Abstract
The role of cortisol as a premorbid vulnerability factor for trauma sequelae remains unclear. Furthermore, the onset of long-term endocrine changes in response to first-time trauma as a function of later psychopathology is not clarified yet. Thus the predictive value of pre- and post-traumatic hair cortisol concentrations (HCCs) for psychological trauma sequelae was investigated in response to motor vehicle crash (MVC). A total of N= 62 MVC survivors participated in this study (46 females, mean age (SD): 43.94(12.95)). Subsequent trauma sequelae were measured with a structured clinical interview and self-report questionnaires to evaluate psychological symptoms (pre-MVC and three months post-MVC). Hair strands were taken immediately after MVC and three months post-MVC, reflecting cumulative cortisol secretion over the three-month period before and after the MVC. A total of 22.6% of the participants developed a trauma sequela with an affective disorder (14.5%) and/or anxiety disorder (16.1%). We observed a significant main effect of group and diagnosis × time interaction with an increase of HCC in those individuals who presented a subsequent psychiatric disorder. Regression analyses revealed that post-MVC increased HCC were significantly predictive of higher levels of subsequent depressiveness, and that pre-MVC increased HCC were predictive of higher levels of subsequent avoidance behavior. Our findings demonstrate that individual differences in long-term cortisol secretion in response to a first-time traumatic event (MVC) contribute to subsequent psychopathology. Specifically, higher long-term cortisol secretion before and after first-time MVC was a risk factor for subsequent development of avoidance behavior and depressiveness, respectively.
Higher cortisol secretion and stress experience before a motor vehicle crash was a risk factor for subsequent development of psychological symptoms.
Lay summary
Introduction
For years, the number of motor vehicle crash (MVC) has been increasing steadily, with an increase of 10.2% severely and 4.8% mildly injured traffic participants in Germany (Destatis, Citation2012). Post-crash, 8–45% of survivors, investigated worldwide, meet the criteria for a syndromal (Heron-Delaney, Kenardy, Charlton, & Matsuoka, Citation2013; Holeva, Tarrier, & Wells, Citation2001; Mayou, Bryant, & Ehlers, Citation2001; Murray, Ehlers, & Mayou, Citation2002) or sub-syndromal post-traumatic stress disorder (PTSD; Berna, Vaiva, Ducrocq, Duhem, & Nandrino, Citation2012). However, PTSD is only one psychiatric sequela that may develop in response to trauma exposure. Besides 23% PTSD in MVC survivors, 22% developed a specific phobia, 17% a general anxiety disorder, and 5% a depression three months post-crash in Britain (Mayou et al., Citation2001). However, the percentage of depressive symptoms is much higher with 10.3% of participants using a self-report (Smith, Mackenzie-Ross, & Scragg, 2007) or 26% using a standardized interview (Kenardy et al., 2018). Therefore, the used method explains an underestimation of depressive symptoms. While the prevalence rates suggest that some of trauma survivors recover from initial consternation and do not develop a psychiatric disorder (Breslau, Davis, Peterson, & Schultz, Citation1997), it is important to identify those individuals who are at high risk for developing a trauma sequela.
Among others, previous research has considered biological risk factors for PTSD development with a particular focus on the body’s stress response systems (for review see Zoladz & Diamond, Citation2013). Immediately after trauma exposure, lower basal cortisol concentrations, as well as a low plasticity of the HPA-axis, are associated with a higher risk for developing symptoms of a PTSD (Delahanty, Raimonde, & Spoonster, Citation2000; Ehlert, Gaab, & Heinrichs, Citation2001; Mouthaan et al., Citation2014). Based on MVC survivors a blunted increase in saliva cortisol two days after a traumatic crash was associated with a higher risk for the development of a PTSD as well (McFarlane, Barton, Yehuda, & Wittert, Citation2011). Yet it is uncertain whether an altered HPA-axis functioning either marks a pre-morbid vulnerability factor or rather an endocrine condition following trauma exposure (Yehuda et al., Citation2000). Therefore, cortisol concentrations before trauma exposure in samples of high-risk for PTSD development were investigated which are indecisive. Hereby, basal cortisol levels failed to reveal PTSD-predictive effects (Heinrichs et al., Citation2005; Inslicht et al., Citation2011; van Zuiden, Geuze, et al., Citation2012; van Zuiden, Kavelaars, et al., Citation2011), although higher pre-traumatic GR numbers (van Zuiden, Geuze, et al., Citation2011, Citation2012) and increased GC sensitivity (van Zuiden, Heijnen, et al., Citation2012) were predictive of PTSD symptom development. These inconsistencies might be explicable by differences in co-morbid mental disorders, and trauma type in the participants included (Heinrichs et al., Citation2005; van Zuiden, Geuze, et al., Citation2011, Citation2012; van Zuiden, Heijnen, et al., Citation2012; van Zuiden, Kavelaars, et al., Citation2011) as well as methodological differences (saliva cortisol, plasma cortisol and glucocorticoid receptors; Meewisse, Reitsma, De Vries, Gersons, & Olff, Citation2007). These traditional cortisol assessment methods provide relatively unreliable estimates of long-term cortisol output, since it is highly irritable and differs as a function of ultradian (Young, Abelson, & Lightman, Citation2004), circadian rhythmicity (Weitzman et al., Citation1971), and acute stress (Kirschbaum, Pirke, & Hellhammer, Citation1993), among others.
