Abstract
Stress during adolescence has profound effects on the onset and severity of substance use later in life. However, not everyone with adverse experiences during this period will go on to develop a substance use disorder in adulthood, and the factors that alter susceptibility to substance use remain unknown. Here, we investigated individual differences in response to stress and drugs of abuse using our selectively bred high-responder (bHR) and low-responder (bLR) rats. These animals model extremes of temperamental tendencies and differ dramatically in both stress responsiveness and addiction-related traits. The present study investigated how environmental interventions in the form of a chronic variable stress (CVS) regimen in early adolescence interact with the bHR/bLR phenotype to alter behavioral sensitization to cocaine in adulthood. We also determined whether accumbal dopamine signaling is involved in the interaction of stress history and cocaine by assessing the mRNA levels of dopamine D1 (D1R) and D2 (D2R) receptors. Our results showed that CVS history alone had enduring and phenotype-specific effects on accumbal dopamine signaling. Importantly, adolescent stress had opposing effects in the two lines- decreasing the locomotor response to cocaine challenge in bHRs but increasing this measure in bLRs. Moreover, these opposing effects on cocaine sensitivity following adolescent CVS were accompanied by parallel effects in the accumbal dopamine system, with prior stress and cocaine exposure interacting to decrease D2R mRNA in bHRs but increase it in bLRs. Overall, these findings indicate that environmental challenges encountered in adolescence interact with genetic background to alter vulnerability to cocaine later in life.
Stress experienced during adolescence affects the onset and severity of drug dependence later in life. However, not everyone with adverse experiences during this period will go on to develop SUD in adulthood. Using a rat model of innate differences in emotional reactivity, this study shows that the interplay between individual temperament and previous experience of adolescent stress/trauma determines whether an individual will be vulnerable or resilient to develop SUDs later in life. In addition, the present study shows that the dopamine D2 receptor in the brain’s reward center, nucleus accumbens, may be implicated in this interplay.
Lay Summary
1. Introduction
Evidence from clinical and preclinical studies demonstrates the connection between stress and substance use disorders (SUD) (Oswald et al., Citation2005; Rovaris et al., Citation2015; Wand et al., Citation2007), and stress experienced during adolescence appears to have particularly profound effects on the onset and severity of drug dependence later in life (Hoffman et al., Citation2000). However, individual differences exist in response to environmental challenges encountered in adolescence (Romeo, Citation2015; Southwick et al., Citation2005), and not everyone with adverse experiences during this period will go on to develop SUD in adulthood. For example, although half of adults are exposed to some kind of stressor during development, only 26% of SUD with onset in young adulthood are predicted by stressful life experiences (Enoch, Citation2012; Green et al., Citation2010). An understanding of the factors that determine individual differences in vulnerability or resilience to stress-provoked substance disorders is critical for our ability to both identify at-risk individuals, as well as target effective therapies
Animal models are essential for investigating the interaction between genetic background and environmental challenges owing to the ability to control these factors. In the present study, we utilized a rat model we selectively breed in house based on differences in locomotor response to a novel environment where bred high-responders (bHRs) display significantly greater exploratory locomotion compared to bred low-responders (bLRs). Similar to the outbred HR/LR rats (Aydin et al., Citation2011; Piazza et al., Citation1989) the bred lines differ in several behavioral traits relevant to addiction in adulthood. Indeed, compared to bLRs, bHRs are more impulsive (Flagel et al., Citation2010), more aggressive (Kerman et al., Citation2011), more likely to sign-track to food- and drug-associated cues (Flagel et al., Citation2010), display higher susceptibility to drug-taking behavior (Davis et al., Citation2008) and responsivity to cocaine, including addiction and relapse (Clinton et al., Citation2012; Flagel et al., Citation2016; Garcia-Fuster et al., Citation2017). Importantly, the bHR/bLR rats also differ in stress reactivity. By contrast, compared to bHRs, bLRs are more vulnerable to the negative effects of chronic stress in adulthood (Stedenfeld et al., Citation2011), although interestingly, they are resilient to chronic stress in adolescence (Rana et al., Citation2016). These differences in addiction-related behaviors and stress reactivity make the bHR/bLR an ideal model to study the interaction of genetic background and environmental challenges in adolescence on addiction liability.
The role of dopaminergic signaling in the nucleus accumbens (Nacc) in addiction-related behaviors has been widely demonstrated (see (Chen et al., Citation2017) for a recent review). Notably, the Nacc is also highly susceptible to stress and is altered by stressful stimuli (Cabib and Puglisi-Allegra, Citation1996; Krishnan et al., Citation2007) due to the ability of cortisol and corticosterone to modulate dopamine release (Oswald et al., Citation2005; Wand et al., Citation2007), making it an important structure for the interaction of stress and drugs of abuse. In the Nacc, the two main dopamine receptor types, D1R and D2R, have been widely implicated in drug sensitization (Cabib et al., Citation1991; Kai et al., Citation2015) as well as in stress-related disorders (Francis and Lobo, Citation2017). However, their contribution to the interaction of these behaviors remain unclear. Previous studies from our laboratory have shown that the levels of D1R and D2R differ basally in the Nacc of the bHR\bLR rats (Clinton et al., Citation2012; Flagel et al., Citation2010). Specifically, lower D2R (Clinton et al., Citation2012; Flagel et al., Citation2010) but higher D1R (Clinton et al., Citation2012) mRNA levels are observed in the Nacc in bHRs compared to bLRs, indicating innate differences in the accumbal dopamine signaling. These differences may be associated with differences in addiction vulnerability, and possibly the differential responsivity to chronic stress observed in these animals. However, whether these differences in accumbal dopamine signaling influence the interaction of chronic stress and drugs of abuse has not been investigated.
In the present study, we investigated the behavioral and neurobiological consequences of chronic stress on addiction vulnerability in individuals with differential stress reactivity using the bHR/bLR rats as a preclinical model. Given the importance of early life events in shaping responses in adulthood (Wilkin et al., Citation2012) we chose weanling through periadolescence (early adolescence) as the period of stress exposure. Subsequently, 1 month after cessation of stress, we determined whether adolescent stress history interacts with adulthood vulnerability to cocaine by exposing the bHR/bLR rats to a chronic cocaine regimen and using expression of locomotor sensitization as an index of addiction liability. Finally, to determine the neurobiological underpinnings of adolescent stress history alone, as well as its interaction with cocaine we assessed the expression levels of two dopamine receptors, D1 and D2, in the Nacc.
