Abstract
Both the autonomic nervous system and the neuroendocrine system are activated by osmotic stimulation (OS) evoking cardiovascular effects. The current study investigated the mechanisms involved in the cardiovascular responses evoked by an acute osmotic stimulus with intraperitoneal (i.p.) injection of either isotonic (0.15 M NaCl) or hypertonic saline (0.6 M NaCl) in conscious rats. Hypertonic saline increased mean arterial pressure (MAP) and heart rate (HR) for 30 min, as well as plasma osmolality and sodium content. Urinary sodium and urinary volume were also increased. Pretreatment with the ganglion blocker pentolinium (i.v.) did not affect the pressor response, but significantly decreased the tachycardic response caused by OS. Pretreatment with the V1-vasopressin receptor antagonist dTyr(CH2)5(Me)AVP (i.v.) reduced the pressor response, without affecting the tachycardic response evoked by the hypertonic OS. Neither the pressor nor the tachycardic response to OS was affected by pretreatment with either the oxytocin receptor antagonist atosiban or the α1-antagonist prazosin. Pretreatment with the β1-antagonist atenolol had no effect on the pressor response, but markedly decreased the tachycardic response evoked by OS. Results indicate that i.p. hypertonic OS-evoked pressor response is mediated by the release of vasopressin, with a minor influence of the vascular sympathetic input.
Increased plasma osmolality, such as that observed during dehydration or salt intake, is a potent stimulus yielding to marked cardiovascular and neuroendocrine responses. The intraperitoneal (i.p.) injection of hypertonic saline solution is a commonly used animal model to cause a sustained increase in plasma osmolality, leading to a cardiovascular response characterized by sustained blood pressure and heart increases, whose systemic mechanisms were presently studied. Our findings indicate that the pressor response to the i.p. osmotic stimulus (OS) is mediated mainly by the release of vasopressin into the blood circulation with a minor or even the noninvolvement of the vascular sympathetic nervous system, whereas activation of the sympathetic-cardiac system mediates the tachycardic response to OS.
LAY SUMMARY
1. Introduction
Changes in plasma osmolality are identified by central and peripheral osmoreceptors (Antunes-Rodrigues, de Castro, Elias, Valenca, & McCann, Citation2004; Bourque, Oliet, & Richard, Citation1994; Haberich, Citation1968). Increases in plasma concentration of NaCl, the main determinant of plasma osmolality and extracellular fluid volume, induce behavioral and neurovegetative adjustments, such as a blood pressure (BP) increase, associated with neurohumoral changes, including sympathetic nervous system activation and vasopressin release (Akins & Bealer, Citation1990; Antunes-Rodrigues et al., Citation2004; Crofton & Share, Citation1989; Garcia-Estan, Carbonell, Garcia-Salom, Salazar, & Quesada, Citation1989; Onaka & Yagi, Citation2001; Sharp, Sagar, Hicks, Lowenstein, & Hisanaga, Citation1991; Weiss, Claassen, Hirai, & Kenney, Citation1996; Xiong & Hatton, Citation1996).
Osmoreceptors are found in forebrain areas, such as the subfornical organ and organum vasculosum of the lamina terminalis (Bourque, 2008; Broadwell & Brightman, Citation1976; McKinley et al., Citation1983). Additionally, there are peripheral osmoreceptors in the liver, mouth, splanchnic circulation, and hepatoportal region (Bisset & Chowdrey, Citation1988; Bourque et al., Citation1994; Hosomi & Morita, Citation1996). When stimulated, they activate the hypothalamic supraoptic nucleus (SON) and the paraventricular nucleus (PVN) to regulate vasopressin (AVP) and oxytocin (OT) secretion, and the central sympathetic neurocircuitry (Hussy, Deleuze, Desarmenien, & Moos, Citation2000; Larsen & Mikkelsen, Citation1995; Onaka & Yagi, Citation2001; Weiss & Hatton, Citation1990), integrating cardiovascular control and body fluid balance (Haberich, Citation1968; Herbert, Moga, & Saper, Citation1990; Kobashi & Adachi, Citation1985; Ricardo & Koh, Citation1978; Saper, Reis, & Joh, Citation1983; Sawchenko & Swanson, Citation1982; Stocker, Osborn, & Carmichael, Citation2008; Toney, Chen, Cato, & Stocker, Citation2003; Torvik, Citation1956; van der Kooy & Koda, Citation1983).
The intraperitoneal (i.p.) injection of a hypertonic NaCl solution, a common stimulus used to increase plasma osmolality, increases c-Fos expression in AVP and OT magnocellular neurons present in SON and PVN (Brimble & Dyball, Citation1977; Dunn, Brennan, Nelson, & Robertson, Citation1973; Onaka & Yagi, Citation2001; Sharp et al., Citation1991; Stricker & Verbalis, Citation1986; Xiong & Hatton, Citation1996).
Acute changes in the body fluid osmolality caused by intragastric or intravascular hypertonic solutions have been reported to evoke persistent increases in sympathetic nerve activity, blood pressure (BP), heart rate (HR), peripheral resistance, and catecholamine secretion (Blanch et al., Citation2013; Bourque et al., Citation1994; Chen, Morris, Key, & Chen, Citation2004; Chen & Toney, Citation2001; Cunningham, Penny, & Murphy, Citation2004; Leng et al., Citation2001; Stocker et al., Citation2008; Toney et al., Citation2003). However, the mechanisms involved in the cardiovascular responses to the i.p. injection of hypertonic saline were not yet fully studied (Brimble & Dyball, Citation1977; Honda, Negoro, Dyball, Higuchi, & Takano, Citation1990; Larsen & Mikkelsen, Citation1995; Onaka & Yagi, Citation2001; Pirnik, Mravec, & Kiss, Citation2004; Zemo & McCabe, Citation2002).
In the present study, we investigated the mechanisms involved in the cardiovascular response to the i.p. injection of hypertonic saline in conscious rats. For that, we pretreated rats with: (1) the ganglion blocker pentolinium, to reduce overall autonomic outflow; (2) V1-vasopressin and oxytocin receptor antagonists to, respectively, assess the participation of AVP and OT release in the cardiovascular response to OS; and (3) pretreatment with either the α1-adrenoceptor antagonist prazosin or the β1-adrenoceptor antagonist atenolol to assess the participation of the sympathetic vascular and sympathetic cardiac outputs in the mediation of the cardiovascular response to OS.
2. Material and methods
2.1. Animal preparation
We used male Wistar rats weighing 250–300 g that were kept in the Animal Care Unit of the Department of Pharmacology of the School of Medicine of Ribeirão Preto, University of São Paulo. The following conditions were provided: they were housed in plastic cages in a temperature-controlled room (20–25 °C), having free access to water and commercial chow. Housing conditions and experimental protocols (no. 151/2015) were approved by the animal ethics committee of the institution.
We implanted polyethylene catheters into the femoral artery and vein under anesthesia with 10% ketamine–2% xylazine (0.09 mL/100g body weight, i.p.). The catheters were exposed on the dorsum of the animal and attached to the skin, allowing respectively physiologic recordings and drug injection in conscious rats. After the surgery, the rats were given poly-antibiotic (Pentabiotico®, Fontoura-Wyeth, SP, Brazil, 80.000 UI i.m.) to prevent infection and nonsteroidal anti-inflammatory flunixin meglumine (Banamine®, ScheringPlough, Brazil) for postoperation analgesia as described in (Fortaleza, Scopinho, & Correa, Citation2012a). Twenty-four hours after surgery, mean arterial pressure (MAP) and heart rate (HR) were recorded, and animals subjected to intravenous treatment with vehicle or drugs.
