Abstract
The aim of this study was to investigate the behavioral, immunological, and neurological effects of long-term isolation in an animal model. Male C3H/eB mice wereraised in either social isolation or standard conditions for 6 weeks. At 10 weeks, each group was further divided into 3 sets. (A) Physical strength and behavior were evaluated with the grip strength, hot plate, staircase, and elevated plus-maze tests. Natural-killer cell activity and lymphocyte proliferation were measured. (B) Half the animals were subjected to electric shock with 3 reminders, and freezing time was evaluated at each reminder. Cortisone levels were evaluated after 16 weeks. (C)Mice were injected with 38 C-13 B lymphoma cells and followed for tumor size and survival. Strength evaluation yielded asignificantly lower body weight and grip strength in the socially isolated mice. Behavioral test results were similar in the two groups. The pattern of reactions to stress conditioning differed significantly, with the socially isolated mice showing an incline in freezing with each successive reminder, and the control mice showing a decline. The socially isolated mice had significantly attenuated tumor growth, with no significant difference in survival from control mice. There were no significant between-group differences in immunological parameters. In conclusion, social isolation serves as a model for chronic stress. It was associated with significant changes in stress conditioning reaction, resembling symptoms of post-traumatic stress disorder, and attenuated tumor development. No differences from controls were found in behavior tests, immune parameters, or survival after tumor cell inoculation.
This article explores biological and behavioral consequences of social isolation in a mice model. Our results show that social isolation leads to changes in the Hypothalamic-hypophyseal-adrenal axis, which in turn alter the response to stress. Additionally, social isolation was shown to impact tumor progression.
Lay summary
1. Introduction
Stress is a constellation of events, comprised of precipitating factors, the stressor, stress perception, andstress response. Acute stress, occurring at a single day and lasting minutes to hours, promotesshort-term physiological, immunological and neurological adaptation and stability. Chronic stress, which lasts several hours every day for a period of weeks or monthsmay have deleterious consequences and is recognized as a risk factor for many diseases and psychiatric disorders (Dhabhar, Citation2014; Dudek, Citation2019; Dhabhar, Citation2009).
Social isolation was found to be a form of chronic psychosocial stress in both animals and humans. Human studies showed an increased risk for mental illness, including depression, anxiety and substance abuse (Chou et al., Citation2011). Additionally, social isolation in humans was shown to predict mortality fromcancer (Fleisch Marcus et al., Citation2017). Immunological changes in socially isolated cancer patients include increased inflammatory burden (Yang et al., Citation2014) and a shift in macrophage differentiation to a prometastatic profile (Bower, Citation2018).Another immunologicaleffect found in socially isolated individuals is the lack of leukocyte distributional response to endogenous glucocorticoids (Cole, Citation2008).In animal models, social isolation is associated with various neurological, endocrinological and immunological alterations. Social isolation results in hyperactivity of the hypothalamus-pituitary-adrenal (HPA) axis, demonstrated by increased adrenocorticotropic hormone (ACTH) and corticosterone levels (Griffin et al., Citation2014), and alterations in pro- and anti-inflammatory cytokinelevels (Corsi-Zuelli et al., Citation2018). Additionally, social isolation modulates tumor growth (Hermes et al., Citation2009).Studies associated these modulations with changes in immunefunction in socially isolated animals. Budiu et. al found social isolation to be associated with a decline in survival of mammary adenocarcinoma bearing mice, a declinethat correlated with the decrease in splenic CD8 cells and activated T-cells (Budiu et al., Citation2017). Wu et al attributed the increase in angiogenesis in colon carcinoma-bearing socially isolated mice to increased TNF-alpha levels (Wu et al., Citation1999).
Immunology and immunotherapy of lymphoma has been one of our main research fields for many years. We have published many studies in this field, including studies on the effects of enriched environment and stress conditioning on lymphoma growth and lymphoma immunotherapy.One may also view social isolation as an impoverished environment. Previous studies in our lab have shown that an enriched environment increased natural killer cells activityand improved B cell lymphoma survival (Benaroya-Milshtein et al., Citation2011). Thus, we would expect opposite results in environmental impoverishment.
Post-Traumatic Stress Disorder is of the most characterized reactions to environmental stress. Its psychological features are accompanied by neuroendocrine changes, one of which is decreased reactivity of the hypothalamic-pituitary-adrenal axis (Sherin & Nemeroff, Citation2011) Etiologically, PTSD may be explained by the’two hit hypothesis’, according to which the first hit occurs inearly life and disrupts normal brain developmental and maturational processes, and a second hit,usually an environmental stressor leads to the onset of the mental disorder (Lim et al., Citation2012). Pynoos et al. demonstrated the applicability of the ‘two hit hypothesis’ to PTSD in an animal model (Pynoos et al., Citation1996). In a previous study, our group used the stress paradigm, in whichelectric shock with reminders were used as a model of post-traumatic stress (Benaroya-Milshtein et al., Citation2004; Pynoos et al., Citation1996).
The aim of the present study was to further explore theimpact of social isolation on physiological, behavioral, and immunological parameters and cancer progression.Lymphocyte proliferation in response to mitogens is a widely used method to assess the overall immune competence. It has been part of the diagnostic immunologic repertoire assessment (in immunodeficiency for instance) for many years. Similarly, NK cell activity was chosen by us as a parameter of the overall (non-antigen specific) cytotoxic potential of the immune system.
In addition, we investigated the interaction between chronic psychosocial stress (social isolation) and acute stress(the stress paradigm) on physiological, behavioral and immunological parameters. We hypothesizedthat social isolation in our model will have three major consequences:
Behavioral and physiological changes, including altered response to a second stressor;
Immunological changes in natural killer (NK)cell activity and lymphocyte proliferation;
Poorer prognosis of B-cell lymphoma.
