Abstract
It is assumed that the production of cortisol is modulated by light exposure. While initial evidence supports this principal effect, the specific effect of light (intensity and wavelength) onto the cortisol stress response is still not completely understood.
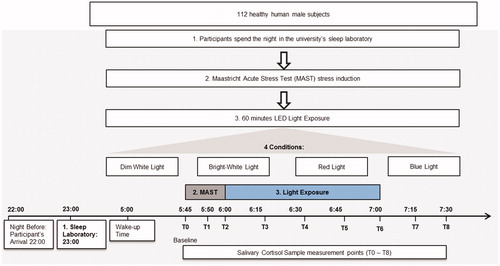
One between-subject experiment was conducted in a standardized sleep laboratory setting to investigate the effect of light intensity (dim white vs. bright white light) and spectral composition (red vs. blue) on the cortisol response after the Maastricht Acute Stress Test (MAST). 112 healthy young males (24.83 ± 4.10 years of age) were randomly assigned to one of the four light conditions. Across conditions, light exposure was conducted for one-hour post-awakening and the light effect was measured based on eight saliva samples.
The analysis indicates significant main effects for time and light condition as well as a significant interaction effect. Notably, bright light exposure evoked the highest cortisol levels when compared to dim white, red, and blue light.
Our findings illustrate the crucial role of light intensity and wavelength for the cortisol stress response, in line with current theoretical knowledge of underlying neurobiological mechanisms.
Effects of different light sources were tested on healthy male adults in the morning after a stress test. Their stress responses showed that a bright light exposure increased the stress hormone level greatest compared to dim white, red or blue light sources. Findings point toward the crucial role of light intensity associated with the hormonal stress response.
LAY SUMMARY
Introduction
Stress generally refers to a situation or a living circumstance in which the body is exposed to an increased load and, depending on the evaluation of the stressor and the resulting stress for the organism, a corresponding adaptation reaction (McEwen, Citation1998) This sets in motion physiological and endocrine processes (Lupien et al., Citation2007), which is influenced by the assessment of the situation, as well as the general state of health (psychobiological stress model by McEwen, Citation2004). According to the evaluation, an adjustment of the homeostatic systems (ph level, body temperature, oxygen content in the blood, etc.) as well as the allostatic system with the activation of the sympathetic nervous system takes place (McEwen, Citation1998).
Hereby, especially for the stress recovery the glucocorticoide (cortisol) play an important role and a relevant influencing factor is the light exposure (Clow et al., Citation2010). Light-induced effects on cortisol secretion could be mediated by retinal projections to the body’s central circadian pacemaker, the hypothalamic suprachiasmatic nucleus (SCN), which, in turn, affects cortisol secretion from the adrenal cortex via different routes (Dickmeis, Citation2009; Ishida et al., Citation2005; Ulrich-Lai et al., Citation2006). Furthermore, light as an influencing factor of the circadian system is detected by a subset of melanopsin-expressing retinal ganglion cells (mRGCs; Berson et al., Citation2002; Brainard et al., Citation2001; Hattar et al., Citation2002, Citation2003; Provencio et al., Citation2002) with a characteristic spectral sensitivity pattern peaking at ∼480 nm, i.e. in the short-wavelength (blue) light spectrum (Berson et al., Citation2002; Dacey et al., Citation2005, 2002; Enezi et al., Citation2011; Hankins et al., Citation2008; Tu et al., Citation2005). Several studies have shown that exposure to light of this wavelength, or to bright light of broader wavelengths, have a stimulatory effect on the hypothalamic-pituitary-adrenal (HPA) axis activity (Hatanaka et al., Citation2008; Ishida et al., Citation2005; Niijima et al., Citation1992), which is dependent on the time of day and the circadian phase (Leproult et al., Citation2001).