2.2. Experimental procedure: drug treatments and acute osmotic stimulation
Throughout the period of experiments, the rats were kept in individual cages in the Animal Care Unit, in which they were transported to the experimental room. Before starting blood pressure and heart rate recording, a period of 1 h was allowed to adapt the animals to the conditions of the experimental room, i.e. sound and illumination. Another 20 min period was allowed for baseline recording before experiments were initiated. Whenever stable blood pressure and heart rate were simultaneously observed, the pretreatment with intravenous (i.v.) injection of vehicle or drug was performed; each animal received only one injection. The injection needle was slowly introduced into the catheter exposed on the dorsum of the animal without touching or restraining it. Ten minutes later, animals were injected i.p. with 0.6 M/NaCl in a volume of 20 mL/kg body weight for about 60 s (Onaka & Yagi, Citation2001). The i.p. injection was performed without removing the animals from their home-cages.
2.3. Measurement of cardiovascular responses
Pulsatile arterial pressure (PAP) of freely moving animals was recorded using an HP-7754A preamplifier (Hewlett Packard, Palo Alto, CA) and an acquisition board (MP100A, Biopac Systems Inc, Goleta, CA) connected to a computer. Transducer signal was recorded at 200 Hz sampling. Heart rate (bpm) was calculated from PAP peak intervals integrated every 6 s using the software Acknowledge (Biopac Systems Inc, Goleta, CA) according to a previous report (Scopinho, Fortaleza, & Correa, Citation2012). BP and HR changes over time were graphically represented as Δ values subtracting the average of MAP and HR recorded during the 1 min period prior to i.v. treatment with either vehicle or drugs. Baseline absolute MAP and HR values are presented in . On graphic time–Δ effect curves, each point represents the average of MAP and HR recorded during the 1 min time period.
Table 1. Baseline means arterial pressure (MAP), heart rate (HR), before treatment (i.v.), and before osmotic stimulation.
2.4. Experimental protocols
Rats were divided into eight experimental groups: (1) OS – isotonic saline (0.15 M, i.p.); (2) OS – hypertonic saline (0.6 M, i.p.); (3) animals pretreated with vehicle (0.9% NaCl, 1 mL/kg, i.v. – control group) 10 min prior to hypertonic OS; (4) animals pretreated with the ganglion blocker pentolinium (5 mg/kg, i.v.); (5) animals pretreated with the α1-adrenoceptor antagonist prazosin (0.5 mg/kg, i.v.); (6) animals pretreated with the β1-adrenoceptor antagonist atenolol (1 mg/kg, i.v.); (7) animals pretreated with the V1 receptor antagonist dTyr(CH2)5(Me)AVP (50 μg/kg, i.v.); (8) animals pretreated with the oxytocin receptor antagonist Atosiban (10 μg/kg, i.v.). All drug treatments were performed 10 min prior to the hypertonic OS.
Drug doses were selected based on previous evidence of their selectivity and effectiveness as: (1) ganglioplegic (pentolinium, 5 mg/kg); α1-adrenoceptor antagonist (prazosin, 0.5 mg/kg); β1-adrenocepotr antagonist (atenolol, 1 mg/kg), (Dos Reis, Fortaleza, Tavares, & Correa, Citation2014); (2) V1-vasopressin receptor antagonist (dTyr(CH2)5(Me)AVP, 50 µg/kg), (Correa, Magro, Peres-Polon, & Antunes-Rodrigues, Citation1985; Fernandes, Crippa, Tavares, Antunes-Rodrigues, & Correa, Citation2003); (3) oxytocin–receptor antagonist (atosinban, 10 µg/kg) (Manning et al., Citation2012; Melin, Trojnar, Johansson, Vilhardt, & Akerlund, Citation1986).
2.5. Drugs
Drugs were dissolved in sterile saline (0.9% NaCl), in a concentration that allowed their i.v. injection in 1 mL/kg. Pentolinium, prazosin, atenolol, and dTyr(CH2)5(Me)AVP were purchased from Sigma-Aldrich Corporation (St. Louis, MO). Atosiban was purchased from Ferring GmbH, Kiel, Germany. Flunixin meglumine (Banamine®, Schering-Plough, RJ, Brazil) and the poly antibiotic preparation of streptomycins and penicillins (Pentabiotico®, Fontoura Wyeth, SP, Brazil) were used as provided.
2.6. Determination of plasma osmolality, plasma sodium, and effect of urinary volume and sodium concentration
Plasma samples were collected at time 30 min. Plasma osmolality was measured using an osmometer (model 5004; Precision Systems, Natick, MA) based on the freezing-point method and expressed as mOsm/kg H2O. Plasma sodium concentration was measured by flame photometry (Micronal b262, São Paulo, Brazil), and results were expressed as mEq/L. Moreover, this protocol also aimed to verify the renal concentration of sodium and metabolic cages were used to collect urine samples of the animals during the 30 min period after the intraperitoneal administration of hypertonic saline solution (0.6 M NaCl) or control (isotonic saline 0.15 M NaCl).
2.7. Statistical analysis
Statistical analysis was performed using the Prism software (GraphPad, La Jolla, CA). Student’s t-test was used to compare basal MAP and HR values before and after vehicle or drug treatment and to compare the isotonic group (saline 0.15 M NaCL) versus hypertonic group (saline 0.6 M NaCl) on plasma osmolality, plasma sodium, urinary volume, and sodium concentration. Two-way analysis of variance (ANOVA) for repeated measures followed by the Fisher-LSD post hoc test was used to compare time curves for MAP (ΔMAP) and HR (ΔHR) after vehicle and drug treatments. Although PAP was recorded throughout the experimental procedure, curves for statistical analysis or illustrative figures were generated with points obtained with different data sampling. For statistical purposes, curves were generated by sampling at 0.07/min to generate five points (experiments with five subjects), or 0.08/min to generate six points (experiments with six subjects). Illustrative curves ( and ) were generated sampling at 0.64/min for more accurate representation.
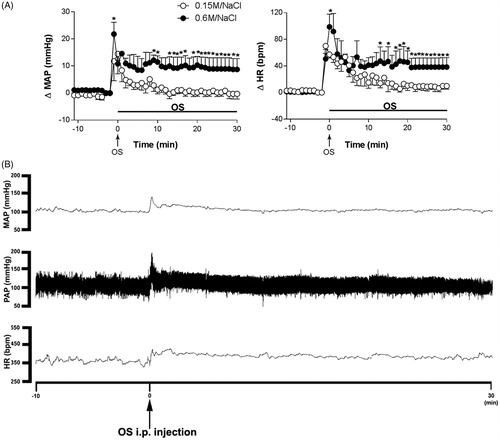
Figure 1. (A) Time course of the mean arterial pressure (ΔMAP) and heart rate (ΔHR) responses observed after intraperitoneal (i.p.) injection of isotonic saline (0.15 M NaCl, n = 4) or hypertonic saline (0.6 M NaCl, n = 4). The onset of osmotic stimulation (OS) was at time 0 min. (*) indicates significantly different from control, Two-way ANOVA, P < 0.05. (B) Representative recordings of mean arterial pressure (MAP), pulsatile arterial pressure (PAP) and heart rate (HR) showing the cardiovascular effects to OS i.p. injection (0.6 M NaCl) in one unanesthetized rat. The onset of OS i.p. injection is indicated by an arrow at time 0 min.
