?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The adaptogenic properties of alkylglycerols (AGs) after 1 month’s treatment were investigated in a rat model of acute immobilization stress (AIS). The animals receiving AGs 157 mg/kg showed a body weight (BW) decrease in addition to a more pronounced increase in the adrenal glands index under stress conditions. Also, AGs at this dose prevented AIS-induced catalase inhibition. In addition, antiulcerative AG effects were already detected at a dose of 15 mg/kg. The data indicate that AGs promote adrenal gland activation in AIS. At the same time, AGs neutralize some of negative effects of stressful conditions, which include restoration of the oxidation–reduction balance, reduction of gastric mucosal stress lesion formation.
The effect of alkylglycerols, ether lipids from marine organisms, was studied in stressed animals. AGs have antioxidant activity and can be useful in the complex therapy of stomach lesions.
LAY SUMMARY
1. Introduction
Mental and neurological disorders, including various types of stress, are common all over the world. They affect every community and every age group in countries with different levels of wealth. These disorders account for 14% of the global burden of disease (World Health Organization Mental Health Program, Citation2018).
Permanent mental and psychoemotional stress and violation of the work regime, rest, and nutrition often lead to disruption of adaptation mechanisms and the development of disease. The inability to cope with the impact of working stressors has been empirically associated with a number of adverse conditions such as gastrointestinal dysfunction, musculoskeletal problems, and cardiovascular diseases (Van der Doef & Maes, Citation1998).
It has been shown that AIS exposure for 6 h resulted in a decrease in the brain glutathione, superoxide dismutase, glutathione S-transferase, and catalase levels in the brain with an increase in the level of products reacting with thiobarbituric acid (Zaidi et al., Citation2014). AIS during 18 h in rats led to a distinct neuronal damage in the cerebral cortex and the hippocampal CA1 region (Gulyaev, Shantanova, & Batotsyrenova, Citation2017).
Studies on the influence of chronic immobilization stress and cold restraint stress in CA3 and СА1 hippocampus regions (Dolzhikov, Tverskoi, Bobyntsev, Kriukov, & Belykh, Citation2015; Jayakumar, Raghunath, Ilango, Vijayakumar, & Vijayaraghavan, Citation2017) revealed the neuronal loss, cytoplasmic, and nuclear changes. The observed morphometrical changes probably reflect a decrease in hippocampal neurons’ functional activity under chronic immobilization stress.
1-O-Alkylglycerols are compounds formed by fatty alcohols and glycerol. On the average, the alkyl radical consists of 12–24 carbon atoms (usually 14–18) and 1–2 unsaturated bonds or more. The alkylglycerol structure is present in various classes of lipids: (1) neutral lipids in alkyldiacylglycerols (AGs) and (2) polar lipids in plasmalogen forms. Various AGs differ within the structure of the alkyl radical. The use of AGs can cause an adequate body response; these compounds enhance the body’s protective functions, including hematopoietic, immunostimulating, and antioxidant activities (Deniau et al., Citation2010; Iannitti & Palmieri, Citation2010; Vadalа, Laurino, Palmieri, & Palmieri, Citation2017). Currently, much attention has been paid to the plasmalogen forms of phospholipids as regulators of the normal activity of nervous tissue and the brain as a whole, preventing the development of different types of dementia, including Alzheimer's disease (Dean & Lodhi, Citation2018; Wood, Citation2013); they affect the structure of lipid rafts and conduction of neurons and, for example, act as potential antioxidants in biological membranes and fluids, reducing oxidative stress in the lungs (Broniec et al., Citation2011; Honsho & Fujiki, Citation2017; Wynalda & Murphy, Citation2010). In a recent survey, Dean and Lodhi (Citation2018) have suggested that plasmalogens’ antioxidant capacities may be contextually dependent. Given that the synthesis of alkylglycerols in peroxisomes decreases with age, the consumption of these substances is essential (Blank, Cress, Smith, & Snyder, Citation1992; Chen & Liu, Citation2013; Wood, Khan, Mankidy, Smith, & Goodenowe, Citation2011). Significant amounts of lipids with a simple ether link were found in marine hydrobionts, which include cartilaginous fish (sharks, skates, and chimeras) (Bakes & Nichols, Citation1995; Magnusson & Haraldsson, Citation2011), mollusks (Hayashi & Kishimura, Citation2002), sea stars (Hayashi & Kishimura, Citation1997), zooplankton (Phleger, Nichols, & Virtue, Citation1997), and other forms, which can be useful for the creation of medical preparations with a wide range of compensatory action.
The purpose of this study was to test adaptogenic properties of AGs. We wondered, whether (1) AGs have an influence on the adaptation of animals to AIS. (2) AGs have an influence on the condition of rats after 1 month treatment.
2. Materials and methods
2.1. Materials
All chemical reagents used in this study were of analytical grade (Sigma-Aldrich, St. Louis, MO). All solvents were of HPLC grade, supplied by Sigma-Aldrich (St. Louis, MO). Sodium thiopental was purchased from Sandoz (Kundl, Austria).