Besides using large-scale prospective designs in high-risk groups to predict the development of psychological symptoms after trauma, another valuable approach constitutes the analysis of cortisol concentrations in human scalp hair. This novel assessment strategy is assumed to provide a reliable and valid retrospective marker of long-term cortisol secretion (Stalder & Kirschbaum, Citation2012). Specifically, since examined hair strands have grown in the past, this approach provides the unique opportunity to investigate cortisol levels before a traumatic event, based on samples collected after the event. Hair cortisol concentrations (HCCs) have been established as an endocrine biomarker of prolonged stress exposure (Kalra, Einarson, Karaskov, Van Uum, & Koren, Citation2007; Stalder et al., Citation2017), as well as of mental disorders such as PTSD (Steudte et al., Citation2011; Citation2013; Steudte-Schmiedgen et al., Citation2017) or major depression (MD; Dettenborn et al., Citation2012). Recently, the predictive value of HCC concerning PTSD symptoms have been established, with diverging results (Luo et al., Citation2012; Pacella, Hruska, Steudte-Schmiedgen, George, & Delahanty, Citation2017; Steudte-Schmiedgen et al., Citation2015). On the one side, no significant associations were revealed between HCC in hair segments reflecting the time before the earthquake and PTSD development (Luo et al., Citation2012). On the other side, decreased HCC in German male soldiers after military deployment (Steudte-Schmiedgen et al., Citation2015), as well as elevated HCC, predicted PTSD symptom development upon traumatization in response to a traumatic physical injury (Pacella et al., Citation2017).
Concerning the prediction of trauma sequela by HCC, shortcomings of the previous studies are the lack of evaluation of a broad range of mental disorders, the investigation of high-risk samples which show specific characteristics (e.g. social class etc.), different time periods of hair sampling (“wash-out” effect) and different measurement points of psychological diagnostics (2, 7, and 18 days after trauma). Therefore, the current study aimed at testing the predictive value of long-term integrated HCC for the development of trauma sequela in a sample of single event trauma-exposed individuals who had never been exposed to a traumatic event before. Specifically, the present study investigated whether pre-MVC integrated HCC differed significantly between those participants who develop trauma sequelae three-month post-MVC as compared to those who do not meet the criteria of a mental disorder.
In the literature, a predictive association between elevated HCC and post-traumatic symptom severity was observed for traumatic physical injury (Pacella et al., Citation2017). Since MVC survivors are also traumatically physically injured, it can be proposed that MVC survivors with pre-crash elevated HCC show a higher severity of traumatic symptoms.
Methods
Description of the study participants
Individuals who had been exposed to a MVC requiring hospital admission were recruited at the Department of Trauma & Reconstructive Surgery of the university hospital in Dresden, Germany. Recruitment took place between October 2010 and June 2014. All study participants had to spend at least one night in hospital. Staff of the Department of Trauma & Reconstructive Surgery provided access to MVC survivors. If patients seemed eligible after first screening of the medical record review (consciousness, mental disorder, chronic somatic illness, medication) and were able to give written consent, they were contacted within the first 10 days either personally or by phone, depending on their preference and admission/discharge, and educated about study purposes. Inclusion criteria were 18–65 years of age and a minimal hair length of 3 cm. Exclusion criteria comprised smoking of more than 10 cigarettes per day, a lifetime or acute mental disorder (including previous trauma exposure) conducted by the screening of the Structured Clinical Interview for DSM-IV (SCID-IV) (Wittchen, Citation2007) and established trauma-specific questionnaire (see below), psychotropic or corticosteroid drug intake (including topical creams and inhalants) within the past three months, chronic or acute illnesses or inflammations (e.g. cancer, diabetes), pregnancy and shift working (night employment, rotating shift schedule).
A total of n = 268 individuals were screened according to the defined inclusion and exclusion criteria. However, nexlusion = 67 individuals were excluded initially from study participation due to failure to meet inclusion criteria, and ndrop out = 139 individuals were not available or declared that they were no longer interested at three-months follow-up, resulting in a final sample of n = 62final participants (46 females, mean age (SD): 43.94 (12.95)). These subsamples (nexclusion, ndrop out, and nfinal) did not differ significantly in terms of age, F(2, 258)= 2.46, p = .088, η2p = 0.019, injury severity as measured by the Injury Severity Scale (ISS), recruitment days, or any of the psychological health indicators (depression, anxiety, stress, and post-traumatic stress symptoms), F ≤ 1.14, p≥.321, η2p ≤ 0.010. However, there were significant differences with regard to participant gender: compared to nfinal, there were more men in both ndrop out (59 vs. 73 women) and nexclusion (44 vs. 23 women), leading to an unequal distribution overall, χ2(2)= 22.394, p<.001.
The remaining 62 participants were invited to the Department of Psychotherapy and Psychosomatic Medicine of the university hospital in Dresden for SCID diagnostics and hair sample collection. There were n = 25 (40.3%) participants who owned university-entrance diploma, n = 33 (53.2%) participants who owned a secondary school certificate and n = 2 (3.2%) participants owning eight classes certificate. The Childhood Trauma Questionnaire (CTQ; Bernstein & Fink, Citation1998; Gast, Rodewald, Benecke, & Driessen, Citation2001) and the Trauma History Questionnaire (THQ; Green, Citation1996; Maercker, Citation2002) ensured that no study participant has experienced an additional distant traumatic event besides the crash. With regard to childhood maltreatment, means (SD) for the CTQ subscales were as follows (each subscale has a range of 5–25): sexual abuse 5.35 (1.41), physical abuse 5.65 (1.64), emotional abuse 6.35 (1.71), physical neglect 6.14 (1.67), and emotional neglect 7.65 (3.44). Overall, these scores are without clinical evidence, compared to patient samples with a (chronic) PTSD diagnosis following interpersonal traumatization (e.g. Wichmann, Kirschbaum, Böhme, & Petrowski, Citation2017). Study participants provided written informed consent and the study procedure was approved by the local Ethics Committee of the Medical Faculty of the Technische Universität Dresden, Germany (EK# 65022010).
