Abstract
One in three adults reports experiencing inadequate or disrupted sleep throughout the night, with the incidence being higher in women than in men. Disturbances in nightly sleep result in physiological alterations that contribute to a number of disorders. Poor sleep quality is believed to contribute to the pathogenesis of these disorders through interactions with the hypothalamic-pituitary-adrenal (HPA) axis. The present study investigated the effect of one and three days of restricted sleep on HPA axis reactivity. Male and female C57BL/6J (n = 8/group) mice were sleep-deprived for a 20 h period for one day or three consecutive days using the modified multiple platform method, and then subjected to acute restraint stress. In response to sleep restriction, males showed blunted restraint-induced rises in CORT relative to controls. After three days of restricted sleep, females showed a similar attenuation in restraint-induced CORT. However, this effect was ablated after a single day of sleep restriction. Analyses of gene expression revealed significant elevations in the expression of pituitary HPA axis regulatory genes proopiomelanocortin and corticotropin releasing factor receptor 1 in both sexes following sleep restriction. In males, but not females, adrenal mRNA expression of 11β-hydroxylase and melanocortin receptor 2 were also increased. Altogether, these data suggest several possible mechanisms are involved in the HPA axis dysregulation following sleep restriction, and that there are sex differences in how the HPA axis responds to sleep loss.
Sleep restriction alters the stress response differently in males and females following varying nights of sleep restriction. These alterations are accompanied by changes in gene expression in the pituitary and adrenal glands.
Lay summary
Introduction
Sleep hygiene is becoming a public health concern, as one in three adults report experiencing inadequate or disrupted sleep throughout the night (Liu et al., Citation2016). Disturbances in sleep result in hormonal, neurochemical and behavioral alterations that contribute to a number of disorders (Andersen, Martins, D’Almeida, Bignotto, & Tufik, Citation2005; Ohayon & Roth, Citation2003). Sleep loss is believed to contribute to the pathogenesis of these disorders through its interaction with the neuroendocrine stress axis, also known as the hypothalamic-pituitary-adrenal (HPA) axis.
Sleep and the stress axis share a bidirectional relationship, wherein stress induces disruptions in sleep quality by altering slow wave sleep (SWS) and rapid eye movement (REM) sleep patterns (Buckley & Schatzberg, Citation2005). In turn, disrupted sleep alters the responsivity of the HPA axis to stressors (Spiegel, Leproult, & Van Cauter, Citation1999; Suchecki, Tiba, & Tufik, Citation2002). Past studies have revealed alterations in glucocorticoid (cortisol in humans, corticosterone in rodents (CORT)) levels immediately following a period of sleep deprivation (Arthaud et al., Citation2015; Machado, Tufik, & Suchecki, Citation2013; Meerlo, Koehl, van der Borght, & Turek, Citation2002). The HPA axis mediates the physiological response to stress via the paraventricular nucleus of the hypothalamus (PVN). Upon detection of a stressor, PVN neurons become activated leading to the release of corticotropin releasing factor (CRF) into the hypophyseal portal vasculature. CRF in turn promotes the release of the adrenocorticotropic hormone (ACTH) from the anterior pituitary into the general circulation, ultimately leading to release of CORT from the cortex of the adrenal glands (Carrasco & Van de Kar, Citation2003; Handa & Weiser, Citation2014).
Past studies have explored the impact of sleep deprivation on HPA axis reactivity (Arthaud et al., Citation2015; Machado et al., Citation2013; Meerlo et al., Citation2002; Sgoifo et al., Citation2006). However, few studies have explored what changes are occurring at the different levels of the HPA axis that might contribute to the alterations in HPA axis reactivity. Furthermore, few studies have investigated how these alterations are further impacted by sex, despite a wealth of evidence suggesting innate sex differences in sleep structure and a higher incidence of sleep disorders affecting women over their lifetime (Koehl, Battle, & Meerlo, Citation2006; Mallampalli & Carter, Citation2014; Shaib & Attarian, Citation2017; Zhang & Wing, Citation2006).
Based on these observations, we hypothesized that HPA axis reactivity would be dysregulated in a sex-dependent manner in response to varying amounts of sleep loss in mice. The first aim of this study was to determine how varying lengths of sleep restriction impact HPA axis reactivity to an acute stressor. Male and female mice underwent a single or multiple day(s) of sleep restriction and were then exposed to mild restraint stress to assess the CORT response. The second aim of this study was to explore changes in HPA axis feedback mechanisms and the biosynthetic pathway of CORT following sleep restriction. This was assessed by quantifying the relative expression of HPA axis-related genes at the level of the PVN, pituitary and adrenal glands. This study revealed a sex-dependent dysregulation of HPA axis reactivity following sleep deprivation that is accompanied by alterations in HPA axis gene expression.
Experimental procedures
Animals
Male and female C57BL/6J mice, aged 6-8 weeks old upon arrival, were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in groups of four with same-sex cage mates. Mice acclimated to the laboratory animal facilities for at least one week prior to experimentation. All animals were maintained on a 12 h/12 h light/dark cycle (lights on 0700–1900 h; Zeitgeber (ZT)0 at 0700 h) under conditions of controlled temperature (22–25 °C) and humidity (30–70%). Mice had ad libitum access to standard rodent chow and water throughout the experimental timeline. Handling and care of animals were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the Uniformed Services University of the Health Sciences, Bethesda, Maryland.