Research supported a stimulatory influence of bright light exposure on the cortisol concentration (Petrowski et al., Citation2019). Building on the notion that mRGCs are most sensitive the short-wavelength (blue) light spectrum (∼460nm), it can be assumed that stimulation at this spectral frequency should exert the strongest influence on the cortisol. To the best of our knowledge, only two studies have investigated such effects to date, with mixed results: Comparing blue (470 nm) and red (625 nm) LED light exposure (40 lux) from 8:00–9:00am, no significant differences could be found as compared to dim white light (<3lux; Figueiro & Rea, Citation2010). In contrast, in sleep restricted adolescents a blue/short-wavelength (470 nm) LED light exposure (40 lux) at 6:00 am led to a significantly enhanced cortisol release (Figueiro & Rea, Citation2012). These results could be recently replicated in the same laboratory showing an increased cortisol awakening response after exposure to bright (vs. dim white) light and following blue and green (vs. red) light exposure (Petrowski et al., Citation2019). To our knowledge it has not yet been investigated whether light exposure influences the stress response of a laboratory standardized stress test. Until now, exclusively the basal cortisol concentration was manipulated by the light exposure.
The few available studies on these topics have jointly produced promising results, but differ methodologically, as in different times of a day for light exposure with different pre-light exposure settings and sleep deprivation. Therefore, the same time of the day, without pre-light exposure and controlled sleep as in the previous study was chosen to insure comparability. A between-subject experimental sleep laboratory study was conducted to examine the influence of one hour post-awakening bright white light vs. dim white light exposure on the cortisol reactivity after stress induction. Based on the effect of light on basal cortisol concentration (Petrowski et al., Citation2019) and the clear neurophysiological rationale (Berson et al., Citation2002) one can hypothesize that (i) bright white light exposure exerts the stimulatory effect on the cortisol concentration in addition to the stress induction compared to dim white light, and (ii) the stimulatory light effect for blue/short-wavelength light exposure heightens the cortisol reactivity strongest compared to red light exposure.
Method
Participants
The sample included N = 112 healthy male human adults. A description of sociodemographic characteristics of the participants is provided in (see Supplementary Appendix). Participants were recruited and tested between November 2017 and October 2018 at the German Sport University Cologne (GSU). They were recruited through an online advert published on the GSU website. Participation was compensated with 50 Euros per night. Prior to participation, individuals completed an online health assessment with respect to defined exclusion criteria (such as chronic illness and/or other influencing factors of the HPA axis). The study protocol was approved by the Ethics Committee of the Medical Faculty of the Technical University of Dresden, Germany (No #EK353092014) and in accordance with the Declaration of Helsinki (1964). All participants provided written informed consent prior to their participation.
Table 1. Sociodemographic information.
Procedure
The study used a between-subject experimental design in a standardized sleep laboratory setting with the independent variable being the light condition (bright white, dim white, blue or red light). HRV and cortisol data were collected as central biological outcome measures throughout the testing period.
Participants were advised via email to refrain from alcohol and any strenuous physical activity/exercise on the day prior to the testing nights and subsequent morning. Upon arrival, participants completed the provided questionnaires, were tested for color vision, fitted with the motion sensor and introduced to the testing procedure for the following day. The participants went to bed in a completely dimmed room at 23:00 and were woken up the next morning at 5:00. The sleep quality as well as earlier wake up was monitored by actigraphy. Participants were instructed to wear dark sunglasses when using the rest rooms in the night and after wake up to prevent light exposure effects. The Maastricht Acute Stress Test (MAST) – lasting 15 min – was used to induce physical and psychological stress at 5:45, followed by 60 min of light exposure. Directly before, during and after the MAST the first three saliva samples were taken and followed by six further samples taken every 15 min until 7:30. Baseline measures for HRV were taken right before the MAST. The different conditions of the light exposure were randomized over the time of testing in order to avoid seasonal effects. During the light exposure period, five saliva samples were collected (T2-T6). In the remaining 30 min after light exposure, two additional saliva samples were taken. Testing was completed by 7:30 (see ).