The squid Berryteuthis magister was fished in the Bering Sea in September 2017. After squid processing, the hepatopancreas was separated and stored for 3 months at −18 °C.
2.2. Animals
The study was carried out on 60 male Wistar rats kept in vivarium on the standard diet with free access to food and water. All procedures were approved by the Animal Ethics Committee at National Scientific Center of Marine Biology Far Eastern Branch, Russian Academy of Sciences, according to the Laboratory Animal Welfare guidelines.
2.3. Preparations
2.3.1. Preparation of the AGs
After extraction of total lipids from the squid hepatopancreas and lipid hydrolysis, AGs were precipitated from saponified lipids by double crystallization in acetone at different temperatures (Ermolenko, Latyshev, Sultanov, & Kasyanov, Citation2015).
2.3.2. Determination of AG composition
Composition of AGs as trimethylsilyl derivatives (TMS-AGs) was determined by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS).
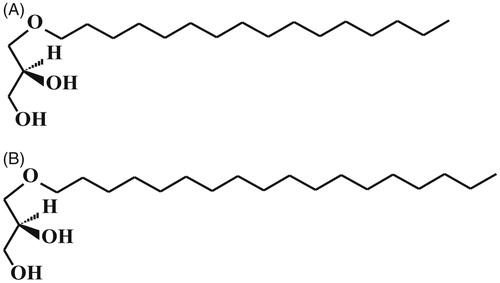
TMS-AGs were prepared by adding 50 μl of N, O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) to 5 mg AGs, and the mixture was then heated to 80 °C for 1 h. After the addition of 200 μl of hexane, 1 μl of each silylated fraction was injected into the GC system. The composition of TMS-AGs was determined by GC using a chromatograph «Shimadzu GC-2010 plus» with a flame ionization detector (Tokyo, Japan) and a capillary column Supelco SLB™-5 ms 30 m × 0.25 mm i.d. (USA). Separation of mixture components was carried out under specific conditions: (1) initial temperature 200 °C; (2) heating rate of 2 °C/min to 260 °C; and (3) the temperature was maintained for 35 min. The injector and detector temperatures were 270 and 260 °C, respectively. AGs were identified by comparison with the available known standards. GC-MS was used to identify the TMS-AGs’ structures. Electronic impact spectra were recorded using an instrument «Shimadzu TQ-8040» (Tokyo, Japan) with the column Supelco SLB™-5 ms (USA) at 70 eV under the same temperature conditions as during GC. Chimyl alcohol () was the main component of AGs, the content of batyl alcohol () was 3.5% ().
Table 1. AG composition after double crystallization.
The resulting AG preparation was a white friable powder, odorless. The total amount of AGs in the preparation was 99%.
2.4. Biological experiment
2.4.1. Animal treatment
For use in animals, the AGs were dispersed in water using IKA ULTRA-TURRAX® T 18 Digital Disperser (Berlin, Germany).
Rats of the experimental groups received AGs for a month orally through a catheter at a dose of 15 or 157 mg/kg. The duration of AG treatment was 1 month. The animals were divided into six groups of 10 animals each:
Group 1 – the control – animals receiving water through a catheter.
Group 2 – animals receiving AGs at a dose of 15 mg/kg.
Group 3 – animals receiving AGs at a dose of 157 mg/kg.
Group 4 – the stress control – animals receiving water and then subjected to stress.
Group 5 – animals receiving AGs at a dose of 15 mg/kg and then subjected to stress.
Group 6 – animals receiving AGs at a dose of 157 mg/kg and then subjected to stress.
Each animal received 1 ml of AGs solution in proper concentration. The last dose was given 24 h before the end of experiment.
2.4.2. Stress procedure
The rats were fixed within a special pen-box, equipped with air holes, which was adjusted to the size of the animal in the position on the back motionless for 16 h once to induce acute immobilization stress (AIS). Before immobilization, rats were food deprived at 24 h.
2.4.3. Collecting of samples
Before the end of the experiment, body weight (BW) of animals was determined before the stress impact. Rats were anesthetized with sodium thiopental intraperitoneally at a dose of 60 mg/kg and sacrificed with decapitation. Blood for biochemical studies was taken at decapitation.
The hippocampus was fixed for histological analysis. The stomach was removed, cut open along the greater curvature, turned out (mucosa out), thoroughly washed with physiological saline, and examined visually. Тhe number of gastric mucosal lesions was counted.
In animals subjected to stress, the adrenal glands, spleen, and thymus gland were weighed. For the weighed organs, the relative weights of the organs per 100 g of the BW were calculated according to the formula:
An additional study was carried out to examine the AG effect on the weight of adrenal glands, thymus and spleen of intact rats. The experiment was performed according to the same scheme as described for groups 1–3 on similar Wistar rats. Organ mass was measured and organ mass index was calculated. The number of animals was 6 in each group.