2. Materials and methods
2.1. Drugs
Cocaine hydrochloride (Mallinckrodt, St Louis, MO) was dissolved in 0.9% sterile saline and administered intraperitoneally at doses of 7.5 and 15 mg/kg. All injections were administered at 1 ml/kg body weight.
2.2. Subjects
Animals were treated in accordance with the National Institute of Health guidelines on laboratory animal use and care and approved by the University of Michigan Committee on the Use and Care of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used. Male Sprague-Dawley rats (N = 72; n = 7-10) from the 42nd and 43rd generations of our in-house bHR/bLR selective-breeding colony were used. A detailed description of the breeding paradigm and characterization of these bred lines has been published previously (Flagel et al., Citation2010; Stead et al., Citation2006). Rats were pair-housed under a 12-hour light-dark cycle with ad libitum access to standard chow and water. Rats were acclimated to the housing room for 2 weeks before the start of the experiments.
2.3. Chronic Variable Stress (CVS) paradigm
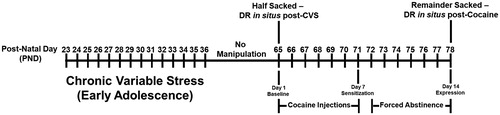
The CVS paradigm was used in bHRs and bLRs during adolescence and contrasted with handling controls. summarizes the order and time of stressors employed in the CVS paradigm. Four stressors that are widely used in the literature and were previously utilized in published work (Isgor et al., Citation2004; Oztan et al., Citation2011) were selected. Stressors were applied in a systematic random order and at varying times of the day to avoid habituation. Rats experienced a total of 14 stress exposures, 1 per day, between postnatal days (PND) 23 and 36, corresponding to the early adolescence stage of rat adolescence (Tirelli et al., Citation2003) when chronic stress produces a broad range of behavioral outcomes in rats (Wilkin et al., Citation2012). The stressors used in the CVS paradigm were as follows: 1) Restraint: Animals were wrapped individually in flexible Teflon and secured with Velcro closures to limit movement for 2 hrs. 2) Isolation: Animals were individually transferred into cages similar to home cages and placed in a room other than the home colony for 2 hrs with free access to food and water. 3) Novel environment: Animals were placed in novel environments with their cage mates for 2 hrs. 4) Cage tilt: Animals were kept in their home cages, which were tilted 45° along the vertical axis for 2 hrs. Following the 2-wk CVS exposure animals were allowed to rest and grow into adulthood (PND 65) for 4 weeks. At this point, half of the rats were euthanized to determine the effects of the CVS history alone on D1R and D2R expression in the Nacc. The remaining half of the rats went through a cocaine sensitization regimen summarized below.
Table 1. Stressors were applied at a systematically randomized fashion for 14 days during the CVS regimen (PN 28–41). Control animals remained untouched during this time aside from routine cage changes.
2.4. Cocaine sensitization
Adult bHR and bLR rats, with or without a history of stress during adolescence, received daily intraperitoneal injections of cocaine (15 mg/kg) for 7 consecutive days. On the first and last days of treatment, rats were placed into activity boxes for 60 minutes to allow for habituation to the testing environment. Activity during the 60-minute habituation period on the first day was recorded to measure locomotor response to this novel environment. All rats then received a cocaine injection and their behaviors were video recorded for 60 minutes. On days 2-6, rats received their cocaine injections in the activity boxes, but the behavior was not recorded. Following this 7-day induction period, rats remained abstinent from cocaine for 7 days. At the end of the abstinence period, all rats were challenged with a low dose cocaine (7.5 mg/kg; i.p) and their behaviors were video-recorded for 60 minutes. Immediately after the challenge session, rats were sacrificed via rapid decapitation. Brains were dissected, snap frozen in isopentane (−30 °C), and stored at −80 °C prior to molecular analyses. A timeline of all experimental procedures as well as tissue collection timepoints for in situ hybridization is presented in .
2.5. Behavioral analysis
Behavior was digitally recorded with overhead cameras on days 1 and 7 of the induction phase, and on the challenge day (for details, see (Isgor et al., Citation2004)). Clever Sys, Inc. (Reston, VA) Drug Effect Scan software was used to analyze locomotor activity in response to cocaine injections (Flagel and Robinson, Citation2007; Flagel et al., Citation2010). In brief, the automated software identified the ‘center’ of the rat in each video frame, and calculated/reported the total distance traveled, in millimeters, throughout the 1-hour video. Standard exclusion criteria employed in all of our experiments, such as excluding any animal who displays a negative reaction to cocaine injection or who fails to move/explore the chamber during either locomotor habituation or testing, were in place. However, no animal was excluded from this particular study as a result of those criteria.
2.6. In situ hybridization histochemistry
Brains were sectioned on a cryostat (−20 °C), and 20-μm-thick coronal sections were mounted on electrostatically charged slides. These slides were kept at −80 °C until processed. On the day of hybridization, sections were fixed in 4% paraformaldehyde at room temperature for 1 hr, followed by three washes in 2x SSC (1x SSC is 150 mM sodium chloride, 15 mM sodium citrate). Sections were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M, pH 8) for 10 min, rinsed in distilled water, dehydrated through graded alcohols (50%, 75%, 85%, 95% and 100%) and air-dried. Rat dopamine D1 (D1R) and D2 (D2R) receptor cDNAs were cloned in our laboratory, antisense linearized, transcribed, and 35S labeled. Reactions were incubated for 90 min at 37 °C and separated from unincorporated nucleotides over Micro Bio-Spin chromatography columns (Bio-Rad, CA). Probes were diluted in hybridization buffer (50% formamide, 10% dextran sulfate, 2x SSC, 50 mM sodium phosphate buffer, pH 7.4, 1x Denhardt’s solution, 0.1 mg/ml yeast tRNA and 10 mM dithiothreitol) to yield 2.5 × 106 dpm/110 µl. Sections were hybridized with probe mixture inside a humidified chamber over night at 55 °C. Next day, sections were washed in 3x SSC for 5 min each, incubated for 1 hr in RNAase (20 mg/ml in Tris buffer containing 0.5 M NaCl, pH 8) at 37 °C. Sections were washed with 2x, 1x and 0.5x SSC, and incubated for 1 hr in 0.1x SSC at 65 °C. After rinsing in distilled water, sections were dehydrated, air dried and exposed to a Kodak XAR film (Eastman Kodak, NY). Section images were captured digitally from x-ray films with a CCD camera, and relative integrated densities were determined using the Image J software. Levels of mRNA for D1R and D2R were quantified in the Nacc. Integrated optical density values were corrected for background and then averaged across multiple sections to produce one data point for each animal. These data points were averaged per group and compared statistically. The Nacc core and shell were analyzed separately but were not different by region; thus, data were collapsed across region.
