Abstract
Numerous studies have shown that the presence of psychosocial stress impairs the ability to retrieve episodic memories, which raise questions about the specific cognitive processes that underlie this impairment. Here, we tested the hypothesis that stress targets retrieval processes needed to reliability discriminate previously learned from new information within episodic memory, pattern separation processing by measuring the effects of retrieval-induced stress on a modified version of the Mnemonic Similarity Task. In a two-part between-subjects design, all participants studied a series of object images in an initial testing session. In a second session, held 24 h later, half of the participants completed a stress induction task (stress group) and half performed a similarly structured but non-stressful task (control group) and all were then given a recognition memory test for the previously studied images which included new images similar to those studied (lures), and images that were completely novel (foils). Both groups performed equally well in terms of overall recognition memory, but the stress group was significantly impaired in discriminating new and similar (lure) items from studied items. This pattern of results suggests that stress specifically targets pattern separation processing when retrieving information from episodic memory. We discuss the implications of this effect, specifically how stress at retrieval reduces the ability to discriminate new from learned information.
Introduction
There is clear evidence that acute psychosocial stress impacts episodic memory processing with differential effects emerging during memory encoding and retrieval (Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, Citation2012). Studies have linked stress to benefits when encoding an episodic memory, particularly for emotional material (Ulrich-Lai & Herman, Citation2009). However, stress is also linked to deficits in memory retrieval, mainly by impairing the ability to recall previously learned material (Gagnon & Wagner, Citation2016; Schwabe, Citation2017; Shields, Sazma, McCullough, & Yonelinas, Citation2017) (however: Domes, Heinrichs, Reichwald, & Hautzinger, Citation2002). A characteristic of successful episodic memory retrieval is the ability to discriminate new and similar experiences from those that have been previously encountered, coined behavioral pattern separation (Yassa & Stark, Citation2011). Among the memory-related brain regions affected by stress are regions in the prefrontal cortex important for executive processes (Shields et al., Citation2016) as well as the hippocampus, which plays a critical role in memory-based processes needed to engage in behavioral pattern separation, also referred to as memory discrimination (Gagnon, Waskom, Brown, & Wagner, Citation2019; O’Reilly & Norman, Citation2002). Here, we focused on testing how the effects of stress at retrieval present as deficits in memory discrimination.
Memory retrieval requires pattern completion processes to reactivate a memory representation in response to a retrieval cue, but pattern separation processes to successfully discriminate or ‘separate’ a correctly reactivated memory from a representation with overlapping features (Hunsaker & Kesner, Citation2013). In this way, pattern separation processes are thought to support mnemonic discrimination during retrieval - the ability to accurately reject a similar but new experience as a previously encoded event (Leal & Yassa, Citation2014). There is established research linking the effects of acute stress to pattern separation processes during encoding (Cunningham, Leal, Yassa, & Payne, Citation2018; Jiang, Tran, Madison, & Bakker, Citation2019; Segal, Stark, Kattan, Stark, & Yassa, Citation2012) and whether these processes are also affected by stress during retrieval is an open question. There are indications from the literature that retrieval-induced stress presents with behavioral impairments indicative of pattern separation deficits. Without proper pattern separation processing to discriminate learned from new information, a person tends to overgeneralize during memory retrieval, which is what happens when retrieving under stress (Bernstein, Kleiman, & McNally, Citation2019; Leal, Tighe, & Yassa, Citation2014). Moreover, clinical studies have shown that chronic stress, as experienced in post-traumatic stress and anxiety disorders, is linked to poor memory discrimination that have been attributed to problems with pattern separation (Balderston et al., Citation2017; Kaczkurkin et al., Citation2017; Kheirbek, Klemenhagen, Sahay, & Hen, Citation2012). In the brain, pattern separation has been linked to the functioning of the dentate gyrus of the hippocampus (Rolls, Citation2016), which is a hippocampal subfield that is particularly sensitive to the effects of acute stress (McEwen, Nasca, & Gray, Citation2016).
