Abstract
In a number of adult tissues, Nestin-positive stem cells/progenitors have been identified and shown to be involved in maintenance and remodeling. Various studies have shown that under stressful conditions, quiescent Nestin-positive progenitor cells are activated. Thereby, they migrate to their target location and differentiate into mature cells. In the current paper, we discuss if Nestin-positive progenitors in the hippocampus and adrenal gland belong to unique cell populations that are responsive to stress. Furthermore, we speculate about the mechanism behind their activation and the clinical importance of this stress-response.
Stress is a state of altered homeostasis, and the endocrine stress system involves communication among different tissues, including among others, the brain and components of the hypothalamic-pituitary-adrenal (HPA) axis. Dysregulation of the stress system can lead to morphologic changes in target organs or epigenetic stem cell changes (Berger et al., Citation2019). These in turn might contribute to the development or exacerbation of various psychiatric disorders.
The adrenal, as part of both the HPA-axis and the sympatho-adrenomedullary system, is an essential regulator of the stress response. The adrenal cortex originates from cells of the celomic epithelium, which at an early stage of development, condense between the urogenital ridge and the dorsal mesentery and form the adrenogonadal primordium. Shortly later during organogenesis, cells of the neural crest (NC) migrate into the fetal adrenal, where they become precursors of the adrenal medulla (Lerario et al., Citation2017; Pignatti et al., Citation2017). The NC represents a migratory population of multipotent cells, transiently present during embryogenesis, which give rise to a diverse variety of differentiated cell types (Butti et al., Citation2014). In addition to neurons and glial cells of the peripheral nervous system, melanocytes and some endocrine derivatives, the NC has the striking capacity of producing a large diversity of mesenchymal cell types in the head and neck (Dupin et al., Citation2018). When late neural crest cells migrate toward the dorsal root ganglion, they acquire a Schwann cell precursor (SCP) fate. SCPs are multipotent progenitors that in addition to mature Schwann cells generate various outcomes (Aquino & Sierra, Citation2018; Lousado et al., Citation2017; Petersen & Adameyko, Citation2017). In the adrenal medulla, more than 50% of the catecholamine-producing chromaffin cells are derived from SOX10-positive SCPs, which have migrated along the nerves toward the medulla primordium (Furlan et al., Citation2017). In addition to chromaffin cells, the postnatal adrenal medulla is composed of neurons stimulating the catecholamine production. Cells of glial nature that express S100B, glial fibrillary acidic protein (GFAP), and Vimentin are found in proximity to both chromaffin cells and nerve terminations, but their function has not been clarified yet (Suzuki & Kachi, Citation1995). A part of these glial cells, corresponding to around 10% of the total adrenomedullary cells, co-expresses Nestin (Rubin de Celis et al., Citation2015). The cytoskeletal type VI intermediate filament Nestin was originally identified as a marker of neural stem and progenitor cells (Cattaneo & McKay, Citation1990; Lendahl et al., Citation1990), but has later been shown to be expressed in a variety of tissues and stem cells or progenitors (Bernal & Arranz, Citation2018). Though the adrenal cortex is not NC-derived, 1–2% glial-like Nestin-positive cells are found in this tissue (Steenblock et al., Citation2017).
Neurons are generated from early embryonic development until early postnatal stages, with only a few neurogenic zones remaining active in the adult (Paridaen & Huttner, Citation2014). In the adult human brain, neurogenesis is restricted to two main niches: the sub-ventricular zone (SVZ) of the lateral ventricles and the sub-granular zone (SGZ) of the dentate gyrus (DG) in the hippocampus. Neural stem cells (NSCs) residing in the SVZ dgenerate neurons that migrate to the olfactory bulb, while in the hippocampal SGZ, radial glia-like NSCs give rise to new dentate granule neurons. These hippocampal neurons contribute to memory and cognitive functions, as well as to the processing of emotions and the regulation of stress responses (Cameron & Schoenfeld, Citation2018; Dranovsky & Leonardo, Citation2012). In addition to their neural fate, in order to constrain and/or prevent tissue damage, NSCs from both SVZ and SGZ may turn into both astroglial and oligodendroglial cells, which means a rather gliogenic than neurogenic response (Butti et al., Citation2014).