Light exposure
Instruments used for light exposure were two half Ulbricht spheres that were indirectly illuminated through LEDs positioned equally on the inside around the opening. LEDs were covered with a spectral selective diffusor to ensure a homogeneous illumination of participants’ retina. The LEDs were precisely regulated by Computer (USB to DMX Controller) and powered by electrical DC-dimming. Four light exposure settings were presented. shows light settings and intensities that were used as well as the luminance in the sphere and the illuminance at the eye. The light exposure consisted of the following lighting conditions: narrow-band LEDs, blue (201 lux; peak wavelength 470–480 nm); red (235 lux; peak wavelength 635 nm) as well as control condition bright white light (1240 lux; mix of blue, green and red); and dim white light (<2 lux; mix of blue, green and red). The two targeted light conditions (blue and red) were set up in such a way that each targeted condition used the same number of photons, resulting in the above mentioned illuminances at the subject’s eye. Standardization by photon density, instead of standardization by illuminance (lux), was chosen since the present study aimed to target the non-visual circadian system/mRGCs rather than the visual system. Hence, in order to investigate whether mRGCs respond differentially to light of different wavelengths and consequently induce a differential influence, it was deemed critical to standardize light conditions by the unit that actually mediates the photo-biological response at the receptor level, i.e. photon density (see also Brainard et al., Citation2001). This differentiates from luminance perception, which is a phenomenon which is dependent on the spectral sensitivity of the visual system and is thus of less interest in this context.
Table 2. Light exposure characteristics.
The light exposure took place in a fully darkened room with stray light levels below 1 lux. Illumination was measured at eyelevel before and after light exposure using an illumination meter (Pocket Lux 2 of the Lichtmesstechnik GmbH Berlin). Participants were positioned in a chair in front of the light source with their chin resting on a chinrest so that their faces were reaching into a half sphere (2PI-Geometrie). Thus the eyelevel was equal for each participant. The light exposure consisted of the following lighting conditions: LEDs, blue (201 lux; peak wavelength 470–480nm); red (235 lux; peak wavelength 635 nm); bright white light (1240 lux; mix of blue, green and red); dim white light (<2 lux; mix of blue, green and red).
MAST
The Maastricht Acute Stress Test (MAST) was used to elicit autonomic and glucocorticoid stress responses, thus activating the HPA axis. The MAST combines physical stress (cold-induced pain) with unpredictability, uncontrollability and social evaluation. The standard MAST procedure developed by Smeets et al. (Citation2012) was implemented with minor alterations to account for the study’s light conditions and unwanted light exposure. In the 5 min preparation phase, participants were informed of the task, followed by the 10 min acute stress phase in which participants would alternate between submerging their non-dominant hand in ice-water (2–8○C; measured with Harbor Digital Thermometer model: HCP1) and the mental arithmetic task that requires participants to count backwards from a random 4-digit number in steps of an odd 2-digit number as fast and accurately as possible. During the tasks, participants experience social-evaluative pressure through negative feed and videotaping (using an ELP web camera with infrared led for day and night, with a Megapixel HD resolution up to 1080 × 720P; ELP-USB100W05MT-DL36).
Cortisol sampling
The saliva samples were obtained using Salivette swabs (Sarstedt, Nümbrecht, Germany). The samples were kept frozen at −20 °C until assay. Before analysis, the samples were centrifuged at 3000 rpm for five minutes to produce a clear supernatant of low viscosity. 50 μl were removed for cortisol analysis using a commercially available immunoassay with chemi-luminescence detection. The lower detection limit of this assay is 0.43 nmol/l. The intra- and inter-assay coefficients of variation were below 8% for low (3 nmol/l) and high (25 nmol/l) cortisol levels, respectively.
Statistical analysis
All statistical analyses were performed using SPSS for Windows, version 23 (IBM, Chicago, Illinois). First, cortisol data were cleaned: Outlying values of more than three standard deviations above or below the mean were deleted in each group. The resulting fifteen missing values were then multiply imputed for a total of 11 participants. Basing the analysis on a sample gained by listwise deletion instead, does not substantially change the results. In initial analyses, differences in baseline cortisol before and during MAST (T0 and T1) values between conditions were examined using ANOVA. For the main analyses, we calculated a 4 (light condition: dim white, bright white, red, blue) x 8 (measurement points T1 to T8 as described in procedure) mixed-measures ANOVA. We further calculated the area under the cortisol curve with respect to increase (AUCI) and ground (AUCG) using the formula described in Pruessner et al. (Citation2003).