To follow these findings, we investigated whether stress at retrieval would alter pattern separation processing. We tested the effects of acute stress during retrieval on a mnemonic discrimination task (Stark, Yassa, Lacy, & Stark, Citation2013), with the expectation that stress would affect the ability to differentiate between previously encoded, and new related items, a measure of mnemonic discrimination known to be rely upon the hippocampally-dependent patterns separation processes (Kirwan & Stark, Citation2007; Stark et al., Citation2013; Yassa & Stark, Citation2011), we hypothesize are targeted by stress.
Methods
Participants
51 participants were recruited from classifieds-ads and online advertisements posted on McGill University forums. Two participants had incomplete data due to testing interruption and three participants were excluded for having made less than 10% correct responses. For one participant no saliva samples were collected. The final sample consisted of 20 men and 26 women (men: mean age = 23.3, SD ± 3.34; women: mean age = 21.4, SD ± 3.46).
Participants were randomly assigned to either the control, or stress group. All participants were fluent in English and were free from factors that could affect stress reactivity (i.e. no previous exposure to the stress task, moderate alcohol (<10 units per week) and tobacco consumption (<5 cigarettes per day), no illicit drug use, and no endorsed symptoms of depression and/or anxiety). All female participants were regularly menstruating and were not on oral or chemical contraceptives. Participants provided informed consent prior to the study and were compensated with $30 CAD. The study was approved by the McGill University Faculty of Science Institutional Review Board.
Procedure
Participants were invited to the laboratory for two separate testing sessions. In order to account for fluctuating levels of diurnal cortisol (Goodman, Janson, & Wolf, Citation2017), all testing sessions took place between 1:00 pm–7:00 pm. On day-1 participants filled out a series of questionnaires before they completed the encoding phase of the mnemonic discrimination task (see below). On day-2 (24 h later), participants filled out a series of questionnaires before they were introduced to the stress task (see below), or the control task. Immediately after the stress (or control) task participants completed the retrieval component of the mnemonic discrimination task. To assess the effect of acute stress on mnemonic discrimination, the recognition test occurred under acute psychosocial stress.
Mnemonic discrimination task
We used a modified version of the mnemonic discrimination task (MDT; Stark et al., Citation2013). The MDT consists of an encoding phase and a retrieval phase (recognition test) which were completed over two testing sessions separated by 24 h. During the encoding session, participants were shown 128 colored photographs of everyday objects against a white background, each presented for 2 seconds on the screen (0.5 second ISI). For each photograph, participants had to indicate if the object in to was an indoor or outdoor item via button press. During the surprise recognition testing session (24 h later) participants were shown 192 photographs of objects. Of these, 64 were previously seen or repeats (“old”), 64 were lures (“similar”), and 64 were foils (“new”). The lures were designed such that there was a range in the degree of overlap in information with repeat objects, assigned to one of five bins of similarity (i.e. bin-1 = similar information that had little overlap; bin-5 = similar information with substantial overlap). For each image, participants identified the presented object as either “old” (i.e. previously seen, or repeat), “similar” (i.e. similar but not identical to previously viewed images), or “new” (i.e. not previously seen) via button press.
Stress paradigm
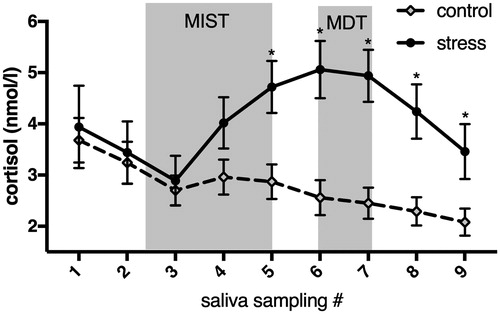
In order to elicit a state of psychosocial stress, we used the Montreal Imaging Stress Task (MIST; Dedovic et al., Citation2005). The stress condition of the MIST consists of a series of mental arithmetic tasks that are performed under time-constraint. Performance is followed by negative feedback meant to induce a state of psychosocial stress in the participants. The task is designed to result in failure, or poor performance, by an algorithm that self-adjusts the time-constraints slightly beyond the ability of each individual participant. In addition the level of difficulty of the arithmetic tasks are also manipulated so that the participant performs at an average success rate of 20% to 30% correct answers. Following this the participant is given negative feedback by the investigator about their poor performance with the instruction to improve their performance to reach a minimum requirement. In the control group, participants solved easy problems with high success rate and did not receive any negative feedback on their performance. To assess the stress response, cortisol levels (nmol/l) were measured using a time-resolved fluorescence immunoassay described by Dressendörfer, Kirschbaum, Rohde, Stahl, and Strasburger (Citation1992) on salivary samples (“Salivette”; Sarstedt AG & Co, Nümbrecht, Germany). We collected a total of 9-salivary samples, taken 10 min apart, throughout the experiment (see ).