In this paper, we will focus on the stem cells in the hippocampal SGZ and in the adrenal cortex and medulla. Especially, due to their known involvement in stress responses, we will discuss the role of Nestin-positive cell populations in these tissues.
Nestin-positive stem cells/progenitors
Stem and progenitor cell populations are required for the successful homeostasis and adaptation of most tissues. Progenitor cells are early descendants of stem cells that can differentiate to form one or more types of cells meaning that progenitor cells lie between stem cells and mature, functional cells in the body. Generally, stem and progenitor cells are in an inactive/quiescent form. Eukaryotic cells can respond to certain environmental cues by arresting the cell cycle and entering a reversible state of quiescence. Quiescent cells do not divide, but can reenter the cell cycle and resume proliferation if exposed to specific signals from the environment. Quiescent cells in mammals and humans include adult stem cells. These cells often exhibit improved stress resistance and enhanced survival ability (Mohammad et al., Citation2019). They can be activated by growth factors or cytokines originating from their niches. Upon activation, particular progenitor cells not only differentiate into their specialized cells types but also migrate to the target locations of the tissue. In order to form the fully functional endocrine stress system, external and internal stressors influence the process of cell differentiation of stem and progenitor cells in the HPA axis (Mariniello et al., Citation2019).
SCPs express markers like SOX10, GAP43 and BLBP (Jessen & Mirsky, Citation2019), but also Nestin might be a potential marker for these multipotent progenitors (Bernal & Arranz, Citation2018). Nestin is related to essential stem cell functions including self-renewal/proliferation, differentiation and migration (Bernal & Arranz, Citation2018), yet is it still unclear exactly how Nestin influences stemness. Intermediate filaments such as Nestin are expressed in a cell type specific manner during development. Its expression is usually transient and does not persist into adulthood. Upon differentiation, Nestin becomes downregulated and is replaced by tissue-specific intermediate filament proteins. During neuro- and gliogenesis, Nestin is replaced by cell type-specific intermediate filaments such as neurofilaments and GFAP. Nestin-positive cells are able to differentiate into cells with very different functions. For example, in testis, Nestin-positive cells differentiate into steroid producing cells (Jiang et al., Citation2014). Similarly, in the murine adrenal cortex glial-like Nestin-positive progenitor cells are able to differentiate into steroid-producing cells (Steenblock et al., Citation2018). However, steroidogenesis in these adrenocortical cells is very slow, and mainly Gli1- and Shh-positive stem cells/progenitors are responsive for homeostasis and regeneration in the adult adrenal cortex under normal circumstances (Lerario et al., Citation2017). In the murine adrenal medulla, a Nestin-positive progenitor population distinct from the adrenocortical Nestin-positive population (Steenblock et al., Citation2018), is able to differentiate into chromaffin, neurons and glial cells (Rubin de Celis et al., Citation2015). In humans, Nestin also marks cells with stem-like properties in both the adrenal medulla (Poli et al., Citation2019; Santana et al., Citation2012) and cortex (Toti et al., Citation2005). In addition, cancer stem cells in the adrenal medulla are occasionally Nestin-positive (Scriba et al., Citation2020). Furthermore, Nestin marks mesenchymal stem cells, which are able to differentiate into cells of the osteoblastic, adipocytic, and chondrocytic lineages (Bernal & Arranz, Citation2018).
In the adult human hippocampus, a small amount of Nestin-positive cells lacking significant proliferative activity has been observed in the SVZ, DG and fimbrio-dentate-junction (Cipriani et al., Citation2018). These quiescent Nestin-positive cells probably belong to different subtypes of radial glial cells as they show a variable degree of expression and co-labeling with GFAP (Cipriani et al., Citation2018).