Paradoxical sleep deprivation protocol
The modified multiple platform method was adapted from previous work to induce paradoxical sleep deprivation (PSD), while minimizing stress due to social isolation or movement restriction, and preserving existing social hierarchies established in the home cage (Ashley, Sams, Brown, & Dumaine, Citation2016; De Lorenzo, de Oliveira Marchioro, Greco, & Suchecki, Citation2015; Suchecki & Tufik, Citation2000). This model reliably produces significant deficits in paradoxical sleep, also known as rapid-eye movement (REM) sleep, and partial deficits in non-REM (NREM) sleep (Machado, Hipólide, Benedito-Silva, & Tufik, Citation2004; Sucheki, Lobo, Hipolide, & Tufik, Citation1998). Animals that begin to enter the REM stage of sleep, characterized by muscle atonia, are awakened when their snouts touch the water.
PSD tanks were constructed based on the tanks described previously (Suchecki & Tufik, Citation2000) and manufactured by the Stoelting Company (Wood Dale, IL). The tanks were made of clear acrylic (430 mm length × 260 mm width × 375 mm height) encased in brown paper to minimize visibility of the outside environment. The interior of each tank contains 12 cylindrical columns (145 mm height × 30 mm diameter) and a built-in food hopper and water bottle. Sets of four socially-familiar cage mates were tested per 12-column tank. The columns were submerged in water approximately 5–10 mm below the surface of the platform. All animals regardless of group assignment were exposed to the sleep deprivation paradigm between 0800 and 1200 h for 30 min daily for three consecutive days prior to testing. The purpose of this acclimation period was to habituate animals to the testing environment and to attenuate stress associated with exposure to a novel environment (Ashley et al., Citation2016; De Lorenzo et al., Citation2015). On the day of the experimental procedure, mice were placed onto the columns 4 h (ZT4) after the onset of lights on (the mouse inactive period), and sleep-deprived for a period of 20 h. Control animals remained in their home cages in the same room as sleep restricted mice.
Experimental design
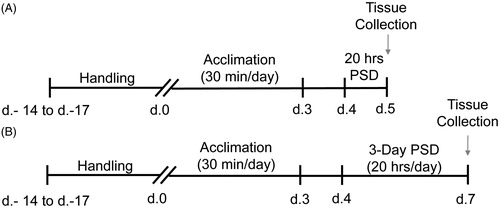
To explore the effect of varying amounts of sleep restriction on the HPA axis, gonadally intact male and freely cycling female mice were randomly assigned to one of 16 groups (n = 8/group). The study was conducted as a 2 × 2 × 2 factorial design for each sleep restriction duration to examine the effects of sleep (home-cage control (HCC) v. paradoxical sleep deprivation (PSD)), sex (male v. female), and restraint (no restraint v. restraint). After the animals arrived at the facility they were given up to 17 days of acclimation to the facility and experimenter handling. The animals were then acclimated to the paradoxical sleep tanks for three consecutive days for 30 min a day, followed by one or three days of sleep deprivation for 20 h per day. They were then sacrificed between ZT8-ZT10, 8 h following the end of the final sleep restriction session ().
Figure 1. Schematic of experimental design. Upon arrival C57BL/6J mice were acclimated to the facility and handled for up to 17 days. All mice were then acclimated to the paradoxical sleep deprivation (PSD) tanks with their cage mates for 30 min per day for three consecutive days. Mice were assigned to one of three groups: 1 day PSD, 3 day PSD or home cage control (HCC). All PSD animals were placed into the PSD tanks for 20 h a day for either (A) 1 day or (B) 3 days. All HCC animals remained in their home cages for the full 20 h. Animals were euthanized 8 h following the end of sleep deprivation and tissue was collected according to the experimental procedures described above.
Restraint
We tested the effect of restricted sleep on the neuroendocrine stress response by using mild restraint stress. Half of the HCC and PSD animals were placed into flat-bottom Plexiglas rodent restrainers (3.8 cm width × 9.5 cm length, Plas-Labs, Inc., Lansing, MI) for 20 min and were sacrificed 10 min following the end of restraint to assess for peak serum CORT levels following restraint.
Tissue collection
Mice were euthanized via CO2 inhalation overdose and immediately decapitated. Brain, trunk blood, pituitary, and adrenals were harvested. Brains were flash frozen in 2-methyl-butane on dry ice, and stored at –80 °C until use. Brains were sectioned at 250 μm (HM525 Cryostat, Thermo Fisher Scientific, Waltham, MA) and the paraventricular nucleus (PVN) of the hypothalamus was microdissected using a 1 mm punch (Integra York PA, Inc., York, PA). The microdissected hypothalamic area containing PVN neurons was collected at –0.58 to –1.22 mm from bregma based on the coordinates and associated structural landmarks in the Franklin and Paxinos Mouse Brain Atlas. Serum was separated from blood by centrifugation at 2000 g at 4 °C for 10 min, and stored at −80 °C until assessed for serum corticosterone concentration. Pituitary and adrenals were stored at –80 °C until use.
Serum CORT assay
Serum CORT levels were measured using a commercially available Corticosterone ELISA kit (Cat. No. ADI-901-097, Enzo, Farmingdale, NY). Samples were diluted to a final concentration of 1:40 to ensure samples fell within the 20–80% range on the linear part of the standard curve. If samples fell outside of the 20–80% range, dilutions were modified to ensure samples fell within the linear part of the standard curve. The intra-assay coefficients of variance for internal standards at high (250 ng/mL) and low (20 ng/mL) concentrations of CORT were 2.12% and 1.99%.
RNA extraction
RNA extractions were performed as previously described (Russell et al., Citation2018). In brief, samples were homogenized in a phenol-chloroform-based reagent (Ribozol; Cat. No. N580, AMRESCO, Solon, OH). Immediately following homogenization, RNA was purified using the Direct-zol RNA Miniprep Kit (Cat. No. R2052, Zymo Research, Irvine, CA) per the manufacturer’s protocol. The concentration and quality of the RNA was then assessed with a spectrophotometer (NanoDrop Lite; Thermo Fisher Scientific). This was followed by reverse transcription to single stranded cDNA using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Cat. No. K1671; Thermo Fisher Scientific).