Analyses plan
Demographics
A one-way (control vs stress) ANOVA was conducted with age as dependent variables to ensure that groups did not differ.
Stress induction
We then tested for group differences in cortisol response over time using a repeated measures ANOVA, with cortisol (all 9 time-points) as within-subject factor, and group (control vs stress) and gender/sex as between subjects factors. Gender/sex was included to insure that conditions did not systematically differ in their stress response. Previous research has shown that acute stress can result in differential cortisol responses for men and women (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, Citation1999; Kudielka, Hellhammer, & Wüst, Citation2009). Cortisol values were logarithmically transformed to account for skewness in the data.
Recognition memory performance
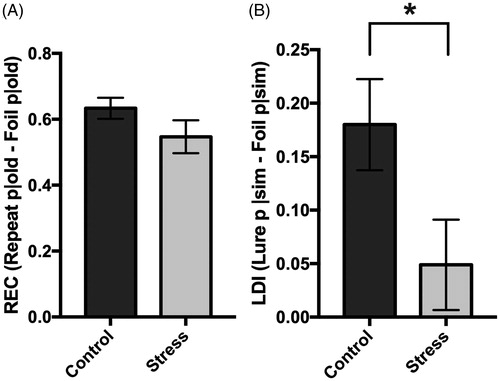
To test for between group differences in correct responses on the recognition memory test, we ran a MANOVA, with group (control versus stress) as a between-subjects factor, and correct response rates as dependent variables (repeat - “old” response; lure - “similar” response; foil - “new” response; reported in the ‘Correct responses by item type’ section). Based on the results of this analysis, we further probed the mnemonic discrimination ability by examining correct responses to the lures (“similar” to lures) as a function of lure-difficulty (bin-1 = least difficult; bin-5 = most difficult). Next, we derived the overall recognition rate (REC) for repeated items by calculating the difference between the rate of “old” responses given to repeated items, minus “old” responses given to foils (O|R- O|F) (cf. Stark, Stevenson, Wu, Rutledge, & Stark, Citation2015). We subsequently ran an ANOVA to test for differences in recognition rates between groups.
Pattern Separation Perfromance (LDI)
Finally, to assess pattern separation performance, we calculated a bias corrected Lure Discrimination Index (LDI; see ). LDI was calculated as the difference between the rate of “similar” responses given to lure items minus “similar” responses given to foils (S|L- S|F) (Stark et al., Citation2015). Lower LDI score indicate poorer pattern separation abilities. The LDI were entered into an ANOVA reported in the ‘Pattern Separation Performance (LDI)’ section.
Table 1. Behavioral data for the mnemonic discrimination task for both groups.
Results
Demographics
There were 23 participants in the control group (13 females, 10 males), and 23 in the stress group (13 females, 10 men) and these groups did not differ in average age, F(1,44) = 1.00, p = 0.32 (stress group: mean age = 21.7, SD ± 3.34; control group: mean age = 22.7, SD ± 3.72).
Stress induction
The repeated measures ANOVA that compared the cortisol response between groups, across time (all 9 time-points) showed a significant time x group interaction, F(8, 222.32) = 4.7, p < 0.001, η2 = 0.09. Pairwise comparisons revealed significant differences in cortisol levels between groups for the time-points 5,6,7,8, and 9 (all ps< 0.05)., indicating a stressful stress induction. See for a depiction of the cortisol response over time between groups.
