citation2010). Although the majority of studies associated early stress to reduced integrity in several WM tracts including corpus callosum, superior and inferior longitudinal fasciculus, and cingulum bundle (Teicher, Samson, Anderson, & Ohashi, Citation2016), other studies found opposite results. Early stress was associated with markers of increased myelination in rodents (Ono et al., Citation2008) and with greater WM volumes (Hanson et al., Citation2010) and integrity (Jensen et al., Citation2018) in humans. These data suggest that increased myelination could follow mild stress, as opposed to severe stress, as an adaptation to a stressful environment. Also, the impact of stress has been shown to depend on the age at which it occurred. Prenatal stress associates with reduced WM integrity in the genu and splenium of the corpus callosum, childhood stress associates with higher integrity in the splenium, and stress during adolescence associates with higher integrity in the genu of corpus callosum (Jensen et al., Citation2018).
The impact of recent stress on the human brain has been less investigated, but a detrimental effect has been suggested by studies showing reduced brain volume in the amygdala of healthy subjects (Sublette et al., Citation2016). In animal studies, decreased numbers of oligodendrocytes and neural/glial antigen positive oligodendrocytes precursor cells have been reported in the cortex and amygdala of rats exposed to social stress (Banasr et al., Citation2007; Czeh et al., Citation2007).
Both WM development and the brain response to stress can differ according to gender although the effect of gender is still unclear. Changes in WM microstructure during adolescence have been reported in both sexes, with males showing larger FA increases than females (Simmonds, Hallquist, Asato, & Luna, Citation2014). In adults, higher FA in men was reported in several tracts including corona radiata, internal and external capsule, cingulum, superior longitudinal fasciculus, cerebral peduncle, corticospinal tracts, and uncinate fasciculus (Inano, Takao, Hayashi, Abe, & Ohtomo, Citation2011; Kanaan et al., Citation2014; Lebel & Beaulieu, Citation2011). Higher FA in women was observed in the column of the fornix (Inano et al., Citation2011) and in the genu of corpus callosum (Kanaan et al., Citation2014) whereas higher FA in the splenium of the corpus callosum was reported both in males (Inano et al., Citation2011) and females (Lebel & Beaulieu, Citation2011).
Males and females also respond differently to stress in terms of activation of the HPA axis, the sympathetic nervous system and of behavioral outcomes. Males show a greater stress hormone response than females with higher levels of cortisol in response to stress but seems also to show a more rapid recovery process (Kudielka & Kirschbaum, Citation2005). However, major differences in the methods used have led to contrasting results. Women seem to respond with increased mobilization of various immune cells and decreased glucocorticoid sensitivity to acute stress and with exaggerated immunosuppression to chronic stress. However, inflammatory biomarkers are more consistently linked to depression in men in agreement with animal studies suggesting that males may be more likely to display stress-induced inflammatory changes (Bekhbat & Neigh, Citation2018).
The long-term consequences of early stress may show in healthy subject by influencing the response to adverse events in adult life by activating stress-response systems and altering their molecular organization to modify their sensitivity and response bias either in a detrimental or in a protective direction. Although several studies reported that severe early stress, when combined to recent stress in adult life, have a detrimental effect on mental health (Bandoli et al., Citation2017; Hammen, Henry, & Daley, Citation2000; Shapero et al., Citation2014) and structural integrity of the brain (Admon et al., Citation2013; Hanson, Knodt, Brigidi, & Hariri, Citation2015), as suggested by Teicher and Samson brain abnormalities, considered as important correlates of different psychopathologies, are also observable in healthy adults with histories of early stress but no signs of depression (Teicher & Samson, Citation2013). Indeed, accumulating evidence shows that early stress exposure may lead also to resilience to subsequent stress experienced in adulthood, measured as diminished increases in salivary cortisol (Gunnar, Wewerka, Frenn, Long, & Griggs, Citation2009), lower levels of corticotropin-releasing-factor (Carpenter et al., Citation2004), and diminished cardiovascular responses (Boyce & Chesterman, Citation1990). Indeed, milder forms of adversity have been suggested to provide a challenge, that when overcome, produces competence in the management of subsequent stressors and enhanced resistance to stress (Garmezy, Masten, & Tellegen, Citation1984). Accordingly, resilient adolescents showed higher WM microstructure in the corpus callosum (Galinowski et al., Citation2015).
Despite a large amount of literature investigating the effects of stress on brain integrity, few studies focused on mild stress and none at our knowledge addressed gender differences. Following this line of reasoning, we explored the effects of early and recent mild stress on WM microstructure in a sample of healthy controls. We hypothesize that (1) both early and recent mild stress will affect WM microstructure, (2) mild stress will affect males and females differently, (3) exposure to early stress will affect the impact of recent stress on WM microstructure.
2. Methods
2.1. Participants
We studied 130 healthy subjects (71 females, 59 males) with mean ± SD age 32.89 ± 12.84. Exclusion criteria were: intellectual disability, pregnancy, major medical, neurological and psychiatric disorders, history of drug or alcohol abuse or dependency. Subjects were recruited from the general population through posts on social networks. After a complete description of the study, approved by the local ethical committee, a written informed consent was obtained.