2.7. Statistical analysis
Data on total distance traveled in response to a novel environment, following the challenge cocaine injection as well as the mRNA levels obtained from in situ hybridization histochemistry were analyzed by two-way ANOVAs: Phenotype (bLR, bHR) X Stress history (CONT, STR). Data on distance traveled following cocaine injections on Day 1 and Day 7 were analyzed by repeated measures ANOVA: Phenotype (bLR, bHR) X Stress history (CONT, STR) X Injection Days (Day 1, Day 7). Interactions were followed by post-hoc (Fisher’s LSD) comparisons and significance was set at p ≤ 0.05. For direct comparisons between groups t tests were used with appropriate α corrections.
3. Results
3.1. The effects of stress history alone on locomotor response to novelty and accumbal D1R and D2R mRNA levels
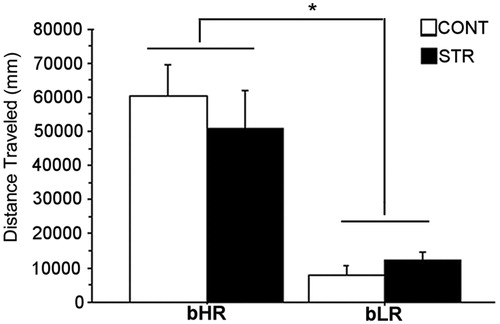
shows the differences in total locomotor response to novelty in bLRs and bHRs at the end of the 60-min habituation period to the activity boxes on day 1 of the cocaine administration regimen (prior to cocaine administration). As expected, a two-way ANOVA revealed a significant main effect of phenotype [F(1,30) = 35.47, p = 0.0001], where stress-naive, as well as stress-exposed bHRs had dramatically higher levels of total distance traveled compared to bLRs. Early adolescent stress exposure did not alter baseline locomotion in either bred line.
Figure 2. Total locomotion in a novel environment measured as distance traveled during the first hour of exposure to the activity boxes. Group means ± SEMs are plotted with the bar graph (*p ≤ 0.05).
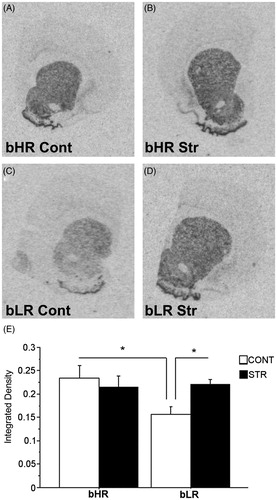
Analyses of D1R mRNA () in the Nacc in non-cocaine exposed adult rats by a two-way ANOVA did not show main effects of Phenotype or Stress history but, revealed an interaction between Phenotype and Stress history [F(1,27) = 4.16, p = 0.050] in this measure. Specific post-hoc comparisons showed that in control (i.e., unstressed) animals, bHRs had higher D1R [p = 0.014] mRNA levels compared to bLRs in the Nacc. Early adolescent CVS resulted in a selective increase in D1R mRNA expression in the bLRs [p = 0.033], such that they became indistinguishable from the bHRs on this measure.
Figure 3. Dopamine D1 receptor mRNA expression in the Nacc 4 weeks after the CVS regimen of a representative stress-naïve bHR rat (A), a CVS-exposed bHR rat (B), a stress-naïve bLR rat (C) and a CVS-exposed bLR rat (D). Panels A, B, C and D show images of coronal hemisections containing the Nacc that were radioactively labeled with an antisense cRNA probe against D1R mRNA and exposed on an x-ray film. Means of quantification results for integrated density ± SEMs are plotted with the bar graph (E; *p ≤ 0.05).
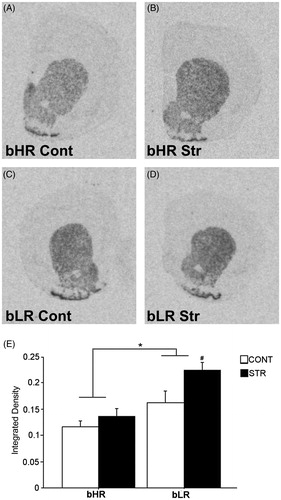
shows D2R mRNA levels in the Nacc in non-cocaine exposed rats. A two-way ANOVA did not reveal an interaction but, showed main effects of Phenotype [F(1,27) = 1.80, p = 0.0002] and Stress history [F(1,27) = 7.04, p = 0.013], indicating that overall, bLRs had higher accumbal D2 mRNA levels compared to bHRs and stress led to an overall upregulation in this measure. Moreover, direct comparisons showed that while the history of early adolescent CVS was without effect in D2R mRNA levels in the Nacc in bHRs, it resulted in a selective increase in bLRs expression levels [p = 0.008].
Figure 4. Dopamine D2 receptor mRNA expression in the Nacc 4 weeks after the CVS regimen of a representative stress-naïve bHR rat (A), a CVS-exposed bHR rat (B), a stress-naïve bLR rat (C) and a CVS-exposed bLR rat (D). Panels A, B, C and D show images of coronal hemisections containing the Nacc that were radioactively labeled with an antisense cRNA probe against D2R mRNA and exposed on an x-ray film. Means of quantification results for integrated density ± SEMs are plotted with the bar graph (E). * represents the main effect of Phenotype, while # represents the direct comparison between stress-exposed and stress-naive bLRs.
