Abstract
Convincing evidence shows that stress is associated with the development and course of psychiatric and metabolic disorders. The hypothalamic-pituitary-adrenal (HPA) axis mediates the stress response, a cascade of events that culminate in the release of glucocorticoids from the adrenal cortex. Chronic hypercortisolism typically characterizes stress-related illnesses, such as depression, anxiety, and metabolic syndrome. Considering previous studies pointing that environmental enrichment (EE) mitigates the deleterious effects of stress on neurobiological systems, we hypothesized that EE can confer resiliency against prolonged glucocorticoid administration-induced behavioral and metabolic alterations in mice. In this regard, three-month-old male Swiss mice were exposed to a four-week period of standard environment (SE) or EE. After this period, still in the respective environments, dexamethasone was administered intraperitoneally (i.p.) at a dose of 4 mg/kg, for 21 consecutive days, in order to generate the emotional-related behavioral outcomes, as previously described. It is demonstrated herein that EE prevents the dexamethasone-induced anxiety-like and passive stress-coping behaviors, as observed in the open field and tail suspension tests. Moreover, EE mitigated the hyperproteinemia and body weight loss induced by excess dexamethasone and decreased basal glucose levels. Taken together, these results support the hypothesis that EE attenuates the effects of chronic administration of synthetic glucocorticoids in mice, a strategy that may be translated to the clinical perspective.
Introduction
The stress-responsive hypothalamic-pituitary-adrenal (HPA) axis is causally implicated in the development and course of major depressive disorder (MDD) (Keller et al., Citation2017), being described that patients with depression present HPA-axis overactivity (de Kloet, Joëls, & Holsboer, Citation2005). The untoward consequences of excess cortisol are manifold, ranging from peripheral effects (e.g. glucose intolerance) to adverse effects on brain function. In this regard, hypercortisolemia, as seen in Cushing’s disease or after prolonged glucocorticoid administration, can result in depression or emotional lability (Brown, Vera, Frol, Woolston, & Johnson, Citation2007; Newell-Price, Bertagna, Grossman, & Nieman, Citation2006). For instance, chronic dexamethasone administration in adult life (Skupio et al., Citation2015) or during intrauterine development (Conti, Spulber, Raciti, & Ceccatelli, Citation2017) induces a range of depressive-like symptoms in rodents, such as anhedonia, behavioral despair, weight loss, and anxiety-like behavior.
The ability to cope with stress is influenced by multiple factors such as early life experiences, gender, or personality traits (Chen & Baram, Citation2016). In fact, many environmental factors seem to influence both the physiological functions of the central nervous system and its ability to counteract pathological changes. For instance, negative life events, such as the death of a parent, maternal deprivation, parental abandonment and separation of parents or divorce, can influence the development of mental disorders, such as depression (Juruena, Citation2013). In laboratory animals, stress reduction may be achieved by enriching the animal’s environment with devices that promote its normal instinctive tendencies (Belz, Kennell, Czambel, Rubin, & Rhodes, Citation2003). In this regard, environmental enrichment (EE) is an animal housing technique composed of increased space, physical activity, and social interactions, which in turn increases sensory, cognitive, motor, and social stimulation (Nithianantharajah & Hannan, Citation2006). EE leads to improved cerebral health as defined by increased neurogenesis, enhanced learning and memory and resistance to external cerebral insults (Young, Lawlor, Leone, Dragunow, & During, Citation1999). Moreover, living in an EE with physical and social stimulation also leads to improved metabolic health (Cao et al., Citation2011). Considering previous studies pointing that EE mitigates the deleterious effects of stress on neurobiological systems and endocrine profiles (Lehmann & Herkenham, Citation2011; Mitra & Sapolsky, Citation2009), the present study hypothesized that EE would prevent the development of behavioral (e.g. depressive and anxiogenic-like effects) and metabolic alterations induced by chronic dexamethasone administration in mice. Thus, in order to evaluate the preventative effects of EE, mice were reared in this condition prior to the dexamethasone exposure.
Methods
Animals
Experiments were carried out on 3-month-old male Swiss albino mice from the animal facility of Universidade Federal de Santa Catarina (UFSC, Florianópolis, Brazil), weighing around 45–55 g at the time of testing. They were kept in groups of 10 animals per cage in a room under controlled temperature (23 ± 1 °C) and subjected to a 12-h light cycle (lights on 6:00 a.m.) with free access to food and water. All procedures comply with the guidelines on animal care of the local Ethics Committee on the Use of Animals (protocol 5497310718).
Drug administration
The synthetic glucocorticoid agonist dexamethasone (Laboratório Teuto, Brazil) was dissolved in saline and administered intraperitoneally (i.p.) at a dose of 4 mg/kg of body weight for 21 consecutive days. The dose of dexamethasone was chosen based on the study by Skupio et al. (Citation2015), which demonstrated that chronic dexamethasone administration induces both a range of depressive-like symptoms, such as behavior despair, weight loss, and anxiety-like behavior. The control group received saline injection (0.1 mL per 10 grams of body weight). The treatments occurred between 7:00 and 9:00 a.m.