The severity of early stress was rated on the Risky Families Questionnaire (RFQ) (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, Citation2006) after the fMRI scanning. The RFQ has been adapted from an instrument originally developed to assess the relation of family stress to mental and physical health outcomes in adulthood (Felitti et al., Citation1998). The instrument is aimed at rating the degree of harsh parenting with overt family conflict and deficient nurturing experienced by the children from 5 to 15 years of age in their familial environment (i.e. Would you say the household you grew up in was chaotic and disorganized? Would you say you were neglected while you were growing up, left on your own to fend for yourself?). Participants rated aspects of their childhood family environment on 4-point scales ranging from 1 (rarely or none of the time) to 4 (most or all of the time). Higher values represented a riskier family environment. Previous research validated this questionnaire against clinical interviews conducted and coded by trained clinical interviewers; the dual assessment (questionnaire and interview) demonstrated high agreement and reliability (Taylor, Lerner, Sage, Lehman, & Seeman, Citation2004). The scores can range from 13 (no stress) to 65 (very high stress). Mean RFQ score suggests only mild early stress.
The Schedule of Recent Experiences (SRE) (Amundson, Citation1981) was used to measure the occurrence of recent (number of events) stressful events in the last three years. The scale focuses on events that led to changes in usual activities that frequently precede illness onsets including positive (holidays or marriage), negative (death of a parent, mortgage), and neutral (change of job, change of house) and yields two different measures of stress: one score obtained as the sum of the number of stressful events that had occurred in the last 3 years; one score obtained as the weighted probability that these stressful events are followed by detrimental effects, such as illness. To measure with both tests the risk for developing stress-related symptoms, the weighted score was used. Again, mean scores suggest only mild stress.
No validated cutoff values exist for RFQ nor SRE, therefore, to test the interaction between early and recent stress each group of participants was divided into two subgroups, using median values as a discriminant between high and low scores of the SRE scale, then each group was further divided according to RFQ scores. This approach has been proven successful in detecting the structural and functional brain correlates of early stress in adult life (Benedetti et al., Citation2011, Citation2014; Taylor et al., Citation2006). With this approach, we identified four groups: low early-low recent stress, low early-mild recent stress, mild early-low recent stress, and mild early-mild recent stress.
2.2. Image acquisition
Diffusion tensor imaging was performed on a 3.0 Tesla scanner (Gyroscan Intera, Philips, Netherlands) using SE Eco-planar imaging (EPI) and the following parameters: TR/TE = 8753.89/58 ms, FoV (mm) 231.43 (ap), 126.50 (fh), 240.00 (l); acquisition matrix 2.14 × 2.71 × 2.31; 55 contiguous, 2.3-mm thick axial slices reconstructed with in-plane pixel size 1.88 × 1.87 mm; SENSE acceleration factor= 2; 1 b0 and 35 non-collinear directions of the diffusion gradients; b value = 900 sec/mm2. Fat saturation was performed` to avoid chemical shift artifacts. On the same occasion and using the same magnet 22 Turbo Spin Echo (TSE), T2 axial slices (TR = 3000 ms; TE = 85 ms; flip angle = 90°; turbo factor 15; 5-mm- thick, axial slices with a 512 × 512 matrix and a 230 × 230 mm2 field of view) were acquired to rule out brain lesions.
2.3. DT-MRI data preprocessing
Whole-brain tract-wise average FA values were extracted in the dataset according to ENIGMA-DTI protocols (available online at http://enigma.ini.usc.edu/protocols/dti-protocols/). DTI images were pre-processed using FSL tools (http://www.fmrib.ox.ac.uk/fsl). By FSL’s “eddy correct” command, all volumes were corrected for eddy current induced distortions and subjects movements (Horsfield, Citation1999). A brain mask was then created using FSL’s Brain Extraction Tool (BET) (Smith, Citation2002), which deletes non-brain tissues from the image. Next, by FSL’s DTIFIT command, included in FMRIB’s Diffusion Toolbox (FDT) (Behrens et al., Citation2003), a voxel-wise diffusion tensor model was fit to the data in order to obtain parametric maps of FA.
Whole brain statistical analyses of all subject’s FA images were conducted using FSL's Tract-Based Spatial Statistics (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) analytic method. All subject’s FA data were aligned to the Montreal Neurological Institute (MNI) space, by use of local deformation procedures performed by FMRIB's Non-Linear Image Registration Tool (FNIRT) (www.fmrib.ox.ac.uk/fsl/fnirt/index.html). The mean of all aligned FA images was then created and a “thinning” process was applied to create a skeletonized mean FA image representing the centers of all common tracts. A threshold of 0.2 was set to this image in order to control for inter-subject variability and reduce the likelihood of partial volume effect. Quality control, including inspections of data, vector gradients, registration, and average skeleton projection distance, were performed according to the ENIGMA-DTI protocol.