2. Materials and methods
2.1. Animals and rearing conditions
Four-week-old C3H/eB male mice were obtained from the animal facility of Tel Aviv University, Israel.Experiments were conducted under permit number M 02-152, authorized by the Animal Care Committee of the Sackler Faculty of Medicine. The experiments were carried out on mice in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with the European Convention for the Protection of Animals Used forExperimental and Other Scientific Purposes.The minimal possible number of animals was used, and all efforts were made to minimize their suffering. Throughout the experiment, the animals were maintained on a 12-h-on:12-h-off lighting schedule (lights on at 06.00 h) in a room thermostatically maintained at 22 ± 1 °C. Food and water were available ad libitum. Control mice were housed in standard plastic cages (35 × 22 × 15 cm3); the housing conditions conformed to the European Economic Community directive 86/609. Isolated mice were housed in standard plastic cages (30 × 20 × 15 cm3), one mouse in each cage. Weight was measured before randomization and was similar in the study and control groups.
2.2. Experimentaldesign
The experimental design and timeline are portrayed in their entirety in . The study and control groups were each further divided into three sets to test the effects of social isolation on different parameters.
Animals from the age of 4 weeks were reared in social isolation or a standard (control) environment. At age 10 weeks, weight was measured, and behavioral tests were performed. At age 15 weeks, immunological tests were performed. The number of mice used for these experiments were 9-10 mice, depending on the group and specific experiment. See for detailed information.
Animals from the age of 4 weeks were reared in social isolation or a standard environment. At age 10 weeks, half the animals in each rearing condition underwent a stress paradigm. At age 16 weeks, all animals were evaluated for biochemical measures of stress. The number of mice used for these experiments were 5-10 mice, depending on the group and specific experiment. See and for detailed information.
Animals were reared in social isolation or a standard environment. We applied the experimental model of murine 38C-13 B-cell lymphoma in our previous studies (Benaroya-Milshtein et al., Citation2007; Haimovich et al., Citation1999) to a C3H/eB male mouse model.At age 10 weeks, tumor cells were injected, and the animals were followed for tumor progression (n = 10 for both isolated and control groups).
Figure 1. Experimental design - timeline and interventions. Timeline for experimental design showing age (weeks) of animals in each stage. At age 4 weeks, mice were divided into two groups: rearing in social isolation and rearing in a standard environment. Each group was then divided into three sets. Set A: At 10 weeks, weight was measured and behavioraltests were performed. At 15 weeks, immunological tests were performed. Set B: At 10 weeks, half the study and control animals were subjected to a stress paradigm with reminders. At 16 weeks, corticosterone levels were measured in all animals (exposed/not exposed to the stress paradigm). Set C: Tumor was injected at 10 weeks of age and animals were followed for tumor growth and survival. W, week; NK, natural killer.
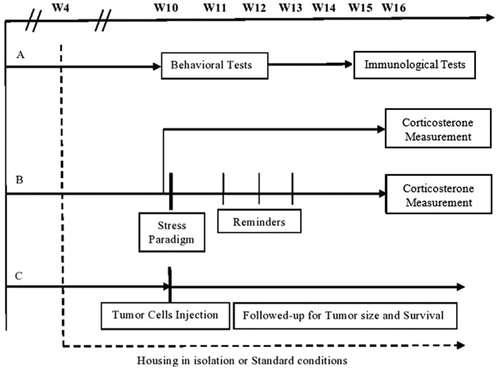
Figure 2. Serum corticosterone levels in isolated and control mice either exposed or not exposed to the stress paradigm. Mice reared in social isolation or standard environment for 12 weeks were exposed or not to a stress paradigm at 10 weeks of age. Levels of corticosterone were measured at 16 weeks. The stress paradigm resulted in a significant increase in corticosterone levels in control mice but not in socially isolated mice. Values are presented as mean ± SEM (isolated, stressed – n = 9, isolated, non-stressed – n = 10, control, stressed – n = 9, control, non-stressed – n = 6, p < .01). **p < .01.
Table 1. Behavioral changes in control and socially isolated mice.
Table 2. Spleen weight and lymphocyte proliferation in socially isolated and control mice.
2.3. Set A: behavioral tests
Mice were subjected to the following behavioral tests, in random order, at the time point noted in : grip strength, elevated plus maze, staircase, and hot plate. The behavioral experiments were conducted between 09.00 h and 12.00 h in the same room in which the mice were housed, which was quiet and without visual stimuli besides the tests’ apparatuses. The equipment was cleaned after each test with an alcohol sponge to eliminate residual odors. Additionally, the experimenter did not use any perfume on experiments’ days.Scoring was performed during the testing period.
2.3.1. Grip strength test
Muscle strength was measured by the number of seconds the mouse was able to hang suspended on a stationary bar. A neurologically normal mouse is able to remain suspended (hang time) for 30 sec or longer (Schreiber et al., Citation2019). The gripstrength test was performed in order to exclude the possibility that behavioral changes are due to higher muscle strength. Each mouse was suspended on a metal bar(15 mm diameter) elevated 20 cm above a foam surface.
The mouse was suspended with the two forelimbs. The latency time before falling was measured and analyzed.The apparatus was cleaned after each test with an alcohol sponge to eliminate residual odors.
2.3.2. Elevated plus maze test
The elevated plus maze was constructed according to the description of Holmes et al. (Citation2002) (see for specifications Benaroya-Milshtein et al. (Citation2011)). Each mouse was placed separately in the center of the maze, facing an open arm, and allowed to freely explore the apparatus for 5 min. Parameters measured included the total number of arm entries, the total closed-arm entries, the percentage of open-arm entries (open/total × 100), and the percentage time spent in open arms of the maze (time in open arms/session duration × 100). The closed-arm entries are an accepted index of motor function, and the latter measures are accepted indices of anxiety-like behavior (Holmes et al., Citation2002; Benaroya-Milshtein et al., Citation2004; Schreiber et al., Citation2019). A four-paw criterion was used for arm entries. At the end of 5 min, the mouse was removed, and the apparatus was cleaned with an alcohol sponge to eliminate residual odors.