3. Results
3.1. Cardiovascular responses to the i.p. injection of isotonic (0.15 M NaCl) or hypertonic (0.6 M NaCl) saline in conscious rats
The i.p. injection of isotonic saline caused short-lasting MAP and HR increase, and parameters returned to control levels 10–15 min after saline injection, . The injection of hypertonic saline caused a persistent blood MAP and HR increase that lasted throughout the 30 min of the recording after the injection, . Significant differences were observed in the MAP and HR (p > 0.05) between the i.p. isotonic saline group (0.15 M NaCl, n = 4) and the group injected i.p. with hypertonic saline (0.6 M NaCl, n = 4). Treatment with hypertonic saline caused persistent pressor (ΔMAP treatment: F1.36 = 9.86, p = 0.003; Time: F5.36 = 6.97, p < 0.0001, n = 4, two-way ANOVA), and tachycardic responses HR (ΔHR treatment: F1.36 = 4.50, p = 0.04; time: F5.36 = 14.76, p < 0.0001, n = 4, two-way ANOVA), significantly higher than that observed in animals that received isotonic saline, .
3.2. Effect of osmotic stimulation on plasma osmolality, plasma sodium, urinary volume, and sodium concentration
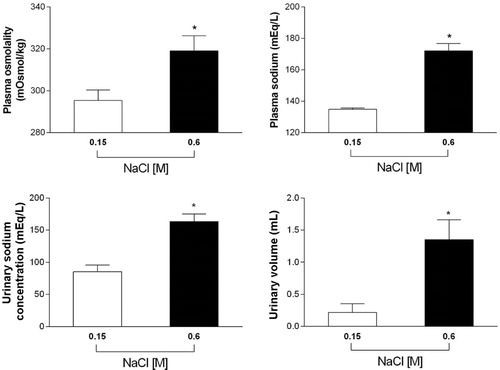
OS with i.p. hypertonic saline (0.6 M NaCl, n = 5) increased both plasma osmolality and sodium, when compared to control animals with i.p. isotonic saline (0.15 M NaCl, n = 5) on time point 30 min after OS onset. Plasma osmolality (isotonic group 295 ± 5mOsmol/kg versus hypertonic group 319 ± 7.2mOsmol/kg, t = 2.69, p < 0.05) and plasma sodium (isotonic group 134 ± 0.8mEq/L versus hypertonic group 172 ± 5mEq/L, t = 7.81, p < 0.05). Moreover, OS with hypertonic saline (n = 6) caused an increase in urinary volume and sodium concentration, when compared with control isotonic saline animals (n = 5) on time point 30 min after OS onset. Urinary sodium concentration (isotonic group 85 ± 10mEq/L versus hypertonic group 163 ± 12mEq/L, t = 4.86, p < 0.05) and urinary volume (isotonic group 0.22 ± 0.14 mL versus hypertonic group 1.35 ± 0.32 mL, t = 3.08, p < 0.05), .
Figure 2. Plasma osmolality, plasma NaCl concentration, urinary volume, and NaCl concentration were measured 30min after the onset of OS with either i.p. injection of isotonic saline (0.15 M NaCl, n = 5) or hypertonic saline (0.6 M NaCl, n = 6) in conscious rats. (*) indicates significant difference, Student's t-test, P < 0.05.
3.3. Effect of i.v. treatment with the ganglion blocker pentolinium on the cardiovascular responses to osmotic stimulation
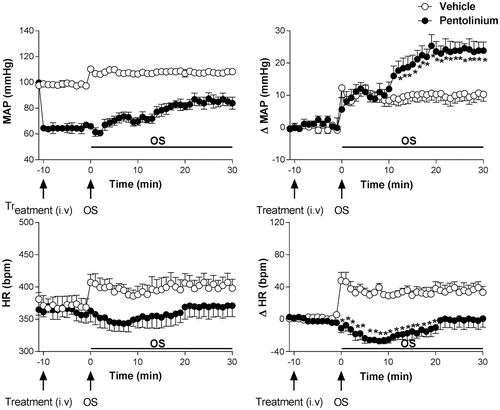
Pretreatment with pentolinium (5 mg/kg, i.v.) significantly reduced baseline values of MAP and did not affect the baseline values of HR, .
Pentolinium significantly increased the blood pressure response and reduced the tachycardic response caused by OS with i.p. hypertonic saline (0.6 M NaCl) when compared to animals receiving vehicle solution (saline 0.9% i.v. n = 8); (ΔMAP, interaction: F5.66= 8.98, p < 0.0001; treatment: F1.66= 27.68, p < 0.0001, time: F5.66 = 32.30, p < 0.0001, two-way ANOVA, followed by the Fisher-LSD post-test). Pentolinium significantly reduced the tachycardic response to OS (ΔHR, interaction: F5.66= 4.72, p < 0.05; treatment: F1.66 = 68.79, p < 0.05; time: F5.66= 2.75, p < 0.05, two-way ANOVA, followed by the Fisher-LSD post-test), .
Figure 3. Left -Time course curves of mean arterial pressure (MAP) and heart rate (HR) responses observed after acute OS i.p. injection (0.6 M NaCl) in animals pretreated with either vehicle solution (1 mL/kg i.v. n = 8) or pentolinium (5 mg/kg, i.v. n = 5). Right - Time course showing absolute mean arterial pressure MAP and HR values. Left - Time course showing changes in mean arterial pressure (ΔMAP) and heart rate (ΔHR) responses. Drugs were injected at the time -10 min. The onset of OS was at time 0min. (*) indicates significantly different from control, Two-way ANOVA, P < 0.05.
3.4. Effect of i.v. pretreatment with the α1 adrenoceptor antagonist prazosin on the cardiovascular responses to osmotic stimulation
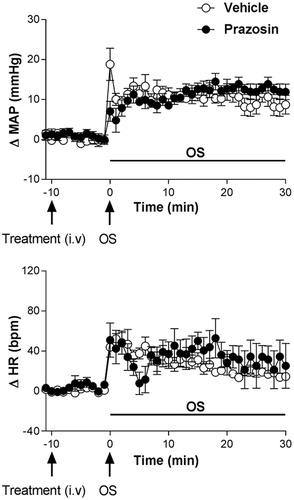
Pretreatment with prazosin (0.5 mg/kg, i.v.) caused no significant changes in baseline values of MAP and HR, .
The α1-adrenoceptor antagonist prazosin caused no changes in the blood pressure and tachycardic responses caused by i.p. OS with hypertonic saline (0.6 M NaCl), when compared with animals pretreated with vehicle (0.9% saline n = 6); (ΔMAP, interaction: F5.54= 1.87, p > 0.05; treatment: F1.54= 0.46, p > 0.05; time: F5.54= 17.83, p < 0.0001, two-way ANOVA, followed by Fisher-LSD post-test) and HR (ΔHR, interaction: F5.54= 0.47, p > 0.05; treatment: F1.54= 0.33, p > 0.05; time: F5.54= 7.12, p < 0.0001, two-way ANOVA, followed by Fisher-LSD post-test), .