Nestin-positive progenitors in stress
Stress is a state of altered homeostasis, and the ability of an individual to cope with this homeostatic challenge relies on activation of the neuroendocrine hypothalamic-pituitary-adrenal (HPA) axis and the sympatho-adrenal system. Interactions between these two systems play a central role in adaptation. When the HPA axis is activated corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) are secreted from the paraventricular nucleus of the hypothalamus. CRH and AVP then activate the anterior pituitary to secrete adrenocorticotropic hormone, which in turn stimulates the adrenal cortex to produce glucocorticoids (Berger et al., Citation2019). Glucocorticoid receptors (GR) are expressed in several areas of the adult brain such as the hypothalamus, pituitary, hippocampus and amygdala, which are all involved in glucocorticoid feedback. Excessive or chronic activation of the endocrine stress axis, which also leads to a disrupted feedback loop, triggers and contributes to numerous psychopathologies in humans, including anxiety, post-traumatic stress disorder, depression and schizophrenia. Patients with stress-related disorders exhibit increased vulnerability to mental illness, even years after the stress experience. Furthermore, there is increasing evidence that prenatal and early life stress may influence health and wellbeing in adulthood (Agorastos et al., Citation2019). This might be due to long-term changes generated in the brain’s architecture and function. Long-term behavioral consequences of early life stress may be due to interference with hippocampal development, in particular with assembly of the DG. A common thread in these disorders appears to be alterations in cognitive processes, such as learning and memory, main functions of the hippocampus. Indeed, it has been shown that the hippocampus is highly susceptible to stress. There are different views on how stress affects hippocampal functions. The prevailing view is that glucocorticoids produced in the adrenal cortex activate type II GR in the hippocampus, thereby altering learning and memory. Another hypothesis is that alterations in the hippocampal function after stress are due to an excessive activity exerted by the amygdala and the prefrontal cortex (Kim et al., Citation2015). Stress-induced lower hippocampal volumes have been shown to be more apparent in males than in females, suggesting a sex-dependent effect (Cameron & Schoenfeld, Citation2018).
We and others have shown that Nestin-positive progenitor cells play an important role in stress. In a recent review, Parfejevs et al. (Citation2018) discuss the role of adult NC-derived cells in injury and stress. The authors report that a growing body of evidence suggests that quiescent SCPs with NC stem cell features are maintained into adulthood and that these cells can be recruited when needed. This implies that these cells are rather stress-responsive than resistant to stress. Furthermore, after stress and injury certain NC-derived cells achieve a stem cell-like state with the capacity to generate new cell types during repair processes, presumably by reprograming of differentiated cells such as mature Schwann cells (Parfejevs et al., Citation2018). Recently, Youssef et al. (Citation2019) showed that in mice, early life stress led to a delay in DG development and diminishing of the Nestin-expressing adult stem cell pool without altering cell proliferation and neurogenesis in young mice. In adult mice, on the other hand, which were subjected to chronic restraint stress, Jung et al. (Citation2020) showed that autophagic death of NSCs resulted in suppressed neurogenesis in hippocampal Nestin-positive cells. Similar results were observed in adult rats, where stress or glucocorticoid administration promoted oligodendrogenesis but decreased neurogenesis in hippocampal Nestin-positive cells (Chetty et al., Citation2014). Conversely, acute, mild stress has been shown to induce hippocampal neurogenesis, which in turn is required for a proper stress response (Snyder et al., Citation2011).
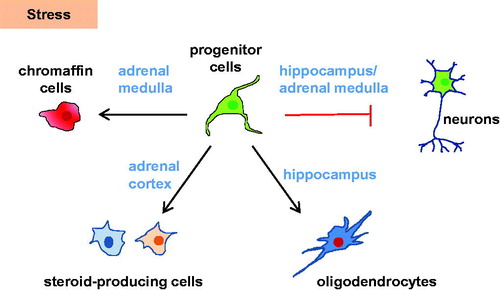
In the adrenal, two separate Nestin-positive progenitor populations co-expressing S100B and GFAP have been identified; one in the adrenal cortex, and one in the medulla (Steenblock et al., Citation2017). Despite the distinct origin and hormone production profiles, the communication between the cortex and medulla is important for the proper function of the gland and for its adaptation to stress (Bornstein et al., Citation2019). Our work on the adrenal cortex suggests that under normal homeostasis the adrenocortical Nestin-positive progenitor population is quiescent (Steenblock et al., Citation2018). However, in a model of restraint stress, we showed that adrenocortical Nestin-positive cells migrate centripetally from the adrenal capsule to the cortex-medulla boundary. Furthermore, they differentiate into steroidogenic cells (Steenblock et al., Citation2018). Similarly, this stress model led to enhanced differentiation of adrenomedullary Nestin-positive progenitors into chromaffin cells, whereas differentiation into neurons or glial cells was decreased or not affected (Rubin de Celis et al., Citation2015).