2.3.3. Staircase test
The staircase was constructed according to the description of Simiand et al. (Citation1984) (see for specifications Benaroya-Milshtein et al. (Citation2011)). Each mouse was placed individually onto the staircase. A step was considered climbed only if the mouse placed all four paws on the stair. The number of stairs climbed is an accepted locomotor and exploratory index. The number of steps descended was not counted. Rearing was recorded when the mouse rose on its hind legs either on the step or against the wall to sniff the air. Rearing is considered an anxiety and exploratory index. At the end of 3 min, the mouse was removed, and the staircase was cleaned with an alcohol sponge to eliminate residual odors (Benaroya-Milshtein et al., Citation2011; Schreiber et al., Citation2019; Simiand et al., Citation1984).
2.3.4. Hotplate test
The nociceptive threshold was tested with the hotplate analgesia meter Model 35 D (IITC Inc., Woodland Hills, CA, USA) as previously described (Pick et al., Citation1991; Schreiber et al., Citation2019). The device consists of a metal plate (40 × 35 cm2) heated to a constant temperature on which a plastic cylinder is placed. In the present study, the analgesimeter was set to a plate temperature of 52 ± 0.5 °C. The latency (in sec) from the time the animal was placed on the hotplate surface until it licked its back paw; suddenly jerked its back paw strongly (as opposed to lifting the paw for purposes other than pain avoidance) off the surface;or jumped out was recorded (Schreiber & Pick, Citation2006). The apparatus was cleaned after each test with an alcohol sponge to eliminate residual odors.
2.4. Set A: immunological tests
2.4.1. NK cytotoxicity assay
This assay was performed in mice, killed at 15 weeks of age, that had been maintained for 11 weeks in either social isolation or standard housing. NK activity was assessed by a standard chromium (Cr) release assay, which measures the anti-tumor cytotoxicity of NK cells. In the Cr release assay, suitable target cells are labeled intracellularly with 51Cr. When NK cells are incubated with these labeled target cells, the effect or NK cellslysethetargetcells, releasingtheintracellular 51Cr which can then be measured. The amount of 51Cr released correlates directly with the number of target cells lysed by the NK cells. For preparation of effector cells, spleens were homogenized, and erythrocytes were removed by hypo-osmolar lysis with Ammonium ChloroKalium (ACK) lysing buffer. The spleen cells were then washed three times,resuspendedinRoswellParkMemorialInstitute(RPMI)medium supplemented with 10% fetal calf serum anddistributed into 96-well Vbottom microplates at different concentrations according to the desired foureffector:target cellsratios (100:1, 50:1, 25:1 and 12.5:1). Different E:T ratios were used to be on the safe side, since we did not know the frequency and activity of the NK cells to start with.The murine lymphoma cell line YAC-1 was used as target in the cytotoxicity assays. YAC-1 cells express very low levels of MHC class I. Thus, they are highly sensitive to the cytotoxic activity of NK cells but resistant to cytotoxic T cells.YAC-1 target cells were labeled by incubation for 1 h with 200 µCi of 51Cr (New England Nuclear). The labeled cells were washed three times, resuspended in medium, andaddedtothemicroplatewells ataconcentrationof1X104 cells per well. Plates were centrifuged for 5 min at 100 g and incubated for 4 h at 37 °C in a humidified incubator containing 5% CO2. After incubation, the plates were centrifuged for 5 min at 300 g, and radioactivity in supernatants was determined by gamma counting. Spontaneous 51Cr release was evaluated by incubation of target cells without effector cells. Maximal releasable radioactivity was evaluated by incubation of targetcells with 1 %TritonX-100. Samples were run in triplicates, and percentage of lysis was calculated using the formula of Zalcman et al. (Citation1991): (Experimental counts per minute (CPM)-Spontaneous CPM/Maximal Releasable CPM-Spontaneous CPM) × 100 (Zalcman et al., Citation1991).
2.4.2. Lymphocyte proliferation measurement
T-cell proliferation was determined in response to concanavalin A (Con A), and B-cell proliferation was determined in response to lipopolysaccharide (LPS). Spleen cells were cultured at 2 × 105 cells/well in flat-bottom microtiter plates in 0.2 ml medium containing no mitogen, 5 µg Con A/ml (Sigma-Aldrich, St. Louis, MO, USA), or 50 µg LPS/ml (Sigma-Aldrich). Cultures were incubated in a humidified 37 °C incubator in an atmosphere of 5% CO2. After 24 h incubation, cultures were pulsed with 1 µCi/well (10 µl) of [3H]thymidine and incubated for an additional 24 h. Cells were harvested using a cell harvester, and the incorporated radioactivity was determined by liquid scintillation counting. The results were expressed as meancounts per minute(CPM) of triplicate cultures. The proliferation index (PI) represents the ratio of CPM of lymphocytes incubated with mitogen divided by CPM of lymphocytes incubated without mitogen (Benaroya-Milshtein et al., Citation2011).
2.5. Set B:stress paradigm
2.5.1. The stress paradigm:
For the stress paradigm, we used a modified time-dependent sensitization model of repeated exposures to situational reminders of a prior exposure to an aversive stimulus, as previously described (Benaroya-Milshtein et al., Citation2011). Shock was produced by a Gemini Avoidance System (San Diego Instruments, San Diego, CA, USA). The animals were placed in a dual-compartment apparatus. After a 10-sec adaptation period, a guillotine door was opened, and a bright lightswitched on in the compartment where the mouse had been placed. The door remained open until the mouse entered the dark compartment. The door then closed, and the mouse received a 1 mA shock for 10 sec. The situational reminderwas achieved by placing the animal in the lighted chamber with the gate closed to prevent it from entering the shock compartment. The situational reminder was experienced once per week for 3 weeks. At each reminder, freezing time was measured. Freezing was defined as the lack of all movement apart from those necessary for respiration, starting from the time the animals were positioned in the illuminated chamber. The stress paradigm was executed the same room in which the mice were housed, which was quiet and without visual stimuli besides the tests’ apparatuses. The equipment was cleaned after each test with an alcohol sponge to eliminate residual odors. Additionally, the experimenter did not use any perfume on experiments’ days. Slope analysis was performed for each group to determine whether there was a pattern to the measured freezing (Houston et al., Citation1999).