Figure 4. Time course curves of mean arterial pressure (ΔMAP) and heart rate (ΔHR) responses observed after acute OS i.p. injection (0.6 M NaCl) in animals pretreated with either vehicle (1 mL/kg i.v. n = 6) or prazosin (0.5 mg/kg, i.v. n = 5). Drugs were injected at the time -10 min. The onset of OS was at time 0 min. (*) indicates significantly different from control, Two-way ANOVA, P < 0.05.
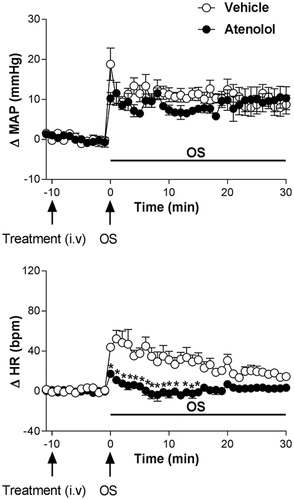
3.5. Effect of pretreatment with the β1 adrenoceptor antagonist atenolol on the cardiovascular responses to osmotic stimulation
Pretreatment with atenolol (1 mg/kg, i.v.) caused no changes in baseline values of MAP and HR, .
The β1-adrenoceptor antagonist atenolol significantly reduced the tachycardic response without any significant effect on the blood pressure response caused by i.p. OS with hypertonic saline (0.6 M NaCl) when compared to animals receiving vehicle (0.9% saline, n = 6); (ΔMAP, interaction: F5.48= 0.77, p > 0.05; treatment: F1.48= 0.45, p > 0.05; time: F5.48= 12.74, p < 0.0001, two-way ANOVA, followed by Fisher-LSD post-test) and HR (ΔHR, interaction: F5.48= 5.01, p < 0.05; treatment: F1.48= 49.35, p < 0.0001; time: F5.48= 9.69, p < 0.0001, two-way ANOVA, followed by Fisher-LSD post-test), .
Figure 5. Time course curves of mean arterial pressure (ΔMAP) and heart rate (ΔHR) responses observed after acute OS i.p. injection (0.6 M NaCl) in animals pretreated with either vehicle (1 mL/kg i.v. n = 6) or atenolol (1 mg/kg, i.v. n = 4). Drugs were injected at the time -10 min. The onset of OS was at time 0 min. (*) indicates significantly different from control, Two-way ANOVA, P < 0.05.
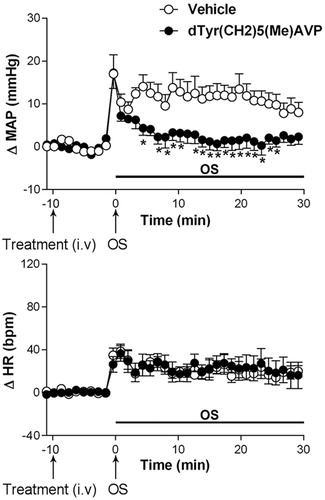
3.6. Effect of i.v. of the V1 receptor antagonist dTyr(CH2)5(me)AVP on the cardiovascular responses to osmotic stimulation
Pretreatment with dTyr(CH2)5(Me)AVP (50 µg/kg, i.v.) caused no changes in baseline values of MAP and HR, .
The V1 receptor antagonist dTyr(CH2)5(Me)AVP significantly reduced the blood pressure response to OS without a significant effect on the tachycardic response to i.p. injection of hypertonic saline (0.6 M NaCl), when compared to animals receiving vehicle solution (0.9% saline n = 6), (ΔMAP, interaction: F5.60= 4.78, p = 0.0010; treatment: F160= 24.11, p < 0.0001; time: F5.60= 10.66, p < 0.0001, two-way ANOVA, followed by Fisher-LSD post-test) and HR (ΔHR, interaction: F5.60 = 0.11, p > 0.98; treatment: F160 = 0.00004, p = 0.99; time: F5.60 = 14.92, p < 0.0001, two-way ANOVA, followed by Fisher-LSD post-test), .
Figure 6. Time course curves of mean arterial pressure (ΔMAP) and heart rate (ΔHR) responses observed after acute OS i.p. injection (0.6 M NaCl) in animals pretreated with either vehicle (1 mL/kg i.v. n = 6) or dTyr(CH2)5(Me)AVP (50 μg/kg, i.v. n = 6). Drugs were injected at the time -10 min. The onset of OS was at time 0 min. (*) indicates significantly different from control, Two-way ANOVA, P < 0.05.
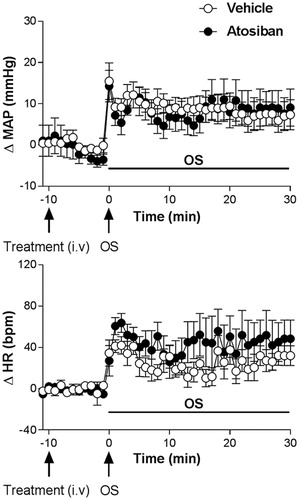
3.7. Effect of the i.v. pretreatment with the oxytocin receptor antagonist Atosiban on the cardiovascular responses to osmotic stimulation
Pretreatment with Atosiban (10 µg/kg, i.v.) caused no changes in baseline values of MAP and HR, .
The oxytocin receptor antagonist Atosiban did not affect the blood pressure and tachycardic responses caused by OS with i.p. hypertonic saline (0.6 M NaCl, n = 5), when compared to animals receiving vehicle (0.9% saline, n = 6); (ΔMAP, interaction: F5.54= 0.27, p > 0.05; treatment: F1.54= 0.30, p > 0.05; time: F5.54= 3.85, p < 0.004, two-way ANOVA, followed by the Fisher-LSD post-test) and HR (ΔHR, interaction: F5.54= 0.42, p > 0.05; treatment: F1.54= 2.38, p > 0.05; time: F5.54= 6.66, p < 0.0001, two-way ANOVA, followed by the Fisher-LSD post-test), .
Figure 7. Time course curves of mean arterial pressure (ΔMAP) and heart rate (ΔHR) responses observed after acute OS i.p. injection (0.6 M NaCl) in animals pretreated with either vehicle (1 mL/kg i.v. n=6) or Atosiban (10 μg/kg, i.v. n = 5). Drugs were injected at the time -10 min. The onset of OS was at time 0 min. (*) indicates significantly different from control, Two-way ANOVA, P < 0.05.
4. Discussion
We report the effect of pretreatment with pentolinium, dTyr(CH2)5(Me)AVP, atosiban, prazosin and atenolol on the pressor and tachycardic response to the i.p. injection of hypertonic saline, in order to study the peripheral mechanisms involved. There is evidence that the cardiovascular changes evoked by i.p. injection of hypertonic saline are due to the hyperosmotic stimulus, and the stressor component related to the discomfort caused by animal handling and the process of injection. To evaluate the contribution of the injection procedure (handling and injection volume), we compared the effects of i.p. injection of an isotonic solution (0.15 M NaCl) with those observed after the injection of hypertonic saline (0.6 M NaCl) in conscious rats.