Finally, all individual FA image was projected onto the skeleton by searching perpendicular from the skeleton for maximum FA values (Smith et al., Citation2006). Average FA values were calculated from voxels in each subject’s WM skeleton within 46 tract-wise regions of interest (ROIs), derived from the Johns Hopkins University (JHU) WM parcellation atlas (Mori et al., Citation2008) (Supplementary Figure S1).
2.4. Statistical analyses
First, a MANCOVA was performed with all 46 WM tracts as dependent variables, early and recent stress, divided in mild and low, as factors, and age and sex as covariates in the model. Second, to investigate possible additive effect a composite measure was created accounting for the levels (mild/low) of early and recent stress. This measure was then added to a further MANCOVA as factor, with WM tracts as dependent variables and age and sex as covariates. If the MANCOVA was significant, we performed a post-hoc univariate ANOVA only in the significant WM tracts.
3. Results
Characteristics of the sample according to gender are resumed in . No difference was observed between males and females in the number of early or recent events. Females showed lower total intracranial volume (t = 9.11; p < .001) and average FA (t = 3.87; p < .001) compared to males. No association was observed between early and recent stress (M: χ = 0.0147, p = .917; F = χ = 0.1635, p = .2).
Table 1. Characteristics of the sample according to gender.
3.1. Extracted FA
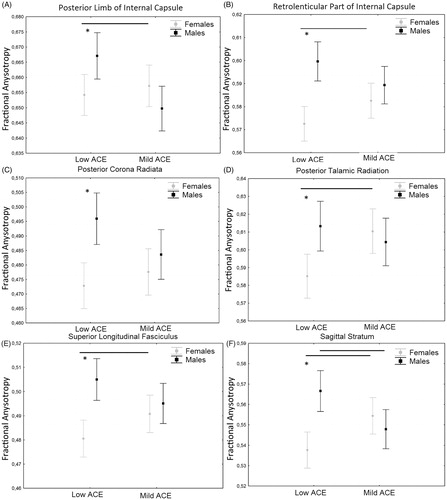
First, the MANCOVA showed a significant interaction between early stress and sex (Roy’s Largest Root V = 0.426, F = 1.767, p = .035) on WM tracts. Inspection of univariates between effects showed a main effect of early stress in the cingulate gyrus (F = 7.83; df = 107; p = .006) where subjects with mild levels of early stress showed reduced FA compared to those with low levels. A significant interaction between sex and early stress was observed in the Internal capsule (F = 10.25; df = 107; p = .002), posterior limb of internal capsule (F = 7.96; df = 107; p = .006. ), retrolenticular part of the internal capsule (F = 6.46; df = 107; p = .012. ), posterior corona radiata (F = 4.16; df = 107; p = .044. ), posterior thalamic radiation (F = 6.77; df = 107; p = .011. ), superior longitudinal fasciculus (F = 6.07; df = 107; p = .015. ), sagittal stratum (F = 14.18; df = 107; p < .001. . In these tracts, females reported reduced FA compared to males in the low early stress group whereas no difference was observed in the mild early stress group. Also, females in the low early stress group showed lower FA than females in the mild early stress group whereas the opposite was observed in males. A main effect of recent stress was observed in corona radiata (F = 4.726; df = 107; p = .032), superior corona radiata (F = 6.44; df = 107; p = .013), posterior corona radiata (F = 7.47; df = 107; p = .007), internal capsule (F = 5.79; df = 107; p = .018), retrolenticular part of internal capsule (F = 8.76; df = 107; p = .004), and superior longitudinal fasciculus (F = 7.97; df = 107; p = .006) where recent stress was negatively associated with FA.
Figure 1. Interaction effect of early stress and sex on FA. Bars are mean and whiskers are standard deviation. *p < .05.
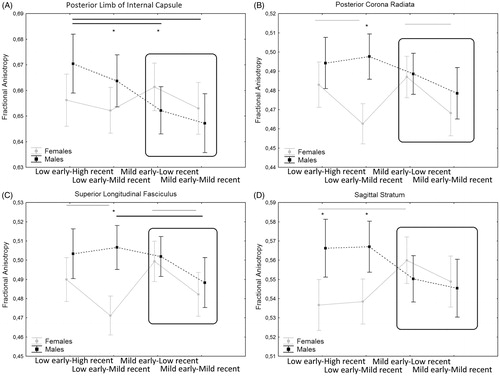
Finally, the second MANOVA showed a significant interaction between the additive variable and sex (Roy’s Largest Root V = 0.508, F = 2.154, p = .006) on WM tracts. Univariate results show an additive effect of early and recent stress that interact with sex in the internal capsule (F = 3.80; df = 107; p = .01), posterior limb of internal capsule (F = 2.78; df = 107; p = .04. ), posterior corona radiata (F = 3.23; df = 107; p = .02. ), superior longitudinal fasciculus (F = 3.61; df = 107; p = .01. ), sagittal stratum (F = 4.99; df = 107; p = .003. ). The additive effect is more evident in females as can be observed in .
Figure 2. Interaction between the additive effect of early and recent stress on FA. Data shown are mean ± standard deviation. *p < .05. Black lines are significant (p > .05) differences in the males groups and gray lines are significant differences in the females groups.