3.2. The effects of early adolescent CVS on locomotor sensitization to cocaine and accumbal D1R and D2R mRNA levels in adulthood
3.2.1. Induction phase
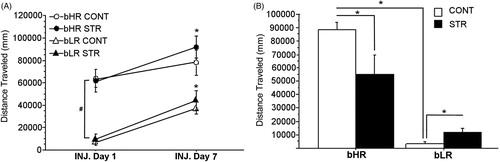
To determine the effects of phenotype and stress history on the locomotor sensitization to cocaine, we compared the total distance traveled following cocaine injections on days 1 and 7 of the induction phase (). Repeated measures ANOVA revealed a significant main effect of Injection Days [F(1,32) = 19.62, p = 0.0001], showing an increase in total distance traveled from Day 1 to Day 7 in both phenotypes. In addition, a main effect of Phenotype [F(1,32) = 73.54, p = 0.0001] was revealed, indicating that overall, bHRs displayed higher rates of locomotion compared to bLRs. On the other hand, there was not a main effect of Stress history or any interaction.
Figure 5. Total locomotor reactivity to cocaine on days 1 and 7 of the induction phase of the sensitization protocol (A), and total locomotor reactivity to a low dose cocaine challenge following 1 week of abstinence (i.e., the expression phase; B). Group means ± SEMs are plotted in line (A) and bar (B) graphs. In panel A, (#) represents the main effect of phenotype in total locomotor reactivity to cocaine on both injection days, whereas (*) represents the main effect of Injection Days in each phenotype.
3.2.2. Expression phase
During the expression phase of the behavioral sensitization to cocaine regimen, when rats were challenged with a low dose of cocaine following 1 week of abstinence (), interesting phenotype-specific stress effects emerged. A two-way ANOVA revealed a main effect of Phenotype [F(1,32) = 73.45, p = 0.0001] on total distance traveled following the challenge injection, in addition to a significant interaction between Phenotype and Stress history [F(1,32) = 7.71, p = 0.009]. Post-hoc comparisons showed that early adolescent CVS history resulted in lower locomotor reactivity to the challenge cocaine in bHRs [p = 0.0271] but higher reactivity in bLRs [p = 0.035] compared to their respective controls. Post-hoc comparisons also showed that stress-naive bHRs displayed dramatically higher locomotor responses to the cocaine challenge compared to stress-naïve bLRs [p = 0.0001] and this effect was preserved in their stress-exposed counterparts [p = 0.0048].
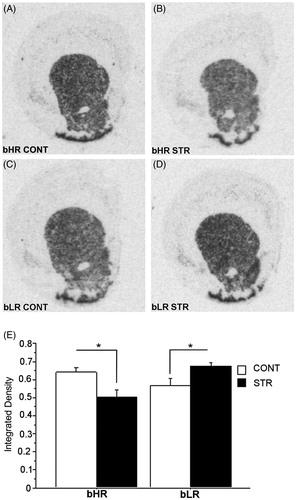
shows D2R mRNA levels in the Nacc in bLRHR rats following the cocaine challenge. A two-way ANOVA indicated a significant interaction between Phenotype and Stress history [F(1,27) = 12.62, p = 0.001]. Moreover, specific post-hoc comparisons showed that the history of early adolescent CVS resulted in decreased D2R mRNA levels in bHRs [p = 0.011] but an increase in this measure in bLRs [p = 0.032] compared to their respective controls. In contrast, no significant changes were observed in D1R mRNA levels in either phenotype relative to what was observed in the absence of cocaine ().
Figure 6. Dopamine D2 receptor mRNA expression in the Nacc following the challenge cocaine injection of a representative stress-naïve bHR rat (A), a stress-exposed bHR rat (B), a stress-naïve bLR rat (C) and a CVS-exposed bLR rat (D). Panels A, B, C and D show images of coronal hemisections of the Nacc that were radioactively labeled with an antisense cRNA probe against D2R mRNA and exposed on an x-ray film. Means of quantification results for integrated density ± SEMs are plotted with the bar graph (E).
4. Discussion
In confirmation of previous reports (Clinton et al., Citation2012; Stead et al., Citation2006), the present data showed that rats that display high levels of novelty-induced locomotion, bHRs, exhibit higher levels of locomotor response to cocaine compared to their counterparts that display low rates of locomotion in novel environments, bLRs. That is to say, the characteristic phenotype is preserved in the presence of a psychostimulant in both strains, rather than one strain being particularly sensitive/insensitive to the effect of cocaine. Also confirming earlier findings (Clinton et al., Citation2012), our data show that the baseline behavioral differences are accompanied by higher D1R and lower D2R mRNA levels in the Nacc in bHRs compared to bLRs, suggesting that dopaminergic tone may underlie the phenotypic differences in response to cocaine observed in these animals. Importantly, the present findings extend on previous work by demonstrating that CVS exposure in adolescence differentially affects behavioral response to a sensitizing cocaine regimen in adulthood depending on the underlying phenotype. Indeed, a history of stress in bHRs decreased locomotor responses to cocaine challenge after abstinence but increased the challenge response in bLRs, relative to the locomotor response to cocaine of animals of the same phenotype without a history of stress. Moreover, these shifts in cocaine sensitivity following adolescent stress were accompanied by parallel shifts in the accumbal dopamine system, with prior stress and cocaine exposure interacting to decrease D2R mRNA in bHRs but increase it in bLRs. Taken together, these data provide novel evidence that the impacts of adolescent stress on subsequent behavioral and neural sensitivity to cocaine are modulated by underlying genetic phenotype, indicating individual variability as a risk factors for SUDs.