Housing conditions
The standard environment (SE) mice were housed in wire-topped clear plastic cages (38 cm × 32 cm × 17 cm). The EE mice were housed in larger plastic boxes (44 cm × 32 cm × 18 cm), containing a variety of stimuli, e.g. a running wheel, plastic tubing, ladders, rubber balls and a wood shelter, as previously established in our laboratory (de Souza et al., Citation2019a). These items were moved to different spatial locations of the cage every 3–4 days. The animals were maintained 24 h per day in groups of 10 animals for both SE and EE conditions until the beginning and throughout the duration of the behavioral experiments. Although in several studies the mice designated to the EE cohort were housed in larger groups to allow more social interactions, we kept the same number of animals per cage, as in previous studies of our research group (de Souza et al., Citation2019a).
Experimental design
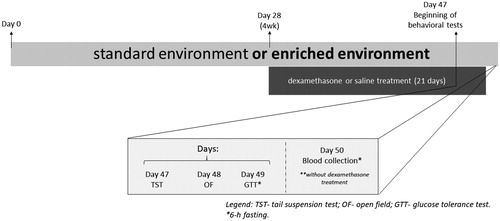
Forty male Swiss adult mice were divided into four experimental groups (10 mice per group): standard environment plus saline (SE Saline), standard environment plus dexamethasone (SE Dexa), environmental enrichment plus saline (EE Saline) and environmental enrichment plus dexamethasone (EE Dexa). Animals were exposed to a 28-days period of SE or EE. After this period, still in the respective environments, the animals received daily saline solution or dexamethasone, as previously described, for 21 consecutive days. Body mass was measured daily. On days 47 and 48, still on the influence of the above-mentioned treatments, the animals were submitted to two behavioral tests: tail suspension test (day 47) and open field test (day 48). One day after the last behavioral test, mice were food-deprived for six hours (starting 7 a.m.) and the glucose tolerance test was performed (day 49). A day after (day 50), mice were again food-deprived for six hours, anesthetized with a mixture of ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and the blood was collected from the heart to determination of plasma triglycerides, cholesterol, and total protein levels. On day 50, no dexamethasone was administered ().
Behavioral tasks
All experiments were conducted during the light phase (1:00–6:00 p.m.). Experiments were conducted in a controlled-temperature room (23 °C, the humidity was between 40% − 60%), with low-light intensity (12 lx). Moreover, they were recorded (webcam Microsoft VX 3000) and the videos were analyzed using the Ethowatcher® software.
Tail suspension test (TST)
The TST measures coping strategy to an acute inescapable stress (Commons, Cholanians, Babb, & Ehlinger, Citation2017). This test was developed by Steru et al. (Citation1985) based on the premise that an animal subjected to a stressful and inescapable situation presents two types of alternating behaviors, the agitation characteristic of the attempt to escape from the stressful situation, and immobility. This pattern of behavior can also be called searching-behavior, characterized by the alternation of intense motor activity and energy expenditure with immobility. Mice both acoustically and visually isolated were suspended 30 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was manually recorded during a 6-min period by an experienced observer. The observer was in the room where experiments were performed and were blind to the animal condition.
Open field test (OFT)
The open field was used to evaluate the locomotor and exploratory activities induced by a novel environment. The natural tendency of the animal in a new environment is to exploit it, despite the stress and conflict caused by the new environment (Prut & Belzung, Citation2003). This test assess unconditioned emotional reactions based on natural approach/avoidance conflict (Ramos, Citation2008). The anxiety-related behavior in the OFT is triggered by two factors: individual testing and agoraphobia. Higher levels of anxiety should mainly lead to decreases in the ratio “number of squares visited in center/number of squares visited on periphery.” The apparatus, made of wood and covered with impermeable Formica, had 50 cm wide × 50 cm deep × 40 cm high. Each mouse was placed in the center of the open field and allowed to freely explore the apparatus for 5 min. The following behavioral parameters were evaluated: % of crosses in the center, total number of crosses and time in the center.
Biochemical analysis
The glucose tolerance test was assessed by i.p. administration of D-(+)-glucose (Sigma Aldrich, St Louis, MO) at a concentration of 2 g/kg body weight. Blood glucose was measured from the tail tip at baseline and 15, 30, 60, and 120 minutes after glucose overload using a glucometer (Accu-Chek Performa®). For the area under the curve (AUC) calculation, it was used the mean baseline value (time 0) for each group (baseline for the area). Total cholesterol, triglycerides, and total proteins were measured in plasma using the enzymatic kit according to the manufacturer’s instructions (Gold Analisa, Belo Horizonte, MG, Brazil). The results were expressed as mg/dL.
Statistical analysis
The results are presented as mean + standard error of the mean (SEM). Statistical analyses were carried out using a two-way or three-way analysis of variance (ANOVA). Following significant ANOVAs, multiple post hoc comparisons were performed using Newman Keul’s test. p values less than .05 (p < .05) were considered as indicative of significance. All tests were performed using the STATISTICA® software package (StatSoft Inc, Tulsa, OK, USA).