Psychopathological assessment
For clinical characterization, the following self-report paper-pencil instruments were handed out to the participants both within the first 10 days after the MVC as well as three months post-MVC (except for the ISS). (1) The severity of depressive symptoms was evaluated using the Beck Depression Inventory II (BDI; Beck, Steer, & Brown, Citation1996; Hautzinger, Keller, & Kühner, Citation2006) that consists of 21 item groups matching the DSM-IV criteria of a major depressive disorder episode. The respondents are asked to rate the way they felt during the past two weeks with the help of each item group within which the items are arranged in increasing intensity (range: 0–63). The internal consistency was .89. (2) Additionally, participants self-rated PTSD symptom severity anchored to the index event (MVC) on the Posttraumatic Diagnostic Scale (PDS; Foa, Citation1995; Foa, Cashman, Jaycox, & Perry, Citation1997). The respondents are asked to rate how much they experienced each of the DSM-IV PTSD core symptoms (intrusion, avoidance, hyperarousal) on a four-point Likert-scale and to indicate which areas of life were impaired due to trauma-related symptoms, e.g. household, occupation, leisure time, or sexuality (range: 0–51). The internal consistency was 0.90. (3) More specifically, the Impact of Event Scale – revised ( IES-R; Horowitz, Wilner, & Alvarez, Citation1979; Weiss, Citation2007 ) assessed the frequency of symptom occurrence with respect to intrusion, avoidance and hyperarousal with the help of 22 items (range for the subscales intrusion and hyperarousal: 0–35; range for the subscale avoidance: 0–40). The internal consistency was for the scale intrusions 0.89, for the scale avoidance 0.77, and for the scale hyper arousal 0.89. All questionnaires were handed out in the German language version. (4) The ISS (Baker, OʼNeill, Haddon, & Long, Citation1974) is an established medical scoring system to assess trauma severity in patients with multiple injuries referring to anatomical regions (head and neck, face, chest, abdomen, extremity; range: 1–75). The ISS rating was evaluated by the trained medical physician of the Department of Trauma & Reconstructive Surgery of the university hospital in Dresden. Three months after the recruitment, participants (none hospitalized at this time) were interviewed by a trained clinical psychologist using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I; Wittchen, Citation1997). Participants were additionally interviewed concerning their medical history including medication intake.
Hair cortisol analysis
Hair strands (2–3 mm in diameter) were taken within the first 10 days after the crash as well as three months post-MVC as close as possible to the scalp from the same posterior vertex region. The scalp-near hair strands were cut into segments of 3 cm length. Human hair growth rate is assumed to be 1 cm per month (Wennig, Citation2000); hence HCC in these segments reflect on average the integrated cortisol levels up to three months prior to sampling (Stalder & Kirschbaum, Citation2012). Specifically, HCC of hair samples taken immediately after trauma exposure reflect integrated cortisol secretion over the three-month period prior to the crash (pre-MVC HCC), and HCC of hair samples taken 3-months post-MVC reflect integrated cortisol secretion over the three-month period after the crash (post-MVC HCC). The determination of HCC was carried out according to the protocol by Gao et al. (Citation2013) using liquid chromatography-tandem mass spectrometry in the endocrinological laboratory of the Technische Universität Dresden (Institute of Biological Psychology, Prof. Clemens Kirschbaum).
Statistical procedure
All statistical analyses were performed using SPSS for Windows, version 23 (IBM, Chicago, IL). Hair cortisol data were subject to log-transformation to normalize skewed data for use in parametric statistics. Data from one participant were excluded due to an outlying HCC value of more than three standard deviations above the mean.
First, group comparisons (at least one vs. none SCID diagnosis) with respect to sociodemographic, HCC, and clinical variables were evaluated using univariate analysis of variance (ANOVA) for continuous and Chi-square-test for dichotomous variables (). In addition, to test whether pre- or post-MVC integrated HCC differed between those individuals who develop trauma sequelae in comparison to those who do not develop trauma sequelae, a 2 (group: at least one SCID diagnosis vs. no diagnosis)×2 (pre-and post-MVC HCC) ANOVA with baseline pre-MVC HCC added as covariate was performed to test for a change in HCC in reference of SCID diagnostics. Alterations in the self-rated clinical measures (PDS, IES-R, BDI) from the first assessment to the three months post-MVC assessment were evaluated using repeated measures ANOVA with Greenhouse–Geisser corrections when necessary (Table S1).
Table 1. Comparison of MVC survivors with at least one vs. no SCID diagnosis. Mean (SD) are listed except where noted.
To analyze the predictive value of the change in post-MVC HCC for psychological trauma sequelae the following statistics were run. First, Pearson’s correlational analyses assessed the relationship between pre- and post-MVC integrated HCC and the initial and follow-up clinical self-rate measures (). Linear regression analyses were performed with trauma-related symptoms (PDS, IES-R subscales: intrusion, avoidance, hyperarousal, BDI) as dependent variables, initial symptom severity in the respective measures, HCC and injury severity added as predictors ().
Table 2. Correlation matrix of the relationship between hair cortisol and clinical measures.
Table 3. Linear regression analyses predicting psychological symptoms 3 months post-crash from hair cortisol concentrations (HCC) and initial psychological symptoms immediately after the motor vehicle crash (MVC).
Results
Sample characteristics and cross-sectional baseline associations
A total of n = 14 (22.6%) MVC survivors fulfilled the criteria of at least one mental disorder according to SCID diagnostics three months post-MVC. Specifically, n = 9 individuals (14.5%) showed an affective disorder (single episode of a MD) and n = 10 individuals (16.1%) showed an anxiety disorder. In detail, n = 1 individual fulfilled the criteria of a PTSD, an adjustment disorder and a panic disorder, respectively, n = 2 of an agoraphobia without panic disorder, n = 2 of a social phobia and n = 4 of a specific phobia. The ISS varied from 1 to 45, with a mean ± SD score of 5.20 ± 7.55. A total of 52.5% (n = 32) had an ISS of ≥3 and therefore at least one moderate injury. Overall, self-rated post-traumatic stress and depressive symptoms decreased from the first 10 days after of the crash to the three months follow-up assessment (p’s≤.008; see Table S1).
illustrates the findings of the group comparisons (SCID diagnosis vs. no SCID diagnosis). There were no significant sex, age, or education differences between those MVC survivors who developed a psychiatric diagnosis and those who did not (p’s≥.155). Further, they showed higher scores in all clinical measures, except for avoidance behavior as measured with the IES-R (p’s≤.031).