Behavioral sensitization to drugs of abuse is thought to occur in two phases: induction and expression. Induction is the immediate neural events that induce behavioral sensitization, and expression is the long-term consequences of these initial events (Kalivas and Stewart, Citation1991). The present data show that although exposure to a CVS regimen in adolescence did not affect behavioral responses during the induction phase of the cocaine sensitization regimen in either phenotype, during the expression phase it increased the sensitivity to the locomotor activating effects of a low dose cocaine in bLRs while decreasing this measure in bHR. A large body of evidence implicates stress in development of addiction-like behaviors in adult animals (Hikida et al., Citation2020; Piazza and Moal, Citation1998). In particular, drug sensitization studies show that whether the stress exposure takes place in adolescence or adulthood determines sensitized responses to psychostimulants. For examples, neither predictable nor unpredictable forms of physical stress in adolescence affect locomotor sensitization to amphetamine or nicotine in adulthood (Cruz et al., Citation2008; Hollis et al., Citation2013; Kabbaj et al., Citation2002) whereas unpredictable physical stress in adulthood enhances sensitization to drugs of abuse (Piazza et al., Citation1990). Similarly, social stress in adulthood induces sensitization to cocaine, whereas in adolescence it exerts the opposite effect against amphetamine sensitization (Kabbaj and Isgor, Citation2007). These findings suggest that stress in adulthood results in sensitization to psychostimulants, but that when the stress occurs in adolescence it may have a protective effect against the sensitizing effects of these drugs; although, contrary findings have also been reported (Hikida et al., Citation2020; Rowson et al., Citation2018). Differences in sex, nature and length of the stress, as well as the drug of choice in these studies may underlie the differential results. To our knowledge, the present study is among the earliest to report that when all these factors are limited and considered, individual differences in the interplay between adolescent stress and vulnerability to the sensitizing effects of drugs are observed.
The expression of behavioral sensitization is thought to occur at the level of the Nacc where dopamine terminals are located (Cador et al., Citation1986; Steketee and Kalivas, Citation2011). Our molecular analyses of the two main dopamine receptor types in this structure showed that in parallel to the behavioral effects observed, D2R mRNA levels were upregulated in stress-exposed bLRs but downregulated in stress-exposed bHRs compared to unstressed controls following the challenge cocaine injection, during the expression phase of the behavioral sensitization to cocaine regimen. The precise role of D2R in the Nacc in the expression of psychostimulant sensitization is unclear. While some studies suggest that psychostimulant sensitization may involve supersensitivity of the postsynaptic D2R (Vanderschuren and Kalivas, Citation2000), others show no specific role for D2R in this process (Kang et al., Citation2017). On the other hand, a specific role for accumbal D2R in the interaction of stress and expression of cocaine sensitization has been reported. In their 2013 study, Sim et al. demonstrated that specific knock-down of D2R in the Nacc confers the ability of stress to inhibit expression of cocaine-induced behavioral sensitization. In line with these findings, our data show that decreased sensitivity to the challenge dose of cocaine observed in bHRs was associated with decreased levels of accumbal D2R mRNA, whereas opposing effects were observed in bLRs. These findings indicate that adolescent stress history affects vulnerability to the sensitizing effects of cocaine in a phenotype-dependent way and affects D2R receptor expression, which points to a D2R- associated mechanism in the Nacc. However, this relationship was not directly tested. Future studies will explore the causal influence of accumbal D2R-signaling on stress-mediated vulnerability to cocaine.
The present data demonstrate that prior to the introduction of cocaine, early adolescent stress alone had long-lasting and phenotype-specific effects in the accumbal dopamine receptor expression and thus likely signaling in the bred lines. Specifically, in bLRs, the CVS history alone upregulated both D1R and D2R mRNA levels in the Nacc, suggesting an increase in the dopaminergic tone in this structure. In contrast, CVS exposure was without an effect in these measures in bHRs. Evidence from clinical and preclinical studies suggests that due to the ability of cortisol and corticosterone to modulate dopamine release (Oswald et al., Citation2005; Wand et al., Citation2007) the mesolimbic dopamine system, which includes the Nacc is highly susceptible to stress and is altered by stressful stimuli (Cabib and Puglisi-Allegra, Citation1996; Krishnan et al., Citation2007). Importantly, the dopamine system goes through transformations within the mesocorticolimbic areas during early adolescence with dopamine receptor (D1-like and D2-like) expression in the Nacc peaking during midadolescence (Tarazi and Baldessarini, Citation2000), marking it as a particularly sensitive period to the effects of stress on the dopaminergic circuitry. Hence, it has been suggested that exposure to stressful events during this time interferes with the programed maturation of the dopamine system, resulting in its altered development (Anderson et al., Citation2009; Burke and Miczek, Citation2014). Indeed, CVS exposure during early adolescence, when the dopamine receptor maturation is highly dynamic, altered accumbal D1R and D2R expression in our animals. Moreover, these alterations were observed in adulthood, long after discontinuation of stress, and in a phenotype-specific manner; without an effect in bHRs but upregulating the mRNA levels of both receptors in bLRs. Furthermore, these long-lasting phenotype-dependent alterations possibly set the stage for a chronic cocaine regimen to further alter this system to result in opposing effects on the expression of behavioral sensitization in these animals. These data indicate that the neural consequences of interfering with the normal maturation of the accumbal dopamine system by way of a CVS regimen in adolescence presents individual variability and may have enduring consequences that can affect vulnerability to the sensitizing effects of cocaine in adulthood.
Notably, although early adolescent CVS exposure alone altered the mRNA levels of both D1R and D2R in bLRs, the interaction of stress history and cocaine was without an effect in D1R mRNA levels in both phenotypes. This is consistent with reports showing that sensitized behavioral responses to D1R agonists in psychostimulant pretreated animals are not observed following either systemic (Levy et al., Citation1988; Vanderschuren et al., Citation1999) or intra-accumbens administration (Pierce and Kalivas, Citation1995), suggesting that D1R in the Nacc may not be directly involved in this process (Vanderschuren and Kalivas, Citation2000). However, it has been suggested that although D1R signaling does not directly mediate behavioral sensitization, it may contribute to the behavioral responses to psychostimulants by altering other relevant receptors in the Nacc, such as D2R (Hu and White, Citation1997; Vanderschuren and Kalivas, Citation2000). Therefore, it is plausible to suggest that the transient increase in D1R in stress-exposed bLRs may be indirectly involved in the heightened sensitivity to the low dose cocaine challenge observed after 1 week of abstinence.