Results
Open field test (OFT)
The two-way ANOVA indicated a significant main effect for treatment [F(1, 36) = 5.18, p < .05] and for housing conditions by treatment interaction [F(1, 36) = 6.93, p < .05] on center time (). Subsequent post hoc comparisons revealed a significant decreased in the time spent in the center of the apparatus in the SE Dexa group in comparison with the SE Saline group and with the EE Dexa group (p < .05). On the other hand, the two-way ANOVA indicated no significant effects on the percentage of center crossings () and on the total crossings ().
Figure 2. EE mitigates the dexamethasone-induced anxiety-like behavior in the open field test. (A) Center time (s), (B) Percentage of center crossings, (C) Total crossings. Data are expressed as mean + SEM (n = 10 animals per group). *p < .05 vs. Saline/SE group and &p < .05 vs. Dexa/SE group (two-way analysis of variance followed by Newman–Keuls post-hoc test). Dexa (dexamethasone); SE (standard environment); EE (environmental enrichment).
Tail suspension test (TST)
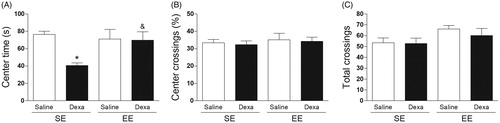
The two-way ANOVA indicated no significant effects on the latency to immobility (). On the other hand, the two-way ANOVA indicated a significant main effect for treatment [F(1, 36) = 8.58, p < .05] and for housing conditions by treatment interaction [F(1, 36) = 9.65, p < .005] on the immobility time in the TST. Subsequent post-hoc analysis revealed a significant increase in the immobility time in the SE Dexa group when compared with the SE Saline group and with the EE Dexa group (p < .05) ().
Figure 3. EE mitigates the dexamethasone-induced behavioral despair in the tail suspension test. (A) Latency to immobility (s) and (B) Immobility time (s). Data are expressed as mean + SEM (n = 10 animals per group). *p < .05 vs. Saline/SE group and &p < .05 vs. Dexa/SE group (two-way analysis of variance followed by Newman–Keuls post-hoc test). Dexa (dexamethasone); SE (standard environment); EE (environmental enrichment).
Metabolic analysis
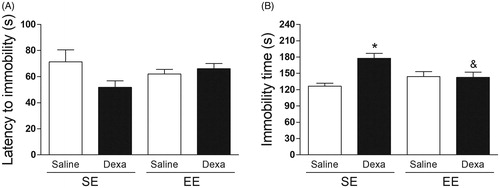
The two-way ANOVA revealed a significant main effect for treatment [F(1, 36) = 27.98, p < .0005] and for housing conditions by treatment interaction [F(1, 36) = 4.06, p < .05] on body mass variation (). Subsequent post-hoc analysis revealed a significant loss of body mass (%) in the SE Dexa group when compared with the SE saline group and with the EE Dexa group (p < .05).
Figure 4. EE effects on dexamethasone-induced metabolic alterations. (A) Body mass variation (%), (B) Plasma cholesterol levels, (C) Plasma triglycerides levels, (D) Plasma total proteins levels, (E) Basal glucose levels, (F) Glucose tolerance test, and (F) Area under curve of GTT. Data are expressed as mean + SEM (n = 10 animals per group). *p < .05 vs. Saline/SE group and &p < .05 vs. Dexa/SE group (two-way analysis of variance followed by Newman–Keuls post-hoc test). £p < .05 treatment factor (two-way analysis of variance followed). #p < .05 environment factor (two-way analysis of variance). Dexa (dexamethasone); SE (standard environment); EE (environmental enrichment).
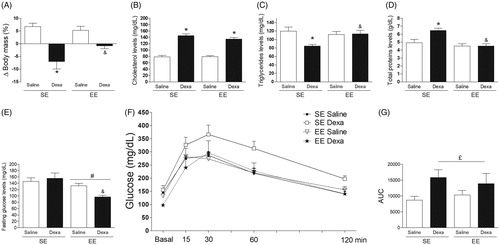
The two-way ANOVA analysis indicated a significant main effect for treatment [F(1, 36) = 153.86, p < .0005] on plasma cholesterol concentration (). Subsequent post-hoc analysis revealed a significant elevation on plasma cholesterol concentrations in both the SE Dexa group and the EE Dexa group when compared with their respective saline control groups (p < .05). On the other hand, the two-way ANOVA indicated a significant main effect for treatment [F(1, 36) = 5.12, p < .05] and for housing conditions by treatment interaction [F(1, 36) = 6.26, p < .05] on plasma triglyceride concentration (). Subsequent post-hoc analysis revealed a significant decrease in triglycerides concentrations in the SE Dexa group when compared with the SE Saline group and with the EE Dexa group (p < .05). Moreover, the two-way ANOVA revealed a significant main effect for treatment [F(1, 36) = 5.85, p < .05] and for housing conditions by treatment interaction [F(1, 36) = 5.61, p < .05] on plasma total protein concentration. Subsequent post-hoc analysis revealed a significant increase in total protein concentration in the SE Dexa group when compared with the SE Saline group and with the EE Dexa group (p < .05) ().