Longitudinal HCC analyses
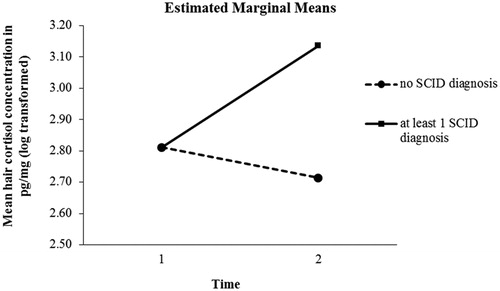
Repeated measures ANCOVA with baseline pre-MVC HCC as covariate resulted in significant main effects of time (F1;60= 23.834, p = .000, η2= 0.288) and group (F1;60= 4.237, p = .044, η2= 0.067) as well as in a significant time × group interaction effect with increasing HCC in those individuals who presented a subsequent psychiatric disorder (F1;60= 4.237, p = .044, η2= 0.067; see ). Univariate ANOVA failed to reveal significant differences in baseline pre-MVC HCC between groups (diagnosis vs. no SCID diagnosis; F1;61= 1.262, p = .266).
Predictive associations between pre- and post-MVC hair cortisol and trauma sequela
Pearson’s correlation analyses revealed a significant relationship between pre-MVC integrated HCC and the three months post-MVC rated depressiveness (p<.05). Further, there was a significant relationship between post-MVC integrated HCC and the three months post-MVC rated depressiveness and hyperarousal symptomatology (p’s<.05; see ).
Overall, the linear regression analyses, performed to predict clinical symptoms rated three months post-MVC, revealed 20.2–54.9% explanation of variance (see for details). Pre-MVC integrated elevated HCC demonstrated a predictive value for subsequent higher avoidance behavior (β = .247, p ≤.05) while post-MVC integrated elevated HCC was predictive of higher depressiveness rated three months post-MVC (β = .212, p ≤.05), respectively.
Discussion
The current study investigated the predictive value of long-term integrated HCC for the development of psychological trauma sequelae following a single, first-time traumatic event (i.e. MVC). Overall, in the present sample of MVC survivors 22.6% developed a trauma sequela as measured 3-month after the crash. Previous research reported prevalence rates from 5% (for depression) up to 23–45% (for anxiety disorders including PTSD; Heron-Delaney et al., Citation2013; Holeva et al., Citation2001; Mayou et al., Citation2001; Murray et al., Citation2002) based on structured interviews. In the present study, the prevalence for depression was higher with 14.5%. These rates are in line with the self-report data suggesting 10.3% moderate-high depressive symptoms as assessed by the HADS 3-month post-MVC (Smith et al., 2007). In this study, based on standardized interview the prevalence rate after 6 months was much higher with 28% (Kenardy et al., 2008). These differences might be explicable by the differences in methodological approach (assessment instruments, follow-up measurement points). However, the prevalence for anxiety disorders was minor than reported with 16.1% and solely one individual met the diagnostic criteria for a PTSD diagnosis. These prevalences of anxiety disorders are much lower compared to the literature (Kenardy et al., 2018) which might be explicable by the lower severity of the injuries (ISS) of the participants. For the hair sample collection, the participants had to be oriented max 10 days after the MVC.
Results showed that individual differences in long-term cortisol secretion in response to a single, first-time traumatic event are associated with subsequent psychopathology, including affective and anxiety disorders according to the SCID. Specifically, those MVC survivors who developed a trauma sequela exhibited an HCC increase from pre- to post-trauma while MVC survivors who did not present subsequent psychological trauma sequelae showed no change in HCC. Dimensional analyses further suggest that higher long-term cortisol secretion before and after first-time MVC is a risk factor for subsequent development of avoidance behavior and depressiveness, respectively. These findings necessitate future studies focusing on larger samples including an additional control group to investigate the potential of HCC as a marker for identifying individuals with a higher risk for the development of a mental disorder.
Research data suggest that stressors briefly result in an increased cortisol secretion, which in the long term is accompanied by a stronger pituitary and hypothalamic negative feedback resulting in a chronic attenuated HPA-axis activity (Fries, Hesse, Hellhammer, & Hellhammer, Citation2005; Heim, Ehlert, & Hellhammer, Citation2000; Steudte-Schmiedgen, Kirschbaum, Alexander, & Stalder, Citation2016). According to the “Theory of Allostasis and Allostatic Load” by McEwen (Citation2004) repeated trauma exposure as well as trauma-related symptoms (e.g. intrusions, flashbacks, panic attacks) represent “repeated hits” onto the HPA-axis leading to repeated activation and thus possibly in the median and long run to an inadequate reaction of the HPA-axis (prolonged response). Our data support these theoretic models of an increased cortisol secretion in the aftermath of a single, first-time traumatic event (MVC) for the survivors developing symptoms.
The present findings further revealed significant predictive power of post-MVC increased HCC for higher levels of subsequent depressiveness as measured three months post-MVC. This finding agrees with data of Goodyer, Tamplin, Herbert, and Altham (Citation2000) as well as LeMoult, Ordaz, Kircanski, Singh, and Gotlib (Citation2015) suggesting that the new onset of a major depressive disorder was predicted by the combined effects of recent stressful life events (e.g. dangers to self or to others, permanent losses) and elevated daily salivary cortisol levels. The present data further agree with previous research that found a higher cortisol awakening response to be a risk factor for the development of a new-onset major depressive disorder (Adam et al., Citation2010; Vrshek-Schallhorn et al., Citation2013).