2.4.4. Biochemical analysis
Catalase activity was investigated by the method described by Aebi (Citation1984); the principle of the method for determination of the concentration of malonic dialdehyde in erythrocytes and the amount of hemoglobin in the erythrocyte mass was developed by Devasagayam, Boloor, and Ramasarma (Citation2003). Determination of the hematological parameters was carried out with the hematology differential analyzer «Mindray BC-3000Plus» (China).
2.4.5. Histological examination
Histological preparations of hippocampus were made according to the standard scheme. The brain was removed and post-fixed overnight in parafor-maldehyde (4% in 0.1 M PBS). Tissue samples were embedded in paraffin and 4 mm sections were cut. Methods of staining with hematoxylin and eosin were used (Day, Citation2014). The preparations were examined with the «ImejerZ2 Zeiss» light microscope, equipped with a «Carl Zeiss AxioCam HRс» camera (Berlin, Germany).
The photos of the CA3 hippocampal region were later processed using the ImageJ program (version 1.51 j1.8.0_112) to increase the sharpness and contrast of the image. The results of the treatment were visually controlled by comparison with the image of the control group. The cells were counted on an area of 150 × 100 μm. At least, three sections were analyzed for each animal.
2.4.6. Statistical analysis
Statistical processing was carried out using the program «Statistica 10.0». To describe the data, the mean (M), the median (Me), the upper, and the lower quartiles (interval 25–75%) are given (Scheff, Citation2016).
Correlation analysis was carried out to determine the regularities. The Kendall correlation coefficient (implemented as Kendall’s tau correlation in the Statistica Program) was used (Scheff, Citation2016). Since the same data were used to test the impact of alkylglycerols and stress, the required level of significance should be at least p = 0.025 (Hoffman, Citation2015). The correlations were calculated separately for different groups of animals subjected to stress and treated with the AG preparation at different doses.
The absolute value of the correlation coefficient from 0 to 0.25 indicates a weak correlation between the studied parameters, the value of the coefficient from 0.26 to 0.74 indicates correlation of average degree, and 0.75–1.0 means a strong correlation. A positive correlation coefficient indicates a direct relationship between the variables and a negative correlation coefficient indicates an inverse relationship.
The Kruskal–Wallis and Mann–Whitney U tests were used for comparison of the groups (Scheff, Citation2016; Nisbet, Miner, & Yale, Citation2017). The minimum number of comparisons for making a conclusion about the nature of the change in the studied characteristic in the group is 3. Then the significance level for the results of the Mann–Whitney U test with Bonferroni correction used in multiple comparisons will be 0.017.
After primary estimation, the data for stress-induced gastric mucosal lesions in stressed animals were compared in with data for stressed animals treated later with AGs.
3. Results
The quantitative description of the obtained results is presented in and Supplementary Tables S1–S4.
Figure 2. Blood catalase activity of non-stressed and stressed rats. The values are reported as the median, [![]()
![Figure 2. Blood catalase activity of non-stressed and stressed rats. The values are reported as the median, [Display full size]- 25–75%,[Display full size]- range without ejection, **p = 0.016 (valid), Mann–Whitney U test (comparison between stressed group AGs 15 mg/kg and stressed group AGs 157 mg/kg); Kendall correlation, K = 0.78 (stressed groups); Kruskal–Wallis test (p = 0.024).](/cms/asset/6ecd2adb-84f3-4f8a-8ea8-df6b515df9ae/ists_a_1660316_f0002_b.jpg)
Figure 3. Hemoglobin level of non-stressed and stressed rats. The values are reported as the median, [![]()
![Figure 3. Hemoglobin level of non-stressed and stressed rats. The values are reported as the median, [Display full size]- 25–75%,[Display full size]- range without ejection, *extreme; Kruskal–Wallis test (p = 0.03).](/cms/asset/8b868d2e-f23c-4cb9-80ee-92cde44431a3/ists_a_1660316_f0003_b.jpg)
Figure 4. Effect of AGs on body weight of non-stressed rats. The values are reported as the median, [![]()
![Figure 4. Effect of AGs on body weight of non-stressed rats. The values are reported as the median, [Display full size]- 25–75%,[Display full size]- range without ejection, °- emissions; **p = 0.00018 (valid) Mann–Whitney U test (comparison between control group and AGs 157 mg/kg); Kendall correlation (K = −0.53); Kruskal–Wallis test (p = 0.001).](/cms/asset/33453455-b753-450f-a217-4a3f328abace/ists_a_1660316_f0004_b.jpg)
Figure 5. The adrenal gland index in stressed rats. The values are reported as the median, [![]()
![Figure 5. The adrenal gland index in stressed rats. The values are reported as the median, [Display full size]- 25–75%,[Display full size]- range without ejection; **p = 0.016 (valid) Mann–Whitney U test (comparison between stressed group AGs 15 mg/kg and stressed group AGs 157 mg/kg); Kendall correlation, K = 0.81; Kruskal–Wallis test (p = 0.01).](/cms/asset/218bec73-d136-4a97-82f0-dc2ee7e8459f/ists_a_1660316_f0005_b.jpg)
3.1. Catalase activity and malondialdehyde concentration
We have revealed a significantly moderate negative correlation of catalase activity with exposure to AIS (K = −0.46). Under the influence of stress, catalase activity shows a significant strong positive correlation with the applied AG dose (Supplementary Table S2), whereas the correlation is not significant among animals not exposed to AIS (Supplementary Table S1). The Kruskal–Wallis test confirmed the significant change of catalase activity (Supplementary Table S1; Н = 12.93, р = 0.024). clearly demonstrates the revealed patterns. It is evident that catalase activity decreases with stress and significantly increases in animals that previously received AGs at a dose of 157 mg/kg and then were subject to AIS. The Mann–Whitney U test revealed the significance of differences between rats receiving AGs at a dose of 157 mg/kg and animals receiving the preparation at the same dose under stress (Z = −2.32, p = 0.016). The test also showed significant differences in the activity of blood catalase for animals receiving AGs at a dose of 15 mg/kg and 157 mg/kg, and then subjected to stress (p = 0.016).