The two-way ANOVA analysis indicated a significant main effect for housing conditions [F(1, 36) = 10.72, p < .005] on basal glucose levels (). Subsequent post-hoc analysis revealed a significant decrease on basal glucose levels specifically in the EE Dexa group when compared with its respective saline control group (p < .05). The three-way ANOVA indicated significant main effects for housing conditions [F(1, 180)= 11.68, p < .001], treatment [F(1, 180)= 4.81, p < .05], time [F(1, 180)= 11.68, p < .0001], and housing conditions by treatment interaction [F(1, 180)= 11.35, p < .0005] (). Subsequent post-hoc comparisons indicated no significant differences among groups at each time point. On the other hand, the two-way ANOVA revealed a significant main effect for treatment [F(1, 36) = 5.81, p < .05] on the area-under-the-glucose-curve (AUC) () that was based on blood glucose values from (.
Discussion
The present study aimed to investigate the effects of EE on dexamethasone-induced behavioral reactivity and metabolic disturbances in mice. Our findings that chronic dexamethasone treatment induces anxiety-like and passive stress-coping behaviors in mice replicate the findings of the study conducted by Skupio et al. (Citation2015), upon which our experimental protocol was based. Regarding the metabolic effects of dexamethasone, mice presented weight loss, decreased plasma triglycerides levels, increased total plasma proteins, and cholesterol levels and glucose intolerance. Of importance, we demonstrated that EE blunted the dexamethasone-induced behavioral reactivity in the TST and OFT, respectively. EE also mitigated dexamethasone-induced weight loss, hypotriglyceridemia, and hyperproteinemia.
Herein, chronic dexamethasone treatment-induced behavioral despair and anxiety-like behavior in mice, as observed in the TST and OFT, respectively. These findings agree with data observed in the literature. There is compelling evidence that dexamethasone administration, in an acute or chronic regimen, at doses varying from 16 µg/kg to 4 mg/kg, induces depressive-like and anxiety-like behavior in mice (de Souza et al., Citation2019b; Skupio et al., Citation2015; Wróbel, Serefko, Wlaź, & Poleszak, Citation2014). Moreover, Pazini et al. (Citation2017) have shown that swiss mice treated with corticosterone, at a dose of 20 mg/kg, for 21 days, showed an increase in the immobility time in the TST. The authors associated such depressive-like effect with decreased hippocampal cell proliferation and neuronal differentiation and increased glial fibrillary acid protein (GFAP) immunostaining (suggestive of astrogliosis) in dentate gyrus (DG) of the hippocampus (Pazini et al., Citation2017). Moreover, Zhang and colleagues have shown that C57Bl/6 mice treated with corticosterone, at a dose of 40 mg/kg, for 35 days showed significantly less time spent in the center zone of the OFT (Zhang et al., Citation2016). The behavioral despair and anxiety-like behavior seen here probably reflect the spectrum of mood disorders observed in patients with hypercortisolemia, as in Cushing’s syndrome (Newell-Price et al., Citation2006) and the common comorbidity of depression and anxiety observed in patients (Wu & Fang, Citation2014). In the present study, it was found that treatment with EE mitigated both behavioral despair and anxiety-like behavior induced by dexamethasone in the TST and OFT, respectively. In this regard, previous studies reported that EE decreases corticosterone concentrations in rodents (Belz et al., Citation2003). In an elegant study, Sztainberg, Kuperman, Tsoory, Lebow, and Chen (Citation2010) demonstrated that EE significantly reduced CRHr-1 mRNA expression in limbic regions, suggesting a molecular mechanism that might explain the EE effects on decreasing corticosterone concentrations: the downregulation of this receptor decreases the release of ACTH by adenohypophysis and, consequently, decreases the release of corticosterone by the adrenal cortex. Furthermore, Hare et al. (Citation2014) showed that four weeks of voluntary wheel running resulted in a shorter time to peak corticosterone concentration and a more rapid decay of plasma corticosterone levels following restrain stress in mice. The authors conclude that exercise renders animals particularly resilient, capable of producing a robust response to stress while limiting the damage that might result from overexposure to glucocorticoids during periods of stress (Hare, Beierle, Toufexis, Hammack, & Falls, Citation2014). Indeed, numerous clinical and experimental studies demonstrated that many environmental factors, such as EE, may affect both the physiological functions of the central nervous system and its ability to counteract pathological changes (van Praag, Kempermann, & Gage, Citation2000). Of note, the changes provided following EE exposure are commonly described as experience-dependent, indicating that they result from active interaction between the animal and the affordances available in the environment (Nithianantharajah & Hannan, Citation2006).