The present results further provide evidence for a predictive value of increased pre-MVC integrated HCC for trauma-related avoidance behavior. The present findings are in accordance with Pacella et al. (Citation2017) who also found a predictive value of pre- and peri-traumatic elevated HCC for trauma-related avoidance behavior assessed post-traumatically within 60-days. However, this finding conflicts with evidence for an association between a pre-traumatic decreased cortisol concentration and an increased risk for developing symptoms of a PTSD (e.g. Steudte-Schmiedgen et al., Citation2015; van Zuiden, Geuze, et al., Citation2012; van Zuiden, Heijnen, et al., Citation2012). These differences might be explicable by the differences in time line for hair cortisol collection as well as psychological diagnostic (17 vs. 3 months).
However, in the present sample, regression analyses failed to reveal a predictive value of pre-MVC integrated HCC for other trauma-related symptoms reported in a previous study (numbing, global severity; Pacella et al., Citation2017). This diversity of the results might be explicable by the fact that most of previous studies did not control for previous trauma history. Studies that controlled for previous traumatization found no predictive value of pre-traumatic cortisol levels for subsequent trauma sequelae (e.g. Delahanty et al., Citation2000; Heinrichs et al., Citation2005; Luo et al., Citation2012; Resnick, Yehuda, Pitman, & Foy, Citation1995; Shalev, Peri, Canetti, & Schreiber, Citation1996; Walsh et al., Citation2013).
A strength of this study is that it extends previous research reports on spot assessments by applying hair cortisol assessment reflecting long-term integrated cortisol secretion over a three-months period relatively unaffected by acute conditions of the assessment context as this is the case in spot measurements (Stalder & Kirschbaum, Citation2012). However, study limitations include the high dropout rate at the follow-up assessment. In the literature, the drop-out rates show a wide and high range between 13 and 66% (see overview Nishi et al., Citation2008). The high attrition rate of the current study might be explicable by the fact that a relatively high rate of MVC victims has been transported to clinics with specialized trauma centers with a long distance to their home. Therefore, a follow-up meeting after three months might have been too time and cost consuming for these participants. Future studies might deal with this problem by conducting home-based hair sampling (by using a video-tutorial) accompanied by online questionnaires. Based on Nishi et al. (Citation2008), the patients with less severe injuries and lower psychological symptoms dropped out of the MVC studies. Therefore, the prediction might not be as affected by the high drop-out rate. In addition, there were significant differences with regard to gender, with men being more likely to drop out. Although this finding is in line with previous research as well (e.g. Nishi et al., Citation2008; Wu et al., Citation2014), it is unclear which factors might have contributed to this.
However, this study investigated a carefully selected sample without past trauma history excluding the confounding impact of potential long-term disruptions on the HPA-axis functionality due to previous traumatization. As a result of the small sample size of individuals who developed trauma sequela, statistical analyses were limited to the prediction of dimensional self-evaluated clinical measures, instead to predict the categorical outcome in terms of a clinical diagnosis. Future studies ought to investigate a larger sample size or survivors of different and more severe trauma types to increase the number of patients who develop full diagnostic criteria. Thus, the generalizability of the present results is limited to survivors of comparatively mild and particularly first-time single traumatic events; results may not extend to patient samples who experienced more severe trauma types or suffer from chronic PTSD. Furthermore, the results need to be replicated in an additional larger prospective study in order to extend our findings of a predictive value of HCC for an increased risk to develop psychopathology. Since this study sample did not include a non-traumatized control group, no conclusion can be drawn whether trauma exposure per se may have an impact long-term endocrine alterations as suggested by prior research (for review: Steudte-Schmiedgen et al., Citation2017). Furthermore, the predominantly female sample is a limiting factor for the generalizability of results. This may be attributable to the fact that men more often did not fulfill the inclusion criterion of a minimum hair length of 3 cm or cigarette consume less than 10 per day. Hence, future studies are needed to replicate our findings in larger mixed samples.
Future research may include additional endocrine markers also having both an impact on the development of trauma sequela and interaction effects with the HPA-axis functionality, e.g. parameters of an overactive inflammation response (Rohleder, Joksimovic, Wolf, & Kirschbaum, Citation2004). In addition, future studies should investigate the potential of HCC to identify high-risk individuals who might benefit from treatment interventions. For example, HCC may effectively complement research into the use of pharmacological glucocorticoid treatment in trauma survivors in the days and weeks following traumatization (e.g. review Hruska, Cullen, & Delahanty, 2014). Here, it is conceivable that HCC may modulate the efficacy of such a hydrocortisone treatment (Yehuda, Bierer, Prachtchett, Lehrner, Koch, Van Manen et al., 2014). The examination of such individual-level predictive relationships holds the promise of the discovery of targeted preventive interventions that could reduce the risk of developing psychopathology in response to trauma exposure.
In conclusion, our findings demonstrate that individual differences in long-term cortisol secretion in response to a single, first-time traumatic event (i.e. MVC) contribute to subsequent psychopathology. In particular, trauma survivors who developed a trauma sequela showed an HCC increase from pre- to post-trauma assessment. Dimensional analyses further revealed that HCC integrated over the three-month period before MVC was found to predict subsequent avoidance behavior. HCC integrated over the three-month period after MVC was found to predict subsequent depressive symptoms. The present study highlights the potential of HCC to retrospectively assess pre- and post-traumatic cortisol levels by taking hair samples in the aftermath of a traumatic event. Given its unique characteristics, hair cortisol analysis holds promise to identify individuals who are at high risk at an early stage for the development of psychological trauma sequela.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Katja Petrowski
Prof. Dr. Katja Petrowski is a full professor for Medical Psychology and Medical Sociology at University Medicine, Johannes Gutenberg University Mainz in Germany. Her research interests include anxiety disorders, psychotherapy, stress, psychophysiology, psychoendocrinology and psychoimmunology. She is a licensed cognitive and behavioral psychologist and conducted several multi-center psychotherapy studies for depression and anxiety disorders.