Real-time quantitative PCR (RT-qPCR)
Relative mRNA expression in the pituitary (proopiomelanocortin (Pomc), corticotropin releasing factor receptor 1 (Crfr1), mineralocorticoid receptor (MR), and glucocorticoid receptor (GR)) and adrenal glands (11β-hydroxylase (11β-OHase)and melanocortin 2 receptor (Mc2r)) was measured by RT-qPCR as previously described (Russell et al., Citation2018). Each sample was assayed in duplicate using the iQ SYBR Green Supermix (Cat. No. 1708884, Bio-Rad, Hercules, CA) and the respective primer pairs on the CFX Connect Real-time System (Bio-Rad). Each run consisted of an initial denaturation and enzyme activation at 95 °C for 3 min, followed by 40 cycles of denaturation (95 °C, 15 s), annealing (60 °C, 30 s) and extension (72 °C, 30 s). Melt curve analysis was performed after each reaction to ensure a single amplicon. All primer sequences for the pituitary and adrenal glands were designed and verified using NCBI BLAST (). Relative expression of each gene was determined using the delta delta Ct (ΔΔCt) method. All data was normalized to the expression of the TATA-binding protein (Livak & Schmittgen, Citation2001; Pfaffl, Citation2001; Schmittgen & Livak, Citation2008) and expressed relative to the male control group.
Table 1. Primer sequences used for real-time PCR and ddPCR.
Droplet digital PCR
Absolute quantification of MR and GR mRNA transcripts in the microdissected hypothalamic area containing PVN neurons were measured via droplet digital PCR. Each sample was assayed in duplicate in a 22 μL reaction, which consisted of 2 ng of cDNA template, 200 nM of each of the respective forward and reverse primer pairs, and EvaGreen Supermix (Cat. No. 1864036, Bio-Rad). Droplets were then generated using the automated droplet generator (Bio-Rad). Droplet generation was followed by PCR amplification using the C1000 Touch Thermal Cycle (Bio-Rad). The PCR amplification consisted of an initial enzyme activation (95 °C for 5 min), followed by 40 cycles of denaturation (95 °C, 30 s), annealing and extension (60 °C, 1 min), and concluded with a final signal stabilization step (4 °C for 5 min then 90 °C for 5 min). Droplets were read using the QX200 Droplet Reader (Bio-Rad) and analyzed with the Quantasoft software (Bio-Rad). The number of copies of mRNA transcript per ng of cDNA template were used for analysis.
Statistical analyses
The interactions between sleep, sex and restraint on serum CORT were analyzed via a three-way (sleep × sex × restraint) analysis of variance (ANOVA). Interactions between sleep and sex were analyzed for the remainder of the data via a two-way ANOVA, followed by tests of simple main effects. If the data failed to pass Levene’s test of equality of variances, the data was log10-transformed prior to analysis, and then the original data were graphed on a log-scaled y-axis. Graphs were generated and data analyzed using GraphPad Prism 7 (GraphPad, La Jolla, CA) and IBM SPSS 24 (IBM, Armonk, NY). A p-value < .05 was considered significant.
Results
Basal and stress-induced CORT responses are altered following sleep restriction
Serum CORT levels were assayed to examine the effect of sleep restriction on HPA axis reactivity. A 3-way ANOVA revealed main effects of sex (F(1, 55) = 5.251, p < .05), sleep (F(1, 55) = 17.711, p < .0001) and restraint (F(1, 55) = 54.313, p < .001) on CORT secretion following a single day of sleep restriction (). Sex × restraint (F(1, 55) = 5.194, p < .05), sleep × restraint (F(1, 55) = 5.260, p < .05), and sex × sleep × restraint (F(1, 55) = 8.604, p < .01) interactions were also found. Pairwise comparisons revealed a main effect of restraint whereby all groups, except PSD females (p > .05), showed significant increases in CORT levels following restraint (p < .05). However, male mice that underwent PSD showed significantly lower restraint induced CORT levels as compared to their home cage counterparts (p < .05), and significantly higher CORT as compared to their female counterparts (p < .05). Furthermore, male mice that underwent PSD showed significantly lower basal CORT levels as compared to their home cage counterparts (p < .05).
Figure 2. Restraint-induced corticosterone (CORT) is dysregulated following PSD. Following a single day of PSD, (A) female mice showed a complete ablation of HPA axis reactivity, while male mice showed blunted restraint induced CORT levels. Following three days of PSD, (B) both males and females showed a blunted restraint induced CORT response. n = 7–8. *Denotes pairwise comparisons where p < .05.
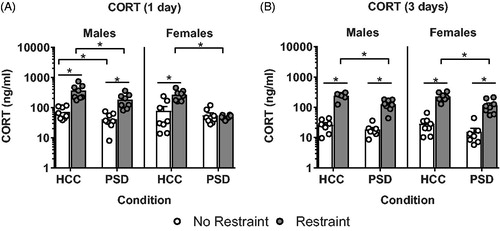
A 3-way ANOVA for three days of sleep restriction revealed main effects of sleep (F(1, 52) = 21.269, p < .001) and restraint (F(1, 52) = 274.203, p < .001) on CORT levels (). No other main effects or interactions were found (all F’s ≤ 0.852).