Previous studies by our group and others showed that three reminders led to a significant increase in circulating corticosterone levels, an accepted level of physiological stress (Benaroya-Milshtein et al., Citation2004; Pynoos et al., Citation1996; Whitaker et al., Citation2014). Therefore, we performed three reminders in the present study as well.
2.5.2. Measurement ofbloodcorticosterone levels:
Blood was drawn at 08:00 AM from the retro‐orbital sinus of the mice at 16 weeks of age. After blood serum was separated by centrifugation (4000 r.p.m. for 10 min), it was deep frozen (−20 °C) until assayed. The corticosterone levels were determined by a radioimmunoassay (RIA) kit (DPC, Los Angeles, CA, USA), according to the manufacturer’s instructions. The procedure is a solid‐phase RIA in which 125I‐labeled rat corticosterone competes with corticosterone in the sample for antibody sites. All samples were run on the same assay in order to avoid inter-assay variability.
2.6. Set C: tumor development and survival
2.6.1. Cell line
Thesyngeneic 38 C-13 cell line, a carcinogen-induced B-cell tumor was obtained from our laboratory. Cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol.
2.6.2. Tumor cell injection
Mice, isolated and control,were injected subcutaneously in the right flank with 1 × 105 38 C-13 tumor cells in 0.1 ml phosphate buffered saline.Tumor size was measured with calipers three times a week. Tumor area was calculated by multiplying the horizontal diameter by the vertical diameter.Follow-up for survival was carried out daily (Benaroya-Milshtein et al., Citation2007).
2.7. Statistical analysis
All results were calculated as mean and standard error of the mean (SEM) and analyzed with the Statistical Package for the Social Sciences (SPSS) version 20 for Windows (SPSS Inc, Chicago, IL, USA). Unpaired Student’s t tests were used to compare stressed and control mice for weight, behavioral parameters,spleen weight,and lymphocyte proliferation. Corticosterone levels were comparedwith one-way analysis of variance (ANOVA). When significant group effects were detected, Tukey’stest was used to identify significant posthoc differences between individual groups.Differences between groups in freezing time, NK cell activity, and tumor size were analyzed by ANOVA with repeated measures. Survival analysis was performed with the log rank test. Significance was set at the p < .05 level.
3. Results
3.1. Effect of social isolation on behavioral and biochemical measures of stress
The behavioral and biochemical parameters were measured in mice reared in social isolation or a standard environment for 6 weeks. At 10 weeks, mean body weight was significantly lower in the socially isolated mice (26.0 ± 0.5 g vs 27.9 ± 0.4 g, t(19) = 2.82, p < .05; see ). Social isolation did not alter the behavior of the mice as evidenced by the lack of a difference from control mice in results of the grip, elevated plus maze, and staircase tests. There was also no between-group difference in pain threshold inthe hotplate test (see ). Serum corticosterone levels were elevated in the socially isolated mice compared to the control mice, but the difference did not reach statistical significance (ANOVA, F(3,33) = 6.69, p < .01; isolated vs control: 38.36 ± 6.46 ng/ml vs 23.49 ± 1.18 ng/ml, p = .93; see ).
3.2. Effect of social isolation on response to the stress paradigm
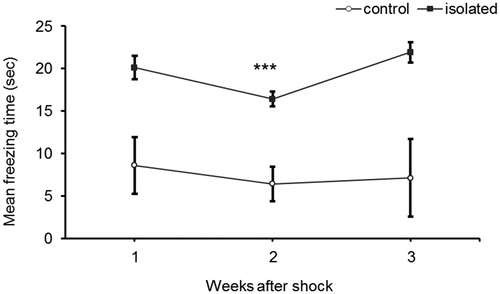
Social isolation was associated with a significant increase in freezing time in response to the stress paradigm (ANOVA with repeated measures F(1,18) = 26.86, p < .001). Additionally, the pattern of freezing time over the course of the test differed, with the control mice displaying a decline in freezing time on consecutive situational reminders, and the socially isolated mice displaying an incline (control: slope = −0.74, r2 = 0.44, isolation: slope = 0.9, r2 = 0.1; see ). A disparity in the change in corticosterone levels following the stress paradigm was noted as well. Within the control group, corticosterone levels were increased in the mice that underwent the stress paradigm compared to the mice that did not (ANOVA, F(3,33) = 6.69, p < .01; control vs. control stressed: 23.49 ± 1.18 ng/ml vs 116.68 ± 20.31 ng/ml, p < .01; ). No such difference was found within the socially isolated group.
Figure 3. Effect of social isolation on freezing time following shock reminders. Mice were subjected to a stress paradigm. Mean freezing time (sec) was measured once a week for 3 weeks following the electric shock and reminders. The socially isolated mice displayed a pattern of significantly increased freezing time over the course of the test. Values are displayed as mean ± SEM (n = 10 for each group, p < .001). ***p < .001.
3.3. Effect of social isolation on spleen weight, lymphocyte proliferation, and NK cell activity
Spleen weight was examined at 15 weeks of age, following 11 weeks of living in either social isolation or a standard environment. Spleens were weighed immediately after harvesting. Spleen weight was significantly lower weight in the socially isolated than the control mice (84 ± 2 mg vs 110 ± 14 mg, t(18) = 5.20, p < .001; see ). There were no between-group differences in splenic NK cell activity for alleffectorcell:target cell ratios (ANOVA with repeated measures: F(1,8) = 4.763, p = .06) or in splenic T- and B- lymphocyte proliferation (see ).
3.4. Tumor development and survival time
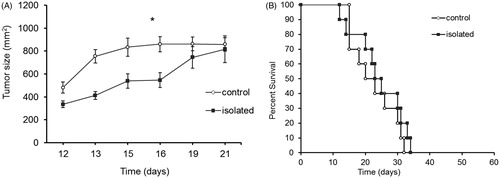
Social isolation resulted in the attenuation of tumor growthcompared to control mice (ANOVA with repeated measures df = 1,18, F = 6.694, p = .02, see ). As can be seen in , the difference is maintained until the final days of the experiment (days 19, 21), when tumor growth reaches the stage of asymptotic course. Despite tumor growth attenuation, no significant difference in overall survival was found between the groups (log rank, χ2 = 0.736, df = 1, p = .39, see ).