Susann Wichmann
Dr. Susann Wichmann is a clinical psychologist and psychotherapist. She went to receive her diploma and doctoral degree from the Technische Universität Dresden. In addition, she had acquired a supplementary qualification as a behavioral therapist and has specialized in the diagnosis und treatment of dissociative and complex posttraumatic stress disorder.
Jaroslav Pyrc
Dr. Jaroslaw Robert Pyrc MD, is an alumnus of human medical University in Wroclaw, Poland. He finished the general and vascular surgery education 2007 in University Dresden, Germany. Afterwards, he graduated the education in trauma and orthopedic surgery and was consultant on the Medical University in Dresden to 2018. He is a member of rescue helicopter team “Christoph 38” Dresden, Germany. Since July 2018 he is chef physician of the trauma, orthopedic and spine surgery in Elblandklinikum Radebeul, Germany.
Susann Steudte-Schmiedgen
Dr. Steudte-Schmiedgen completed her doctoral work and began her postgraduate fellowship in the working group of Biopsychology at the Technische Universität Dresden. Since April 2018, she is head of the Psychobiology of Stress Unit at the Department of Psychotherapy and Psychosomatic Medicine, University Hospital Carl Gustav Carus, Technische Universität Dresden.
Clemens Kirschbaum
Prof. Dr. Clemens Kirschbaum is the Chairman of the School of Science and full professor at TU Dresden in Germany. His research interests include stress, psychoendocrinology, psychoimmunology and molecular genetics. He is an author of more than 400 peer-reviewed scientific papers.
References
- Adam, E.K., Doane, L.D., Zinbarg, R.E., Mineka, S., Craske, M.G., & Griffith, J.W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35, 921–931. doi:10.1016/j.psyneuen.2009.12.007
- Baker, S.P., OʼNeill, B., Haddon, W., & Long, W.B. (1974). The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. Journal of Trauma and Acute Care Surgery, 14, 187–196. doi:10.1097/00005373-197403000-00001
- Beck, A.T., Steer, R.A., & Brown, G.K. (1996). Beck depression inventory-II. San Antonio: The Psychological Corporation.
- Berna, G., Vaiva, G., Ducrocq, F., Duhem, S., & Nandrino, J. (2012). Categorical and dimensional study of the predictive factors of the development of a psychotrauma in victims of car accidents. Journal of Anxiety Disorders, 26, 239–245. doi:10.1016/j.janxdis.2011.11.011
- Bernstein, D.P., & Fink, L. (1998). Childhood trauma questionnaire: A retrospective self-report: Manual. Orlando: Psychological Corporation.
- Breslau, N., Davis, G.C., Peterson, E.L., & Schultz, L. (1997). Psychiatric sequelae of posttraumatic stress disorder in women. Archives of General Psychiatry, 54, 81–87. doi:10.1001/archpsyc.1997.01830130087016
- Delahanty, D.L., Raimonde, A.J., & Spoonster, E. (2000). Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry, 48, 940–947. doi:10.1016/S0006-3223(00)00896-9
- Destatis. (2012). Fachserie 8 Reihe 7 Verkehr. Verkehrsunfälle. 2011. Wiesbaden: Statistisches Bundesamt.
- Dettenborn, L., Muhtz, C., Skoluda, N., Stalder, T., Steudte, S., Hinkelmann, K., … Otte, C. (2012). Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress, 15, 348–353. doi:10.3109/10253890.2011.619239
- Ehlert, U., Gaab, J., & Heinrichs, M. (2001). Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus–pituitary–adrenal axis. Biological Psychology, 57, 141–152. doi:10.1016/S0301-0511(01)00092-8
- Foa, E.B. (1995). Posttraumatic stress diagnostic scale (PDS). Minneapolis: National Computer Systems.
- Foa, E.B., Cashman, L., Jaycox, L., & Perry, K. (1997). The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychological Assessment, 9, 445–451. doi:10.1037/1040-3590.9.4.445
- Fries, E., Hesse, J., Hellhammer, J., & Hellhammer, D.H. (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30, 1010–1016. doi:10.1016/j.psyneuen.2005.04.006
- Gao, W., Stalder, T., Foley, P., Rauh, M., Deng, H., & Kirschbaum, C. (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC–APCI–MS/MS assay. Journal of Chromatography B, 928, 1–8. doi:10.1016/j.jchromb.2013.03.008
- Gast, U., Rodewald, F., Benecke, H.-H., & Driessen, M. (2001). Deutsche Bearbeitung des Childhood Trauma Questionnaire (unautorisiert). Unveröffentlichtes Manuskript. Medizinische Hochschule Hannover.
- Goodyer, I.M., Tamplin, A., Herbert, J., & Altham, P. (2000). Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry, 177, 499–504. doi:10.1192/bjp.177.6.499
- Green, B.L. (1996). Trauma History Questionnaire. In B. H. Stamm (Ed.), Measurement of Stress, Trauma, and Adaptation (p. 366–369). Lutherville, MD: Sidran Press.
- Hautzinger, M., Keller, F., & Kühner, C. (2006). Beck Depression Inventory (BDI-II). Frankfurt: Harcourt Test Services.