Significant WM tracts are shown in Supplementary Figure S1.
4. Discussion
An overall effect of mild early stress and an additive effect of early and recent mild stress was observed on WM microstructure, with a different impact on males and females. We found a significant interaction between sex and mild early stress on FA in several WM tracts including the internal capsule, posterior corona radiata, posterior thalamic radiation, and superior longitudinal fasciculus. In these tracts, we observed that females had lower FA than males in the low early stress group whereas no difference was present in the mild early stress group. Also, females in the low early stress group showed lower FA than those in the mild early stress group, whereas, the opposite was observed in males. The fiber tracts in which a reduced FA has been observed are mainly projection fibers connecting cortical areas including parietal, occipital, cingulate, and temporal regions to subcortical destinations in the thalamus. The thalamus has been related to cortisol-induced changes in fear acquisition (Merz et al., Citation2010), to the recollection of traumatic events (Lanius et al., Citation2003), to vigilance, and sustained attention and it showed enhanced activation after stress (Sarter, Givens, & Bruno, Citation2001; Wang et al., Citation2005). Accordingly, early stress may sharpen our senses, create a state of increased arousal (de Kloet, Joels, & Holsboer, Citation2005), strengthen the memories of stressful experiences and sensitize to further stressful events.
Lower FA in females compared to males, as observed here in the low early stress group, is in agreement with the literature showing higher FA in males compared with females in several tracts including corona radiata, internal capsule, and superior longitudinal fasciculus (Huster, Westerhausen, Kreuder, Schweiger, & Wittling, Citation2009; Inano et al., Citation2011; Oh et al., Citation2007).
Animal studies suggested that sex differences in WM could be related to the exposure to sex hormones, specifically estrogen and testosterone (Cerghet, Skoff, Swamydas, & Bessert, Citation2009). Testosterone may induce growth in axon caliber leading, in turn, to larger volumes of WM (Perrin et al., Citation2009) as observed in the low early stress group. On the other side, estrogen receptors are expressed on oligodendrocytes (Santagati, Melcangi, Celotti, Martini, & Maggi, Citation2002) and steroid hormones, especially estrogen, seem to be involved in the proliferation of myelin and the protection of myelin during development (Gerstner et al., Citation2007; Nunez, Nelson, Pych, Kim, & Juraska, Citation2000) but also in the prevention or reduction of neurodegeneration (Arevalo, Azcoitia, & Garcia-Segura, Citation2015). The effects of testosterone could then explain higher FA in males whereas the protective effect of estrogen could explain why in the mild early stress group females showed no difference in FA compared to males. A protective effect of estrogen could also explain our finding of higher FA in females exposed to mild stress compared to those exposed to low stress. This finding is in agreement with previous results from our group showing that in healthy controls mild early stress can induce neural efficacy in circuitry associated with social cognition (Vai et al., Citation2018). Animal studies showing that chronic stress exposure decreases cell proliferation in male rats, while it enhanced neurogenesis in females (Westenbroek, Den Boer, Veenhuis, & Ter Horst, Citation2004), could suggest a protective effect of estrogen against stress-induced alterations. Also, diarylpropionitrile, a selective generic estrogen receptor β agonist has been shown to stimulate endogenous myelination (Khalaj, Hasselmann, Augello, Moore, & Tiwari-Woodruff, Citation2016). Moreover, estrogen levels have been suggested to modulate cortisol levels, so that higher estrogen levels stimulate the production of cortisol-binding globulin, resulting in the removal of free cortisol from circulation (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, Citation1999). Accordingly, elevated cortisol during infancy was associated with reduced FA in posterior occipital regions in adulthood (Howell et al., Citation2013).
As suggested by Teicher (Teicher et al., Citation2016), developmental modifications may occur during sensitive stages in response to stress in order to adapt to the environment. These changes seem to be prevalent in females and not in males. Furthermore, these adaptive changes seem to be confined to the development period as suggested by a lack of sex-based differences observed in response to recent stress. This is in agreement with the literature showing heightened HPA axis reactivity during puberty (Gunnar et al., Citation2009) leading to increase the vulnerability of the brain to stress during early adolescence (Andersen & Teicher, Citation2009). Also, it is likely that adult differences in neuroendocrine regulation between males and females begin to emerge with the pubertal transition.
When considering the additive effect of early and recent events, we observed that the additive effect is present mainly in females which show the lowest levels of FA when exposed to both early and recent stress.
The questionnaires used in our study to estimate the exposure to stress during childhood and adulthood measure different aspects of stress. Indeed, while the Risky Family questionnaire is a measure of a dysfunctional environment and therefore of chronic stress, the Schedule of Recent Experiences measure the number of events (i.e. acute stress). Considering this difference, a possible mechanism explaining the additive effect of early and recent stress in males and female could be a different regulation of the inflammatory response to stress among sexes. In females, estrogen leads to higher systemic baseline levels of glucocorticoids compared to males due to the transcriptional regulation of corticoid releasing hormone (Cutolo et al., Citation2006). Glucocorticoids downregulate acute-phase mediators like IL-1, IL-6, and CRP that are released in response to acute stress thereby maintaining homeostasis. Whereas severe stress may lead to glucocorticoid resistance of immune cells and brain alterations, mild stress can promote future resilience to stress as suggested by previous reports of beneficial effects of predictable repeated mild stress (Parihar, Hattiangady, Kuruba, Shuai, & Shetty, Citation2011; Suo et al., Citation2013).