Results
Baseline cortisol levels did not differ between light conditions for either T0, F(3, 107) = 0.378, p = .769, η2p = .010, or T1, F(3, 107) = 0.466, p = .706, η2p = .013. However, there was a significant increase in cortisol from T0 to T1 for all conditions as a result of the MAST, F(1, 107) = 14.43, p < .001, η2p = .119. The main effect for light condition, F(4, 107) = 0.93, p = .451, η2p = .033, and the interaction effect, F(4, 107) = 0.88, p = .478, η2p = .032, were not significant. In addition, we controlled participants’ chronotype using the MEQ: There were no meaningful differences between the light conditions, F(3, 86) = 0.58, p = .632, η2 = .021.
In the main analysis, a mixed-measures ANOVA revealed a significant main effect of light exposure condition (bright white vs. dim white vs. red vs. blue light) on overall cortisol levels, F(3, 107) = 3.564, p = .017, η2p = .091. Across measurement points, the bright light condition was associated with the highest cortisol levels (see for descriptive statistics). Apart from an effect of time, F(2.99, 320.22) = 91.446, p < .001, η2p = .461, there was also an interaction effect of measurement point and light exposure condition, F(8.98, 320.22) = 2.739, p = .004, η2p = .071. illustrates this effect, which was characterized by a more pronounced increase in cortisol levels in the bright white light condition. Analyses for individual measurement points revealed significant differences in cortisol levels between light exposure conditions on measurement points T2 to T6 (p < .05, adjusted for multiple comparisons), but not for the remaining four points. For measurement points T2 and T3, all light conditions were significantly lower than bright white light. For the measurement points T4 to T6 red as well as dim white light was significantly lower than bright white light. There was a significant effect for AUCG, F(3, 107) = 4.025, p = .009, η2p = .101, but not for AUCI, F(3, 107) = 1.797, p = .152, η2p = .048.
Figure 2. Mean (± SE) salivary cortisol levels across measurement points for dim white, bright white, blue and red light conditions.
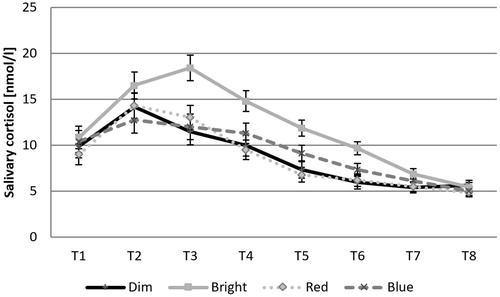
Table 3. Means and standard deviations for cortisol levels across measurement points by light condition.
Discussion
In one between-subject experimental sleep laboratory studies the principal influence of 1 h post-awakening bright light vs. dim white light exposure on the cortisol reactivity after stress induction as well as the specifically enhanced stimulatory effect (mRGCs) of blue and an attenuated effect for red light on the cortisol reactivity was investigated.
To our knowledge, the present study is the first study investigating the light effect on stress induced cortisol reactivity applying a highly standardized method and procedure. To proof the high standardization, baseline values were not significant between the conditions, stress induction successfully activated cortisol release in all conditions and chronotype variation was statistically controlled. As hypothesized the present results showed that bright white light led to a significant increase in cortisol reactivity after the stress induction compared to dim white light (i). In contrast to our (ii) hypotheses there are only higher descriptive cortisol values in the blue light compared to the red light condition. In particular, the cortisol level in the blue condition elevated for longer and decreased later compared to red light and dim white light (see ). Based on our current results we do not find evidence for hypothesis (ii). In addition the red light led to significant blunted cortisol reactivity after the stress induction compared to bright white light.
Therefore, the present results are in contrast to the literature: In sleep restricted adolescents a blue/short-wavelength (470 nm) LED light exposure at 6:00 led to a significantly enhanced cortisol reaction (Figueiro & Rea, Citation2012). These results could be recently replicated in the same laboratory showing an increased cortisol awakening response after exposure at 5:00 to blue (vs. red) light exposure (Petrowski et al., Citation2019). However, the non-effect of light exposure at 8:00–9:00 by Figueiro and Rea (Citation2010) in normal adults without sleep deprivation could not be replicated.