The concentration of malonic dialdehyde in erythrocytes did not depend significantly on AIS (K = −0.3) or AG administration (Supplementary Tables S1 and S2), this may indicate an increase in the antioxidant defense of the organism in acute stress. The Kruskal–Wallis test also revealed no significant differences between the groups (Supplementary Table S2). However, there is a tendency to increased level of malondialdehyde as a function of the dose of AGs in non-stressed rats.
3.2. Hemoglobin level
A significant moderate negative correlation was found between the level of hemoglobin and the impact of AIS (K = −0.59). The Kruskal–Wallis test also confirms the significance of differences (Н = 12.12, p = 0.03) in hemoglobin concentration between groups of animals (Supplementary Table S2). shows that the hemoglobin level is decreasing in stress groups; this reduction under stress can be explained by hemolysis.
The figure reflects also a clear tendency toward an increase in hemoglobin levels after AG administration at a dose of 157 mg/kg. However, according to the Mann–Whitney U test, the difference between animals receiving AG preparation at a dose of 15 mg/kg and 157 mg/kg does not reach a statistically significant level (p = 0.34). This increase corresponds well to the literature data on higher erythrocyte and hemoglobin levels in sows after intake of shark liver oil (Mitre et al, Citation2005), and a greater number of erythrocytes, platelets, and lymphocytes and on a higher hemoglobin level in rats after the use of AGs at a dose of 0.4 g/kg BW for a month (Karaman, Novgorodtseva, Gvozdenko, & Kasynov, Citation2013). The dose in our study was almost three times (2.5) lower, so we can conclude that the effect was dose-dependent, or probably that more time was needed for accumulation of the preparation in the animal’s body. As the levels of erythrocytes and hemoglobin are important components of adaptation to hypoxia (Sinex & Chapman, Citation2015), this effect of the preparation should be considered as adaptogenic.
3.3. Body weight
BW shows a statistically significant moderate negative correlation with the dose of the preparation (Supplementary Table S1). The Kruskal–Wallis test confirmed a significant difference in body weights between groups of animals receiving AGs in different doses and those not treated with AGs (Н = 13.9, p = 0.001). The Mann–Whitney U test showed no difference between intact animals and animals receiving AGs at a dose of 15 mg/kg. On the contrary, the body weight of animals treated with AGs at a dose of 157 mg/kg differed significantly from the BW of both rats of the intact group (Z = 3.28, p = 0.00018) and from the animals treated with AGs at a dose of 15 mg/kg (Z = 2.93, p = 0.0016). shows that the BW was reduced in the group receiving AGs at a dose of 157 mg/kg.
3.4. Stress-induced gastric mucosal lesions
We observed no gastric mucosal lesions in unstressed animals. The application of the selected AIS model caused the formation of gastric mucosal lesions in animals; this is well known from the literature (Selye, Citation1955). In our study, point and linear lesions of the stomach bottom under stress were also observed. Their total number and ratio of lesion types are given in the table (Supplementary Table S3). The lesions were not observed in the non-stressed animals. The appearance of gastric mucosal lesions in our experiments on rats is evidence of the adequacy of the chosen model of stress. The number of damage to the gastric mucosa showed a strong positive correlation with AIS (K = 0.96) and a significant moderate negative correlation with the fact of AGs administration before exposure to AIS (Supplementary Table S3). In animals treated with AGs, stress-induced gastric mucosal lesions were less common than in the stress control group, this is confirmed by the Mann–Whitney U test (Z = 2.03, p = 0.036). We have concluded that even an AG dose of 15 mg/kg showed an antiulcerative effects.
3.5. Adrenal glands and lymphoid organs
AGs did not have a significant effect on the weight of the adrenal glands and thymus of intact animals (Supplementary Table S4). The Kruskal–Wallis test (H = 9.04; p = 0.011) confirmed differences of spleen index in control and AG treated groups (Supplementary Table S4).