There are several limitations to the present study. The lack of information about the exact age at which early stress occurred potentially limits any specific inferences that might be made about the effect of early stress on the developmental pathway of FA. However, being the Risky Family a measure of family environment we can hypothesize certain constancy through time. The retrospective reporting of childhood experiences may be associated with difficulties in recalling certain events. However, this likely results in misclassification (classifying persons exposed to early stress as unexposed) that would bias our results toward the null (Della Femina, Yeager, & Lewis, Citation1990). Finally, only FA was investigated in this article but previous reports on rodents showed that resilience was paralleled by the decreases in mean, radial, and axial diffusivity despite an overall increase in FA (Magalhaes et al., Citation2017). Also, further studies are needed to investigate if the effect observed on FA is paralleled by changes in other indices of WM microstructure and if the same effect can be observed for other measures of brain structure and function such as brain volume, cortical thickness, or resting state.
However, these limitations do not bias the main finding of gender-specific consequences of mild early and recent stressful experiences on WM microstructure.
supplementary_figure1.tif
Download TIFF Image (1.8 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Sara Poletti
Sara Poletti has a PhD in Neuroscience and is a lecturer in physiology
Elisa Melloni
Elisa Melloni is a post-hoc fellow with a PhD in Molecular Medicine-neuroscience.
Elena Mazza
Elena Mazza is a Phd Student in Molecular Medicine-neuroscience
Benedetta Vai
Benedetta Vai is a post-hoc fellow with a PhD in Evolutionary Psychopathology.
Francesco Benedetti
Francesco Benedetti is professor of Psychiatry and is the head of the Psychiatry and Clinical Psychobiology Unit of San Raffaele Hospital in Milan.
References
- Admon, R., Leykin, D., Lubin, G., Engert, V., Andrews, J., Pruessner, J., & Hendler, T. (2013). Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Human Brain Mapping, 34, 2808–2816. doi:10.1002/hbm.22100
- Amundson, M.E., Hart, C.A., & Holmes, T.H. (1981). University of Washington Press, Stress (Psychology).
- Anda, R.F., Felitti, V.J., Bremner, J.D., Walker, J.D., Whitfield, C., Perry, B.D., … Giles, W.H. (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. doi:10.1007/s00406-005-0624-4
- Andersen, S.L., & Teicher, M.H. (2009). Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neuroscience & Biobehavioral Reviews, 33, 516–524. doi:10.1016/j.neubiorev.2008.09.009
- Arevalo, M.A., Azcoitia, I., & Garcia-Segura, L.M. (2015). The neuroprotective actions of oestradiol and oestrogen receptors. Nature Reviews Neuroscience, 16, 17–29. doi:10.1038/nrn3856
- Banasr, M., Valentine, G.W., Li, X.Y., Gourley, S.L., Taylor, J.R., & Duman, R.S. (2007). Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biological Psychiatry, 62, 496–504. doi:10.1016/j.biopsych.2007.02.006
- Bandoli, G., Campbell-Sills, L., Kessler, R.C., Heeringa, S.G., Nock, M.K., Rosellini, A.J., … Stein, M.B. (2017). Childhood adversity, adult stress, and the risk of major depression or generalized anxiety disorder in US soldiers: A test of the stress sensitization hypothesis. Psychological Medicine, 47, 2379–2392. doi:10.1017/S0033291717001064
- Bartzokis, G., Lu, P.H., Heydari, P., Couvrette, A., Lee, G.J., Kalashyan, G., … Altshuler, L.L. (2012). Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biological Psychiatry, 72, 1026–1034. doi:10.1016/j.biopsych.2012.07.010
- Behrens, T.E., Woolrich, M.W., Jenkinson, M., Johansen-Berg, H., Nunes, R.G., Clare, S., … Smith, S.M. (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine, 50, 1077–1088. doi:10.1002/mrm.10609
- Bekhbat, M., & Neigh, G.N. (2018). Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain, Behavior and Immunity, 67, 1–12. doi:10.1016/j.bbi.2017.02.006
- Ben-Ari, Y., & Spitzer, N.C. (2010). Phenotypic checkpoints regulate neuronal development. Trends in Neurosciences, 33, 485–492. doi:10.1016/j.tins.2010.08.005
- Benedetti, F., & Bollettini, I. (2014). Recent findings on the role of white matter pathology in bipolar disorder. Harvard Review of Psychiatry, 22, 338–341. doi:10.1097/HRP.0000000000000007
- Benedetti, F., Bollettini, I., Radaelli, D., Poletti, S., Locatelli, C., Falini, A., … Colombo, C. (2014). Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychological Medicine, 44, 3069–3082. doi:10.1017/S0033291714000506
- Benedetti, F., Radaelli, D., Poletti, S., Falini, A., Cavallaro, R., Dallaspezia, S., … Smeraldi, E. (2011). Emotional reactivity in chronic schizophrenia: Structural and functional brain correlates and the influence of adverse childhood experiences. Psychological Medicine, 41, 509–519. doi:10.1017/S0033291710001108
- Boretius, S., Escher, A., Dallenga, T., Wrzos, C., Tammer, R., Bruck, W., … Stadelmann, C. (2012). Assessment of lesion pathology in a new animal model of MS by multiparametric MRI and DTI. NeuroImage, 59, 2678–2688. doi:10.1016/j.neuroimage.2011.08.051
- Boyce, W. T., & Chesterman, E. (1990). Life Events, Social support and cardiovascular reactivity in adolescence. Developmental and Behavioral Pediatrics, 11, 105–111.