Figure 4. Effect of social isolation on tumor progression. Mice were injected subcutaneously with 1×10538C-13 lymphoma cells at 10 weeks of age following 6 weeks’ rearing in social isolation or a standard environment. (A) Tumor size was measured from day 12 following injection. Social isolation significantly attenuated tumor progression. Values are displayed as averages of tumor size ± SEM, (n = 10 for each group, p < .05 ANOVA repeated measures). (B) Mice were monitored for survival time. No significant difference was found in survival time between stressed and control mice (n = 10 in each group).
4. Discussion
The presentstudy investigated the effects of social isolation in several disciplines using a mouse model. We demonstrated that social isolation was associated with prominent physiological and cellular changes, namely, lower body weight and attenuated tumor development. Social isolation, as a single chronic stressor, did not lead to behavioral changes. However, it did alter the response to a second (acute) stressor, both behaviorally and physiologically: the decline in freezing time over the course of the stress test was attenuated, and there was no significant increase in corticosterone levels.
Overall, our data show that social isolation serves as a chronic stressor in mice, andincreases vulnerability for the development of PTSD following an exposure to the second stressor, the stress paradigm, thus lending support to the ‘two hits hypothesis’ of PTSD (Pynoos et al., Citation1996). In humans, post-traumatic stress disorder (PTSD) has a deleterious effect on many endocrine systems, one of the which is the HPA axis. Data show that there is a blunted response to corticotropin-releasing hormone. In the present study, following the stress paradigm, there was a significant increase in the corticosterone levels of the control mice, as expected, but this was not replicated in the socially isolated mice. In the isolated group, exposure of the mice to two stressors (social isolation and electric shock), resulted in a physiological profile resembling PTSD. Thus, it might be postulated that the HPA axis was dysregulated by the longstanding chronic stress followed by the stress paradigm, and like in PTSD, there may be a need for two traumatic events to cause physiological changes (Griffin et al., Citation2014; Whitaker et al., Citation2014). This theorization may also explain why we and others (Rodgers & Cole, Citation1993) did not find differences in behavioral tests following social isolation alone.
The HPA axis activitywas shown to beaffected by the intensity and duration of the different stressors. For example, social defeat, forced swim, and restraint were all associated with high plasma corticosterone stress responses (300–600 ng/ml) in animal models (Heinrichs et al., Citation1994; Koob et al., Citation1993; Koolhaas et al., Citation1997) whereas mild foot shock was associated with a low response (100–200 ng/ml) (Benaroya-Milshtein et al., Citation2004; Korte et al., Citation1992). Foot shock, unlike the other stressors, also failed to potentiate fear in the plus-maze test (Korte & De Boer, Citation2003).In current study, social isolation mildly increasedcorticosterone levels compared to control mice.However, it did not reach statistical significance. Supporting the notion that social isolation is only a mild stressor is the lack of differences in behavioral tests between socially isolated and control mice seen in our study and by others (Rodgers & Cole, Citation1993). Of note, some studies found opposing effects whereby social isolation lead to a significant change in mice behavior (Koike et al., Citation2009).
Another result supporting our line of thought that social isolation acts as a mild stressor only is the lack of alteration in immune function between socially isolated and control animals in the present study. The disparity between the short-term and long-term effects of stress is demonstrated by theimplications for the immune system. In the short term, theprimary release of stress hormones allows the immune system to prepare for potential immunological challenges, with redistribution of leukocytes, increased macrophage activation, increased dendritic cell and T-cell mobilization, and increased activity of splenic NK cells and inflammatory cytokine and chemokines (Bigler et al., Citation2015; Dhabhar et al., Citation2000; Sharify et al., Citation2007; Viswanathan & Dhabhar, Citation2005). Accordingly, in our previous study, acute stress induced by electric foot shock and weekly exposures to situational reminders resulted in higher NK cell activity, increased humoral immune response, and increased splenocyte proliferation rate (Benaroya-Milshtein et al., Citation2011). Acute stress also potentiates the secondary immune response. One deleterious result of acute stress may be autoimmune and hypersensitivity phenomena due to increased sensitization and activation of the immune system (Dhabhar, Citation2014).
Unlike acute stress, prolonged orchronic stress leads to immune suppression and dysfunction. For example, chronic stress has been found to decrease the response to antigen exposure, decrease antibody production, inhibit NK cell activity and leukocyte proliferation, and decrease mycobacteria clearance by macrophages (Li et al., Citation2005).
Finally, our study shows that the growth of B-cell lymphoma tumor injected subcutaneously was attenuated until the stage of asymptotic growth in socially isolated mice. These results are similar to our pervious reported finding thatshowed that exposure to the stress paradigm led to anti-tumor activity and attenuated growth of the same type of tumor used in our current study (Benaroya-Milshtein et al., Citation2011). Previous studies on the effect of stress on tumor growth and development yielded conflicting results. Justice (Citation1985) reviewed the effects of stress on cancer in laboratory animals, and pointed out the importance of the time of stress application and the type of tumor. More specifically, it has been suggested that non-viral tumors, such as used in our study, exhibited attenuated growth during periods of stress (Bhattacharyya & Pradhan, Citation1979). These changes were related to alterations in the function of the autonomic system (Justice, Citation1985). Other studies reported a deleterious impact of stress (Sumis et al., Citation2016), and specifically of social isolation (Dawson, Citation2018). For example, in mice maintained in a low-stress environment rather than in a conventional animal room, transplanted tumors showed slower growth (Riley, Citation1981). In rats, chronic stress led to a decrease in the activation of NK cells following exposure to IL-12, which attenuated IL-12-mediated resistance to lung cell adenocarcinoma (Levi et al., Citation2011). Furthermore, a high-anxious behavioral phenotype in mice lead to increased susceptibility to squamous cell carcinoma and suppressed anti-tumor activity (Dhabhar et al., Citation2012). Of note, in the present study, while tumor growth was attenuated in socially isolated mice, no difference from controls was foundin survival.