Under the influence of stress, the adrenal gland weights (both relative and absolute values) showed a positive correlation with the dose of the preparation, which agrees with the data of Selye (Citation1955). For the index of the adrenal glands, this correlation was strong (Supplementary Table S2). The adrenal gland index () was higher in animals that received AGs before they were subjected to AIS. The Kruskal–Wallis test confirmed the significant differences for the adrenal gland (Н = 9.08, p = 0.01). The Mann–Whitney U test revealed significant differences between animals that received AGs at a dose of 15 and 157 mg/kg, respectively, and then subjected to stress (Z = −2.32, p = 0.016).
Our study did not reveal statistically significant effects of AG doses on thymus and spleen weights in animals exposed to AIS (Supplementary Table S2). However, there was a tendency to restore the thymus index (Supplementary Table S2).
3.6. Hippocampus
The number of cells in the CA3 region of hippocampus did not significantly depend either on AIS (K = 0.3) or on the dose of the AG preparation (Supplementary Tables S1 and S2). Morphological changes of the hippocampus were not observed also. However, in the stress-control group, we revealed an animal with a reduced number of cells (≤ 10) in two sections (Supplementary Figure S1(A)). This animal had an area in which the number of cells was visually reduced and the average number of cells was 13.8. In the group of animals given AGs at a dose of 15 mg/kg and then stressed, the number of cells in the CA3 region of one of the rats varied from 12 to 14, and the average number of cells was 13 (Supplementary Figure S1(B)). For comparison, see the photo of the CA3 hippocampal region of the animal from the intact-control group (Supplementary Figure S1(C)).
4. Discussions
Oxidative stress is considered to be an important component of various diseases, including cardiovascular, neurological, and mental disorders (Aschbacher et al., Citation2013; Frijhoff et al., Citation2015) such as major depression (Lindqvist et al., Citation2017).
Under oxidative stress, changes in the mitochondrial membrane potential and permeabilization (or rupture) of the outer membrane lead to release of cytochrome c. Cytochrome c possesses a high redox potential (+260 mV) and a compact tertiary structure containing covalently bound heme iron. Upon interaction with negatively charged phospholipids, especially cardiolipin, cytochrome c undergoes a conformational alteration with subsequent displacement of the axial Met-80 that is accompanied by a dramatic decrease in its redox potential to –400 mV. Cardiolipin-activated cytochrome c (or plasmalogenase) catalyzes the oxidatively enabled hydrolytic cleavage of the vinyl ether linkage of plasmenylcholine and plasmenylethanolamine in the presence of hydrogen peroxide (H2O2). The reaction products are 2-acyl-lysophospholipids and highly reactive α-hydroxy fatty aldehydes (Jenkins et al., Citation2018).
Additionally, the increase in β-amyloid (Aβ) levels associated with Alzheimer’s disease has been shown to promote oxidative stress in the brain, leading to a loss of peroxisomal function. This in turn decreases the activity of alkylglycerone phosphate synthase (AGPS – the rate-limiting enzyme of ether lipid synthesis) and ultimately reduces the plasmalogen levels (Dean & Lodhi, Citation2018).
Shock-induced acute gastric mucosal lesions (stress ulceration) are attributed to multiple factors including gastric hyperacidity, impaired gastric mucus secretion, and an abnormal gastric mucosal permeability to hydrogen ions, stress-promoted tumor necrosis factor (TNF)-α, interleukin (IL)-1β, cytokine-induced neutrophil chemoattractants, and other inflammatory substances. Moreover, it is a well-documented fact that the presence of bile salts in the stomach during shock renders the mucosa more vulnerable to stress-induced ulceration (Jia et al., Citation2007; Menguy & Masters, Citation1974).
The gastric mucosa is rich in peroxisomes and plasmalogens, the ether linkage is synthesized in peroxisomes (Abugila, Connock, & Burdett, Citation1995). However, as already mentioned, oxidative stress leads to a collapse of plasmalogen levels in all body tissues, so it might be reasonable to view this process as the primary stage in the development of stomach ulcers precisely under acute stress, along with the above-described causes.
It is known that dietary AGs are precursors in the biosynthesis of plasmalogens, they allow to omit the stage of the formation of a simple ether linkage in peroxisomes (Watschinger & Werner, Citation2013). It was previously shown that AG-enriched diet leads to an increase in the level of plasmalogens in erythrocytes, kidneys, liver, and heart in WT-mice and in mice deficient in phospholipids with a simple ether linkage (Brites et al., Citation2011). It is plasmalogens that are antioxidants (Zoeller, Morand, & Raetz, Citation1988) and participate in binding of the reactive oxygen species (Skaff, Pattison, & Davies, Citation2008). The mechanism of plasmalogen’s antioxidant actions depends on the fact that the simple vinyl ether bond has relatively low dissociation energy and is preferentially oxidized by various free radicals and reactive oxygen species (Broniec et al., Citation2011). Thus, AGs increase the level of plasmalogens and so indirectly contribute to the protection of cells from lipid peroxidation.