- Carpenter, L. L., Tyrka, A. R., Mcdougle, C. J., Malison, R. T., Owens, M. J., Nemeroff, C. B., & Price, L. H. (2004). Cerebrospinal Fluid Corticotropin-Releasing Factor and Perceived Early-Life Stress in Depressed Patients and Healthy Control Subjects. Neuropsychopharmacology, 29, 777–784. 10.1038/sj.npp.1300375
- Cerghet, M., Skoff, R.P., Swamydas, M., & Bessert, D. (2009). Sexual dimorphism in the white matter of rodents. Journal of the Neurological Sciences, 286, 76–80. doi:10.1016/j.jns.2009.06.039
- Choi, J., Jeong, B., Polcari, A., Rohan, M.L., & Teicher, M.H. (2012). Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage, 59, 1071–1079. doi:10.1016/j.neuroimage.2011.09.033
- Choi, J., Jeong, B., Rohan, M.L., Polcari, A.M., & Teicher, M.H. (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65, 227–234. doi:10.1016/j.biopsych.2008.06.022
- Cutolo, M., Capellino, S., Sulli, A., Serioli, B., Secchi, M.E., Villaggio, B., & Straub, R.H. (2006). Estrogens and autoimmune diseases. Annals of the New York Academy of Sciences, 1089, 538–547. doi:10.1196/annals.1386.043
- Czeh, B., Muller-Keuker, J.I., Rygula, R., Abumaria, N., Hiemke, C., Domenici, E., & Fuchs, E. (2007). Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: Hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology, 32, 1490–1503. doi:10.1038/sj.npp.1301275
- de Kloet, E.R., Joels, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews. Neuroscience, 6, 463–475. doi:10.1038/nrn1683
- Dean, D.C. III, O'Muircheartaigh, J., Dirks, H., Waskiewicz, N., Lehman, K., Walker, L., … Deoni, S.C. (2014). Modeling healthy male white matter and myelin development: 3 through 60months of age. NeuroImage, 84, 742–752. doi:10.1016/j.neuroimage.2013.09.058
- Della Femina, D., Yeager, C.A., & Lewis, D.O. (1990). Child abuse: Adolescent records vs. adult recall. Child Abuse & Neglect, 14, 227–231. doi:10.1016/0145-2134(90)90033-P
- Edwards, V.J., Holden, G.W., Felitti, V.J., & Anda, R.F. (2003). Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. American Journal of Psychiatry, 160, 1453–1460. doi:10.1176/appi.ajp.160.8.1453
- Eluvathingal, T.J., Chugani, H.T., Behen, M.E., Juhasz, C., Muzik, O., Maqbool, M., … Makki, M. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics, 117, 2093–2100. doi:10.1542/peds.2005-1727
- Felitti, V.J., Anda, R.F., Nordenberg, D., Williamson, D.F., Spitz, A.M., Edwards, V., … Marks, J.S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14, 245–258. doi:10.1016/S0749-3797(98)00017-8
- Galinowski, A., Miranda, R., Lemaitre, H., Martinot, M.-L.P., Artiges, E., Vulser, H., … Barbot, A. (2015). Resilience and corpus callosum microstructure in adolescence. Psychological Medicine, 45, 2285–2294. doi:10.1017/S0033291715000239
- Garmezy, N., Masten, A.S., & Tellegen, A. (1984). The study of stress and competence in children: A building block for developmental psychopathology. Child Development, 55, 97–111. doi:10.2307/1129837
- Gerstner, B., Sifringer, M., Dzietko, M., Schuller, A., Lee, J., Simons, S., … Felderhoff-Mueser, U. (2007). Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Annals of Neurology, 61, 562–573. doi:10.1002/ana.21118
- Gunnar, M.R., Wewerka, S., Frenn, K., Long, J.D., & Griggs, C. (2009). Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology, 21, 69–85. doi:10.1017/S0954579409000054
- Hammen, C., Henry, R., & Daley, S.E. (2000). Depression and sensitization to stressors among young women as a function of childhood adversity. Journal of Consulting and Clinical Psychology, 68, 782–787. doi:10.1037//0022-006X.68.5.782
- Hanson, J.L., Chung, M.K., Avants, B.B., Shirtcliff, E.A., Gee, J.C., Davidson, R.J., & Pollak, S.D. (2010). Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30, 7466–7472. doi:10.1523/JNEUROSCI.0859-10.2010