Recognition memory performance
The results of the MANOVA for average proportion of correct responses between groups, by item type, revealed no significant differences between groups for repeat items, F(1,44) = 1.02, p = 0.32; or foils F(1,44) = 0.03, p = 0.85, but a significant difference in correct responses for lure items, F(1,44) = 8.59, p < 0.001, η2 = 0.16. See for mean accuracies. The control group had a significantly higher proportion of correct responses, compared to the stress group, for lure items. The ANOVA testing for group differences (control versus stress) for bias corrected memory recognition scores (REC)(O|R - O|F) revealed no significant differences between the control and the stress groups F(1,44) = 2.129, p = 0.154. Indicating that control and stressed individuals performed similarly when recognizing repeat-items ().
Table 2. Recognition and LDI scores.
Lure Discrimination Index (LDI)
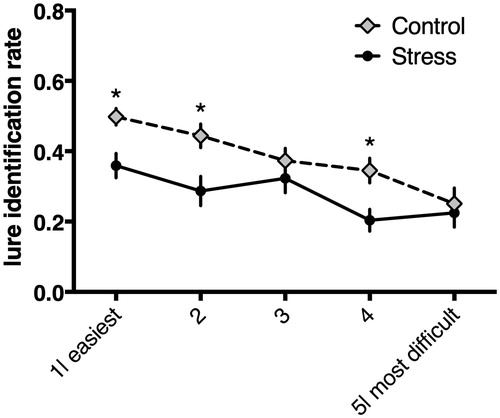
The ability to correctly respond to repeat items and discriminating them from lures, LDI, was significantly different across group, F(1,44) = 5.03, p < 0.029, η2 = 0.10; control: mean = 0.183, SEM= 0.025.06; stress: mean = 0.049, SEM = 0.025. See and . Individuals in the control group had a greater sensitivity for discriminating repeated from lure information, than the stress group. Individuals in the control group had a greater sensitivity for discriminating repeated from lure information, than the stress group. See and . Following this result, we then ran a repeated measures ANOVA with correct responses to lures as dependent variable, lure difficulty (bin) as within-subject factor and group as between-subjects factor. Results revealed significant main effects of bin (difficulty of lures), F(184, 4) = 35.82, p < 0.001, η2 = 0.42, and group, F(45, 1) = 4.44, p = 0.04, η2 = 0.02. These results show, more difficult bins were associated with lower accuracy, compared to easier bins, and stressed individuals had a lower accuracy for lures, compared to unstressed participants. See . In an additional step we tested for an interaction between group and bin, however, the interaction was not significant (p = 0.1). Pairwise comparisons were conducted to test for group differences in correct responses to lures as a function of lure-difficulty. Here we found that the proportion of correct responses between stressed and non-stressed individuals were significantly different for: bin-1, bin-2, and bin-4; all Fs > 6, ps < 0.02. See .
Discussion
In the current study, we used a validated mnemonic discrimination task (Stark et al., Citation2013) to investigate the effects of acute psychosocial stress at retrieval on pattern separation processing. Young healthy participants encoded pictures of objects on day 1, and then on day 2 (24 h later) they completed a recognition memory test that included the previously encoded objects (repeated), similar but new objects (lures) as well as distinct and new objects (foils). Shortly before the recognition memory test, half of the participants (the stress group) underwent a psychosocial stress induction, and the other half (the control group) completed a matched non-stressful task. Our main finding was that individuals in the stress group were significantly impaired when discriminating new information from previously learned similar information, as indicated by lower LDI scores in the stress group compared to the control group.
In first comparing our results to previous studies examining the effects of stress on retrieval, we note that, like our results, some work has indicated stress at retrieval does not impact recognition memory (e.g. Domes et al., Citation2002; Nater et al., Citation2007; Payne et al., Citation2006; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, Citation2001), yet other work has reported stress at retrieval impaired recognition memory performance using tasks like the DRM (Smeets, Otgaar, Candel, & Wolf, Citation2008). These conflicting results underlie a growing acceptance that the effects of stress on retrieval is complicated by a number of factors, such as whether high or low levels of stress are experienced (Domes et al., Citation2002; Nater et al., Citation2007) and how the stressor is administered and the type of material that is being used (Stock & Merz, Citation2018). We further consider that the type of material used in our task could have prevented overall recognition memory deficits. Research has indicated that retrieval tasks that provide elaborate cues are less impacted by stress because these types of memory tasks are less reliant on the hippocampus to perform accurately (Gagnon & Wagner, Citation2016). The MDT uses multi-element visualized object as study and test items, which we think serve as elaborate cues because these objects can cue recognition in many different ways (shape, color, size) during retrieval. This is unlike a test that uses words as stimuli (e.g. the DRM) that serves as less elaborate retrieval cues, a discrepancy that could help explain why we did not find overall recognition memory deficits.