Endogenous antioxidant enzymes including catalase, glutathione peroxidase, and superoxide dismutase constitute the major part of the enzymatic antioxidant defense system against oxidative stress by their actions to neutralize reactive oxygen species. Oishi and Machida (Citation2002) concluded that the antioxidant state of peripheral tissues is affected by immobilization stress and that rats have tissue-specific mechanisms regulating antioxidant enzymes.
A significant decrease in catalase activity in erythrocytes in response to immobilization stress shows an increase in the rate of lipid peroxidation (Gümüşlü, Sarikçioğlu, Sahin, Yargiçoğlu, & Ağar, Citation2002). In groups that previously received AGs at a dose of 157 mg/kg, we observe the restoration of catalase activity to the values of the control group and above that; this confirms the antioxidant activity of AGs. This corresponds well to the data of Karaman et al. (Citation2013), who revealed that AGs improved the oxidation-reduction status in experimental dyslipidemia by increasing the level of catalase.
In our research, the response of the adrenal glands to AIS was stronger in rats treated with AGs at a dose of 157 mg/kg before they were subjected to stress.
It can also be assumed that administration of the AGs affects a decrease in BW indirectly by acting on the adrenal glands or causing a redistribution of lipids with dependence on the current state of metabolism.
The AGs treatment has a dose-dependent effect, which is a protective action on the gastric mucosa is observed at a dose of 15 mg/kg; 157 mg AGs/kg caused an increase in catalase activity and in the adrenal gland weight under stress, and a dose higher than 157 mg/kg was required to cause a significant increase in the hemoglobin level, and presumably, the recovery of the spleen weight.
5. Conclusions and perspectives
Based on our results, we can conclude that alkylglycerols have the following effects:
Antioxidant activity.
Antiulcerative effects.
Also, according to literature data, AGs increased hematopoiesis (Karaman et al., Citation2013; Mitre et al., Citation2005) and stimulated of immunity (Iannitti & Palmieri, Citation2010).
Thus, the presented data indicate that AGs promote the adrenal gland activation in acute stress. At the same time, AGs neutralize some of the negative effects of stressful conditions: restoration of the oxidative status and reduction of gastric ulcer formation. Though our work is the first attempt in the study of the antistress effect of AGs and the obtained observations need further research of the mechanism of the AG action, our results show that further investigations appear rather promising. Perhaps, AGs can help athletes achieve high performance results. AGs can be useful in the complex therapy of stomach and duodenum lesions and ulcers of other etiology.
Note on Contributions
Poleschuk T. S.: setting up an experiment and medical research; Sultanov R. M.: preparation of AGs; Ermolenko E. V.: gas-chromatography and mass-spectrometry analysis of AGs; Shulgina L. V.: medical research; Kasyanov S. P.: conceptualization of the experiment.
Polyschuk_et_al.__Supplementary_Material.doc
Download MS Word (6.3 MB)Acknowledgements
The authors thank I. A. Barsegova for help in preparing this manuscript for publication, Yu. K. Denisenko for his assistance in the biochemical analysis, E. I. Drobot and V. V. Chaika for participation in the morphological studies.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Hoffman, J.I.E. (2015). Biostatistics for medical and biomedical practitioners. New York: Elsevier Inc. doi:10.1016/B978-0-12-802387-7.00029-9
- Abugila, M.A., Connock, M.J., & Burdett, K. (1995). Gastric mucosal peroxisomes and plasmalogen biosynthesis. Biochemical Society Transactions, 23, 578s. doi:10.1042/bst023578s
- Aebi, H. (1984). Catalase in vitro assay. Methods in Enzymology, 105, 121–126. doi:10.1016/S0076-6879(84)05016-3
- Aschbacher, K., O’Donovan, A., Wolkowitz, O.M., Dhabhar, F.S., Su, Y., & Epel, E. (2013). Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38, 1698–1708. doi:10.1016/j.psyneuen.2013.02.004
- Bakes, M.J., & Nichols, P.D. (1995). Lipid, fatty acid and squalene composition of liver oil from six species of deep-sea sharks collected in southern Australian waters. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 110, 267–275. doi:10.1016/0305-0491(94)00083-7
- Blank, M.L., Cress, E.A., Smith, Z.L., & Snyder, F. (1992). Meats and fish consumed in the American diet contain substantial amounts of ether-linked phospholipids. The Journal of Nutrition, 122, 1656–1661. doi:10.1093/jn/122.8.1656
- Brites, P., Ferreira, A.S., da Silva, T.F., Sousa, V.F., Malheiro, A.R., Duran, M., … Wanders, R.J.A. (2011). Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One, 6, e28539. doi:10.1371/journal.pone.0028539
- Broniec, A., Klosinski, R., Pawlak, A., Wrona-Krol, M., Thompson, D., & Sarna, T. (2011). Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radical Biology and Medicine, 50, 892–898. doi:10.1016/j.freeradbiomed.2011.01.002
- Chen, S., & Liu, C. (2013). Ether glycerophospholipids and their potential as therapeutic agents. Current Organic Chemistry, 17, 802–811. doi:10.2174/1385272811317080006
- Day C.E. (Eds.) (2014). Histopathology: Methods and protocols. New York, NY: Springer Science, Business Media. doi:10.1007/978-1-4939-1050-2_3
- Dean, J.M., & Lodhi, I.J. (2018). Structural and functional roles of ether lipids. Protein and Cell, 9, 196–206. doi:10.1007/s13238-017-0423-5
- Deniau, A.-L., Mosset, P., Pédrono, F., Mitre, R., Le Bot, D., & Legrand, A.B. (2010). Multiple beneficial health effects of natural alkylglycerols from shark liver oil. Marine Drugs, 8, 2175–2184. doi:10.3390/md8072175
- Devasagayam, T.P.A., Boloor, K.K., & Ramasarma, T. (2003). Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian Journal of Biochemistry and Biophysics, 40, 300–308.