The present study examined only locomotor sensitization to cocaine. However, the results seen here following adolescent stress imply that the bHR/bLR phenotypes are differentially affected by adolescent stress. Furthermore, and importantly, this indicates that adolescent experience of stress will likely differentially impact the motivation for cocaine in a phenotype-specific manner. Namely, it can be predicted that the experience of adolescent stress would render the bLRs, normally poor drug seekers/takers in I.V. self-administration paradigms, more likely to quickly acquire self-administration. The experience may perhaps also alter the incentive salience of drug-related cues, making bHRs and bLRs more susceptible to cue-induced reinstatement following extinction. Such influences of adolescent stress on cocaine motivation have been explored in outbred rat models, including traditional HR/LR models (Kabbaj et al., Citation2001), but should be examined using the bred lines.
It should be noted that the present work was conducted in males only. Sex differences are known to be present in adult stress reactivity (Weintraub et al., Citation2010) with females exhibiting heightened hormonal and behavioral stress responses in adulthood following a history of adolescent social isolation, relative to males. Likewise, adolescent stress results in greater alcohol seeking reinstatement in females than males (Bertholomey et al., Citation2016). These findings parallel our observations which find that the bHR/bLR phenotypic difference in anxiety-like behavior are magnified in females in preliminary characterizations (unpublished data). Thus, it is predicted that the differences observed here would be magnified in females. Future studies will examine the effect of adolescent stress on adult addictive-like behaviors in female bHR/bLR rats.
It should also be noted that the behavioral response to cocaine and the effect on D2R were not observed immediately following cocaine sensitization but were observed following 1 week of withdrawal/abstinence following. Thus, the role of the stress of withdrawal itself cannot be distinguished from the interaction between prior stress and cocaine. Future studies will need to be conducted to tease these two mechanisms apart.
In sum, the present study shows that a chronic variable stress regimen in early adolescence results in opposing outcomes on vulnerability to cocaine in adulthood in rats that display innate differences in stress reactivity and psychostimulant propensity. These results suggest that response to drugs of abuse in adulthood following stressful life events in adolescence presents individual variability and depend on the interaction of developmental events and environmental challenges with the genetic make-up of the individual, highlighting the need to consider individual variability in assessing risk factors for SUDs. While it is generally accepted in the substance abuse field that inborn genetic differences and early life experiences are, separately, important factors to consider when regarding treatment, these findings add to a growing body of evidence that the interplay between individual temperament and previous experience of stress/trauma is an important factor to consider when developing treatments for patients with SUDs. Specifically, individuals with ‘internalizing’ personality types who have experienced significant early life adversity may be at considerably higher risk for psychostimulant use/abuse in adulthood. This stands contrary to the conventional wisdom (and empirical findings) that such individuals are more resilient to drug abuse in the absence of such adversity. Alternatively, our results indicate that the experience of adolescent stress may confer a protective effect to bHRs, reflective of individuals with ‘externalizing’ personality traits, high degrees of sensation-seeking and novelty-seeking. Our results also showed that cocaine interacts with stress history through a likely-D2R-mediated mechanism in the Nacc, further implicating the mesolimbic dopamine system in the interaction of stress and drugs of abuse. Future studies will examine the causal relationship between the molecular and behavioral changes observed in the present study.
Author contributions
C. A and H.A designed the experiments. P.B.J oversaw the breeding of the bHR/bLR rats. C.A and K.F conducted the experiments and analyzed the data. C.A, M.E and H.A wrote the manuscript. All authors critically reviewed the content and approved the final version for publication.
Acknowledgements
The authors thank Dr. Pamela Maras for her valuable comments in preparation of the manuscript, and James Stewart and Katelyn Roberts for their technical assistance.
Disclosure statement
Authors have no potential conflict of interest to declare.
Additional information
Funding
References
- Anderson, J. G., Fordahl, S. C., Cooney, P. T., Weaver, T. L., Colyer, C. L., & Erikson, K. M. (2009). Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Research, 1281, 1–14. https://doi.org/10.1016/j.brainres.2009.05.050
- Aydin, C., Oztan, O., & Isgor, C. (2011). Vulnerability to nicotine abstinence-related social anxiety-like behavior: Molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neuroscience Letters, 490(3), 220–225. https://doi.org/10.1016/j.neulet.2010.12.056
- Bertholomey, M. L., Nagarajan, V., & Torregrossa, M. M. (2016). Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology, 233(12), 2277–2287. https://doi.org/10.1007/s00213-016-4278-x
- Burke, A. R., & Miczek, K. A. (2014). Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology, 231(8), 1557–1580. https://doi.org/10.1007/s00213-013-3369-1
- Cabib, S., & Puglisi-Allegra, S. (1996). Stress, depression and the mesolimbic dopamine system. Psychopharmacology, 128(4), 331–342. https://doi.org/10.1007/s002130050142
- Cabib, S., Castellano, C., Cestari, V., Filibeck, U., & Puglisi-Allegra, S. (1991). D1 and D2 receptor antagonists differently affect cocaine-induced locomotor hyperactivity in the mouse. Psychopharmacology, 105(3), 335–339. https://doi.org/10.1007/BF02244427
- Cador, M., Kelley, A. E., Le Moal, M., & Stinus, L. (1986). Ventral tegmentalarea infusions of substance P, neurotensin and enkephalin: Differ-ential effects on feeding behavior. Neuroscience, 18(3), 659–669. https://doi.org/10.1016/0306-4522(86)90061-8
- Chen, W., Nong, Z., Li, Y., Huang, J., Chen, C., & Huang, L. (2017). Role of dopamine signaling in drug addiction. Current Topics in Medicinal Chemistry, 17(21), 2440–2455. https://doi.org/10.2174/1568026617666170504100642
- Clinton, S. M., Turner, C. A., Flagel, S. B., Simpson, D. N., Watson, S. J., & Akil, H. (2012). Neonatal fibroblast growth factor treatment enhances cocaine sensitization. Pharmacology, Biochemistry, and Behavior, 103(1), 6–17. https://doi.org/10.1016/j.pbb.2012.07.006
- Cruz, F. C., DeLucia, R., & Planeta, C. S. (2008). Effects of chronic stress on nicotine-induced locomotor activity and corticosterone release in adult and adolescent rats. Addiction Biology, 13(1), 63–69. https://doi.org/10.1111/j.1369-1600.2007.00080.x