- Hanson, J.L., Knodt, A.R., Brigidi, B.D., & Hariri, A.R. (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Development and Psychopathology, 27, 1611–1619. doi:10.1017/S0954579415000978
- Horsfield, M.A. (1999). Mapping eddy current induced fields for the correction of diffusion-weighted echo planar images. Magnetic Resonance Imaging, 17, 1335–1345. doi:10.1016/S0730-725X(99)00077-6
- Howell, B.R., McCormack, K.M., Grand, A.P., Sawyer, N.T., Zhang, X., Maestripieri, D., … Sanchez, M.M. (2013). Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: Associations with high cortisol during infancy. Biology of Mood & Anxiety Disorders, 3, 21. doi:10.1186/2045-5380-3-21
- Huster, R.J., Westerhausen, R., Kreuder, F., Schweiger, E., & Wittling, W. (2009). Hemispheric and gender related differences in the midcingulum bundle: A DTI study. Human Brain Mapping, 30, 383–391. doi:10.1002/hbm.20509
- Inano, S., Takao, H., Hayashi, N., Abe, O., & Ohtomo, K. (2011). Effects of age and gender on white matter integrity. AJNR. American Journal of Neuroradiology, 32, 2103–2109. doi:10.3174/ajnr.A2785
- Jensen, S.K.G., Pangelinan, M., Bjornholm, L., Klasnja, A., Leemans, A., Drakesmith, M., … Paus, T. (2018). Associations between prenatal, childhood, and adolescent stress and variations in white-matter properties in young men. NeuroImage, 182, 389–397. doi:10.1016/j.neuroimage.2017.10.033
- Kanaan, R.A., Chaddock, C., Allin, M., Picchioni, M.M., Daly, E., Shergill, S.S., & McGuire, P.K. (2014). Gender influence on white matter microstructure: A tract-based spatial statistics analysis. PLoS One, 9, e91109. doi:10.1371/journal.pone.0091109
- Khalaj, A.J., Hasselmann, J., Augello, C., Moore, S., & Tiwari-Woodruff, S.K. (2016). Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects. The Journal of Steroid Biochemistry and Molecular Biology, 160, 43–52. doi:10.1016/j.jsbmb.2016.01.006
- Kirschbaum, C., Kudielka, B.M., Gaab, J., Schommer, N.C., & Hellhammer, D.H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162. doi:10.1097/00006842-199903000-00006
- Kudielka, B.M., & Kirschbaum, C. (2005). Sex differences in HPA axis responses to stress: A review. Biological Psychology, 69, 113–132. doi:10.1016/j.biopsycho.2004.11.009
- Lanius, R.A., Williamson, P.C., Hopper, J., Densmore, M., Boksman, K., Gupta, M.A., … Menon, R.S. (2003). Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biological Psychiatry, 53, 204–210. doi:10.1016/S0006-3223(02)01466-X
- Lebel, C., & Beaulieu, C. (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience, 31, 10937–10947. doi:10.1523/JNEUROSCI.5302-10.2011
- Lebel, C., Gee, M., Camicioli, R., Wieler, M., Martin, W., & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60, 340–352. doi:10.1016/j.neuroimage.2011.11.094
- Leonard, C.M., Towler, S., Welcome, S., Halderman, L.K., Otto, R., Eckert, M.A., & Chiarello, C. (2008). Size matters: Cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex, 18, 2920–2931. doi:10.1093/cercor/bhn052
- Magalhaes, R., Bourgin, J., Boumezbeur, F., Marques, P., Bottlaender, M., Poupon, C., … Cachia, A. (2017). White matter changes in microstructure associated with a maladaptive response to stress in rats. Translational Psychiatry, 7, e1009. doi:10.1038/tp.2016.283
- Merz, C.J., Tabbert, K., Schweckendiek, J., Klucken, T., Vaitl, D., Stark, R., & Wolf, O.T. (2010). Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology, 35, 33–46. doi:10.1016/j.psyneuen.2009.07.009
- Moffitt, T.E., Caspi, A., & Rutter, M. (2005). Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry, 62, 473–481. doi:10.1001/archpsyc.62.5.473
- Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K., … Mazziotta, J. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage, 40, 570–582. doi:10.1016/j.neuroimage.2007.12.035
- Nunez, J.L., Nelson, J., Pych, J.C., Kim, J.H., & Juraska, J.M. (2000). Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Research. Developmental Brain Research, 120, 87–90.