Effects of sleep restriction on gene expression in the microdissected hypothalamic area containing PVN neurons
The expression of MR and GR mRNA was assayed in the microdissected hypothalamic area containing PVN neurons to investigate the integrity of HPA axis feedback mechanisms following sleep restriction at the level of the hypothalamus. No significant main effects or interactions were found following one or three days of sleep deprivation (all F’s≤ 3.04) ().
Table 2. Gene expression in the paraventricular nucleus of the hypothalamus following paradoxical sleep deprivation.
Effects of sleep restriction on pituitary gene expression
The expression of Pomc, Crfr1, MR, and GR mRNA was assessed in the pituitary. Following a single day of sleep restriction, a main effect of sleep was found on Pomc (F(1, 28) = 32.07, p < .0001; ) and Crfr1 (F(1, 28) = 5.188, p < .05; ) mRNA expression. While a main effect of sex was found on MR (F(1, 28) = 12.92, p < .01; ) and GR (F(1, 28) = 40.27, p < .0001; ) mRNA expression following a single day of sleep restriction, no other main effects or interactions were found (all F’s ≤ 1.26).
Figure 3. Alterations in HPA axis regulatory genes in the pituitary following PSD. Following a single (A) and three days (B) of PSD both male and female mice showed an increase in proopiomelanocortin (Pomc) expression. Following both a single (C) and three nights (D) of PSD, female mice showed an increase in corticotropin releasing factor receptor 1 (Crfr1) expression. No changes were found in mineralocortcoid (MR) expression following a single night of PSD (E) however, following three nights of PSD (F) females showed a decrease in MR expression. No differences in GR mRNA expression was seen following (C) a single day or three days (D) of PSD. However, males showed greater GR expression compared to their female counterparts at both a single and three days of sleep restriction. n = 6–8. *Denotes main effects where p < .05.
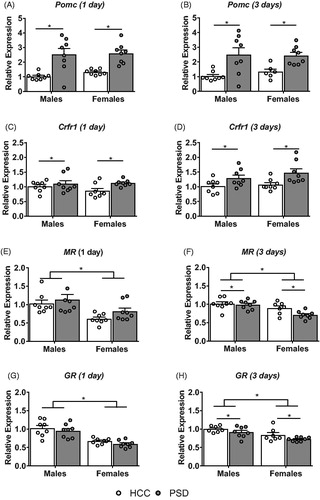
Similarly, following three days of sleep restriction, there was a main effect of sleep on Pomc (F(1, 26) = 18.57, p < .001; ), Crfr1 (F(1, 28) = 11.37, p < .01; ), MR (F(1, 27) = 4.339, p < .05; ) and GR (F(1, 26) = 5.103, p < .05; ) mRNA expression. In addition, a significant main effect of sex was also found on MR (F(1, 27) = 15.19, p < .001; ) and GR (F(1, 26) = 14.83, p < .001; ) mRNA expression. No other main effects or interactions were found (all F’s≤ 1.34). While none of the genes showed a significant sleep × sex interaction, some figures suggest that for some variables the effect of sleep may differ slightly between males and females. However, these differences did not reach statistical significance.
Effects of sleep restriction on adrenal gene expression
To assess the integrity of adrenal function and glucocorticoid biosynthesis and metabolism following one day and three days of sleep restriction, mRNA expression of melanocortin receptor 2 (Mc2r) and11β-hydroxylase (CYP11β1) were analyzed. An ANOVA revealed main effects of sex (F(1, 28) = 66.82, p < .0001) and sleep (F(1, 28) = 14.79, p < .001) on the relative expression of 11β-hydroxylase () following one day of sleep restriction. Furthermore, analysis of adrenal Mc2r expression revealed a significant main effect of sex (F(1, 28) = 132.7, p < .0001) as well as a significant sex × sleep interaction (F(1, 28) = 21.07, p < .0001; ). Pairwise comparisons revealed a main effect of sleep in males, whereby sleep-restricted male mice showed a significant increase in relative expression of Mc2r compared to their home cage controls (p < .05), while female mice showed no significant differences from their HCC counterparts (p > .05). Furthermore, female mice showed greater Mc2r mRNA expression relative to their male counterparts. No other significant main effects or interactions were found (all F’s ≤ 2.34).
Figure 4. Alterations in adrenal gene expression following paradoxical sleep deprivation. Male mice showed an increase in 11β-hydroxylase (11β-OHase) following both a single day (A) and three days (B) of sleep deprivation. Males also showed an increase in melanocortin receptor 2 (Mc2r) expression following both a single (C) and three days (D) of PSD. n = 7–8. * Denotes pairwise comparisons where p < .05.
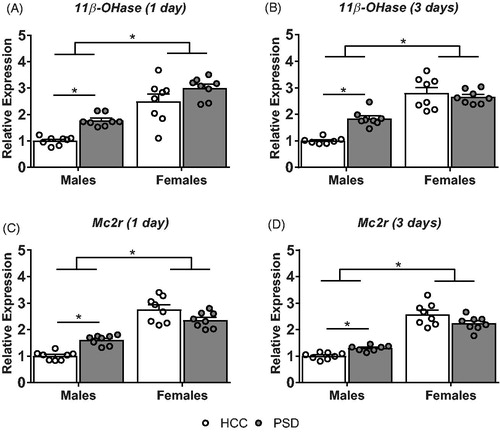
Analysis of 11β-hydroxylase expression in mice that underwent 3 days of sleep restriction showed main effects of sex (F(1, 26) = 98.54, p < .0001) and sleep (F(1, 26) = 6.894, p < .05), as well as a sex × sleep interaction (F(1, 26) = 14.12, p < .001; ). Pairwise comparisons revealed a main effect of sleep in male mice, whereby male mice that underwent three days of sleep restriction showed significantly higher expression of 11β-hydroxylase compared to home cage controls (p < .05). Female mice showed no significant differences in 11β-hydroxylase expression between home cage controls and sleep-restricted animals (p > .05). However, female mice regardless of condition showed greater expression of 11β-hydroxylase compared to their male counterparts (p < .05). Similarly to 11β-hydroxylase, analysis of Mc2r () expression also showed a main effect of sex (F(1, 27) = 174.1, p < .001), as well as a significant sex × sleep interaction (F(1, 27) = 11.19, p < .01). Pairwise comparisons revealed a main effect of sleep on Mc2r expression in male mice, whereby, sleep-restricted male mice showed significantly higher levels of Mc2r expression as compared to home cage controls (p < .05). This effect was absent in female mice (p > .05), but, female mice regardless of condition had significantly greater Mc2r expression relative to their male counterparts (p < .05). No other significant main effects or interactions were found (all F’s ≤ 3.3).