These results show that both in the hippocampus and in the adrenal glial-like stress-responsive Nestin-positive progenitor cell populations are present. Under normal conditions, they seem to be quiescent or slowly contributing to organ homeostasis. However, in these tissues the distinct subtypes of Nestin-positive cell populations are able to adapt to stressful situations by entering the cell cycle leading to differentiation into mature lineages (). How exactly stress affects Nestin-positive stem/progenitor cells is still elusive. One way could be through GR signaling, which has previously been shown to be the case in NSCs though the results on the influence on neurogenesis have been opposing (Koutmani & Karalis, Citation2015). Another way could be through CRH receptor signaling. Proliferating neuronal progenitors express higher levels of CRH receptors and are enhanced in the human fetal brain (Koutmani et al., Citation2013). Moreover, CRH-deficient mice show reduced proliferation and increased apoptosis among neural progenitors (Koutmani et al., Citation2013). GR signaling has been linked to the Hippo pathway as activation of GRs results in elevated YAP protein levels and subsequently to enhanced transcriptional activity (Sorrentino et al., Citation2017). In addition, upstream influence of the canonical Wnt/β-catenin signaling on the Hippo pathway has been described (Azzolin et al., Citation2012). Furthermore, under stress the Hippo pathway might be impacted through neuropeptides and neurotransmitters binding to G protein coupled receptors (Stepan et al., Citation2018). In the hippocampus, the Wnt/β-catenin and Shh signaling pathways play a fundamental role in adult neurogenesis (Tayyab et al., Citation2018), whereas the Hippo and Notch signaling pathways are important for Schwann cell development (Jessen & Mirsky, Citation2019). The mentioned pathways are known to be involved in stem cell regulation in the adrenal as well (Finco et al., Citation2019), but if this is also the case in Nestin-positive progenitors still needs to be elucidated.
Figure 1. Nestin-positive cells in the brain-HPA axis and their role in stress. Under normal conditions, Nestin-positive stem cell/progenitor populations in the hippocampus and adrenal are quiescent. Under stress, differentiation into chromaffin cells (adrenal medulla), steroid-producing cells (adrenal cortex), or oligodendrocytes (hippocampus) is enhanced. Conversely, neurogenesis in the hippocampus and adrenal medulla is decreased under stress.
Conclusion
Progenitor and stem cell populations are required for successful homeostasis and adaptation in most tissues. Stem cells are dynamically regulated by signals originating from their niches, helping to regulate their appropriate proliferation and differentiation. The effects of stress on stem/progenitor cells from a variety of tissues during the early stages of postnatal development may predispose to adult disease (Bornstein et al., Citation2019). Stress seems to “prime” the brain-HPA axis and increasing evidence suggests that this is happening at least partly in the stem/progenitor cells of the hippocampus thereby leading to morphological changes. However, also in the adult adrenal stress-responsive stem cells are present, which suggests that cells can be recruited when needed and that such stress-responsive cells may serve to limit stress responses. This might also explain the promotion to oligodendrogenesis instead of neurogenesis in hippocampal Nestin-positive cells under stress. Altering stem/progenitor cell fate might be a way to adapt to environmental challenges by buffering the response to ongoing stressors. This might be helpful for regaining homeostasis after acute stress. However, in chronic stress such changes might lead to long-term consequences in the form of mental disorders, thereby showing the clinical importance of such stress-responsive stem cells. Furthermore, it has to be considered that medications influencing GRs or beta-adrenergic receptors might lead to epigenetic changes in the stress-responsive stem cells thereby contributing to an increased risk of developing mental disorders.
A number of studies have shown that stress-responsive stem cells are often Nestin-positive. The remaining question is, if Nestin-positive progenitors are generally responsive to stress or just in organs related to stress including the hippocampus, the HPA axis, and the adrenal medulla. Studying stress-responsive progenitor cell populations may be an essential task for the advancement of our understanding of stress and its effects on the endocrine system, neuroendocrine regulation and brain plasticity.