- Heim, C., Ehlert, U., & Hellhammer, D.H. (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25, 1–35. doi:10.1016/S0306-4530(99)00035-9
- Heinrichs, M., Wagner, D., Schoch, W., Soravia, L.M., Hellhammer, D.H., & Ehlert, U. (2005). Predicting posttraumatic stress symptoms from pretraumatic risk factors: A 2-year prospective follow-up study in firefighters. American Journal of Psychiatry, 162, 2276–2286. doi:10.1176/appi.ajp.162.12.2276
- Heron-Delaney, M., Kenardy, J., Charlton, E., & Matsuoka, Y. (2013). A systematic review of predictors of posttraumatic stress disorder (PTSD) for adult road traffic crash survivors. Injury, 44, 1413–1422. doi:10.1016/j.injury.2013.07.011
- Holeva, V., Tarrier, N., & Wells, A. (2001). Prevalence and predictors of acute stress disorder and PTSD following road traffic accidents: Thought control strategies and social support. Behavior Therapy, 32, 65–83. doi:10.1016/S0005-7894(01)80044-7
- Horowitz, M., Wilner, N., & Alvarez, W. (1979). Impact of event scale: A measure of subjective stress. Psychosomatic Medicine, 41, 209–218. doi:10.1097/00006842-197905000-00004
- Hruska, B., Cullen, P. K., & Delahanty, D. L. (2014). Pharmacological modulation of acute trauma memories to prevent PTSD: considerations from a developmental perspective. Neurobiology of Learning and Memory, 112, 122–129. doi: 10.1016/j.nlm.2014.02.001
- Inslicht, S.S., Otte, C., McCaslin, S.E., Apfel, B.A., Henn-Haase, C., Metzler, T., … Marmar, C.R. (2011). Cortisol awakening response prospectively predicts peritraumatic and acute stress reactions in police officers. Biological Psychiatry, 70, 1055–1062. doi:10.1016/j.biopsych.2011.06.030
- Kalra, S., Einarson, A., Karaskov, T., Van Uum, S., & Koren, G. (2007). The relationship between stress and hair cortisol in healthy pregnant women. Clinical & Investigative Medicine, 30, 103–107. doi:10.25011/cim.v30i2.986
- Kenardy, J., Edmed, S. L., Shourie, S., Warren, J., Crothers, A., Brown, E. A., Cameron, C. M., & Heron-Delaney, M. (2018). Changing patterns in the prevalence of posttraumatic stress disorder, major depressive episode and generalized anxiety disorder over 24 months following a road traffic crash: Results from the UQ SuPPORT study. Journal of Affective Disorders, 236, 172–179. doi: 10.1016/j.jad.2018.04.090
- Kenardy, J., Thompson, K., Le Brocque, R., & Olsson, K. (2008). Information-provision intervention for children and their parents following pediatric accidental injury. European Child & Adolescent Psychiatry, 17(5), 316–325. doi: 10.1007/s00787-007-0673-5
- Kirschbaum, C., Pirke, K.-M., & Hellhammer, D.H. (1993). The ‘Trier Social Stress Test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. doi:10.1159/000119004
- LeMoult, J., Ordaz, S.J., Kircanski, K., Singh, M.K., & Gotlib, I.H. (2015). Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology, 124, 850–859. doi:10.1037/abn0000087
- Luo, H., Hu, X., Liu, X., Ma, X., Guo, W., Qiu, C., … Li, T. (2012). Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biological Psychiatry, 72, 65–69. doi:10.1016/j.biopsych.2011.12.020
- Maercker, A. (2002). Deutsche Übersetzung des Trauma History Questionnaire. Unveröffentliches Manuskript. Universität Zürich.
- Mayou, R., Bryant, B., & Ehlers, A. (2001). Prediction of psychological outcomes one year after a motor vehicle accident. American Journal of Psychiatry, 158, 1231–1238. doi:10.1176/appi.ajp.158.8.1231
- McEwen, B.S. (2004). Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032, 1–7. doi:10.1196/annals.1314.001
- McFarlane, A.C., Barton, C.A., Yehuda, R., & Wittert, G. (2011). Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology, 36, 720–727. doi:10.1016/j.psyneuen.2011.05.002
- Meewisse, M.-L., Reitsma, J.B., De Vries, G.-J., Gersons, B.P., & Olff, M. (2007). Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. The British Journal of Psychiatry, 191, 387–392. doi:10.1192/bjp.bp.106.024877
- Mouthaan, J., Sijbrandij, M., Luitse, J.S., Goslings, J.C., Gersons, B.P., & Olff, M. (2014). The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology, 45, 179–186. doi:10.1016/j.psyneuen.2014.04.001
- Murray, J., Ehlers, A., & Mayou, R.A. (2002). Dissociation and post-traumatic stress disorder: Two prospective studies of road traffic accident survivors. British Journal of Psychiatry, 180, 363–368. doi:10.1192/bjp.180.4.363
- Nishi, D., Matsuoka, Y., Nakajima, S., Noguchi, H., Kim, Y., Kanba, S., & Schnyder, U. (2008). Are patients after severe injury who drop out of a longitudinal study at high risk of mental disorder? Comprehensive Psychiatry, 49(4), 393–398. doi: 10.1016/j.comppsych.2008.02.003
- Pacella, M.L., Hruska, B., Steudte-Schmiedgen, S., George, R.L., & Delahanty, D.L. (2017). The utility of hair cortisol concentrations in the prediction of PTSD symptoms following traumatic physical injury. Social Science & Medicine, 175, 228–234. doi:10.1016/j.socscimed.2016.12.046
- Resnick, H.S., Yehuda, R., Pitman, R.K., & Foy, D.W. (1995). Effect of previous trauma on acute plasma cortisol level following rape. The American Journal of Psychiatry, 152, 1675–1677. doi:10.1176/ajp.152.11.1675
- Rohleder, N., Joksimovic, L., Wolf, J.M., & Kirschbaum, C. (2004). Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biological Psychiatry, 55, 745–751. doi:10.1016/j.biopsych.2003.11.018
- Smith, B., Mackenzie-Ross, S., & Scragg, P. (2007). Prevalence of poor psychological morbidity following a minor road traffic accident (RTA): The clinical implications of a prospective longitudinal study. Counselling Psychology Quarterly, 20(2), 149–155. doi: 10.1080/09515070701403679