Discussion
The present study adds to a growing literature demonstrating that paradoxical sleep deprivation (PSD) alters HPA axis function, and furthermore illustrates that these changes vary in a sex-dependent manner. Male mice displayed blunted restraint-induced CORT levels following both one day and three consecutive days of restricted sleep. In contrast, female mice showed a complete ablation of HPA axis reactivity following a single day of sleep restriction, but displayed blunted restraint-induced CORT levels similar to males following three days of PSD. These data suggest that there might be a sex-mediated difference in the HPA axis response to varying amounts of sleep restriction.
Crfr1 expression was measured in the pituitary to explore the potential impact of variable periods of sleep restriction on CRF feedback. CRF is believed to play a circadian-dependent role in sleep-wake cyclicity via its interactions with the suprachiasmatic nucleus of the hypothalamus (Buckley & Schatzberg, Citation2005), as well as its role in HPA axis activation. Following both one and three days of PSD, male and female mice displayed an increase in Crfr1 mRNA levels. CRF receptor density has been previously shown to be altered in a region-specific manner following PSD in rats (Fadda & Fratta, Citation1997). Therefore, the increase in Crfr1 expression could be a plasticity driven mechanism resulting from changes in CRF activity or higher order changes in limbic HPA axis feedback. Further experiments will need to explore CRF activation/expression post-PSD to fully understand how sleep loss impacts the CRF system.
In addition, CRF has been shown to drive ACTH release (Aguilera, Citation1994), which is an important component in the production of CORT. Previous studies have demonstrated that stress-induced ACTH levels are increased following PSD (Meerlo et al., Citation2002; Sgoifo et al., Citation2006) but, drop below that of home-cage controls following a period of recovery (Andersen et al., Citation2005). To indirectly measure ACTH activity, Pomc and Mc2r expression was assayed to explore alterations in the bioavailability and actions of ACTH, respectively. Male and female mice showed an increase in Pomc expression in the pituitary following both one and three days of sleep restriction. Perhaps, following sleep deprivation, males and females experience a shift in the bioavailability of ACTH to overcompensate for a dysregulation either upstream or downstream of POMC. Mc2r expression was similarly increased in the adrenals of males following both one and three days of PSD, while this increase was absent in females. The increases in Mc2r expression may suggest a sex-dependent change in ACTH sensitivity at the level of the adrenal glands. Interestingly, female mice in the HCC group showed greater levels of Mc2r mRNA expression relative to their male counterparts, suggestive of an innate sex difference in Mc2r expression. This innate difference could be caused by the sex-dependent alterations in circadian control of the adrenal glands due to the presence or absence of androgens (Kloehn et al., Citation2016). The adrenal glands rely upon circadian rhythms centrally mediated by the suprachiasmatic nucleus of the hypothalamus (SCN) to produce the diurnal rhythms in CORT secretion (Nader, Chrousos, & Kino, Citation2010; Son, Cha, Chung, & Kim, Citation2018; Son et al., Citation2008). Mc2r itself has been shown to display circadian oscillations in expression (Oster et al., Citation2006) contributing to shifts in adrenal sensitivity over the course of the day. In the present study, following sleep deprivation, male mice show an increased sensitivity to ACTH via the upregulation of Mc2r expression that is absent in female mice, which may be indicative of a possible sex-dependent strategy that helps upregulate CORT following sleep loss in male mice. Further studies examining ACTH following sleep deprivation are necessary to fully understand the implications of these changes.
To assess the effect of PSD on the integrity of CORT-mediated feedback mechanisms, MR and GR mRNA expression was assayed in the PVN and the pituitary. While no significant changes were found in MR or GR mRNA expression at the level of the PVN, some changes were found at the level of the pituitary. Following three days of sleep restriction, male and female mice showed a decrease in MR and GR mRNA expression that was absent following a single night of sleep restriction. These data suggest that there are no major alterations in glucocorticoid feedback following a single night of sleep restriction, however following consecutive nights of PSD changes may emerge in glucocorticoid feedback. Past studies have indicated an absence of the homeostatic sleep response characteristic of a single bout of sleep loss, whereby animals do not show the traditional increases in REM and non-REM sleep following consecutive days of sleep restriction, despite accumulating sleep debt (Kim, Laposky, Bergmann, & Turek, Citation2007). It has also been shown that changes in glucocorticoid receptor trafficking are dependent upon the length of an applied stressor (ter Heegde, De Rijk, & Vinkers, Citation2015). It is possible that consecutive days of PSD could be interacting with the stress axis in a similar capacity, resulting in a dysregulation of HPA axis reactivity through alterations in glucocorticoid feedback due to a loss of the homeostatic “coping” mechanism . However, it is less understood how these alterations in MR and GR receptor expression might be contributing to the differences in HPA axis reactivity following varying lengths of sleep restriction. It has previously been shown that following adrenalectomy, where there is a loss of CORT, GR receptor expression is shown to increase, indicative of a loss of CORT feedback (Herman & Spencer, Citation1998). However, in this case there is an opposite effect. It could be that other forms of feedback on the HPA axis are interacting to produce the differences in HPA axis reactivity, or that higher level feedback from the limbic-HPA axis might be superseding HPA axis centric mechanisms. Further studies looking into CORT feedback in the limbic structures are therefore necessary to understand how sleep restriction impacts CORT feedback.