Our study had several limitations. Due to our focus on the effects of social isolation, we did not examine the effects of behavioral tests themselves on endocrine and immune function. In addition, we were not able to differentiate motivation from strength in the grip test. Blinded scoring in behavioral tests was impossible since the experimenter who took the animals out of the cage was the same experimenter who performed the various testsIn addition, measurements of tumor growth did not include depth, thus, we were only able to accurately measure tumor area and not tumor volume (by ellipsoidal formula). Finally, we had only a single measurement of corticosterone, thus we were not able to observe diurnal patterns.
5. Conclusions
Previous studies support the notion that social isolation serves as a model for stress, as evidenced by behavioral and physiological changes.Our results suggest that social isolation acts as a primary mild stressor,that mayenhancesvulnerability for the development of a physiological and behavioral profile resembling PTSD following exposure to a second stressorin the form ofa stress paradigm. Additionally, we found that social isolation attenuates tumor progression until the stage of asymptotic growth, but this attenuation does not result in prolonged survival. In our study, social isolation did not result inany immune changes. More studies are needed to expand our knowledge on the physiological,behavioral, and immunologicalchanges following exposure to social isolation and a secondary stressor, and the influence of social isolation on tumor growth.
Author contributions
Dan Farbstein: Analyzed data; wrote the paper; Nurit Hollander: Conception and designed research; performed research; critical reading of manuscript; contributed reagents and analytic tools. Orit Peled: Critical reading of manuscript; contribution to the statistical analyses; Alan Apter: Conception and designed research; critical reading of manuscript; Silvana Fennig: Critical reading of manuscript; Yael Haberman: Performed research; Hila Gitman: Performed research; Isaac Yaniv: Conception and designed research; VeredShkalim: Critical reading of manuscript; Chaim G. Pick: Conception and designed research; critical reading of manuscript; contributed reagents and analytic tools; Noa Benaroya-Milshtein: Conception and designed research; performed research; wrote the paper; analyzed data.
Acknowledgements
The authors thank Gloria Ginzach for her help in proof-reading the article. No funding was procured for this work.
Disclosure statement
The authors have no conflicts of interest to disclose.
Data availability statement
The data are available upon request from the corresponding authors. Please direct all email requests [email protected], [email protected]
Additional information
Notes on contributors
Dan Farbstein
Dan Farbstein MD,PhD. Completed PhD in 08/2015 under the supervision of Prof. Andrew P. Levy. Completed MD studies in the Technion, Cum Laude, in 01/2016. Currently completing residency in Child and Adolescent Psychiatry in Schneider's Children Medical Center.
Nurit Hollander
Nurit Hollander Professor of Immunology, Department of Clinical Microiology and Immunology, Sackler Faculty of Medicine, Tel Aviv University. Ph.D. in Immunology (1975). The Weizmann Institute of Science, Rehovot, Israel. Postdoctoral fellow (1977-1980), Stanford University, Stanford, USA.
Orit Peled
Orit Peled Pharm D. (2017), Hadassah school of pharmacy, at the faculty of medicine. Head of clinical pharmacy services, Schneider Children's Medical Centre of Israel. A researcher and expert in pediatric clinical pharmacy.
Alan Apter
Alan Apter Prof. Alan Apter, former director of the Department of Psychological Medicine at Schneider Children's Medical Centre, and one of the leading child psychiatrists in Israel. Prof. Alan Apter, has conducted research for the past 30 years, published scores of articles and book chapters.
Silvana Fennig
Silvana Fennig Professor Fennig serves as the director of child psychiatry department and the director of Crisis Intervention Unit (Pediatrics “D”) at Schneider Children's. Dr. Fennig is a graduate of the Sackler School of Medicine, Tel Aviv University (TAU).
Yael Haberman
Yael Haberman MSc. Clinical embryologist, IVF Unit, at Assuta, previously a clinical embryologist at IVF Fertility Unit, department of Obstetrics and Gynecology, Chaim Sheba Medical Centre.
Hila Gitman
Hila Gitman Msc., Works at Infertility and IVF unit, department of Obstetrics and Gynecology, Chaim Sheba Medical Centre.
Isaac Yaniv
Isaac Yaniv Professor of Pediatrics at the Tel Aviv University. Established the first Pediatric Bone Marrow Transplantation unit in Israel, served as Chairman of Pediatric Hematology Oncology at the Schneider Children's Medical Center of Israel.
Vered Shkalim
Vered Shkalim Dr. Vered Shkalim is a pediatrician and pediatric hemato-oncologist. She worked at Schnieder Childrens' Medical Center of Israel for 14 years and nowadays works at pediatric clinic Petah Tikva, Israel.
Chaim G. Pick
Chaim G. Pick Professor, Chairman Department of Anatomy, Sackler Faculty of Medicine, Tel Aviv University. Expert in animal models for neuroscience. Published numerous publications in the fields of Traumatic brain injury, animal behavior and Pain Medicines.
Noa Benaroya-Milshtein
Noa Benaroya-Milshtein MD, PhD. Senior Child and Adolescent Psychiatrist. Unit director of the Psychiatric Outpatient Clinic, and Director of Tourette's Clinic, at Schneider Children's Medical Center of Israel. A lecturer in Tel-Aviv University, and an active researcher in neuroscience and child psychiatry.