We did find that lure discrimination – and the underlying processes - are sensitive to stress effects when using such multi-dimensional objects. Previous work has suggested the lure discrimination as measured by the LDI is sensitive to hippocampal function, and particularly in supporting pattern separation processes (Kirwan & Stark, Citation2007; Rolls, Citation2016; Stark et al., Citation2013; Yassa & Stark, Citation2011), thus we interpret this result as indicating that stress, at least when it occurs during retrieval, impacts pattern separation processing. Since memory retrieval requires a balance between pattern completion and pattern separation processes to reactivate past memories, and discriminate overlapping mnemonic representations, respectively (Hunsaker & Kesner, Citation2013), our results suggest that stress most sensitively targets the pattern separation processes to correctly identify newly encountered information as novel (Hunsaker & Kesner, Citation2013). However, we found somewhat inconsistent results to the interpretation of data as stress decreasing the ability to pattern separate similar (lure) items during memory retrieval when we analyzed the lures as function of difficulty. Here, we found that stress impaired lure discrimination across all levels of difficulty when we might expect that stress would negatively impact lures that are most similar to the targets (i.e. highest difficulty). One reason we did not finding this result could be because the reduced number of lures within each of these bins are likely not sufficient to provide a reliable estimate of the effects of stress of discriminability (Stark et al., Citation2015), however another reason could be because the effects of stress on lure discrimination is insensitive to the amount of perceptual distinction between the lure and target items and more bluntly affects this aspect of recognition memory.
To consider the mechanism by which stress affected lure discrimination, we turn to the finding that stress effects on memory retrieval are due to the release of cortisol targeting the hippocampus and involved pathways (Gagnon et al., Citation2019). Although both pattern competition and separation rely on the hippocampus, it could be that these processes are dependent to different extents on the integrity of hippocampal subfields, which are targeted in differently ways by cortisol. Specifically, pattern separation has been linked to the functioning of the dentate gyrus (Rolls, Citation2016), an area particularly vulnerable to the pathophysiological changes in response to cortisol release (McEwen et al., Citation2016). Thus, we speculate that stress during retrieval is impacted functioning of this subfield, an interpretation that fits with work reporting that deficits to hippocampal function in chronic stress disorders alter pattern separation abilities from dentate gyrus alterations (Aimone, Deng, & Gage, Citation2011; Yassa et al., Citation2011). Since we did not collected measure of hippocampal function in this study, future neuroimaging studies can test if stress induced cortisol release at retrieval targets specific aspects of the hippocampus. More generally, the stress cortisol response is known to affect areas outside the hippocampus known to be involved in executive functioning also important in successful retrieval (e.g. Shields, Sazma, & Yonelinas, Citation2016). Thus, future studies can establish the specificity of neurocognitive processes underlying the reported mnemonic discrimination deficit.
Overall, the finding that stress affects lure discrimination, possibly via targeting retrieval-based pattern separation processing, is consistent with reported behavioral effects of stress. Foremost, studies have found that stressful scenarios leads to retrieving generalized and less discriminable memory representations (Brewin, Citation2014; Payne et al., Citation2006; Payne, Nadel, Allen, Thomas, & Jacobs, Citation2002). In accord, a recent study by Dandolo and Schwabe (Citation2016) used an acquired equivalence test to show that while stress did not impact the overall learning performance on this task, it reduced participants ability to effectively transfer – generalize – information across situations, which could mean stress led to an inability to identify and differentiate similar but new information. Other work has found that when stressed, an individual tends to be less discriminatory in what they remember – potentially as a survival mechanism – rather than differentiating nuances when recalling information (Wirz, Bogdanov, & Schwabe, Citation2018), which lends well to our results. However, another possibility is that the mnemonic discrimination effect reported in our study is simply a result of this LDI estimate representing a more cognitively taxing task that overall recognition. Behaviorally, and stress has been linked to a shift toward less effortful processing strategies (Schwabe, Citation2017), which relates to our results.