- Shalev, A.Y., Peri, T., Canetti, L., & Schreiber, S. (1996). Predictors of PTSD in injured trauma survivors: A prospective study. The American Journal of Psychiatry, 153, 219–225. doi:10.1176/ajp.153.2.219
- Stalder, T., & Kirschbaum, C. (2012). Analysis of cortisol in hair – State of the art and future directions. Brain, Behavior, and Immunity, 26, 1019–1029. doi:10.1016/j.bbi.2012.02.002
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., … Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. doi:10.1016/j.psyneuen.2016.12.017
- Steudte-Schmiedgen, S., Kirschbaum, C., Alexander, N., & Stalder, T. (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience & Biobehavioral Reviews, 69, 124–135. doi:10.1016/j.neubiorev.2016.07.015
- Steudte-Schmiedgen, S., Stalder, T., Schönfeld, S., Wittchen, H.-U., Trautmann, S., Alexander, N., … Kirschbaum, C. (2015). Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology, 59, 123–133. doi:10.1016/j.psyneuen.2015.05.007
- Steudte-Schmiedgen, S., Wichmann, S., Stalder, T., Hilbert, K., Muehlhan, M., Lueken, U., & Beesdo-Baum, K. (2017). Hair cortisol concentrations and cortisol stress reactivity in generalized anxiety disorder, major depression and their comorbidity. Journal of Psychiatric Research, 84, 184–190. doi:10.1016/j.jpsychires.2016.09.024
- Steudte, S., Kirschbaum, C., Gao, W., Alexander, N., Schönfeld, S., Hoyer, J., & Stalder, T. (2013). Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biological Psychiatry, 74, 639–646. doi:10.1016/j.biopsych.2013.03.011
- Steudte, S., Stalder, T., Dettenborn, L., Klumbies, E., Foley, P., Beesdo-Baum, K., & Kirschbaum, C. (2011). Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Research, 186, 310–314. doi:10.1016/j.psychres.2010.09.002
- van Zuiden, M., Geuze, E., Willemen, H.L.D.M., Vermetten, E., Maas, M., Amarouchi, K., … Heijnen, C.J. (2012). Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: A prospective study. Biological Psychiatry, 71, 309–316. doi:10.1016/j.biopsych.2011.10.026
- van Zuiden, M., Geuze, E., Willemen, H.L., Vermetten, E., Maas, M., Heijnen, C.J., & Kavelaars, A. (2011). Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. American Journal of Psychiatry, 168, 89–96. doi:10.1176/appi.ajp.2010.10050706
- van Zuiden, M., Heijnen, C.J., Maas, M., Amarouchi, K., Vermetten, E., Geuze, E., & Kavelaars, A. (2012). Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: A prospective study. Psychoneuroendocrinology, 37, 1822–1836. doi:10.1016/j.psyneuen.2012.03.018
- van Zuiden, M., Kavelaars, A., Rademaker, A.R., Vermetten, E., Heijnen, C.J., & Geuze, E. (2011). A prospective study on personality and the cortisol awakening response to predict posttraumatic stress symptoms in response to military deployment. Journal of Psychiatric Research, 45, 713–719. doi:10.1016/j.jpsychires.2010.11.013
- Vrshek-Schallhorn, S., Doane, L., Mineka, S., Zinbarg, R., Craske, M., & Adam, E. (2013). The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine, 43, 483–493. doi:10.1017/S0033291712001213
- Walsh, K., Nugent, N.R., Kotte, A., Amstadter, A.B., Wang, S., Guille, C., … Resnick, H.S. (2013). Cortisol at the emergency room rape visit as a predictor of PTSD and depression symptoms over time. Psychoneuroendocrinology, 38, 2520–2528. doi:10.1016/j.psyneuen.2013.05.017
- Weiss, D.S. (2007). The Impact of Event Scale: Revised. In J. P. Wilson & C. S.-k. Tang (Eds.), International and cultural psychology. Cross-cultural assessment of psychological trauma and PTSD (p. 219–238). New York: Springer Science + Business Media.
- Weitzman, E.D., Fukushima, D., Nogeire, C., Roffwarg, H., Gallagher, T., & Hellman, L. (1971). Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. The Journal of Clinical Endocrinology & Metabolism, 33, 14–22. doi:10.1210/jcem-33-1-14
- Wennig, R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Science International, 107, 5–12. doi:10.1016/S0379-0738(99)00146-2
- Wichmann, S., Kirschbaum, C., Böhme, C., & Petrowski, K. (2017). Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology, 83, 135–141. doi:10.1016/j.psyneuen.2017.06.005
- Wittchen, H.-U. (1997). Strukturiertes klinisches Interview für DSM-IV: SKID. Achse I: Psychische Störungen: Interviewheft und Beurteilungsheft; eine deutschsprachige, erweiterte Bearbeitung der amerikanischen Originalversion des SCID-I: Hogrefe, Verlag für Psychologie.
- Wittchen, H.-U. (2007). Diagnostisches Expertensystem für Psychiche Störungen: DIA-X Interviews: Harcourt Test Servives.
- Wu, K. K., Li, F. W., & Cho, V. W. (2014). A randomized controlled trial of the effectiveness of brief-CBT for patients with symptoms of posttraumatic stress following a motor vehicle crash. Behavioural and Cognitive Psychotherapy, 42(1), 31–47. doi: 10.1017/S1352465812000859
- Yehuda, R., Bierer, L.M., Schmeidler, J., Aferiat, D.H., Breslau, I., & Dolan, S. (2000). Low cortisol and risk for PTSD in adult offspring of holocaust survivors. American Journal of Psychiatry, 157, 1252–1259. doi:10.1176/appi.ajp.157.8.1252
- Young, E.A., Abelson, J., & Lightman, S.L. (2004). Cortisol pulsatility and its role in stress regulation and health. Frontiers in Neuroendocrinology, 25, 69–76. doi:10.1016/j.yfrne.2004.07.001
- Zoladz, P.R., & Diamond, D.M. (2013). Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neuroscience & Biobehavioral Reviews, 37, 860–895. doi:10.1016/j.neubiorev.2013.03.024