Concerning the underlying neurobiological mechanisms, light-induced effects on cortisol secretion are assumed to be mediated by retinal projections to the body’s central circadian pacemaker, the hypothalamic suprachiasmatic nucleus (SCN), which, in turn, affects cortisol secretion from the adrenal cortex via different routes (Dickmeis, Citation2009; Ishida et al., Citation2005; Ulrich-Lai et al., Citation2006). The light is detected by a subset of melanopsin-expressing retinal ganglion cells (mRGCs; Berson et al., Citation2002; Brainard et al., Citation2001; Hattar et al., Citation2002, Citation2003; Provencio et al., Citation2002) with a characteristic spectral sensitivity pattern light spectrum. In order to test the sensitivity pattern of the ipRGCs, the light exposure was chosen with narrow-band illumination (blue, peak wavelength 470–480 nm, in order to specifically stimulate ipRGC). As a control, red was presented with the same “number of photons”, whereby red does not stimulate ipRGC. Red showed no effect because this radiation does not stimulate ipRGC.
White light contains all the spectral components (blue, green, red and everything else) and thus should show an effect, but the magnitude of the effect is still unknown. In the present study, the white light with higher number of photons is also perceived as brighter by the visual system. Brighter for the visual system meant more cortisol stimulation based on the present data and is in line with hypothesis (i) comparing bright white against dim white light. More cortisol stimulation due to brighter light might be explicable by an expected interaction with the visual receptors of the eye. The visual receptors are also coupled to ipRGC and thus probably also involved in the effect. The magnitude of this effect one can currently only speculate. Besides the interactions between ipRGC and the visual system (i.e. the blue radiation will not have a cortisol effect alone), there might be thresholds. In a future study, it has to be investigated how exactly the interaction between all the receptors looks like and where the threshold is located. Since in the brightness the stimulation of the ipRGC is not taken into account (lux on the eye would therefore not be the correct size), the brightness alone (evaluation of the visual system) might not a suitable predictor of the effect in a future study since interactions are expected.
Furthermore, several studies (Berson et al., Citation2002; Dacey et al., Citation2005; Enezi et al., Citation2011; Hankins et al., Citation2008; Tu et al., Citation2005) have shown that exposure to light of a certain wavelength (∼480 nm, such as the short-wavelength blue or bright white light of broader wavelengths), has a stimulatory effect of these cells and therefore on the HPA axis activity (Hatanaka et al., Citation2008; Ishida et al., Citation2005; Niijima et al., Citation1992). The present results demonstrate exclusively the stimulating effects of bright white light of broader wavelengths of these cells and therefore on the HPA axis activity (see , ). However, the stimulatory effect of the short wavelength/blue light did not reach the level of significance (against proposed hypothesis ii), but the red light exposure showed the expected attenuating effect on the cortisol reactivity. Hereby it is still unclear whether the mRGCs get inhibited or that the mRGCs receive synaptic input from visual photoreceptors (chiefly cones) which might also be important for the detection of light information of circadian systems (e.g. Hattar et al., Citation2003; Lall et al., Citation2010; Tähkämö et al., Citation2019).
The present study has strengths and limitations which need to be considered. Strengths include the high level of standardization of the test protocol, such as sleep in the laboratory, standardized wake-up times, and standardized stress induction. The sleep laboratory setting also allowed us to control for non-experimental light sources; i.e. before experimental light exposure, the participants were in a completely dimmed room (<2 lux) which, amongst other factors, minimizes the possibility of seasonal effects. The specifically manufactured half spheres allowed a highly accurate light setting identical for each participant.
Limitations of the present study include the fact that the present study design did not include an acclimatization night for participants. This could have led to influences on the cortisol awakening response, e.g. due to novelty effects during the first night. However, it is unlikely that this induced any systematic influences on our results since there is not an increased error variance and it was present in every participant. Hence, future research may be well-advised to include an acclimatization night in the sleep laboratory, if possible. Finally, besides the influences of circadian rhythmicity, the dosage of light might further be investigated to better understand dose-response relationships and to elucidate whether a point of receptor saturation might be reached when even more intense light stimulation leads to no additional effect on the cortisol concentration. Furthermore, the melatonin concentration at night and in the morning was not measured as an additional reference. Therefore, in future studies the melatonin concentration should be specified the night before and in the morning.