Finally, the current findings have to be considered alongside their limitations. The stress task used in this study, the MIST, is thought to elicit a weaker stress response than the ‘gold standard’ stress induction, the Trier Social Stress Test (cf. Ali, Nitschke, Cooperman, & Pruessner, Citation2017). Whether we would find similar effects with other forms of stress induction that come with varying levels of stress is a worthy avenue for future research. Furthermore, stressors experienced in daily life are often relatively mild (Almeida, Wethington, & Kessler, Citation2002). Notably, there is some evidence for u-shaped functions (rather than linear) of stress on memory performances (e.g. Dandolo & Schwabe, Citation2016; Nitschke, Chu, Pruessner, Bartz, & Sheldon, Citation2019). Here, we concentrated on testing hypotheses about the physiological response of cortisol release when stressed. It would be interesting to see how markers of the autonomic nervous system, which have been associated with improvements in pattern discrimination when encoding emotional stimuli (Segal et al., Citation2012). In addition, the current study focused on non-emotional stimuli (i.e. everyday objects), and it might be important to extend this line of research to emotional stimuli (Sheldon, Chu, Nitschke, Pruessner, & Bartz, Citation2018), in particular since differences between neutral and emotional stimuli have been reported in the context of the MDT (Leal & Yassa, Citation2014). Furthermore, it could be that the less discriminable memory processing we attribute to impaired pattern separation abilities are due to other processes, such as impaired attentional processes, which we reference in the above sections. More specifically, shifts in decision making, or attentional processes (Shields et al., Citation2016; Vedhara, Hyde, Gilchrist, Tytherleigh, & Plummer, Citation2000) could have alerted how a person responded to the three choice responses (“old”, “similar”, and “new”) and this could have led to the discrimination differences between the stress and control group. Future studies will need to explore these topics.
Even with these limitations, the current study highlights that acute psychosocial stress at retrieval targets pattern separation processing when performing a mnemonic discrimination task. This extends previous research by elucidating the specific effects that acute stress can have on memory retrieval.
Author contributions
SS, OZ, and JPN developed the study concept and study design. LG and OZ conducted the data collection. JPN conducted the data analyses. JPN and SS drafted the manuscript. All authors provided critical revisions and approved the final version of the manuscript for submission.
Acknowledgments
We want to thank everyone involved in assistance with data collection, in particular Kelly Cool.
Disclosure statement
The authors have no financial or other conflicts of interest to declare.
Additional information
Funding
References
- Aimone, J.B., Deng, W., & Gage, F.H. (2011). Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron, 70, 589–596. doi:10.1016/j.neuron.2011.05.010
- Ali, N., Nitschke, J.P., Cooperman, C., & Pruessner, J.C. (2017). Suppressing the endocrine and autonomic stress systems does not impact the emotional stress experience after psychosocial stress. Psychoneuroendocrinology, 78, 125–130. doi:10.1016/j.psyneuen.2017.01.015
- Almeida, D.M., Wethington, E., & Kessler, R.C. (2002). The daily inventory of stressful events: An interview-based approach for measuring daily stressors. Assessment, 9, 41–55. doi:10.1177/1073191102091006
- Balderston, N.L., Mathur, A., Adu-Brimpong, J., Hale, E.A., Ernst, M., & Grillon, C. (2017). Effect of anxiety on behavioural pattern separation in humans. Cognition and Emotion, 31, 238–248. doi:10.1080/02699931.2015.1096235
- Bernstein, E.E., Kleiman, E.M., & McNally, R.J. (2019). Mnemonic discrimination under stress and its clinical relevance for anxiety. Clinical Psychological Science, 7, 1014–1031. doi:10.1177/2167702619834562
- Brewin, C.R. (2014). Episodic memory, perceptual memory, and their interaction: Foundations for a theory of posttraumatic stress disorder. Psychological Bulletin, 140, 69–97. doi:10.1037/a0033722
- Cunningham, T.J., Leal, S.L., Yassa, M.A., & Payne, J.D. (2018). Post-encoding stress enhances mnemonic discrimination of negative stimuli. Learning & Memory, 25, 611–619. doi:10.1101/lm.047498.118
- Dandolo, L.C., & Schwabe, L. (2016). Stress-induced cortisol hampers memory generalization. Learning & Memory, 23, 679–683. doi:10.1101/lm.042929.116
- Dedovic, K., Renwick, R., Mahani, N.K., Engert, V., Lupien, S.J., & Pruessner, J.C. (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry & Neuroscience, 30, 319–325.