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
Notes on contributors
Stefan R. Bornstein
Professor Stefan Richard Bornstein is Chair and Director of the Department of Internal Medicine of the University Clinic Carl Gustav Carus Dresden and Chair of Endocrinology and Diabetes as well as TransCampus Dean at King’s College London, UK. Prof. Bornstein is member of the German Academy of Science, has been collaborating with several Nobel Laureates and published over 600 peer reviewed publications and book chapters. He received the Medal of Honor of Germany (Bundesverdienstkreuz 1. Klasse). Moreover, he is an Honorary Professor at different universities in Europe, United States and Asia.
Ilona Berger
Ilona Berger obtained her master's degree in Biology from the University of Regensburg in Germany in 2017. She is currently a Ph.D. student under the supervision of Prof. Stefan R. Bornstein and Dr. Charlotte Steenblock at TU Dresden. Her research is centered on stem cell regulating signaling pathways and their signature during stress in the HPA axis.
Charlotte Steenblock
Dr.Charlotte Steenblock finished her PhD in Molecular Biology from Aarhus University and Novozymes A/S in Denmark in 2003. Since 2016 she has been working at the University Clinic Carl Gustav Carus Dresden as a principal investigator. Her research is centered on stem cells of the HPA axis and their role in stress and metabolic diseases.
References
- Agorastos, A., Pervanidou, P., Chrousos, G. P., & Baker, D. G. (2019). Developmental trajectories of early life stress and trauma: A narrative review on neurobiological aspects beyond stress system dysregulation. Frontiers in Psychiatry, 10, 118. https://doi.org/https://doi.org/10.3389/fpsyt.2019.00118
- Aquino, J. B., & Sierra, R. (2018). Schwann cell precursors in health and disease. Glia, 66(3), 465–476. https://doi.org/https://doi.org/10.1002/glia.23262
- Azzolin, L., Zanconato, F., Bresolin, S., Forcato, M., Basso, G., Bicciato, S., Cordenonsi, M., & Piccolo, S. (2012). Role of TAZ as mediator of Wnt signaling. Cell, 151(7), 1443–1456. https://doi.org/https://doi.org/10.1016/j.cell.2012.11.027
- Berger, I., Werdermann, M., Bornstein, S. R., & Steenblock, C. (2019). The adrenal gland in stress – adaptation on a cellular level. The Journal of Steroid Biochemistry and Molecular Biology, 190, 198–206. https://doi.org/https://doi.org/10.1016/j.jsbmb.2019.04.006
- Bernal, A., & Arranz, L. (2018). Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cellular and Molecular Life Sciences : CMLS, 75(12), 2177–2195. https://doi.org/https://doi.org/10.1007/s00018-018-2794-z
- Bornstein, S. R., Berger, I., Scriba, L., Santambrogio, A., & Steenblock, C. (2019). Adrenal cortex–medulla interactions in adaptation to stress and disease. Current Opinion in Endocrine and Metabolic Research, 8, 9–14. https://doi.org/https://doi.org/10.1016/j.coemr.2019.06.005
- Bornstein, S. R., Steenblock, C., Chrousos, G. P., Schally, A. V., Beuschlein, F., Kline, G., Krone, N. P., Licinio, J., Wong, M. L., Ullmann, E., Ruiz-Babot, G., Boehm, B. O., Behrens, A., Brennand, A., Santambrogio, A., Berger, I., Werdermann, M., Sancho, R., Linkermann, A., … Andoniadou, C. L. (2019). Stress-inducible-stem cells: A new view on endocrine, metabolic and mental disease? Molecular Psychiatry, 24(1), 2–9. https://doi.org/https://doi.org/10.1038/s41380-018-0244-9