References
- Benaroya-Milshtein, N., Apter, A., Yaniv, I., Kukulansky, T., Raz, N., Haberman, Y., Halpert, H., Pick, C. G., Hollander, N. (2007). Environmental enrichment augments the efficacy of idiotype vaccination for B-cell lymphoma. Journal of Immunotherapy (Hagerstown, Md.: 1997), 30(5), 517–522. https://doi.org/10.1097/CJI.0b013e31804efc5e
- Benaroya-Milshtein, N., Hollander, N., Apter, A., Kukulansky, T., Raz, N., Wilf, A., Yaniv, I., Pick, C.G. (2004). Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. The European Journal of Neuroscience, 20(5), 1341–1347. https://doi.org/10.1111/j.1460-9568.2004.03587.x
- Benaroya-Milshtein, N., Hollander, N., Apter, A., Yaniv, I., & Pick, C.G. (2011). Stress conditioning in mice: alterations in immunity and tumor growth. Stress (Amsterdam, Netherlands), 14(3), 301–311. https://doi.org/10.3109/10253890.2010.545845
- Bhattacharyya, A. K., & Pradhan, S.N. (1979). Effects of stress on DMBA-induced tumor growth, plasma corticosterone and brain biogenic amines in rats. Research Communications in Chemical Pathology and Pharmacology, 23(1), 107–116.
- Bigler, M. B., Egli, S. B., Hysek, C. M., Hoenger, G., Schmied, L., Baldin, F. S., Marquardsen, F. A., Recher, M., Liechti, M. E., Hess, C., Berger, C. T. (2015). Stress-induced in vivo recruitment of human cytotoxic natural killer cells favors Subsets with distinct receptor profiles and associates with increased epinephrine levels. PLoS One, 10(12), e0145635. https://doi.org/10.1371/journal.pone.0145635
- Bower, J.E. (2018). Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr, 2(3): pky029. https://doi.org/10.1093/jncics/pky029
- Budiu, R. A., Vlad, A. M., Nazario, L., Bathula, C., Cooper, K. L., Edmed, J., Thaker, P. H., Urban, J., Kalinski, P., Lee, A. V., Elishaev, E. L., Conrads, T. P., Flint, M.S. (2017). Restraint and social isolation stressors differentially regulate adaptive immunity and tumor angiogenesis in a breast cancer mouse model. Cancer Clin Oncol, 6(1), 12–24. https://doi.org/10.5539/cco.v6n1p12
- Chou, K.-L., Liang, K., & Sareen, J. (2011). The association between social isolation and DSM-IV mood, anxiety, and substance use disorders. The Journal of Clinical Psychiatry, 72(11), 1468–1476. https://doi.org/10.4088/JCP.10m06019gry
- Cole, S.W. (2008). Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun, 22(7), 1049–1055. https://doi.org/10.1016/j.bbi.2008.02.006
- Corsi-Zuelli, F., Fachim, H. A., Loureiro, C. M., Shuhama, R., Bertozi, G., Joca, S.R. L., Menezes, P. R., Louzada-Junior, P., Del-Ben, C.M. (2018). Prolonged periods of social isolation from weaning reduce the anti-inflammatory cytokine IL-10 in blood and brain. Frontiers in Neuroscience, 12, 1011. https://doi.org/10.3389/fnins.2018.01011
- Dawson, E.H. (2018). Social environment mediates cancer progression in Drosophila. Nature Communications, 9(1), 1–7.
- Dhabhar, F.S. (2009). Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation, 16(5), 300–317. https://doi.org/10.1159/000216188
- Dhabhar, F.S. (2014). Effects of stress on immune function: the good, the bad, and the beautiful. Immunologic Research, 58(2-3), 193–210. https://doi.org/10.1007/s12026-014-8517-0
- Dhabhar, F. S., Satoskar, A. R., Bluethmann, H., David, J. R., & McEwen, B.S. (2000). Stress-induced enhancement of skin immune function: A role for gamma interferon. Proceedings of the National Academy of Sciences of the United States of America, 97(6), 2846–2851. https://doi.org/10.1073/pnas.050569397
- Dhabhar, F. S., Saul, A. N., Holmes, T. H., Daugherty, C., Neri, E., Tillie, J. M., Kusewitt, D., Oberyszyn, T.M. (2012). High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS One, 7(4), e33069. https://doi.org/10.1371/journal.pone.0033069
- Dudek, K.A. (2019). Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. European Journal of Neuroscience, 0, 1–39. https://doi.org/10.1111/ejn.14547
- Fleisch Marcus, A., Illescas, A. H., Hohl, B. C., & Llanos, A.A.M. (2017). Relationships between social isolation, neighborhood poverty, and cancer mortality in a population-based study of US adults. PLoS One, 12(3), e0173370. https://doi.org/10.1371/journal.pone.0173370
- Griffin, G. D., Charron, D., & Al-Daccak, R. (2014). Post-traumatic stress disorder: revisiting adrenergics, glucocorticoids, immune system effects and homeostasis. Clinical & Translational Immunology, 3(11), e27. https://doi.org/10.1038/cti.2014.26
- Haimovich, J., Kukulansky, T., Weissman, B., & Hollander, N. (1999). Rejection of tumors of the B cell lineage by idiotype-vaccinated mice. Cancer Immunology, Immunotherapy: Cii, 47(6), 330–336. https://doi.org/10.1007/s002620050538
- Heinrichs, S. C., Menzaghi, F., Pich, E. M., Baldwin, H. A., Rassnick, S., Britton, K. T., & Koob, G. F. (1994). Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 11(3), 179–186. https://doi.org/10.1038/sj.npp.1380104
- Hermes, G. L., Delgado, B., Tretiakova, M., Cavigelli, S. A., Krausz, T., Conzen, S. D., & McClintock, M.K. (2009). Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22393–22398. https://doi.org/10.1073/pnas.0910753106
- Holmes, A., Yang, R. J., & Crawley, J.N. (2002). Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. Journal of Molecular Neuroscience, 18(1–2), 151–166. https://doi.org/10.1385/JMN:18:1-2:151
- Houston, F. P., Stevenson, G. D., McNaughton, B. L., & Barnes, C.A. (1999). Effects of age on the generalization and incubation of memory in the F344 rat. Learning & Memory (Cold Spring Harbor, N.Y.), 6(2), 111–119.