Herein it was observed that chronic dexamethasone treatment induced a significant metabolic dysfunction, characterized by glucose intolerance, decreased body weight and plasma triglyceride levels, as well as increased plasma total protein and cholesterol levels. The increase in plasma total protein concentrations is related to the catabolic properties of glucocorticoids, i.e. increasing protein degradation and decreasing protein synthesis in skeletal muscle (Morgan et al., Citation2016), which may also support the weight loss observed in dexamethasone-treated rodents (Noh et al., Citation2014; Tonolo, Fraser, Connell, & Kenyon, Citation1988). This reduction in body weight during dexamethasone treatment is also related to a negative water balance (Thunhorst, Beltz, & Johnson, Citation2007) and higher levels of anorexigenic insulin levels (Protzek et al., Citation2014). The dexamethasone treatment also induced a significant decrease in plasma triglyceride concentration. However, in this case, our results diverge from prior findings (Barbosa et al., Citation2016). For instance, dexamethasone treatment, at a dose of 0.5 mg/kg for 15 days, resulted in a significant increase in triglyceride levels in rats (Barbosa et al., Citation2016). Although most data have shown that glucocorticoids induce lipolysis and consequently, plasma free fatty acid increases (Divertie, Jensen, & Miles, Citation1991), the effects of glucocorticoids on lipid mobilization are still controversial. For instance, some studies report an inhibitory effect of cortisol on lipolytic activity (Ottosson, Lönnroth, Björntorp, & Edén, Citation2000). Of note Chen et al. (Citation2018) found a significant decrease of triglyceride in plasma and in the liver of goats after intramuscular administration of dexamethasone, at a concentration of 0.2 mg/kg for 21 days. Finally, the significant increase in plasma cholesterol concentration here observed is in accordance with previous studies. For instance, Kumar, Inamdar, Nayeemunnisa, and Viswanatha (Citation2011) showed that dexamethasone administration, at a dose of 10 mg/kg for 8 days, increased the plasma cholesterol levels in rats. Most importantly herein, we demonstrated that EE mitigated the dexamethasone effects on body weight, plasma triglycerides, and total protein levels. EE has a general tendency to increase activity and activity energy expenditure in rodents, which has downstream effects on other aspects of energy balance (Novak, Burghardt, & Levine, Citation2012). In this regard, it is noteworthy that our EE protocol included a running wheel, and since physical exercise is associated with increased protein synthesis (Tipton & Wolfe, Citation2001), one may associate with the maintenance of body mass observed in EE dexamethasone-treated mice. After ∼ 3 weeks in running well, skeletal muscles (gastrocnemius, tibialis anterior, triceps) of male mice show enhanced citrate synthase activity, a validated biomarker for mitochondrial density in skeletal muscle, resulting in better endurance capacity (Manzanares, Brito-da-Silva, & Gandra, Citation2019). Moreover, Barel and colleagues showed that run on a treadmill attenuated the dexamethasone-induces muscular glycogen loss, muscular atrophy and body weight loss in rats (Barel et al., Citation2010).
Glucocorticoids, per se, are considered diabetogenic agents, as they may promote an increase in hepatic glucose production and a decrease in peripheral uptake (e.g. in muscle and adipose tissues) (Pasieka & Rafacho, Citation2016). Because of such properties, up to 60% of patients with Cushing’s syndrome, characterized by hypercortisolemia, have glucose intolerance (Newell-Price et al., Citation2006). The glucose intolerance caused by dexamethasone treatment in our study is in agreement with previous studies (Lee, Choi, Choi, & Nam, Citation2018; Pandey et al., Citation2014). The reduction of insulin-stimulated protein kinase B phosphorylation in skeletal muscle seems to be consensual at this chronic dexamethasone exposure (Pandey et al., Citation2014, Lee et al., Citation2018), which can imply in reduced glucose disposal. Despite to be not effective in preventing the glucose intolerance caused by glucocorticoids, EE significantly improved the basal glycemic values and kept blood glucose levels bellow of that observed in dexamethasone-treated mice in SE during all period of the test. In this regard, McMurphy et al. (Citation2018) observed that EE exposure induces a robust reduction of brown and white adipose tissue, in addition to decreasing hepatic steatosis, hepatic glucose production and increasing glucose uptake by the liver and adipose tissue, contributing to glycemic control in mice. Specifically, the authors demonstrated that EE induced a significant reduction in the expression of two important enzymes involved in the gluconeogenesis pathway, phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (G6PC) (McMurphy et al., Citation2018). The apparent lack of effect of EE upon beta cell function may be due to the genomic impact of dexamethasone on insulin secretory machinery of beta cells (Lambillotte, Gilon, & Henquin, Citation1997). Although we do not discard new approaches of EE aiming to be more effective, we consider EE as a valuable tool for improvement of neurometabolic outcomes as previously demonstrated in a model of glucose intolerance associated to hypercholesterolemic diet where EE mitigated the glucose intolerance (de Souza et al., Citation2019a).
An inevitable limitation of our study is that multiple sensory, social, and physical activity factors are typically combined in the EE treatment, making it difficult to determine which of the various enrichment factors or the interaction contributes to the observed effects on behavioral and metabolic outcomes. In this regard, it is not possible to distinguish between the effects of EE vs exercise alone, since a running wheel was provided. Of note, McMurphy et al. (Citation2018) evaluated the extent to which physical activity is the responsible for the EE-induced healthy aging. In that study, the gene expression profile of the major organs involved in systemic glucose homeostasis (liver, fat and muscle) revealed distinct patterns between EE and running wheel animals, where animals exposed to running wheel showed minor changes in these parameters (McMurphy et al., Citation2018). Thus, it is noteworthy that no social interaction, novelty, or any other single variable can account for all of the effects of EE (van Praag et al., Citation2000).