- Butti, E., Cusimano, M., Bacigaluppi, M., & Martino, G. (2014). Neurogenic and non-neurogenic functions of endogenous neural stem cells. Frontiers in Neuroscience, 8, 92. https://doi.org/https://doi.org/10.3389/fnins.2014.00092
- Cameron, H. A., & Schoenfeld, T. J. (2018). Behavioral and structural adaptations to stress. Frontiers in Neuroendocrinology, 49, 106–113. https://doi.org/https://doi.org/10.1016/j.yfrne.2018.02.002
- Cattaneo, E., & McKay, R. (1990). Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature, 347(6295), 762–765. https://doi.org/https://doi.org/10.1038/347762a0
- Chetty, S., Friedman, A. R., Taravosh-Lahn, K., Kirby, E. D., Mirescu, C., Guo, F., Krupik, D., Nicholas, A., Geraghty, A., Krishnamurthy, A., Tsai, M. K., Covarrubias, D., Wong, A., Francis, D., Sapolsky, R. M., Palmer, T. D., Pleasure, D., & Kaufer, D. (2014). Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Molecular Psychiatry, 19(12), 1275–1283. https://doi.org/https://doi.org/10.1038/mp.2013.190
- Cipriani, S., Ferrer, I., Aronica, E., Kovacs, G. G., Verney, C., Nardelli, J., Khung, S., Delezoide, A. L., Milenkovic, I., Rasika, S., Manivet, P., Benifla, J. L., Deriot, N., Gressens, P., & Adle-Biassette, H. (2018). Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer’s disease adults. Cerebral Cortex, 28(7), 2458–2478. https://doi.org/https://doi.org/10.1093/cercor/bhy096
- Dranovsky, A., & Leonardo, E. D. (2012). Is there a role for young hippocampal neurons in adaptation to stress? Behavioural Brain Research, 227(2), 371–375. https://doi.org/https://doi.org/10.1016/j.bbr.2011.05.007
- Dupin, E., Calloni, G. W., Coelho-Aguiar, J. M., & Le Douarin, N. M. (2018). The issue of the multipotency of the neural crest cells. Developmental Biology, 444 (Suppl 1), S47–S59. https://doi.org/https://doi.org/10.1016/j.ydbio.2018.03.024
- Finco, I., Mohan, D. R., Hammer, G. D., & Lerario, A. M. (2019). Regulation of stem and progenitor cells in the adrenal cortex. Current Opinion in Endocrine and Metabolic Research, 8, 66–71. https://doi.org/https://doi.org/10.1016/j.coemr.2019.07.009
- Furlan, A., Dyachuk, V., Kastriti, M. E., Calvo-Enrique, L., Abdo, H., Hadjab, S., Chontorotzea, T., Akkuratova, N., Usoskin, D., Kamenev, D., Petersen, J., Sunadome, K., Memic, F., Marklund, U., Fried, K., Topilko, P., Lallemend, F., Kharchenko, P. V., Ernfors, P., & Adameyko, I. (2017). Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science, 357(6346), eaal3753. https://doi.org/https://doi.org/10.1126/science.aal3753
- Jessen, K. R., & Mirsky, R. (2019). Schwann cell precursors; Multipotent glial cells in embryonic nerves. Frontiers in Molecular Neuroscience, 12, 69. https://doi.org/https://doi.org/10.3389/fnmol.2019.00069
- Jiang, M. H., Cai, B., Tuo, Y., Wang, J., Zang, Z. J., Tu, X., Gao, Y., Su, Z., Li, W., Li, G., Zhang, M., Jiao, J., Wan, Z., Deng, C., Lahn, B. T., & Xiang, A. P. (2014). Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Research, 24(12), 1466–1485. https://doi.org/https://doi.org/10.1038/cr.2014.149
- Jung, S., Choe, S., Woo, H., Jeong, H., An, H. K., Moon, H., Ryu, H. Y., Yeo, B. K., Lee, Y. W., Choi, H., Mun, J. Y., Sun, W., Choe, H. K., Kim, E. K., & Yu, S. W. (2020). Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy, 16(3), 512–530. https://doi.org/https://doi.org/10.1080/15548627.2019.1630222
- Kim, E. J., Pellman, B., & Kim, J. J. (2015). Stress effects on the hippocampus: A critical review. Learning & Memory, 22(9), 411–416. https://doi.org/https://doi.org/10.1101/lm.037291.114