- Oh, J.S., Song, I.C., Lee, J.S., Kang, H., Park, K.S., Kang, E., & Lee, D.S. (2007). Tractography-guided statistics (TGIS) in diffusion tensor imaging for the detection of gender difference of fiber integrity in the midsagittal and parasagittal corpora callosa. NeuroImage, 36, 606–616. doi:10.1016/j.neuroimage.2007.03.020
- Ono, M., Kikusui, T., Sasaki, N., Ichikawa, M., Mori, Y., & Murakami-Murofushi, K. (2008). Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience, 156, 1103–1110. doi:10.1016/j.neuroscience.2008.07.078
- Parihar, V.K., Hattiangady, B., Kuruba, R., Shuai, B., & Shetty, A.K. (2011). Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Molecular Psychiatry, 16, 171–183. doi:10.1038/mp.2009.130
- Paus, T. (2010). Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition, 72, 26–35. doi:10.1016/j.bandc.2009.06.002
- Perrin, J. S., Leonard, G., Perron, M., Pike, G. B., Pitiot, A., Richer, L., …Paus, T. (2009). Sex differences in the growth of white matter during adolescence. Neuroimage, 45, 1055–1066.
- Rutter, M. (2002). The interplay of nature, nurture, and developmental influences: The challenge ahead for mental health. Archives of General Psychiatry, 59, 996–1000. doi:10.1001/archpsyc.59.11.996
- Santagati, S., Melcangi, R.C., Celotti, F., Martini, L., & Maggi, A. (2002). Estrogen receptor is expressed in different types of glial cells in culture. Journal of Neurochemistry, 63, 2058–2064. doi:10.1046/j.1471-4159.1994.63062058.x
- Sarter, M., Givens, B., & Bruno, J.P. (2001). The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Research Reviews, 35, 146–160. doi:10.1016/S0165-0173(01)00044-3
- Shapero, B.G., Black, S.K., Liu, R.T., Klugman, J., Bender, R.E., Abramson, L.Y., & Alloy, L.B. (2014). Stressful life events and depression symptoms: The effect of childhood emotional abuse on stress reactivity. Journal of Clinical Psychology, 70, 209–223. doi:10.1002/jclp.22011
- Simmonds, D.J., Hallquist, M.N., Asato, M., & Luna, B. (2014). Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage, 92, 356–368. doi:10.1016/j.neuroimage.2013.12.044
- Smith, S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. doi:10.1002/hbm.10062
- Smith, S.M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T.E., Mackay, C.E., … Behrens, T.E. (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31, 1487–1505. doi:10.1016/j.neuroimage.2006.02.024
- Song, S.K., Sun, S.W., Ramsbottom, M.J., Chang, C., Russell, J., & Cross, A.H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17, 1429–1436. doi:10.1006/nimg.2002.1267
- Sublette, M.E., Galfalvy, H.C., Oquendo, M.A., Bart, C.P., Schneck, N., Arango, V., & Mann, J.J. (2016). Relationship of recent stress to amygdala volume in depressed and healthy adults. Journal of Affective Disorders, 203, 136–142. doi:10.1016/j.jad.2016.05.036
- Suo, L., Zhao, L., Si, J., Liu, J., Zhu, W., Chai, B., … Lu, L. (2013). Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology, 38, 1387–1400. doi:10.1038/npp.2013.67
- Taylor, S.E., Eisenberger, N.I., Saxbe, D., Lehman, B.J., & Lieberman, M.D. (2006). Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry, 60, 296–301. doi:10.1016/j.biopsych.2005.09.027
- Taylor, S.E., Lerner, J.S., Sage, R.M., Lehman, B.J., & Seeman, T.E. (2004). Early environment, emotions, responses to stress, and health. Journal of Personality, 72, 1365–1393. doi:10.1111/j.1467-6494.2004.00300.x
- Teicher, M.H., & Samson, J.A. (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry, 170, 1114–1133. doi:10.1176/appi.ajp.2013.12070957
- Teicher, M.H., Samson, J.A., Anderson, C.M., & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17, 652–666. doi:10.1038/nrn.2016.111
- Teicher, M.H., Samson, J.A., Sheu, Y.S., Polcari, A., & McGreenery, C.E. (2010). Hurtful words: Association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. American Journal of Psychiatry, 167, 1464–1471. doi:10.1176/appi.ajp.2010.10010030
- Vai, B., Riberto, M., Ghiglino, D., Bollettini, I., Falini, A., Benedetti, F., & Poletti, S. (2018). Mild adverse childhood experiences increase neural efficacy during affective theory of mind. Stress, 21, 84–89. doi:10.1080/10253890.2017.1398231
- Wang, J., Rao, H., Wetmore, G.S., Furlan, P.M., Korczykowski, M., Dinges, D.F., & Detre, J.A. (2005). Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences USA, 102, 17804–17809. doi:10.1073/pnas.0503082102
- Wermter, A.K., Laucht, M., Schimmelmann, B.G., Banaschweski, T., Sonuga-Barke, E.J., Rietschel, M., & Becker, K. (2010). From nature versus nurture, via nature and nurture, to gene x environment interaction in mental disorders. European Child & Adolescent Psychiatry, 19, 199–210. doi:10.1007/s00787-009-0082-z
- Westenbroek, C., Den Boer, J.A., Veenhuis, M., & Ter Horst, G.J. (2004). Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Research Bulletin, 64, 303–308. doi:10.1016/j.brainresbull.2004.08.006