Relative expression of 11β-hydroxylase, which is an enzyme involved in the biosynthesis of CORT via the conversion of deoxycorticosterone to the active corticosterone (White, Pascoe, Curnow, Tannin, & Rösler, Citation1992), was measured in the adrenal glands to assess for adrenal insufficiency following PSD. Adrenal insufficiency is a form of adrenal fatigue or exhaustion believed to be caused by a period of overactivation of the adrenal glands, whereby the adrenals are unable to produce CORT due to either a change in local enzymatic activity, or due to a disruption in higher level systems involved in CORT release (Guilliams & Edwards, Citation2010). 11β-hydroxylase expression, was increased in males but not females following both one and three days of sleep deprivation. While, females showed greater 11β-hydroxylase expression overall compared to their male counterparts. These findings suggests that it is not adrenal insufficiency caused by changes in enzymes that is responsible for the dysregulation in HPA axis reactivity, and that there might be sex-specific differences in baseline enzymatic activity that might contribute to overall sex differences in the HPA axis reported in the literature (Rao & Androulakis, Citation2017). Therefore, the alterations seen at the level of the adrenal glands might be due to changes in higher-order systems such as the adrenal-sympathetic system or they might be sex-specific strategies employed by the adrenals to combat disruptions elsewhere in the HPA axis.
The splanchnic nerve is known to promote the release of corticosterone from the adrenal glands via its vasoactive intestinal peptide (VIP)-ergic projections (Bornstein, Ehrhart-Bornstein, Scherbaum, Pfeiffer, & Holst, Citation1990; Ehrhart-Bornstein, Bornstein, Scherbaum, Pfeiffer, & Holst, Citation1991). Similarly, autonomic noradrenergic projections have also been shown to elicit the release of CORT from the adrenal glands (Bornstein et al., Citation1990). Past studies have reported increases in catecholamines such as norepinephrine following a prolonged period of sleep deprivation, which drop below control levels following a period of sleep recovery (Andersen et al., Citation2005). Therefore this dysregulation might be occurring at a higher level through changes in catecholaminergic and/or VIP-ergic projections from the sympathetic nervous system. The changes in enzyme mRNA expression seen here might be a sex-dependent compensatory mechanism by the adrenals to increase the bioavailability of CORT to the animal’s system post-PSD. Further studies exploring splanchnic nerve activation and VIP and norepinephrine expression/release in the adrenals are necessary to fully understand how the sympathetic system might be contributing to the blunted HPA axis reactivity observed in the present study.
In conclusion, the above experiments illustrate that HPA axis reactivity is attenuated several hours following one or multiple bouts of sleep restriction in a sex-dependent manner. Past studies have reported an increase in basal CORT levels immediately following PSD (Arthaud et al., Citation2015; Machado et al., Citation2013; Meerlo et al., Citation2002; Sgoifo et al., Citation2006). However, following a period of sleep recovery, characterized by REM rebound, basal CORT levels show a return to baseline as soon as 4 h following the end of the sleep deprivation period (Arthaud et al., Citation2015; Meerlo et al., Citation2002). Interestingly, past studies in rodents have shown no significant changes in restraint-induced CORT levels following sleep restriction (Meerlo et al., Citation2002; Novati et al., Citation2008; Sgoifo et al., Citation2006), while some human studies have revealed a blunted CORT response to stressors (Capaldi, Handwerger, Richardson, & Stroud, Citation2005; Vargas & Lopez-Duran, Citation2017; Wright, Valdimarsdottir, Erblich, & Bovbjerg, Citation2007). These divergent findings could be due to species-specific differences or more likely reflect variations in sleep restriction protocols. Our findings put forth several considerations for possible mechanisms by which sleep loss may contribute to the changes seen in HPA axis reactivity (). Plasticity driven changes in Pomc and Crfr1 might be indicative of alterations in the bioavailability and release of ACTH following sleep restriction. The sex difference in HPA axis reactivity could also be the result of sex-dependent changes in adrenal sensitivity exhibited by alterations in adrenal Mc2r expression in males. Furthermore, alterations in CORT feedback may mediate the differential responses to varying amounts of sleep restriction. Altogether, these findings provide a framework for better understanding how PSD can alter HPA axis function and reactivity, and possible neurobiological mechanisms by which this dysregulation occurs.
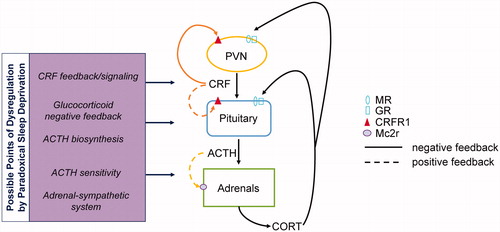
Figure 5. A schematic representation of the feedback loops involved in HPA axis function that are altered following sleep restriction. Animals that underwent sleep restriction showed changes in the CRF and ACTH pathways via alterations in their cognate receptors. Changes in CRFR1 in females might be indicative of changes up or downstream of the PVN altering HPA axis reactivity (top left). Alterations in POMC and Mc2r might represent a dysregulation of ACTH secretion or alterations in ACTH sensitivity at the level of the adrenal (bottom left). Furthermore, some possible changes were found in glucocorticoid feedback via alterations in MR and GR expression at the level of the pituitary (right). These suggest possible pathways that might be contributing to the dysregulation in HPA axis reactivity following sleep restriction. MR: Mineralocorticoid receptor; GR: glucocorticoid receptor; Mc2r: melanocortin receptor 2; ACTH: adrenocorticotropic hormone; CRFR1: corticotropin releasing factor receptor 1; POMC: proopiomelanocortin.