- Koutmani, Y., & Karalis, K. P. (2015). Neural stem cells respond to stress hormones: Distinguishing beneficial from detrimental stress. Frontiers in Physiology, 6, 77. https://doi.org/https://doi.org/10.3389/fphys.2015.00077
- Koutmani, Y., Politis, P. K., Elkouris, M., Agrogiannis, G., Kemerli, M., Patsouris, E., Remboutsika, E., & Karalis, K. P. (2013). Corticotropin-releasing hormone exerts direct effects on neuronal progenitor cells: Implications for neuroprotection. Molecular Psychiatry, 18(3), 300–307. https://doi.org/https://doi.org/10.1038/mp.2012.198
- Lendahl, U., Zimmerman, L. B., & McKay, R. D. (1990). CNS stem cells express a new class of intermediate filament protein. Cell, 60(4), 585–595. https://doi.org/https://doi.org/10.1016/0092-8674(90)90662-X
- Lerario, A. M., Finco, I., LaPensee, C., & Hammer, G. D. (2017). Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Frontiers in Endocrinology, 8, 52. https://doi.org/https://doi.org/10.3389/fendo.2017.00052
- Lousado, L., Prazeres, P., Andreotti, J. P., Paiva, A. E., Azevedo, P. O., Santos, G. S. P., Filev, R., Mintz, A., & Birbrair, A. (2017). Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death & Disease, 8(10), e3072. https://doi.org/https://doi.org/10.1038/cddis.2017.456
- Mariniello, K., Ruiz-Babot, G., McGaugh, E. C., Nicholson, J. G., Gualtieri, A., Gaston-Massuet, C., Nostro, M. C., & Guasti, L. (2019). Stem cells, self-renewal, and lineage commitment in the endocrine system. Frontiers in Endocrinology, 10, 772. https://doi.org/https://doi.org/10.3389/fendo.2019.00772
- Mohammad, K., Dakik, P., Medkour, Y., Mitrofanova, D., & Titorenko, V. I. (2019). Quiescence entry, maintenance, and exit in adult stem cells. International Journal of Molecular Sciences, 20, 2158.
- Parfejevs, V., Antunes, A. T., & Sommer, L. (2018). Injury and stress responses of adult neural crest-derived cells. Developmental Biology, 444, S356–S365. https://doi.org/https://doi.org/10.1016/j.ydbio.2018.05.011
- Paridaen, J. T., & Huttner, W. B. (2014). Neurogenesis during development of the vertebrate central nervous system. EMBO Reports, 15(4), 351–364. https://doi.org/https://doi.org/10.1002/embr.201438447
- Petersen, J., & Adameyko, I. (2017). Nerve-associated neural crest: Peripheral glial cells generate multiple fates in the body. Current Opinion in Genetics & Development, 45, 10–14. https://doi.org/https://doi.org/10.1016/j.gde.2017.02.006
- Pignatti, E., Leng, S., Carlone, D. L., & Breault, D. T. (2017). Regulation of zonation and homeostasis in the adrenal cortex. Molecular and Cellular Endocrinology, 441, 146–155. https://doi.org/https://doi.org/10.1016/j.mce.2016.09.003
- Poli, G., Sarchielli, E., Guasti, D., Benvenuti, S., Ballerini, L., Mazzanti, B., Armignacco, R., Cantini, G., Lulli, M., Chortis, V., Arlt, W., Romagnoli, P., Vannelli, G. B., Mannelli, M., & Luconi, M. (2019). Human fetal adrenal cells retain age-related stem- and endocrine-differentiation potential in culture. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 33(2), 2263–2277. https://doi.org/https://doi.org/10.1096/fj.201801028RR
- Rubin de Celis, M. F., Garcia-Martin, R., Wittig, D., Valencia, G. D., Enikolopov, G., Funk, R. H., Chavakis, T., Bornstein, S. R., Androutsellis-Theotokis, A., & Ehrhart-Bornstein, M. (2015). Multipotent glia-like stem cells mediate stress adaptation. Stem Cells (Dayton, Ohio), 33(6), 2037–2051. https://doi.org/https://doi.org/10.1002/stem.2002