The present findings have some implications for future research. First, the principal finding that light levels influence cortisol reactivity provides further support for the previously raised notion that ambient light levels need to be considered as a potential source of confounding in cortisol research (Stalder et al., Citation2016). Second, the knowledge arising from the present study that differential light exposure provides an effective way to manipulate expression of the cortisol concentration could be used in future experimental research seeking to investigate the effects of the stress induced cortisol reactivity later during the day, e.g. on cognitive functioning.
Supplementary_LED_Specification_and_layout.docx
Download MS Word (295.9 KB)Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
Notes on contributors
Katja Petrowski
Prof. Dr. Katja Petrowski is a full professor for Medical Psychology and Medical Sociology at University Medicine, Johannes Gutenberg University Mainz in Germany. Her research interests include anxiety disorders, psychotherapy, stress, psychophysiology, psychoendocrinology and psychoimmunology. She is a licensed cognitive and behavioral psychologist and conducted several multi-center psychotherapy studies for depression and anxiety disorders.
Stefan Buehrer
Stefan Buehrer M.Sc. is a doctoral student, researcher and teacher for Medical Psychology and Medical Sociology at the University Medical Center of the Johannes Gutenberg University Mainz, Germany. His main research interests include resilience, stress, the assessment of mental and physical health, and transferability into practice utilizing new technologies such as virtual reality.
Mathias Niedling
Dr. Mathias Niedling is head of the Research Institute for Automotive Lighting and Mechatronics – L-LAB in Lippstadt. His research interests include all topics on lighting technology and the influence of light on visual and non-visual effects on humans. The focus of previous research activities was on glare from street and automotive lighting and on the effects of lighting on the circadian rhythm. Besides he works as a lecturer for lighting technology.
Bjarne Schmalbach
Dr. Bjarne Schmalbach is a postdoctoral researcher at the University Medical Center of the Johannes Gutenberg University Mainz, Germany. His main research interests include structural equation models, psychometrics, survey methodology, and the assessment of mental health.
References
- Berson, D. M., Dunn, F. A., & Takao, M. (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science, 295(5557), 1070–1073. https://doi.org/https://doi.org/10.1126/science.1067262
- Brainard, G. C., Hanifin, J. P., Greeson, J. M., Byrne, B., Glickman, G., Gerner, E., & Rollag, M. D. (2001). Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. The Journal of Neuroscience, 21(16), 6405–6412. https://doi.org/https://doi.org/10.1523/JNEUROSCI.21-16-06405.2001
- Clow, A., Hucklebridge, F., Stalder, T., Evans, P., & Thorn, L. (2010). The cortisol awakening response: More than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews, 35(1), 97–103. https://doi.org/https://doi.org/10.1016/j.neubiorev.2009.12.011
- Dacey, D. M., Liao, H.-W., Peterson, B. B., Robinson, F. R., Smith, V. C., Pokorny, J., Yau, K.-W., & Gamlin, P. D. (2005). Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature, 433(7027), 749–754. https://doi.org/https://doi.org/10.1038/nature03387
- Dickmeis, T. (2009). Glucocorticoids and the circadian clock. Journal of Endocrinology, 200(1), 3–22.
- Enezi, J. a., Revell, V., Brown, T., Wynne, J., Schlangen, L., & Lucas, R. (2011). Melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. Journal of Biological Rhythms, 26(4), 314–323. https://doi.org/https://doi.org/10.1177/0748730411409719
- Figueiro, M. G., & Rea, M. S. (2010). Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro Endocrinology Letters, 31(1), 92–96.
- Figueiro, M. G., & Rea, M. S. (2012). Short-wavelength light enhances cortisol awakening response in sleep-restricted adolescents. International Journal of Endocrinology, 16, 301935.