Plasma osmolality, plasma sodium as well as sodium urinary content and urinary volume were significantly increased after the i.p. injection of hypertonic saline when compared to animals injected with isotonic saline, the latter group being within normal values, as expected. These results are in agreement with data from the literature indicating that i.p. injection of hypertonic saline evokes a rapid increase in plasma osmolality (Ma, Lightman, & Aguilera, Citation1999; Shoji et al., Citation1994; Zemo & McCabe, Citation2002). Additionally, it has been reported that i.p. injection of hypertonic saline does not change hematocrit values (Dunn et al., Citation1973), indicating no effects on blood volume.
The i.p. injection of isotonic saline caused a short-lasting increase in MAP and HR; parameters returned to control values 10–15 min after the injection. The magnitude and the duration of the cardiovascular response were similar to that observed after exposure of rats to a novel environment (novelty stress), when animals are transferred from their home cages to a new clean cage (Resstel, Joca, Guimaraes, & Correa, Citation2006), characterizing the effect of the stressor component associated with the i.p. injection procedure per se. However, the present i.p. injection protocol did not control for differences in pain level associated with the i.p. injection of isotonic and hypertonic solutions. Krause et al. (Citation2011) have addressed this issue by pretreating rats subcutaneously (s.c.) with lidocaine prior to the injection of either isotonic or hypertonic saline into the same area of the skin to reduce pain stimuli. The issue of pain intensity associated with the injection of hypertonic solutions has permeated all the studies in the literature involving i.v. or i.p. injection/infusion of hypertonic saline solutions. Both the i.v. and i.p. injections of hypertonic are nociceptive stimuli. However, we are not aware of any report of the previous injection of a local anesthetic either i.v. or into the peritoneal cavity, prior to osmotic stimulation. Concerning the present study, since animal discomfort, such as abdominal twitch, whenever observed after the i.p. injection of hypertonic saline (0.6 M NaCl) lasted less than approximately 10 s, it would be reasonable to assume that differences in pain level would be maximized on the first minute, and still reflect throughout the 5–15 min period after the injection. This idea is favored by previous report on the occurrence of stress-induced analgesia evoked by intraperitoneal injection of hypertonic saline with maximum intensity observed at time 30 s after the injection of hypertonic saline (Wright & Lincoln, Citation1985). Nonetheless, because no procedure to minimize skin and peritoneal pain or irritation was performed in the present experiment, the exact impact of the painful stimulus cannot be correctly estimated.
The i.p. injection of hypertonic saline, in addition to increasing plasma osmolality, caused sustained MAP and HR increase that lasted throughout the 30 min of experiment, and was significantly higher than that observed after the i.p. injection of isotonic saline. Furthermore, the comparison of the results from i.p. injection of isotonic and hypertonic saline indicates that cardiovascular effects (MAP and HR increase) observed from time 15 min after the i.p. injection up to the end of the experiment should the net result from the OS-evoked increase in plasma osmolality
On the other hand, the i.v. infusion of hypertonic saline has been also reported to increase blood volume in addition to plasma osmolality, causing pressor response and reflex bradycardia due to activation of baroreflex, and subsequently, it initiates secretion of AVP and OT (Antunes, Yao, Pickering, Murphy, & Paton, Citation2006; Bealer, Citation2000; Stocker et al., Citation2008; Weiss et al., Citation1996).
To investigate the systemic mechanisms involved in the mediation of the cardiovascular response to the i.p. injection of hypertonic saline in conscious rats, we pretreated the animals with pharmacological antagonists.
First, we tested the involvement of the sympathetic nervous system in these responses by pretreating animals with the ganglion blocker pentolinium that affects transmission in both sympathetic and parasympathetic nervous systems. Treatment with pentolinium significantly reduced baseline blood pressure values, confirming ganglion blockade effectiveness. Pentolinium abolished the initial phasic component of the pressor response to the OS, but did not affect the subsequent OS-evoked sustained blood pressure increase.
As a first approach, the result showing that ganglion blockade only affected the initial component of the pressor response to i.p. OS indicated a minor involvement of the vascular sympathetic system acting mainly on its initial component, which is under influence of the psychological stress due to handling and injection procedure, as well as the no sympathetic involvement in the subsequent long-lasting pressor response concomitant to plasma osmolality increase caused by the OS. In agreement, ganglion blockade was reported to reduce markedly the pressor response to restraint stress (RS), a predominantly psychologic stimulus (Dos Reis et al., Citation2014).
On the other hand, treatment with pentolinium completely abolished the OS-evoked tachycardic response, and even reversed it into a bradycardic response. This result indicates that OS-evoked tachycardic response results from cardiac sympathetic activation. Furthermore, because the cardiac parasympathetic system is less affected by ganglion blockers than the cardiac sympathetic one, it indicates that i.p. OS-evoked blood pressure increase may also activate the baroreflex parasympathetic component. A balance between parasympathetic and sympathetic influences is involved in autonomic cardiac control. Parasympathetic stimulation reduces both the force and rate of cardiac contraction, while sympathetic activation increases it. Although a balanced control between cardiac vagal and sympathetic baroreflex activity may be observed under normal physiologic conditions, a concomitant activation of both autonomic limbs can be observed during chemoreceptor as well as noxious and defensive responses (Paton, Boscan, Pickering, & Nalivaiko, Citation2005).
Enhanced blood pressure and heart rate are common features in cardiovascular responses to different stressors. For instance, exposure to a predominantly psychological stimulus, such as the RS, triggers a cardiovascular response characterized by sustained elevation of blood pressure and tachycardia. In a previous study, we reported that tachycardic response to RS is mediated by an activation of the cardiac sympathetic, once it was abolished by pretreatment with atenolol, while the RS-evoked increase of blood pressure resulted from an activation of both the sympathovascular and the sympathoadrenal components, since it was abolished by pretreatment with either pentolinium or the α1-antagonist prazosin (Dos Reis et al., Citation2014).
Considering that, we further tested the possible noninvolvement of the sympathetic vascular system in the pressor response to i.p. OS by pretreating animals with prazosin at a dose reported to effective to block the sustained pressor response evoked by RS (Dos Reis et al., Citation2014). The blockade of α1-adrenoceptors only affected the initial portion of the pressor response to i.p. OS, whereas the sustained subsequent pressor increase was not significantly affected. This result, together with that of pentolinium, indicate that vascular sympathetic system is only involved the initial stress-related component of the i.p. OS, but not in the sustained pressor response caused by the increase in plasma osmolality resulting from the i.p. OS. As expected, the blockade of α1-adrenoceptors had no effect on the OS-evoked tachycardic response. However, a larger involvement of the vascular sympathetic system was reported to occur after the i.v. injection of hypertonic saline, since pretreatment with prazosin had a marked effect on the blood response, changing it from a pressor response into a biphasic depressor/pressor response (Antunes et al., Citation2006). The main difference between i.p. and i.v. osmotic stimuli derives from the volume expansion associated with the latter one. Although changes in osmolality can be positively correlated with sympathetic outflow (Scrogin, Grygielko, & Brooks, Citation1999), blood volume expansion is inversely correlated with sympathetic baroreflex activity (Wenner, Rose, Delaney, Stillabower, & Farquhar, Citation2007). Contrarily to the i.p. OS, the i.v. infusion of hypertonic saline has been reported to cause either no HR effect or bradycardic responses in conscious rats (Antunes et al., Citation2006; Bealer, Citation2000; Weiss et al., Citation1996). The bradycardia observed during i.v. infusion of hypertonic saline is related to a withdraw of cardiac sympathetic tonus instead of activation of heart parasympathetic input, like observed during infusion of phenylephrine (Bealer, Citation2000). Since no volume changes were reported after i.p. injection of hypertonic saline (Dunn et al., Citation1973), the increase in HR presently observed after i.p. hypertonic saline is in agreement with the idea of a positive correlation between plasma osmolality and sympathetic outflow (Scrogin et al., Citation1999; Weiss et al., Citation1996). In agreement, blood pressure increase and tachycardia were observed after the subcutaneous (s.c.) injection of hypertonic saline, an OS that also increases the plasma osmolality without changes in blood volume (Krause et al., Citation2011).