- Domes, G., Heinrichs, M., Reichwald, U., & Hautzinger, M. (2002). Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology, 27, 843–853. doi:10.1016/S0306-4530(01)00085-3
- Dressendörfer, R.A., Kirschbaum, C., Rohde, W., Stahl, F., & Strasburger, C.J. (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology, 43, 683–692. doi:10.1016/0960-0760(92)90294-S
- Gagnon, S.A., & Wagner, A.D. (2016). Acute stress and episodic memory retrieval: Neurobiological mechanisms and behavioral consequences. Annals of the New York Academy of Sciences, 1369, 55–75. doi:10.1111/nyas.12996
- Gagnon, S.A., Waskom, M.L., Brown, T.I., & Wagner, A.D. (2019). Stress impairs episodic retrieval by disrupting hippocampal and cortical mechanisms of remembering. Cerebral Cortex, 29, 2947–2964. doi:10.1093/cercor/bhy162
- Goodman, W.K., Janson, J., & Wolf, J.M. (2017). Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology, 80, 26–35. doi:10.1016/j.psyneuen.2017.02.030
- Hunsaker, M.R., & Kesner, R.P. (2013). The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and Biobehavioral Reviews, 37, 36–58. doi:10.1016/j.neubiorev.2012.09.014
- Jiang, A., Tran, T.T., Madison, F.N., & Bakker, A. (2019). Acute stress-induced cortisol elevation during memory consolidation enhances pattern separation. Learning & Memory, 26, 121–127. doi:10.1101/lm.048546.118
- Kaczkurkin, A.N., Burton, P.C., Chazin, S.M., Manbeck, A.B., Espensen-Sturges, T., Cooper, S.E., … Lissek, S. (2017). Neural substrates of overgeneralized conditioned fear in PTSD. American Journal of Psychiatry, 174, 125–134. doi:10.1176/appi.ajp.2016.15121549
- Kheirbek, M.A., Klemenhagen, K.C., Sahay, A., & Hen, R. (2012). Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nature Neuroscience, 15, 1613–1620. doi:10.1038/nn.3262
- Kirschbaum, C., Kudielka, B.M., Gaab, J., Schommer, N.C., & Hellhammer, D.H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61, 154–162. doi:10.1097/00006842-199903000-00006
- Kirwan, C.B., & Stark, C.E.L. (2007). Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning & Memory, 14, 625–633. Vol. doi:10.1101/lm.663507
- Kudielka, B.M., Hellhammer, D.H., & Wüst, S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34, 2–18. doi:10.1016/j.psyneuen.2008.10.004
- Leal, S.L., Tighe, S.K., & Yassa, M.A. (2014). Asymmetric effects of emotion on mnemonic interference. Neurobiology of Learning and Memory, 111, 41–48. doi:10.1016/j.nlm.2014.02.013
- Leal, S.L., & Yassa, M.A. (2014). Effects of aging on mnemonic discrimination of emotional information. Behavioral Neuroscience, 128, 539–547. doi:10.1037/bne0000011
- McEwen, B.S., Nasca, C., & Gray, J.D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41, 3–23. doi:10.1038/npp.2015.171
- Nater, U.M., Moor, C., Okere, U., Stallkamp, R., Martin, M., Ehlert, U., & Kliegel, M. (2007). Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology, 32, 758–763. doi:10.1016/j.psyneuen.2007.05.006
- Nitschke, J.P., Chu, S., Pruessner, J.C., Bartz, J.A., & Sheldon, S. (2019). Post-learning stress reduces the misinformation effect: Effects of psychosocial stress on memory updating. Psychoneuroendocrinology, 102, 164–171. doi:10.1016/j.psyneuen.2018.12.008
- O’Reilly, R.C., & Norman, K.A. (2002). Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends in Cognitive Sciences, 6, 505–510. doi:10.1016/S1364-6613(02)02005-3