- Justice, A. (1985). Review of the effects of stress on cancer in laboratory animals: Importance of time of stress application and type of tumor. Psychological Bulletin, 98(1), 108–138. https://doi.org/10.1037/0033-2909.98.1.108
- Koike, H., Ibi, D., Mizoguchi, H., Nagai, T., Nitta, A., Takuma, K., Nabeshima, T., Yoneda, Y., Yamada, K. (2009). Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behavioural Brain Research, 202(1), 114–121. https://doi.org/10.1016/j.bbr.2009.03.028
- Koob, G. F., Heinrichs, S. C., Pich, E. M., Menzaghi, F., Baldwin, H., Miczek, K., & Britton, K.T. (1993). The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Foundation Symposium, 172, 277–289. discussion 290–5. https://doi.org/10.1002/9780470514368.ch14
- Koolhaas, J. M., De Boer, S. F., De Rutter, A. J., Meerlo, P., & Sgoifo, A. (1997). Social stress in rats and mice. Acta Physiologica Scandinavica. Supplementum, 640, 69–72.
- Korte, S. M., & De Boer, S.F. (2003). A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. European Journal of Pharmacology, 463(1-3), 163–175. https://doi.org/10.1016/S0014-2999(03)01279-2
- Korte, S. M., Buwalda, B., Bouws, G. A., Koolhaas, J. M., Maes, F. W., & Bohus, B. (1992). Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiology & Behavior, 51(4), 815–822. https://doi.org/10.1016/0031-9384(92)90120-Q
- Levi, B., Benish, M., Goldfarb, Y., Sorski, L., Melamed, R., Rosenne, E., & Ben-Eliyahu, S. (2011). Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behavior and Immunity, 25(4), 727–735. https://doi.org/10.1016/j.bbi.2011.01.014
- Li, Q., Liang, Z., Nakadai, A., & Kawada, T. (2005). Effect of electric foot shock and psychological stress on activities of murine splenic natural killer and lymphokine-activated killer cells, cytotoxic T lymphocytes, natural killer receptors and mRNA transcripts for granzymes and perforin. Stress (Amsterdam, Netherlands), 8(2), 107–116. https://doi.org/10.1080/10253890500140972
- Lim, A. L., Taylor, D. A., & Malone, D.T. (2012). A two-hit model: behavioural investigation of the effect of combined neonatal MK-801 administration and isolation rearing in the rat. Journal of Psychopharmacology (Oxford, England), 26(9), 1252–1264. https://doi.org/10.1177/0269881111430751
- Pick, C. G., Cheng, J., Paul, D., & Pasternak, G.W. (1991). Genetic influences in opioid analgesic sensitivity in mice. Brain Research, 566(1–2), 295–298. https://doi.org/10.1016/0006-8993(91)91712-A
- Pynoos, R. S., Ritzmann, R. F., Steinberg, A. M., Goenjian, A., & Prisecaru, I. (1996). A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biological Psychiatry, 39(2), 129–134. https://doi.org/10.1016/0006-3223(95)00088-7
- Riley, V. (1981). Psychoneuroendocrine influences on immunocompetence and neoplasia. Science (New York, N.Y.), 212(4499), 1100–1109. https://doi.org/10.1126/science.7233204
- Rodgers, R. J., & Cole, J.C. (1993). Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiology & Behavior, 54(4), 729–736. https://doi.org/10.1016/0031-9384(93)90084-S
- Schreiber, S., & Pick, C.G. (2006). From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 16(6), 464–468. https://doi.org/10.1016/j.euroneuro.2005.11.013
- Schreiber, S., Bader, M., Lenchinski, T., Meningher, I., Rubovitch, V., Katz, Y., Cohen, E., Gabet, Y., Rotenberg, M., Wolf, E., Pick, C.G. (2019). Functional effects of synthetic cannabinoids versus Δ9 -THC in mice on body temperature, nociceptive threshold, anxiety, cognition, locomotor/exploratory parameters and depression. Addiction Biology, 24(3), 414–425. https://doi.org/10.1111/adb.12606
- Sharify, A., Mahmoudi, M., Izad, M. H., Hosseini, M.-J., & Sharify, M. (2007). Effect of acute pain on splenic NK cell activity, lymphocyte proliferation and cytokine production activities. Immunopharmacology and Immunotoxicology, 29(3–4), 465–476. https://doi.org/10.1080/08923970701619877
- Sherin, J. E., & Nemeroff, C.B. (2011). Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues in Clinical Neuroscience, 13(3), 263–278.
- Simiand, J., Keane, P. E., & Morre, M. (1984). The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology, 84(1), 48–53. https://doi.org/10.1007/BF00432023
- Sumis, A., Cook, K. L., Andrade, F. O., Hu, R., Kidney, E., Zhang, X., Kim, D., Carney, E., Nguyen, N., Yu, W., Bouker, K. B., Cruz, I., Clarke, R., Hilakivi-Clarke, L. (2016). Social isolation induces autophagy in the mouse mammary gland: Link to increased mammary cancer risk. Endocr Relat Cancer, 23(10), 839–856. https://doi.org/10.1530/ERC-16-0359
- Viswanathan, K., & Dhabhar, F.S. (2005). Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proceedings of the National Academy of Sciences of the United States of America, 102(16), 5808–5813. https://doi.org/10.1073/pnas.0501650102
- Whitaker, A. M., Gilpin, N. W., & Edwards, S. (2014). Animal models of post-traumatic stress disorder and recent neurobiological insights. Behavioural Pharmacology, 25(5-6), 398–409. https://doi.org/10.1097/FBP.0000000000000069
- Wu, W., Yamaura, T., Murakami, K., Ogasawara, M., Hayashi, K., Murata, J., Saiki, I. (1999). Involvement of TNF-alpha in enhancement of invasion and metastasis of colon 26-L5 carcinoma cells in mice by social isolation stress. Oncol. Res, 11, 461–469.
- Yang, Y. C., Li, T., & Frenk, S.M. (2014). Social network ties and inflammation in U.S. adults with cancer. Biodemography and Social Biology, 60(1), 21–37. https://doi.org/10.1080/19485565.2014.899452
- Zalcman, S., Irwin, J., & Anisman, H. (1991). Stressor-induced alterations of natural killer cell activity and central catecholamines in mice. Pharmacology, Biochemistry, and Behavior, 39(2), 361–366. https://doi.org/10.1016/0091-3057(91)90192-5