To test the involvement of the cardiac sympathetic system in the tachycardic response to i.p. OS, animals were pretreated with the selective β1-adrenergic receptor antagonist atenolol at a dose reported to effective to block the tachycardic response evoked by RS (Dos Reis et al., Citation2014). Blockade of β1-adrenergic receptor had no effect on the pressor response to OS, but blocked the OS-evoked tachycardic response, indicating that an increase in plasma osmolality caused by the i.p. injection of hypertonic saline is associated with increase cardiac sympathetic activity, resulting in the tachycardic response.
Hypertonic stimulus evokes activation of magnocellular neurons in the SON and PVN nuclei that receive projections from areas involved in the osmoreceptor pathway that regulates body fluid balance, such as the subfornical organ, the organum vasculosum of the lamina terminalis and median preoptic nucleus, as well as limbic structures such as the medial nucleus of the amygdala that sends and receives projections from SON and PVN (Fortaleza, Scopinho, & Correa, Citation2012b; Fortaleza, Tavares, & Correa, Citation2009; Kremarik, Freund-Mercier, & Stoeckel, Citation1993; Larsen & Mikkelsen, Citation1995; Xiong & Hatton, Citation1996). Notably, the hypertonic stimulus is a potent releaser of AVP from the posterior pituitary (Dunn et al., Citation1973; Larsen & Mikkelsen, Citation1995; Onaka & Yagi, Citation2001; Shoji et al., Citation1994; Verney, Citation1947; Zemo & McCabe, Citation2002).
To test the role played by peripherally released AVP in the modulation of the cardiovascular response to the acute OS caused by i.p. injection of hypertonic saline, we studied the effect of i.v. pretreatment with the V1-vasopressin receptor antagonist dTyr(CH2)5(Me)AVP on the responses at a dose reported to be effective to block the sustained pressor response evoked by RS (Correa et al., Citation1985; Fernandes et al., Citation2003). The pressor response observed after blockade of V1-vasopressin receptors was very similar to that observed after the injection of isotonic saline that characterizes the cardiovascular changes evoked by the handling and injection procedure. Blockade of V1-vasopressin receptors markedly reduced the subsequent sustained pressor response associated with the increased osmolality. It is interesting to note the complementarity with the results from the pretreatment with prazosin. Whereas pretreatment with the vasopressin antagonist did not affect the phasic component of the pressor response that is observed during the first minutes of OS, the stress-evoked response was blocked by pretreatment with prazosin. In agreement, the cardiovascular response to RS was not affected when animals were treated i.v. with the V1 vasopressin receptor antagonist dTyr-AVP (unpublished data), indicating the noninvolvement of AVP in the mediation of the pressor response to psychologic stress.
The reverse situation was observed concerning the subsequent component of the OS-evoked pressor response associated with the increase in plasma osmolality. Together, the results from the pretreatment with prazosin and the V1-vasopressin antagonist indicate that OS-evoked sustained pressor response caused by i.p. injection of hypertonic saline is mediated by vasopressin release into circulation, without any significant involvement of the vascular sympathetic system.
Additionally, there is evidence in the literature indicating a difference between conscious and anesthetized rats in the relative contribution of the two components, i.e. psychological (handling and injection procedure) and physical (OS) in the induction of neuronal c-Fos protein expression in OT and AVP neurons in the SON; higher activation of OT neurons being observed in conscious animals, whereas activation of AVP neurons was similar under both conditions (Xiong & Hatton, Citation1996).
Increases in ACTH and corticosterone are a common response to stress. Yet, by increasing the magnitude of the stressor stimulus, from a purely psychological stimulus such as the novelty stress, in which release of corticosterone is observed, to a stimulus that is considered to be predominantly psychological but involving a physical component such as RS, in addition to corticosterone, there is a release of OT. Finally, in response to essentially physical stimuli such as exposure to ether or hemorrhage, one can also observe, in addition to corticosterone and OT, the release of AVP into circulation (Gibbs, Citation1986; Ota, Crofton, & Share, Citation1994; Share, Citation1988).
Because of i.p. OS is a physical stimulus, in addition to AVP, it is also known to cause OT release into peripheral circulation. Since activation of OT receptors has been reported to cause negative inotropic and chronotropic effects (Mukaddam-Daher, Yin, Roy, Gutkowska, & Cardinal, Citation2001), we tested whether OT release caused by osmotic stimulation modulated the cardiovascular response to i.p. injection of hypertonic saline, and pretreated animals i.v. with atosiban, an oxytocin receptor antagonist, 10 min prior to OS. Blockade of OT receptors neither affected the pressor nor the tachycardic response to OS. This result rules out the idea of an involvement of OT in the modulation of the OS-evoked cardiovascular response.
Conclusion
The present results indicate that release of AVP, but not of OT, into the peripheral circulation has a preponderant role in the mediation of the pressor response to i.p. OS. Results also indicate a minor involvement of the vascular sympathetic nervous system, mainly in the initial portion of the pressor response to OS. In addition, they indicate the involvement of the sympathetic–cardiac component in the tachycardic response to OS, through an activation of β1 adrenergic receptors.
Acknowledgements
The authors wish to thank Ivanilda A. C. Fortunato for technical help. E. A. T. Fortaleza is a post-doctoral fellow in the Department of Pharmacology of the School of Medicine of Ribeirão Preto-USP [São Paulo Research Foundation (FAPESP) 2012/18556-2]; [National Council for Scientific and Technological Development (CNPq) 167443/2017-8; PDJ 405584/2017-2]. Ivaldo Jesus Almeida Belém-Filho is a PhD student [FAPESP 2016/25502-7]. Cristiane Busnardo is a post-doctoral fellow in the Department of Pharmacology of the School of Medicine of Ribeirão Preto-USP [Postdoctoral National Program (PNPD) of Coordination for the Improvement of Higher Education Personnel (CAPES); Gislaine Almeida-Pereira is a post-doctoral fellow in the Department of Physiology of the School of Medicine of Ribeirão Preto-USP [FAPESP 2014/25005-8].
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Akins, V.F., & Bealer, S.L. (1990). Brain histamine regulates pressor responses to peripheral hyperosmolality. American Journal of Physiology, 259, R507–R513. doi:10.1152/ajpregu.1990.259.3.R507
- Antunes, V.R., Yao, S.T., Pickering, A.E., Murphy, D., & Paton, J.F. (2006). A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. The Journal of Physiology, 576, 569–583. doi:10.1113/jphysiol.2006.115766
- Antunes-Rodrigues, J., de Castro, M., Elias, L.L., Valenca, M.M., & McCann, S.M. (2004). Neuroendocrine control of body fluid metabolism. Physiological Reviews, 84, 169–208. doi:10.1152/physrev.00017.2003
- Bealer, S.L. (2000). Central control of cardiac baroreflex responses during peripheral hyperosmolality. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 278, R1157–R1163. doi:10.1152/ajpregu.2000.278.5.R1157
- Bisset, G.W., & Chowdrey, H.S. (1988). Control of release of vasopressin by neuroendocrine reflexes. Quarterly Journal of Experimental Physiology (Cambridge, England), 73, 811–872.