Acknowledgments
The authors thank Dr. Cara Olsen for her statistical expertise.
Disclosure statement
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences. The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Aguilera, G. (1994). Regulation of pituitary ACTH secretion during chronic stress. Frontiers in Neuroendocrinology, 15, 321–350. doi:10.1006/frne.1994.1013
- Andersen, M.L., Martins, P.J.F., D’Almeida, V., Bignotto, M., & Tufik, S. (2005). Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. Journal of Sleep Research, 14, 83–90. doi:10.1111/j.1365-2869.2004.00428.x
- Arthaud, S., Varin, C., Gay, N., Libourel, P.-A., Chauveau, F., Fort, P., … Peyron, C. (2015). Paradoxical (REM) sleep deprivation in mice using the small-platforms-over-water method: Polysomnographic analyses and melanin-concentrating hormone and hypocretin/orexin neuronal activation before, during and after deprivation. Journal of Sleep Research, 24, 309–319. doi:10.1111/jsr.12269
- Ashley, N.T., Sams, D.W., Brown, A.C., & Dumaine, J.E. (2016). Novel environment influences the effect of paradoxical sleep deprivation upon brain and peripheral cytokine gene expression. Neuroscience Letters, 615, 55–59. doi:10.1016/j.neulet.2016.01.013
- Bornstein, S.R., Ehrhart-Bornstein, M., Scherbaum, W.A., Pfeiffer, E.F., & Holst, J.J. (1990). Effects of splanchnic nerve stimulation on the adrenal cortex may be mediated by chromaffin cells in a paracrine manner. Endocrinology, 127, 900–906. doi:10.1210/endo-127-2-900
- Buckley, T.M., & Schatzberg, A.F. (2005). On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. The Journal of Clinical Endocrinology & Metabolism, 90, 3106–3114. doi:10.1210/jc.2004-1056
- Capaldi, I.I.V.F., Handwerger, K., Richardson, E., & Stroud, L.R. (2005). Associations between sleep and cortisol responses to stress in children and adolescents: A pilot study. Behavioral Sleep Medicine, 3, 177–192. doi:10.1207/s15402010bsm0304_1
- Carrasco, G.A., & Van de Kar, L.D. (2003). Neuroendocrine pharmacology of stress. European Journal of Pharmacology, 463, 235–272. doi:10.1016/S0014-2999(03)01285-8
- De Lorenzo, B.H.P., de Oliveira Marchioro, L., Greco, C.R., & Suchecki, D. (2015). Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology, 57, 134–143. doi:10.1016/j.psyneuen.2015.04.006
- Ehrhart-Bornstein, M., Bornstein, S.R., Scherbaum, W.A., Pfeiffer, E.F., & Holst, J.J. (1991). Role of the vasoactive intestinal peptide in a neuroendocrine regulation of the adrenal cortex. Neuroendocrinology, 54, 623–628. doi:10.1159/000125969
- Fadda, P., & Fratta, W. (1997). Stress-induced sleep deprivation modifies corticotropin releasing factor (CRF) levels and CRF binding in rat brain and pituitary. Pharmacological Research, 35, 443–446. doi:10.1006/phrs.1997.0155
- Guilliams, T.G., & Edwards, L. (2010). Chronic stress and the HPA axis: Clinical assessment and therapeutic considerations. The review of natural & neutraceutical therapies for clinical practice. Standard, 9, 1–12.