Conclusion
Collectively, our results reproduce the depressive- and anxiety-like behavior caused by supraphysiologic doses of dexamethasone in Swiss male mice, as well as metabolic disturbances (glucose intolerance, hyperproteinemia, hypercholesterolemia, and body weight loss). Most importantly, EE mitigates the depressive- and anxiety-like behaviors. Moreover, our findings suggest that EE is able to promote healthier metabolic function in mice, even in situations of chronic administration of synthetic glucocorticoids. It is noteworthy, however, that although our results reinforce the positive infuences of EE on stress response, the interpretation of our data should be seen as prophylactic rather than therapeutic since EE started before the dexamethasone treatment. Taken together, our results support the hypothesis that EE enhances resilience to stressors, such as the chronic administration of synthetic glucocorticoids used herein, a strategy that may be translated to the clinical perspective. Both, behavioral and metabolic improvements, are of particularly importance, since the prevalence of metabolic disturbance is 58% higher in psychiatric patients than in the general population, which suggests that metabolic disturbance is a general comorbidity seen in different psychiatric patient groups, including patients with major depressive disorder (Vancampfort et al., Citation2015) and anxiety disorder (Tang, Wang, & Lian, Citation2017).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Eslen Delanogare
Eslen Delanogare PhD candidate in Neuroscience at the Federal University of Santa Catarina. He has obtained his master's degree in Neuroscience at the Federal University of Santa Catarina, in 2019, and has obtained his Bachelor's degree in Psychology at the Regional Integrated University of Upper Uruguay and Missions (URI) in 2017.
Raul Marin de Souza
Raul Marin de Souza He received his Bachelor of Physical Education Degree from the Unisalesiano Lins University (São Paulo, Brazil) in 2015 and subsequently received his master's degree in Neuroscience from the Federal University of Santa Catarina in 2019.
Giovana Karoline Rosa
Giovana Karoline Rosa Undergraduate student in Pharmacy at the Federal University of Santa Catarina. She is currently granted with as scholarship of the scientific initiative of CNPq.
Fernando Garcia Guanabara
Fernando Garcia Guanabara Pharmacist at the University Hospital of the Federal University of Santa Catarina. Currently pursuing a professional master's degree in Pharmacology.
Alex Rafacho
Alex Rafacho PhD in Physiology (2009, Campinas State University, Brazil). Currently he is an assistance professor of Physiology at the Biological Sciences Center of the Federal University of Santa Catarina and the Head of the Laboratory of Investigation in Chronic Diseases - LIDoC. His main research interests include the glucose homeostasis alterations under conditions of insulin resistance and/or glucose intolerance, especially those induced by glucocorticoid excess.
Eduardo Luiz Gasnhar Moreira
Eduardo Luiz Gasnhar Moreira MSc in Pharmacology (2010), PhD in Neuroscience (2013, Federal University of Santa Catarina, Florianopolis, Brazil). Currently he is an assistance professor of Physiology at the Biological Sciences Center of the Federal University of Santa Catarina and the Head of the Neuroscience Coworking Laboratory. His main research interests include the mechanisms underlying Alzheimer's disease and other neurodegenerative disorders, with a specific focus on neuroprotective strategies, such as physical exercise.
References
- Barbosa, A.M., Francisco, P.C., Motta, K., Chagas, T.R., Dos Santos, C., Rafacho, A., & Nunes, E.A. (2016). Fish oil supplementation attenuates changes in plasma lipids caused by dexamethasone treatment in rats. Applied Physiology, Nutrition, and Metabolism, 41, 382–390. doi:10.1139/apnm-2015-0487
- Barel, M., Perez, O.A., Giozzet, V.A., Rafacho, A., Bosqueiro, J.R., & do Amaral, S.L. (2010). Exercise training prevents hyperinsulinemia, muscular glycogen loss and muscle atrophy induced by dexamethasone treatment. European Journal of Applied Physiology, 108, 999–1007. doi:10.1007/s00421-009-1272-6
- Belz, E.E., Kennell, J.S., Czambel, R.K., Rubin, R.T., & Rhodes, M.E. (2003). Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacology, Biochemistry and Behavior., 76, 481–486. doi:10.1016/j.pbb.2003.09.005
- Brown, E.S., Vera, E., Frol, A.B., Woolston, D.J., & Johnson, B. (2007). Effects of chronic prednisone therapy on mood and memory. Journal of Affective Disorders, 99, 279–283. doi:10.1016/j.jad.2006.09.004
- Cao, L., Choi, E.Y., Liu, X., Martin, A., Wang, C., Xu, X., & During, M.J. (2011). White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metabolism, 14, 324–338. doi:10.1016/j.cmet.2011.06.020