- Blanch, G.T., Freiria-Oliveira, A.H., Murphy, D., Paulin, R.F., Antunes-Rodrigues, J., Colombari, E., … Colombari, D.S.A. (2013). Inhibitory mechanism of the nucleus of the solitary tract involved in the control of cardiovascular, dipsogenic, hormonal, and renal responses to hyperosmolality. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 304, R531–R542. [pii] doi:10.1152/ajpregu.00191.2012
- Bourque, C.W., Oliet, S.H., & Richard, D. (1994). Osmoreceptors, osmoreception, and osmoregulation. Frontiers in Neuroendocrinology, 15, 231–274. [pii] doi:10.1006/frne.1994.1010
- Brimble, M.J., & Dyball, R.E. (1977). Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. The Journal of Physiology, 271, 253–271. doi:10.1113/jphysiol.1977.sp011999
- Broadwell, R.D., & Brightman, M.W. (1976). Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. The Journal of Comparative Neurology, 166, 257–283. doi:10.1002/cne.901660302
- Chen, H., Morris, M., Key, M.P., & Chen, Y. (2004). Rapid neurosecretory and cardiovascular response to osmotic stimulation in conscious mice. Neuroendocrinology, 80, 225–232. doi:10.1159/000082751
- Chen, Q.H., & Toney, G.M. (2001). AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. American Journal of Physiology – Regulatory, Intergrative and Comparative Physiology, 281, R1844–R1853. doi:10.1152/ajpregu.2001.281.6.R1844
- Correa, F., Macro, I., Perespolon, V., & Antunesrodrigues, J. (1985). Mechanism of the CNS-mediated pressor response to intracerebroventricular injection of noradrenaline in unanaesthetized rats. Neuropharmacology, 24, 831–837. doi:10.1016/0028-3908(85)90033-4
- Crofton, J.T., & Share, L. (1989). Osmotic control of vasopressin in male and female rats. The American Journal of Physiology, 257, R738–R743. doi:10.1152/ajpregu.1989.257.4.R738
- Cunningham, J.T., Penny, M.L., & Murphy, D. (2004). Cardiovascular regulation of supraoptic neurons in the rat: Synaptic inputs and cellular signals. Progress in Biophysics and Molecular Biology, 84, 183–196. doi:10.1016/j.pbiomolbio.2003.11.004
- Dos Reis, D.G., Fortaleza, E.A., Tavares, R.F., & Correa, F.M. (2014). Role of the autonomic nervous system and baroreflex in stress-evoked cardiovascular responses in rats. Stress, 17, 362–372. doi:10.3109/10253890.2014.930429
- Dunn, F.L., Brennan, T.J., Nelson, A.E., & Robertson, G.L. (1973). The role of blood osmolality and volume in regulating vasopressin secretion in the rat. Journal of Clinical Investigation, 52, 3212–3219. doi:10.1172/JCI107521
- Fernandes, K.B., Crippa, G.E., Tavares, R.F., Antunes-Rodrigues, J., & Correa, F.M. (2003). Mechanisms involved in the pressor response to noradrenaline injection into the cingulate cortex of unanesthetized rats. Neuropharmacology, 44, 757–763. doi:10.1016/S0028-3908(03)00067-4
- Fortaleza, E.A., Scopinho, A.A., & Correa, F.M. (2012a). Beta-adrenoceptors in the medial amygdaloid nucleus modulate the tachycardiac response to restraint stress in rats. Neuroscience, 227, 170–179. doi:10.1016/j.neuroscience.2012.09.048
- Fortaleza, E.A., Scopinho, A.A., & Correa, F.M. (2012b). Paraventricular and supraoptic nuclei of the hypothalamus mediate cardiovascular responses evoked by the microinjection of noradrenaline into the medial amygdaloid nucleus of the rat brain. Neuroscience, 219, 157–165. doi:10.1016/j.neuroscience.2012.05.051
- Fortaleza, E.A., Tavares, R.F., & Correa, F.M. (2009). The medial amygdaloid nucleus modulates cardiovascular responses to acute restraint in rats. Neuroscience, 159, 717–726. doi:10.1016/j.neuroscience.2009.01.003
- Garcia-Estan, J., Carbonell, L.F., Garcia-Salom, M., Salazar, F.J., & Quesada, T. (1989). Hemodynamic effects of hypertonic saline in the conscious rat. Life Sciences, 44, 1343–1350. doi:10.1016/0024-3205(89)90391-3
- Gibbs, D.M. (1986). Vasopressin and oxytocin: hypothalamic modulators of the stress response: A review. Psychoneuroendocrinology, 11, 131–139. doi:10.1016/0306-4530(86)90048-X
- Haberich, F.J. (1968). Osmoreception in the portal circulation. Federation Proceedings, 27, 1137–1141.
- Herbert, H., Moga, M.M., & Saper, C.B. (1990). Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. The Journal of Comparative Neurology, 293, 540–580. doi:10.1002/cne.902930404
- Honda, K., Negoro, H., Dyball, R.E., Higuchi, T., & Takano, S. (1990). The osmoreceptor complex in the rat: Evidence for interactions between the supraoptic and other diencephalic nuclei. The Journal of Physiology, 431, 225–241. doi:10.1113/jphysiol.1990.sp018328
- Hosomi, H., & Morita, H. (1996). Hepatorenal and hepatointestinal reflexes in sodium homeostasis. Physiology, 11, 103–107. doi:10.1152/physiologyonline.1996.11.3.103
- Hussy, N., Deleuze, C., Desarmenien, M.G., & Moos, F.C. (2000). Osmotic regulation of neuronal activity: A new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Progress in Neurobiology, 62, 113–134. doi:10.1016/S0301-0082(99)00071-4
- Kobashi, M., & Adachi, A. (1985). Convergence of hepatic osmoreceptive inputs on sodium-responsive units within the nucleus of the solitary tract of the rat. Journal of Neurophysiology, 54, 212–219. doi:10.1152/jn.1985.54.2.212
- Krause, E.G., de Kloet, A.D., Flak, J.N., Smeltzer, M.D., Solomon, M.B., Evanson, N.K., … Herman, J.P. (2011). Hydration state controls stress responsiveness and social behavior. Journal of Neuroscience, 31, 5470–5476. doi:10.1523/JNEUROSCI.6078-10.2011
- Kremarik, P., Freund-Mercier, M.J., & Stoeckel, M.E. (1993). Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat. Journal of Comparative Neurology, 333, 343–359. doi:10.1002/cne.903330304
- Larsen, P.J., & Mikkelsen, J.D. (1995). Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. The Journal of Neuroscience, 15, 2609–2627. doi:10.1523/JNEUROSCI.15-04-02609.1995
- Leng, G., Brown, C.H., Bull, P.M., Brown, D., Scullion, S., Currie, J., … Ludwig, M. (2001). Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: An experimental and theoretical analysis. The Journal of Neuroscience, 21, 6967–6977.