- Hankins, M. W., Peirson, S. N., & Foster, R. G. (2008). Melanopsin: An exciting photopigment. Trends in Neurosciences, 31(1), 27–36. https://doi.org/https://doi.org/10.1016/j.tins.2007.11.002
- Hatanaka, M., Tanida, M., Shintani, N., Isojima, Y., Kawaguchi, C., Hashimoto, H., Kakuda, M., Haba, R., Nagai, K., & Baba, A. (2008). Lack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice. Neuroscience Letters, 444(2), 153–156. doi:https://doi.org/10.1016/j.neulet.2008.08.030
- Hattar, S. (2002). Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science, 295(5557), 1065–1070. https://doi.org/https://doi.org/10.1126/science.1069609
- Hattar, S., Lucas, R. J., Mrosovsky, N., Thompson, S., Douglas, R. H., Hankins, M. W., Lem, J., Biel, M., Hofmann, F., Foster, R. G., & Yau, K.-W. (2003). Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature, 424(6944), 75–81. https://doi.org/https://doi.org/10.1038/nature01761
- Ishida, A., Mutoh, T., Ueyama, T., Bando, H., Masubuchi, S., Nakahara, D., Tsujimoto, G., & Okamura, H. (2005). Light activates the adrenal gland: Timing of gen expression and glucocorticoid release. Cell Metabolism, 2(5), 297–307. https://doi.org/https://doi.org/10.1016/j.cmet.2005.09.009
- Lall, G. S., Revell, V. L., Momiji, H., Al Enezi, J., Altimus, C. M., Güler, A. D., … Lucas, R. J. (2010). Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron, 66(3), 417–428. https://doi.org/10.1016/j.neuron.2010.04.037
- Leproult, R., Colecchia, E. F., L'Hermite-Balériaux, M., & Van Cauter, E. (2001). Transition from dim to bright light in the morning induces an immediate elevation of cortisol levels. The Journal of Clinical Endocrinology and Metabolism, 86(1), 151–157. https://doi.org/https://doi.org/10.1210/jcem.86.1.7102
- Lupien, S. J., Maheu, F., Tu, M., Fiocco, A., & Schramek, T. E. (2007). The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition, 65(3), 209–237. https://doi.org/https://doi.org/10.1016/j.bandc.2007.02.007
- McEwen, B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. https://doi.org/https://doi.org/10.1111/j.1749-6632.1998.tb09546.x
- McEwen, B. S. (2004). Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032(1), 1–7. https://doi.org/https://doi.org/10.1196/annals.1314.001
- Niijima, A., Nagai, K., Nagai, N., & Nakagawa, H. (1992). Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus eliminate these changes in rats. Journal of the Autonomic Nervous System, 40(2), 155–160. https://doi.org/https://doi.org/10.1016/0165-1838(92)90026-D
- Petrowski, K., Schmalbach, B., Niedling, M., & Stalder, T. (2019). The effects of post-awakening light exposure on the cortisol awakening response in healthy male individuals. Psychoneuroendocrinology, 108, 28–34. https://doi.org/https://doi.org/10.1016/J.PSYNEUEN.2019.05.016
- Provencio, I., Rollag, M. D., & Castrucci, A. M. (2002). Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature, 415(6871), 493–493. https://doi.org/https://doi.org/10.1038/415493a
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/https://doi.org/10.1016/S0306-4530(02)00108-7
- Smeets, T., Cornelisse, S., Quaedflieg, C. W., Meyer, T., Jelicic, M., & Merckelbach, H. (2012). Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology, 37(12), 1998–2008. https://doi.org/https://doi.org/10.1016/j.psyneuen.2012.04.012
- Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wüst, S., Dockray, S., Smyth, N., Evans, P., Hellhammer, D. H., Miller, R., Wetherell, M. A., Lupien, S. J., & Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. https://doi.org/https://doi.org/10.1016/j.psyneuen.2015.10.010
- Tähkämö, L., Partonen, T., & Pesonen, A. K. (2019). Systematic review of light exposure impact on human circadian rhythm. Chronobiology International, 36(2), 151–170. https://doi.org/10.1080/07420528.2018.1527773
- Tu, B. P., Kudlicki, A., Rowicka, M., & McKnight, S. L. (2005). Logic of the yeast metabolic cycle: Temporal compartmentalization of cellular processes. Science, 310(5751), 1152–1158. https://doi.org/https://doi.org/10.1126/science.1120499
- Ulrich-Lai, Y. M., Arnold, M. M., & Engeland, W. C. (2006). Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 290, 1128–1135.