- Santana, M. M., Chung, K. F., Vukicevic, V., Rosmaninho-Salgado, J., Kanczkowski, W., Cortez, V., Hackmann, K., Bastos, C. A., Mota, A., Schrock, E., Bornstein, S. R., Cavadas, C., & Ehrhart-Bornstein, M. (2012). Isolation, characterization, and differentiation of progenitor cells from human adult adrenal medulla. Stem Cells Translational Medicine, 1(11), 783–791. https://doi.org/https://doi.org/10.5966/sctm.2012-0022
- Scriba, L. D., Bornstein, S. R., Santambrogio, A., Mueller, G., Huebner, A., Hauer, J., Schedl, A., Wielockx, B., Eisenhofer, G., Andoniadou, C. L., & Steenblock, C. (2020). Cancer stem cells in pheochromocytoma and paraganglioma. Frontiers in Endocrinology, 11, 79. https://doi.org/https://doi.org/10.3389/fendo.2020.00079
- Snyder, J. S., Soumier, A., Brewer, M., Pickel, J., & Cameron, H. A. (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature, 476(7361), 458–461. https://doi.org/https://doi.org/10.1038/nature10287
- Sorrentino, G., Ruggeri, N., Zannini, A., Ingallina, E., Bertolio, R., Marotta, C., Neri, C., Cappuzzello, E., Forcato, M., Rosato, A., Mano, M., Bicciato, S., & Del Sal, G. (2017). Glucocorticoid receptor signalling activates YAP in breast cancer. Nature Communications, 8, 14073. https://doi.org/https://doi.org/10.1038/ncomms14073
- Steenblock, C., Rubin de Celis, M. F., Androutsellis-Theotokis, A., Sue, M., Delgadillo Silva, L. F., Eisenhofer, G., Andoniadou, C. L., & Bornstein, S. R. (2017). Adrenal cortical and chromaffin stem cells: Is there a common progeny related to stress adaptation? Molecular and Cellular Endocrinology, 441, 156–163. https://doi.org/https://doi.org/10.1016/j.mce.2016.09.011
- Steenblock, C., Rubin de Celis, M. F., Delgadillo Silva, L. F., Pawolski, V., Brennand, A., Werdermann, M., Berger, I., Santambrogio, A., Peitzsch, M., Andoniadou, C. L., Schally, A. V., & Bornstein, S. R. (2018). Isolation and characterization of adrenocortical progenitors involved in the adaptation to stress. Proceedings of the National Academy of Sciences of the United States of America, 115(51), 12997–13002. https://doi.org/https://doi.org/10.1073/pnas.1814072115
- Stepan, J., Anderzhanova, E., & Gassen, N. C. (2018). Hippo signaling: Emerging pathway in stress-related psychiatric disorders? Frontiers in Psychiatry, 9, 715. https://doi.org/https://doi.org/10.3389/fpsyt.2018.00715
- Suzuki, T., & Kachi, T. (1995). Immunohistochemical studies on supporting cells in the adrenal medulla and pineal gland of adult rat, especially on S-100 protein, glial fibrillary acidic protein and vimentin. Kaibogaku Zasshi. Journal of Anatomy, 70(2), 130–139.
- Tayyab, M., Shahi, M. H., Farheen, S., Mariyath, M. P. M., Khanam, N., Castresana, J. S., & Hossain, M. M. (2018). Sonic hedgehog, Wnt, and brain-derived neurotrophic factor cell signaling pathway crosstalk: Potential therapy for depression. Journal of Neuroscience Research, 96(1), 53–62. https://doi.org/https://doi.org/10.1002/jnr.24104
- Toti, P., Regoli, M., Nesi, G., Occhini, R., Bartolommei, S., Fonzi, L., & Bertelli, E. (2005). Nestin expression in normal adrenal gland and adrenocortical tumors. Histology and Histopathology, 20(4), 1115–1120. https://doi.org/https://doi.org/10.14670/HH-20.1115
- Youssef, M., Atsak, P., Cardenas, J., Kosmidis, S., Leonardo, E. D., & Dranovsky, A. (2019). Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Scientific Reports, 9(1), 4120. https://doi.org/https://doi.org/10.1038/s41598-019-40868-0