- Ma, X.M., Lightman, S.L., & Aguilera, G. (1999). Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology, 140, 3623–3632. doi:10.1210/endo.140.8.6943
- Manning, M., Misicka, A., Olma, A., Bankowski, K., Stoev, S., Chini, B., … Guillon, G. (2012). Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology, 24, 609–628. doi:10.1111/j.1365-2826.2012.02303.x
- McKinley, M.J., Denton, D.A., Leventer, M., Penschow, J., Weisinger, R.S., & Wright, R.D. (1983). Morphology of the organum vasculosum of the lamina terminalis (OVLT) of the sheep. Brain Research Bulletin, 11, 649–657. doi:10.1016/0361-9230(83)90007-2
- Melin, P., Trojnar, J., Johansson, B., Vilhardt, H., & Akerlund, M. (1986). Synthetic antagonists of the myometrial response to vasopressin and oxytocin. Journal of Endocrinology, 111, 125–131. doi:10.1677/joe.0.1110125
- Mukaddam-Daher, S., Yin, Y.L., Roy, J., Gutkowska, J., & Cardinal, R. (2001). Negative inotropic and chronotropic effects of oxytocin. Hypertension, 38, 292–296. doi:10.1161/01.HYP.38.2.292
- Onaka, T., & Yagi, K. (2001). Involvement of N-methyl-D-aspartic acid receptor activation in oxytocin and vasopressin release after osmotic stimuli in rats. Journal of Neuroendocrinology, 13, 166–174. doi:jne607 [pii] doi:10.1046/j.1365-2826.2001.00607.x
- Ota, M., Crofton, J.T., & Share, L. (1994). Hemorrhage-induced vasopressin release in the paraventricular nucleus measured by in vivo microdialysis. Brain Research, 658, 49–54. doi:10.1016/S0006-8993(09)90009-9
- Paton, J.F., Boscan, P., Pickering, A.E., & Nalivaiko, E. (2005). The yin and yang of cardiac autonomic control: Vago-sympathetic interactions revisited. Brain Research Reviews, 49, 555–565.
- Pirnik, Z., Mravec, B., & Kiss, A. (2004). Fos protein expression in mouse hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei upon osmotic stimulus: Colocalization with vasopressin, oxytocin, and tyrosine hydroxylase. Neurochemistry International, 45, 597–607. doi:10.1016/j.neuint.2004.04.003
- Resstel, L.B., Joca, S.R., Guimaraes, F.G., & Correa, F.M. (2006). Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience, 143, 377–385. doi:10.1016/j.neuroscience.2006.08.002
- Ricardo, J.A., & Koh, E.T. (1978). Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research, 153, 1–26. [pii] doi:10.1016/0006-8993(78)91125-3
- Saper, C.B., Reis, D.J., & Joh, T. (1983). Medullary catecholamine inputs to the anteroventral third ventricular cardiovascular regulatory region in the rat. Neuroscience Letters, 42, 285–291. [pii] doi:10.1016/0304-3940(83)90276-8
- Sawchenko, P.E., & Swanson, L.W. (1982). The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Research, 257, 275–325. doi:10.1016/0165-0173(82)90010-8
- Scopinho, A.A., Fortaleza, E.A., & Correa, F.M. (2012). The medial amygdaloid nucleus is involved in the cardiovascular pathway activated by noradrenaline into the lateral septal area of rats. European Journal of Neuroscience, 36, 3059–3065. doi:10.1111/j.1460-9568.2012.08230.x
- Scrogin, K.E., Grygielko, E.T., & Brooks, V.L. (1999). Osmolality: A physiological long-term regulator of lumbar sympathetic nerve activity and arterial pressure. American Journal of Physiology, 276, R1579–R1586. doi:10.1152/ajpregu.1999.276.6.R1579
- Share, L. (1988). Role of vasopressin in cardiovascular regulation. Physiological Reviews, 68, 1248–1284. doi:10.1152/physrev.1988.68.4.1248
- Sharp, F.R., Sagar, S.M., Hicks, K., Lowenstein, D., & Hisanaga, K. (1991). c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. The Journal of Neuroscience, 11, 2321–2331. doi:10.1523/JNEUROSCI.11-08-02321.1991
- Shoji, M., Kimura, T., Kawarabayasi, Y., Ota, K., Inoue, M., Yamamoto, T., … Sonoyama, T. (1994). Effects of acute salt loading on vasopressin mRNA level in the rat brain. The American Journal of Physiology, 266, R1591–R1595. doi:10.1152/ajpregu.1994.266.5.R1591
- Stocker, S.D., Osborn, J.L., & Carmichael, S.P. (2008). Forebrain osmotic regulation of the sympathetic nervous system. Clinical and Experimental Pharmacology and Physiology, 35, 695–700.
- Stricker, E.M., & Verbalis, J.G. (1986). Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. The American Journal of Physiology, 250, R267–R275. doi:10.1152/ajpregu.1986.250.2.R267
- Toney, G.M., Chen, Q.H., Cato, M.J., & Stocker, S.D. (2003). Central osmotic regulation of sympathetic nerve activity. Acta Physiologica Scandinavica, 177, 43–55.
- Torvik, A. (1956). Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. The Journal of Comparative Neurology, 106, 51–141.
- van der Kooy, D., & Koda, L.Y. (1983). Organization of the projections of a circumventricular organ: The area postrema in the rat. The Journal of Comparative Neurology, 219, 328–338. doi:10.1002/cne.902190307
- Verney, E.B. (1947). The antidiuretic hormone and the factors which determine its release. Proceedings of the Royal Society of London. Series B, Biological Sciences, 135, 25–106.
- Weiss, M.L., & Hatton, G.I. (1990). Collateral input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Research Bulletin, 24, 231–238. doi:10.1016/0361-9230(90)90210-Q
- Weiss, M.L., Claassen, D.E., Hirai, T., & Kenney, M.J. (1996). Nonuniform sympathetic nerve responses to intravenous hypertonic saline infusion. Journal of the Autonomic Nervous System, 57, 109–115. doi:10.1016/0165-1838(95)00108-5
- Wenner, M.M., Rose, W.C., Delaney, E.P., Stillabower, M.E., & Farquhar, W.B. (2007). Influence of plasma osmolality on baroreflex control of sympathetic activity. American Journal of Physiology-Heart and Circulatory Physiology, 293, H2313–H2319. doi:10.1152/ajpheart.01383.2006
- Wright, D.M., & Lincoln, D.W. (1985). Stress-induced analgesia evoked by intraperitoneal injection of hypertonic saline: Evidence for its occurrence in vasopressin deficient rats. Physiology and Behavior, 34, 691–695. doi:10.1016/0031-9384(85)90366-X
- Xiong, J.J., & Hatton, G.I. (1996). Differential responses of oxytocin and vasopressin neurons to the osmotic and stressful components of hypertonic saline injections: A Fos protein double labeling study. Brain Research, 719, 143–153. doi:10.1016/0006-8993(95)01466-7
- Zemo, D.A., & McCabe, J.T. (2002). Transcriptional responses of the rat vasopressin gene to acute and repeated acute osmotic stress. Neuroscience Research, 44, 45–50. doi:10.1016/S0168-0102(02)00079-2