- Chen, Q., Niu, L., Hua, C., Geng, Y., Cai, L., Tao, S., … Zhao, R. (2018). Chronic dexamethasone exposure markedly decreased the hepatic triglyceride accumulation in growing goats. General and Comparative Endocrinology, 259, 115–121. doi:10.1016/j.ygcen.2017.11.011
- Chen, Y., & Baram, T.Z. (2016). Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology, 41, 197–206. doi:10.1038/npp.2015.181
- Commons, K.G., Cholanians, A.B., Babb, J.A., & Ehlinger, D.G. (2017). The rodent forced swin test measures stress-coping strategy, not depression-like behavior. ACS Chemical Neuroscience, 8, 955–960. doi:10.1021/acschemneuro.7b00042
- Conti, M., Spulber, S., Raciti, M., & Ceccatelli, S. (2017). Depressive-like phenotype induced by prenatal dexamethasone in mice is reversed by desipramine. Neuropharmacology, 126, 242–249. doi:10.1016/j.neuropharm.2017.09.015
- de Kloet, E.R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6, 463–475. doi:10.1038/nrn1683
- de Souza, R.M., de Souza, L., Machado, A.E., de Bem Alves, A.C., Rodrigues, F.S., Aguiar, A.S., Jr., … Moreira, E.L.G. (2019a). Behavioural, metabolic and neurochemical effects of environmental enrichment in high-fat cholesterol-enriched diet-fed mice. Behavioural Brain Research., 359, 648–656. doi:10.1016/j.bbr.2018.09.022
- de Souza, I.B.M.B., Costa, L.R.F., Tiago, P.R.F., Cagni, F.C., Lima, R.H., Silva Junior, E.D., & Gavioli, E.C. (2019b). Venlafaxine and nortriptyline reverse acute dexamethasone-induced depressive-like behaviors in male and female mice. Experimental and Clinical Psychopharmacology, 27, 433–442. doi:10.1037/pha0000263
- Divertie, G.D., Jensen, M.D., & Miles, J.M. (1991). Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes, 40, 1228–1232. doi:10.2337/diabetes.40.10.1228
- Hare, B.D., Beierle, J.A., Toufexis, D.J., Hammack, S.E., & Falls, W.A. (2014). Exercise-associated changes in the corticosterone response to acute restraint stress: Evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology, 39, 1262–1269. doi:10.1038/npp.2013.329
- Juruena, M.F. (2013). Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy & Behavior, 38, 148–159. doi:10.1016/j.yebeh.2013.10.020
- Keller, J., Gomez, R., Williams, G., Lembke, A., Lazzeroni, L., Murphy, G.M., Jr., & Schatzberg, A.F. (2017). HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Molecular Psychiatry, 22, 527–536. doi:10.1038/mp.2016.120
- Kumar, V.R., Inamdar, M.N., Nayeemunnisa, , & Viswanatha, G.L. (2011). Protective effect of lemongrass oil against dexamethasone induced hyperlipidemia in rats: Possible role of decreased lecithin cholesterol acetyl transferase activity. Asian Pac J Trop Med, 4, 658–660. doi:10.1016/S1995-7645(11)60167-3
- Lambillotte, C., Gilon, P., & Henquin, J.C. (1997). Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. Journal of Clinical Investigation, 99, 414–423. doi:10.1172/JCI119175
- Lee, M.K., Choi, J.W., Choi, Y.H., & Nam, T.J. (2018). Pyropia yezoensis protein supplementation prevents dexamethasone-induced muscle atrophy in C57BL/6 mice. Marine Drugs, 16, 328. doi:10.3390/md16090328
- Lehmann, M.L., & Herkenham, M. (2011). Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. Journal of Neuroscience, 31, 6159–6173. doi:10.1523/JNEUROSCI.0577-11.2011
- Manzanares, G., Brito-da-Silva, G., & Gandra, P.G. (2019). Voluntary wheel running: Patterns and physiological effects in mice. Brazilian Journal of Medical and Biological Research, 52, e7830. doi:10.1590/1414-431x20187830
- McMurphy, T., Huang, W., Queen, N.J., Ali, S., Widstrom, K.J., Liu, X., … Cao, L. (2018). Implementation of environmental enrichment after middle age promotes healthy aging. Aging, 10, 1698–1721. doi:10.18632/aging.101502
- Mitra, R., & Sapolsky, R.M. (2009). Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress, 12, 305–312. doi:10.1080/10253890802379955
- Morgan, S.A., Hassan-Smith, Z.K., Doig, C.L., Sherlock, M., Stewart, P.M., & Lavery, G.G. (2016). Glucocorticoids and 11β-HSD1 are major regulators of intramyocellular protein metabolism. Journal of Endocrinology, 229, 277–286. doi:10.1530/JOE-16-0011
- Newell-Price, J., Bertagna, X., Grossman, A., & Nieman, L. (2006). Cushing’s syndrome. Lancet (London, England), 367, 1605–1617. doi:10.1016/S0140-6736(06)68699-6
- Nithianantharajah, J., & Hannan, A.J. (2006). Enriched environments, experience dependent plasticity and disorders of the nervous system. Nature Reviews Neuroscience, 7, 697–709. doi:10.1038/nrn1970
- Noh, K.K., Chung, K.W., Choi, Y.J., Park, M.H., Jang, E.J., Park, C.H., … Chung, H.Y. (2014). β-Hydroxy β-methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS One., 9, e102947. doi:10.1371/journal.pone.0102947