- Davis, B. A., Clinton, S. M., Akil, H., & Becker, J. B. (2008). The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacology, Biochemistry, and Behavior, 90(3), 331–338. https://doi.org/10.1016/j.pbb.2008.03.008
- Enoch, M. A. (2012). The Influence of gene-environment interactions on the development of alcoholism and drug dependence. Current Psychiatry Reports, 14(2), 150–158. https://doi.org/10.1007/s11920-011-0252-9
- Flagel, S. B., & Robinson, T. E. (2007). Quantifying the psychomotor activating effects of cocaine in the rat. Behavioural Pharmacology, 18(4), 297–302. https://doi.org/10.1097/FBP.0b013e3281f522a4
- Flagel, S. B., Chaudhury, S., Waselus, M., Kelly, R., Sewani, S., Clinton, S. M., Thompson, R. C., Watson, S. J. Jr., & Akil, H. (2016). Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proceedings of the National Academy of Sciences of the United States of America, 113(20), E2861–70. https://doi.org/10.1073/pnas.1520491113
- Flagel, S. B., Robinson, T. E., Clark, J. J., Clinton, S. M., Watson, S. J., Seeman, P., Phillips, P. E. M., & Akil, H. (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology : official Publication of the American College of Neuropsychopharmacology, 35(2), 388–400. https://doi.org/10.1038/npp.2009.142
- Francis, T. C., & Lobo, M. K. (2017). Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biological Psychiatry, 81(8), 645–653. https://doi.org/10.1016/j.biopsych.2016.09.007
- Garcia-Fuster, M. J., Parsegian, A., Watson, S. J., Akil, H., & Flagel, S. B. (2017). Adolescent cocaine exposure enhances goal-tracking behavior and impairs hippocampal cell genesis selectively in adult bred low-responder rats. Psychopharmacology, 234(8), 1293–1305. https://doi.org/10.1007/s00213-017-4566-0
- Green, J. G., McLaughlin, K. A., Berglund, P. A., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., & Kessler, R. C. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. https://doi.org/10.1001/archgenpsychiatry.2009.186
- Hikida, T., Morita, M., Kuroiwa, M., Macpherson, T., Shuto, T., Sotogaku, N., Niwa, M., Sawa, A., & Nishi, A. (2020). Adolescent psychosocial stress enhances sensitization to cocaine exposure in genetically vulnerable mice. Neuroscience Research, 151, 38–45. https://doi.org/10.1016/j.neures.2019.02.007
- Hoffman, J. H., Barnes, G. M., Welte, J. W., & Dintcheff, B. A. (2000). Trends in combinational use of alcohol and illicit drugs among minority adolescents, 1983-1994. The American Journal of Drug and Alcohol Abuse, 26(2), 311–324. https://doi.org/10.1081/ADA-100100607
- Hollis, F., Isgor, C., & Kabbaj, M. (2013). The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience, 249, 232–241. https://doi.org/10.1016/j.neuroscience.2012.09.018
- Hu, X. T., & White, F. J. (1997). Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neuroscience Letters, 224(1), 61–65. https://doi.org/10.1016/S0304-3940(97)13443-7
- Isgor, C., Kabbaj, M., Akil, H., & Watson, S. J. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus, 14(5), 636–648. https://doi.org/10.1002/hipo.10207
- Kabbaj, A., & Isgor, C. (2007). Effects of chronic environmental and social stimuli during adolescence on mesolimbic dopaminergic circuitry markers. Neuroscience Letters, 422(1), 7–12. https://doi.org/10.1016/j.neulet.2007.04.088
- Kabbaj, M., Isgor, C., Watson, S. J., & Akil, H. (2002). Stress during adolescence alters behavioral sensitization to amphetamine. Neuroscience, 113(2), 395–400. https://doi.org/10.1016/S0306-4522(02)00188-4
- Kabbaj, M., Norton, C. S., Kollack-Walker, S., Watson, S. J., Robinson, T. E., & Akil, H. (2001). Social defeat alters the acquisition of cocaine self-administration in rats: Role of individual differences in cocaine-taking behavior. Psychopharmacology, 158(4), 382–387. https://doi.org/10.1007/s002130100918
- Kai, N., Nishizawa, K., Tsutsui, Y., Ueda, S., & Kobayashi, K. (2015). Differential roles of dopamine D1 and D2 receptor-containing neurons of the nucleus accumbens shell in behavioral sensitization. Journal of Neurochemistry, 135(6), 1232–1241. https://doi.org/10.1111/jnc.13380
- Kalivas, P. W., & Stewart, J. (1991). Dopamine transmission in the initiation and expression of drug-induced and stress-induced sensitization of motor-activity. Brain Research Reviews, 16(3), 223–244. https://doi.org/10.1016/0165-0173(91)90007-U
- Kang, B. J., Song, S. S., Wen, L., Hong, K. P., Augustine, G. J., & Baik, J. H. (2017). Effect of optogenetic manipulation of accumbal medium spiny neurons expressing dopamine D2 receptors in cocaine-induced behavioral sensitization. The European Journal of Neuroscience, 46(4), 2056–2066. https://doi.org/10.1111/ejn.13648
- Kerman, I. A., Clinton, S. M., Bedrosian, T. A., Abraham, A. D., Rosenthal, D. T., Akil, H., & Watson, S. J. (2011). High novelty-seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain Research, 1419, 34–45. https://doi.org/10.1016/j.brainres.2011.08.038
- Krishnan, V., Han, M. H., Graham, D. L., Berton, O., Renthal, W., Russo, S. J., LaPlant, Q., Graham, A., Lutter, M., Lagace, D. C., Ghose, S., Reister, R., Tannous, P., Green, T. A., Neve, R. L., Chakravarty, S., Kumar, A., Eisch, A. J., Self, D. W., Lee, F. S., Tamminga, C. A., Cooper, D. C., Gershenfeld, H. K., & Nestler, E. J. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell, 131(2), 391–404. https://doi.org/10.1016/j.cell.2007.09.018
- Levy, A. D., Kim, J. J., & Ellison, G. D. (1988). Chronic amphetamine alters D-2 but not D-1 agonist-induced behavioral responses in rats. Life Sciences, 43(15), 1207–1213. https://doi.org/10.1016/0024-3205(88)90210-X
- Oswald, L. M., Wong, D. F., McCaul, M., Zhou, Y., Kuwabara, H., Choi, L., Brasic, J., & Wand, G. S. (2005). Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 30(4), 821–832. https://doi.org/10.1038/sj.npp.1300667
- Oztan, O., Aydin, C., & Isgor, C. (2011). Chronic variable physical stress during the peripubertal-juvenile period causes differential depressive and anxiogenic effects in the novelty-seeking phenotype: Functional implications for hippocampal and amygdalar brain-derived neurotrophic factor and the mossy fibre plasticity. Neuroscience, 192, 334–344. https://doi.org/10.1016/j.neuroscience.2011.06.077
- Piazza, P. V., & Moal, M. L. (1998). The role of stress in drug self-administration. Trends in Pharmacological Sciences, 19(2), 67–74. https://doi.org/10.1016/S0165-6147(97)01115-2
- Piazza, P. V., Deminière, J. M., Le Moal, M., & Simon, H. (1989). Factors that predict Individual vulnerability to amphetamine self-administration. Science (New York, N.Y.).), 245(4925), 1511–1513. https://doi.org/10.1126/science.2781295
- Piazza, P. V., Deminiere, J. M., Le Moal, M., & Simon, H. (1990). Stress-induced and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Research, 514(1), 22–26. https://doi.org/10.1016/0006-8993(90)90431-A
- Pierce, R. C., & Kalivas, P. W. (1995). Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus-accumbens shell of rats administered repeated cocaine. The Journal of Pharmacology and Experimental Therapeutics, 275(2), 1019–1029.