- Dolzhikov, A.A., Tverskoi, A.V., Bobyntsev, I.I., Kriukov, A.A., & Belykh, A.E. (2015). Morphometric study of hippocampal neurons in chronic immobilization stress. Research Result. «Medicine and Pharmacy» Series, 1, 62–65. doi:10.18413/2313-8955-2015-1-4-62-65
- Ermolenko, E.V., Latyshev, N.A., Sultanov, R.M., & Kasyanov, S.P. (2015). Technological approach of 1-O-alkyl-sn-glycerols separation from Berryteuthis magister squid liver oil. The Journal of Food Science and Technology, 5, 1722–1726. doi:10.1007/s13197-015-2148-x
- Frijhoff, J., Winyard, P.G., Zarkovic, N., Sean, S., Davies, S.S., Stocker, R., … Ghezzi, P. (2015). Clinical relevance of biomarkers of oxidative stress. Antioxidants & Redox Signaling, 23, 1144–1170. doi:10.1089/ars.2015.6317
- Gulyaev, S.M., Shantanova, L.N., & Batotsyrenova, E.T. (2017). Morphometric evaluation of the neuroprotective action of an extract of astragalus membranaceous in the brains of rats during restraint stress. Neuroscience and Behavioral Physiology, 47, 608–611. doi:10.1007/s11055-017-0441-z
- Gümüşlü, S., Sarikçioğlu, S.B., Sahin, E., Yargiçoğlu, P., & Ağar, A. (2002). Influences of different stress models on the antioxidant status and lipid peroxidation in rat erythrocytes. Free Radical Research, 36, 1277–1282. doi:10.1080/1071576021000016508
- Hayashi, K., & Kishimura, H. (2002). Amount and composition of diacyl glyceryl ethers in various tissue lipids of the deep-sea squid Berryteuthis magister. Journal of Oleo Science, 51, 523–529. doi:10.5650/jos.51.523
- Hayashi, K., & Kishimura, H. (1997). Content and composition of diacyl glyceryl ethers in the pyloric ceca and ovaries of the asteroids Solaster paxillatus and Asterias amurensis. Fisheries Science, 63, 945–949. doi:10.2331/fishsci.63.945
- Honsho, M., & Fujiki, Y. (2017). Plasmalogen homeostasis – Regulation of plasmalogen biosynthesis and its physiological consequence in mammals. *Febs Letters, 591, 2720–2729. doi:10.1002/1873-3468.12743
- Iannitti, T., & Palmieri, B. (2010). An update on the therapeutic role of alkylglycerols. Marine Drugs, 8, 2267–2300. doi:10.3390/md8082267
- Jayakumar, S., Raghunath, G., Ilango, S., Vijayakumar, J., & Vijayaraghavan, R. (2017). Effect of fluoxetine on the hippocampus of Wistar albino rats in cold restraint stress model. Journal of Clinical and Diagnostic Research, 11, AF01–06. doi:10.7860/JCDR/2017/26958.9953
- Jenkins, C.M., Yang, K., Liu, G., Moon, S.H., Dilthey, B.G., & Gross, R.W. (2018). Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. Journal of Biological Chemistry, 293, 8693–8709. http://www.jbc.org/cgi/doi/10.1074/jbc.RA117.001629. doi:10.1074/jbc.RA117.001629
- Jia, Y.-T., Wei, W., Ma, B., Xu, Y., Liu, W.-J., Wang, Y., … Xia, Z.-F. (2007). Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. The Journal of Immunology, 179, 7808–7819. doi:10.4049/jimmunol.179.11.7808
- Karaman, Y.K., Novgorodtseva, T.P., Gvozdenko, T.A., & Kasynov, S.P. (2013). Effect of 1-O-alkylglycerols from sea hydrobionts on the metabolic status of rats with alimentary dyslipidemia. Functional Foods in Health and Disease, 3, 103–110. doi:10.31989/ffhd.v3i4.61
- Lindqvist, D., Dhabhar, F.S., James, S.J., Hough, C.M., Jain, F.A., Bersani, F.S., … Mellon, S.H. (2017). Oxidative stress, inflammation and treatment response in major derression. Psychoneuroendocrinology, 76, 197–205. doi:10.1016/j.psyneuen.2016.11.031
- Magnusson, C.D., & Haraldsson, G.G. (2011). Ether lipids. Chemistry and physics of lipids, 164, 315–340. doi:10.1016/j.chemphyslip.2011.04.010