- Novak, C.M., Burghardt, P.R., & Levine, J.A. (2012). The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neuroscience & Biobehavioral Reviews., 36, 1001–1014. doi:10.1016/j.neubiorev.2011.12.012
- Ottosson, M., Lönnroth, P., Björntorp, P., & Edén, S. (2000). Effects of cortisol and growth hormone on lipolysis in human adipose tissue. Journal of Clinical Endocrinology and Metabolism., 85, 799–803. doi:10.1210/jcem.85.2.6358
- Pandey, J., Maurya, R., Raykhera, R., Srivastava, M.N., Yadav, P.P., & Tamrakar, A.K. (2014). Murraya koenigii (L.) Spreng. ameliorates insulin resistance in dexamethasone-treated mice by enhancing peripheral insulin sensitivity. Journal of the Science of Food and Agriculture, 94, 2282–2288. doi:10.1002/jsfa.6555
- Pasieka, A.M., & Rafacho, A. (2016). Impact of glucocorticoid excess on glucose tolerance: Clinical and preclinical evidence. Metabolites, 6, 24. doi:10.3390/metabo6030024
- Pazini, F.L., Cunha, M.P., Azevedo, D., Rosa, J.M., Colla, A., de Oliveira, J., … Rodrigues, A.L.S. (2017). Creatine prevents corticosterone-induced reduction in hippocampal proliferation and differentiation: Possible implication for its antidepressant effect. Molecular Neurobiology, 54, 6245–6260. doi:10.1007/s12035-016-0148-0
- Protzek, A.O., Costa-Júnior, J.M., Rezende, L.F., Santos, G.J., Araújo, T.G., Vettorazzi, J.F., & Boschero, A.C. (2014). Augmented β-cell function and mass in glucocorticoid-treated rodents are associated with increased islet Ir-β/AKT/mTOR and decreased AMPK/ACC and AS160 signaling. International Journal of Endocrinology, 2014:983453. doi:10.1155/2014/983453
- Prut, L., & Belzung, C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology, 463, 3–33. doi:10.1016/S0014-2999(03)01272-X
- Ramos, A. (2008). Animal models of anxiety: Do I need multiple tests? Trends in Pharmacological Sciences, 29, 493–498. doi:10.1016/j.tips.2008.07.005
- Skupio, U., Tertil, M., Sikora, M., Golda, S., Wawrzczak-Bargiela, A., & Przewlocki, R. (2015). Behavioral and molecular alterations in mice resulting from chronic treatment with dexamethasone: Relevance to depression. Neuroscience, 286, 141–150. doi:10.1016/j.neuroscience.2014.11.035
- Steru, L., Chermat, R., Thierry, B., & Simon, P. (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Psychopharmacology), 85, 367–370. doi:10.1007/BF00428203
- Sztainberg, Y., Kuperman, Y., Tsoory, M., Lebow, M., & Chen, A. (2010). The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Molecular Psychiatry, 15, 905–917. doi:10.1038/mp.2009.151
- Tang, F., Wang, G., & Lian, Y. (2017). Association between anxiety and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Psychoneuroendocrinology, 77, 112–121. doi:10.1016/j.psyneuen.2016.11.025
- Thunhorst, R.L., Beltz, T.G., & Johnson, A.K. (2007). Glucocorticoids increase salt appetite by promoting water and sodium excretion. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293, R1444–R1451. doi:10.1152/ajpregu.00294.2007
- Tipton, K.D., & Wolfe, R.R. (2001). Exercise, protein metabolism, and muscle growth. International Journal of Sport Nutrition and Exercise Metabolism, 11, 109–132. doi:10.1123/ijsnem.11.1.109
- Tonolo, G., Fraser, R., Connell, J.M., & Kenyon, C. (1988). Chronic low-dose infusions of dexamethasone in rats: Effects on blood pressure, body weight and plasma atrial natriuretic peptide. Journal of Hypertension, 6, 25–31.
- van Praag, H., Kempermann, G., & Gage, F.H. (2000). Neural consequences of environmental enrichment. Nature Reviews Neuroscience, 1, 191–198. doi:10.1038/35044558
- Vancampfort, D., Stubbs, B., Mitchell, A.J., De Hert, M., Wampers, M., Ward, P.B., … Correll, C.U. (2015). Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psychiatry, 14, 339–347. doi:10.1002/wps.20252
- Wróbel, A., Serefko, A., Wlaź, P., & Poleszak, E. (2014). The depressogenic-like effect of acute and chronic treatment with dexamethasone and its influence on the activity of antidepressant drugs in the forced swim test in adult mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 54, 243–248. doi:10.1016/j.pnpbp.2014.06.008
- Wu, Z., & Fang, Y. (2014). Comorbidity of depressive and anxiety disorders: Challenges in diagnosis and assessment. Shanghai Archives of Psychiatry, 26, 227–231. doi:10.3969/j.issn.1002-0829.2014.04.006
- Young, D., Lawlor, P.A., Leone, P., Dragunow, M., & During, M.J. (1999). Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature Medicine, 5, 448–453.
- Zhang, K., Yang, J., Wang, F., Pan, X., Liu, J., Wang, L., … Wu, C. (2016). Antidepressant-like effects of Xiaochaihutang in a neuroendocrine mouse model of anxiety/depression. Journal of Ethnopharmacology, 194, 674–683. doi:10.1016/j.jep.2016.10.028