- Rana, S., Nam, H., Glover, M. E., Akil, H., Watson, S. J., Clinton, S. M., & Kerman, I. A. (2016). Protective effects of chronic mild stress during adolescence in the low-novelty responder rat. Stress (Amsterdam, Netherlands)), 19(1), 133–138. https://doi.org/10.3109/10253890.2015.1108304
- Romeo, R. D. (2015). Perspectives on stress resilience and adolescent neurobehavioral function. Neurobiology of Stress, 1, 128–133. https://doi.org/10.1016/j.ynstr.2014.11.001
- Rovaris, D. L., Mota, N. R., Bertuzzi, G. P., Aroche, A. P., Callegari-Jacques, S. M., Guimaraes, L. S. P., Pezzi, J. C., Viola, T. W., Bau, C. H. D., & Grassi-Oliveira, R. (2015). Corticosteroid receptor genes and childhood neglect influence susceptibility to crack/cocaine addiction and response to detoxification treatment. Journal of Psychiatric Research, 68, 83–90. https://doi.org/10.1016/j.jpsychires.2015.06.008
- Rowson, S. A., Foster, S. L., Weinshenker, D., & Neigh, G. N. (2018). Locomotor sensitization to cocaine in adolescent and adult female Wistar rats. Behavioural Brain Research, 349, 158–162. https://doi.org/10.1016/j.bbr.2018.04.035
- Sim, H. R., Choi, T. Y., Lee, H. J., Kang, E. Y., Yoon, S., Han, P. L., Choi, S. Y., & Baik, J. H. (2013). Role of dopamine D2 receptors in plasticity of stress-induced addictive behaviours. Nature Communications, 4, 1579https://doi.org/10.1038/ncomms2598
- Southwick, S. M., Vythilingam, M., & Charney, D. S. (2005). The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annual Review of Clinical Psychology, 1, 255–291. https://doi.org/10.1146/annurev.clinpsy.1.102803.143948
- Stead, J. D. H., Clinton, S., Neal, C., Schneider, J., Jama, A., Miller, S., Vazquez, D. M., Watson, S. J., & Akil, H. (2006). Selective breeding for divergence in novelty-seeking traits: Heritability and enrichment in spontaneous anxiety-related behaviors. Behavior Genetics, 36(5), 697–712. https://doi.org/10.1007/s10519-006-9058-7
- Stedenfeld, K. A., Clinton, S. M., Kerman, I. A., Akil, H., Watson, S. J., & Sved, A. F. (2011). Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & Behavior, 103(2), 210–216. https://doi.org/10.1016/j.physbeh.2011.02.001
- Steketee, J. D., & Kalivas, P. W. (2011). Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Reviews, 63(2), 348–365. https://doi.org/10.1124/pr.109.001933
- Tarazi, F. I., & Baldessarini, R. J. (2000). Comparative postnatal development of dopamine D-1, D-2 and D-4 receptors in rat forebrain. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 18(1), 29–37. https://doi.org/10.1016/S0736-5748(99)00108-2
- Tirelli, E., Laviola, G., & Adriani, W. (2003). Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neuroscience and Biobehavioral Reviews, 27(1-2), 163–178. https://doi.org/10.1016/S0149-7634(03)00018-6
- Vanderschuren, L. J. M. J., & Kalivas, P. W. (2000). Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology, 151(2-3), 99–120. https://doi.org/10.1007/s002130000493
- Vanderschuren, L. J. M. J., Wardeh, G., De Vries, T. J., Mulder, A. H., & Schoffelmeer, A. N. M. (1999). Opposing role of dopamine D1 and D2 receptors in modulation of rat nucleus accumbens noradrenaline release. The Journal of Neuroscience, 19(10), 4123–4131. https://doi.org/10.1523/JNEUROSCI.19-10-04123.1999
- Wand, G. S., Oswald, L. M., McCaul, M. E., Wong, D. F., Johnson, E., Zhou, Y., Kuwabara, H., & Kumar, A. (2007). Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology: official Publication of the American College of Neuropsychopharmacology, 32(11), 2310–2320. https://doi.org/10.1038/sj.npp.1301373
- Weintraub, A., Singaravelu, J., & Bhatnagar, S. (2010). Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Research, 1343, 83–92. https://doi.org/10.1016/j.brainres.2010.04.068
- Wilkin, M. M., Waters, P., McCormick, C. M., & Menard, J. L. (2012). Intermittent physical stress during early- and mid-adolescence differentially alters rats' anxiety- and depression-like behaviors in adulthood. Behavioral Neuroscience, 126(2), 344–360. https://doi.org/10.1037/a0027258