- Payne, J.D., Jackson, E.D., Ryan, L., Hoscheidt, S., Jacobs, J.W., & Nadel, L. (2006). The impact of stress on neutral and emotional aspects of episodic memory. Memory, 14, 1–16. doi:10.1080/09658210500139176
- Payne, J.D., Nadel, L., Allen, J.J.B., Thomas, K.G.F., & Jacobs, W.J. (2002). The effects of experimentally induced stress on false recognition. Memory, 10, 1–6. doi:10.1080/09658210143000119
- Rolls, E.T. (2016). Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiology of Learning and Memory, 129, 4–28. doi:10.1016/j.nlm.2015.07.008
- Schwabe, L. (2017). Memory under stress: From single systems to network changes. European Journal of Neuroscience, 45, 478–489. doi:10.1111/ejn.13478
- Schwabe, L., Joëls, M., Roozendaal, B., Wolf, O.T., & Oitzl, M.S. (2012). Stress effects on memory: An update and integration. Neuroscience & Biobehavioral Reviews, 36, 1740–1749. doi:10.1016/j.neubiorev.2011.07.002
- Segal, S.K., Stark, S.M., Kattan, D., Stark, C.E., & Yassa, M.A. (2012). Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiology of Learning and Memory, 97, 465–469. doi:10.1016/j.nlm.2012.03.010
- Sheldon, S., Chu, S., Nitschke, J.P., Pruessner, J.C., & Bartz, J.A. (2018). The dynamic interplay between acute psychosocial stress, emotion and autobiographical memory. Scientific Reports, 8, 8684. doi:10.1038/s41598-018-26890-8
- Shields, G.S., Sazma, M.A., McCullough, A.M., & Yonelinas, A.P. (2017). The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological Bulletin, 143, 636–675. doi:10.1037/bul0000100
- Shields, G.S., Sazma, M.A., & Yonelinas, A.P. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience & Biobehavioral Reviews, 68, 651–668. doi:10.1016/j.neubiorev.2016.06.038
- Smeets, T., Otgaar, H., Candel, I., & Wolf, O.T. (2008). True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology, 33, 1378–1386. doi:10.1016/j.psyneuen.2008.07.009
- Stark, S.M., Stevenson, R., Wu, C., Rutledge, S., & Stark, C.E.L. (2015). Stability of age-related deficits in the mnemonic similarity task across task variations. Behavioral Neuroscience, 129, 257–268. doi:10.1037/bne0000055
- Stark, S.M., Yassa, M.A., Lacy, J.W., & Stark, C.E.L. (2013). A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia, 51, 2442–2449. doi:10.1016/j.neuropsychologia.2012.12.014
- Stock, L.-M., & Merz, C.J. (2018). Memory retrieval of everyday information under stress. Neurobiology of Learning and Memory, 152, 32–38. doi:10.1016/j.nlm.2018.05.008
- Ulrich-Lai, Y.M., & Herman, J.P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10, 397–409. doi:10.1038/nrn2647
- Vedhara, K., Hyde, J., Gilchrist, I.D., Tytherleigh, M., & Plummer, S. (2000). Acute stress, memory, attention and cortisol. Psychoneuroendocrinology, 25, 535–549. doi:10.1016/S0306-4530(00)00008-1
- Wirz, L., Bogdanov, M., & Schwabe, L. (2018). Habits under stress: Mechanistic insights across different types of learning. Current Opinion in Behavioral Sciences, 20, 9–16. doi:10.1016/j.cobeha.2017.08.009
- Wolf, O.T., Schommer, N.C., Hellhammer, D.H., McEwen, B.S., & Kirschbaum, C. (2001). The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology, 26, 711–720. doi:10.1016/S0306-4530(01)00025-7
- Yassa, M.A., Lacy, J.W., Stark, S.M., Albert, M.S., Gallagher, M., & Stark, C.E.L. (2011). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus, 21, 968–979. doi:10.1002/hipo.20808
- Yassa, M.A., & Stark, C.E.L. (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34, 515–525. doi:10.1016/j.tins.2011.06.006