- Menguy, R., & Masters, Y.F. (1974). Gastric mucosal energy metabolism and “Stress Ulceration”. Annals of Surgery, 180, 538–546. doi:10.1097/00000658-197410000-00018
- Mitre, R., Etienne, M., Martinais, S., Salmon, H., Allaume, P., Legrand, P., & Legrand, A.B. (2005). Humoral defence improvement and haematopoiesis stimulation in sows and offspring by oral supply of shark-liver oil to mothers during gestation and lactation. British Journal of Nutrition, 94, 753–762. doi:10.1079/BJN20051569
- Nisbet, R., Miner, G., & Yale, K. (2017). Handbook of statistical analysis and data mining applications (2nd ed.). London, UK: Academic Press. doi:10.1016/B978-0-12-416632-5.00004-9
- Oishi, K., & Machida, K. (2002). Different effects of immobilization stress on the mRNA expression of antioxidant enzymes in rat peripheral organs. Scandinavian Journal of Clinical and Laboratory Investigation, 62, 115–122. doi:10.1080/003655102753611735
- Phleger, C.F., Nichols, P.D., & Virtue, P. (1997). Lipids and buoyancy in Southern Ocean pteropods. Lipids, 32, 1093–1100. doi:10.1007/s11745-997-0141-x
- Scheff, S.W. (2016). Fundamental statistical principles for the neurobiologist. New York, NY: Elsevier Inc. doi:10.1016/B978-0-12-804753-8.00005-1
- Selye, H. (1955). Stress and disease. Science, 122, 625–631. doi:10.1126/science.122.3171.625
- Sinex, J.A., & Chapman, R.F. (2015). Hypoxic training methods for improving endurance exercise performance. Journal of Sport and Health Science, 4, 325–332. doi:10.1016/j.jshs.2015.07.005
- Skaff, O., Pattison, D.I., & Davies, M.J. (2008). The vinyl ether linkages of plasmalogens are favored targets for myeloperoxidase-derived oxidants: A kinetic study. Biochemistry, 47, 8237–8245. doi:10.1021/bi800786q
- Vadalа, M., Laurino, C., Palmieri, L., & Palmieri, B. (2017). Shark derivatives (Alkylglycerols, Squalene, Cartilage) as putative nutraceuticals in oncology. European Journal of Oncology, 22, 5–20.
- Van der Doef, M., & Maes, C. (1998). The job demand-control (-support) model and physical health outcomes: A review of the strain and buffer hypothesis. Psychology and Health, 13, 909–936. doi:10.1080/08870449808407440
- Watschinger, K., & Werner, E.R. (2013). Orphan enzymes in ether lipid metabolism. Biochimie, 95, 59–65. doi:10.1016/j.biochi.2012.06.027
- Wood, P.L. (2013). Alkylglycerol lipid precursors: A review of therapeutic strategies for plasmalogen replacement. Current Organic Chemistry, 17, 786–792. doi:10.2174/1385272811317080004
- Wood, P.L., Khan, M.A., Mankidy, R., Smith, T., & Goodenowe, D.B. (2011). *Chapter 24. Plasmalogen deficit: A new and testable hypothesis for the etiology of Alzheimer's disease. In S. De La Monte (Ed.), Alzheimer's disease pathogenesis-core concepts, shifting paradigms and therapeutic targets. London: InTechOpen Ltd. (pp. 561–588). doi:10.5772/17630
- World Health Organization Mental Health Program. 2018. Retrieved from http://www.who.int/mental_health/mhgap/en
- Wynalda, К.М., & Murphy, R.C. (2010). Low concentration ozone reacts with plasmalogen glycerophosphoethanolamine lipids in lung surfactant. Chemical Research in Toxicology, 23, 108–117. doi:10.1021/tx900306p
- Zaidi, S.K., Hoda, M.N., Tabrez, S., Ansari, S.A., Jafri, M.A., Khan, M.S., … Banu, N. (2014). Protective effect of Solanum nigrum leaves extract on immobilization stress induced changes in rat’s brain. Evidence-Based Complementary and Alternative Medicine, 2014, 1. doi:10.1155/2014/912450
- Zoeller, R.A., Morand, O.H., & Raetz, C.R. (1988). A possible role for plasmalogens in protecting animal cells against photosensitized killing. Journal of Biological Chemistry, 263, 11590–11596.