- Handa, R.J., & Weiser, M.J. (2014). Gonadal steroid hormones and the hypothalamo–pituitary–adrenal axis. Frontiers in Neuroendocrinology, 35, 197–220. doi:10.1016/j.yfrne.2013.11.001
- Herman, J.P., & Spencer, R. (1998). Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. The Journal of Neuroscience, 18, 7462–7473. doi:10.1523/JNEUROSCI.18-18-07462.1998
- Kim, Y., Laposky, A.D., Bergmann, B.M., & Turek, F.W. (2007). Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proceedings of the National Academy of Sciences of Sciences, 104, 10697–10702. doi:10.1073/pnas.0610351104
- Kloehn, I., Pillai, S.B., Officer, L., Klement, C., Gasser, P.J., & Evans, J.A. (2016). Sexual differentiation of circadian clock function in the adrenal gland. Endocrinology, 157, 1895–1904. doi:10.1210/en.2015-1968
- Koehl, M., Battle, S., & Meerlo, P. (2006). Sex differences in sleep: The response to sleep deprivation and restraint stress in mice. Sleep, 29, 1224–1231. doi:10.1093/sleep/29.9.1224
- Liu, Y., Wheaton, A.G., Chapman, D.P., Cunningham, T.J., Lu, H., & Croft, J.B. (2016). Prevalence of healthy sleep duration among adults. Morbidity and Mortality Weekly Report, 65, 137–141. doi:10.15585/mmwr.mm6506a1
- Livak, K.J., & Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods, 25, 402–408. doi:10.1006/meth.2001.1262
- Machado, R.B., Hipólide, D.C., Benedito-Silva, A.A., & Tufik, S. (2004). Sleep deprivation induced by the modified multiple platform technique: Quantification of sleep loss and recovery. Brain Research, 1004, 45–51. doi:10.1016/j.brainres.2004.01.019
- Machado, R.B., Tufik, S., & Suchecki, D. (2013). Role of corticosterone on sleep homeostasis induced by REM sleep deprivation in rats. PLoS One, 8, e63520. doi:10.1371/journal.pone.0063520
- Mallampalli, M.P., & Carter, C.L. (2014). Exploring sex and gender differences in sleep health: A society for women’s health research report. Journal of Women’s Health (Health), 23, 553–562. doi:10.1089/jwh.2014.4816
- Meerlo, P., Koehl, M., van der Borght, K., & Turek, F.W. (2002). Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. Journal of Neuroendocrinology, 14, 397–402. doi:10.1046/j.0007-1331.2002.00790.x
- Nader, N., Chrousos, G.P., & Kino, T. (2010). Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology & Metabolism, 21, 277–286. doi:10.1016/j.tem.2009.12.011
- Novati, A., Hagewoud, R., Cetin, T., Luiten, P.G.M., Roman, V., Meerlo, P., & den Boer, J.A. (2008). Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep, 31, 1579–1585. doi:10.1093/sleep/31.11.1579
- Ohayon, M.M., & Roth, T. (2003). Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of Psychiatric Research, 37, 9–15. doi:10.1016/S0022-3956(02)00052-3
- Oster, H., Damerow, S., Kiessling, S., Jakubcakova, V., Abraham, D., Tian, J., … Eichele, G. (2006). The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metabolism, 4, 163–173. doi:10.1016/j.cmet.2006.07.002
- Pfaffl, M.W. (2001). A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Research, 29, e45–e45. doi:10.1093/nar/29.9.e45
- Rao, R.T., & Androulakis, I.P. (2017). Modeling the sex differences and interindividual variability in the activity of the hypothalamic-pituitary-adrenal axis. Endocrinology, 158, 4017–4037. doi:10.1210/en.2017-00544
- Russell, A.L., Richardson, M.R., Bauman, B.M., Hernandez, I.M., Saperstein, S., Handa, R.J., & Wu, T.J. (2018). Differential responses of the HPA axis to mild blast traumatic brain injury in male and female mice. Endocrinology, 159, 2363–2375. doi:10.1210/en.2018-00203
- Schmittgen, T.D., & Livak, K.J. (2008). Analyzing real-time PCR data by the comparative CT method. Nature Protocols, 3, 1101–1108. doi:10.1038/nprot.2008.73
- Sgoifo, A., Buwalda, B., Roos, M., Costoli, T., Merati, G., & Meerlo, P. (2006). Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology, 31, 197–208. doi:10.1016/j.psyneuen.2005.06.009
- Shaib, F., & Attarian, H. (2017). Chapter 40 - sex and gender differences in sleep disorders: An overview. In M.J. Legato (Ed.), Principles of gender-specific medicine (3rd ed., pp. 585–601). San Diego, CA: Academic Press.
- Son, G.H., Cha, H.K., Chung, S., & Kim, K. (2018). Multimodal regulation of Circadian glucocorticoid rhythm by central and adrenal clocks. Journal of the Endocrine Society, 2, 444–459. doi:10.1210/js.2018-00021
- Son, G.H., Chung, S., Choe, H.K., Kim, H.-D., Baik, S.-M., Lee, H., … Kim, K. (2008). Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proceedings of the National Academy of Sciences of Sciences, 105, 20970–20975. doi:10.1073/pnas.0806962106
- Spiegel, K., Leproult, R., & Van Cauter, E. (1999). Impact of sleep debt on metabolic and endocrine function. The Lancet, 354, 1435–1439. doi:10.1016/S0140-6736(99)01376-8
- Suchecki, D., Tiba, P.A., & Tufik, S. (2002). Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. Journal of Neuroendocrinology, 14, 549–554. doi:10.1046/j.1365-2826.2002.00812.x
- Suchecki, D., & Tufik, S. (2000). Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiology & Behavior, 68, 309–316. doi:10.1016/S0031-9384(99)00181-X
- Sucheki, D., Lobo, L., Hipolide, D., & Tufik, S. (1998). Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. Journal of Sleep Research, 7, 276–281. doi:10.1046/j.1365-2869.1998.00122.x
- ter Heegde, F., De Rijk, R.H., & Vinkers, C.H. (2015). The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology, 52, 92–110. doi:10.1016/j.psyneuen.2014.10.022
- Vargas, I., & Lopez-Duran, N. (2017). Investigating the effect of acute sleep deprivation on hypothalamic-pituitary-adrenal-axis response to a psychosocial stressor. Psychoneuroendocrinology, 79, 1–8. doi:10.1016/j.psyneuen.2017.01.030
- White, P.C., Pascoe, L., Curnow, K.M., Tannin, G., & Rösler, A. (1992). Molecular biology of 11β-hydroxylase and 11β-hydroxysteroid dehydrogenase enzymes. The Journal of Steroid Biochemistry and Molecular Biology, 43, 827–835. doi:10.1016/0960-0760(92)90309-7
- Wright, C.E., Valdimarsdottir, H.B., Erblich, J., & Bovbjerg, D.H. (2007). Poor sleep the night before an experimental stress task is associated with reduced cortisol reactivity in healthy women. Biological Psychology, 74, 319–327. doi:10.1016/j.biopsycho.2006.08.003
- Zhang, B., & Wing, Y.-K. (2006). Sex differences in insomnia: A meta-analysis. Sleep, 29, 85–93. doi:10.1093/sleep/29.1.85
