Abstract
Pathways by which inflammatory stimuli influence behaviors can involve changes in neuronal plasticity, however, the evidence for this is still insufficient. This study aimed to evaluate the effects of chronic lipopolysaccharide (LPS) injected alone or together with tetracycline antibiotic doxycycline (Dox) on the levels of Iba-1, BDNF, Bcl-xL and MMP-9 in brain regions in relation to stress-induced behaviors in the elevated plus-maze (EPM). LPS injected to adult rats every 2 days for a total of 7 injections reduced body weight gain, increased spleen and adrenal weights, decreased locomotor activity, and increased anxiety-like behavior. These effects were associated with increased expression of Iba-1, a well-known marker for activated microglia, in most brain regions investigated. Co-treatment of LPS with Dox attenuated LPS-induced microglial activation and behavioral changes, supporting their relation to the neuroinflammation. LPS administration also produced pro-apoptotic changes in the brain. In the hypothalamus and striatum, the levels of anti-apoptotic protein Bcl-xL were decreased, whereas in the amygdala, a significant increase in MMP-9 protein levels was observed. The levels of Iba-1 as well as MMP-9 in the amygdala positively correlated with the numbers of defecation. The data suggest that mechanisms of anxiety associated with neuroinflammation may involve the increase in MMP-9 levels in the amygdala.
Introduction
It has been suggested that neuroinflammation resulted from stressful or bacterial experiences is a risk factor for mood disorders including anxiety. In animal studies, chronic stress was shown to increase the levels of cytokines in the hippocampus (Costa-Nunes et al., Citation2020; Mograbi et al., Citation2020) and induce an anxiety-like behavior (Costa-Nunes et al., Citation2020). The neuroinflammatory responses are mediated mainly by the microglia, the resident immune cells of the brain; the commonly used marker for the activated microglia is the ionized calcium-binding adaptor molecule 1 (Iba-1) (Hoogland et al., Citation2015). Peripheral injections of lipopolysaccharide (LPS), a component of Gram-negative bacterial cell wall, to experimental animals mimic stress-induced neuroinflammation evidenced by an increase in Iba-1 expression in the central nervous system (Khan et al., Citation2017), and induce the behavioral signs of sickness (Dantzer et al., Citation2008). Similar to the effects of LPS on adult animals, the administration of endotoxin to healthy volunteers also increased anxious symptoms (Reichenberg et al., Citation2001) and induced microglial activation in the brain (Sandiego et al., Citation2015). Therefore, the administration of LPS became widely used approach for examining mechanisms, by which an activation of the systemic inflammation induces psychopathological effects.
Among the proposed pathways of the influence of the inflammatory agents on behaviors are the changes in expression of neurotrophic and apoptotic regulators however supporting in vivo evidence for this is still insufficient. Reported LPS effects on brain-derived neurotrophic factor (BDNF) that plays an important role in the survival and growth of neurons are conflicting and ranged from the decrease (Ma et al., Citation2017; Golia et al., Citation2019) to no alteration (Shaw et al., Citation2001) or even increase (Miwa et al., Citation1997) in the neurotrophin expression. LPS can induce apoptosis partly by decreasing the expression of anti-apoptotic proteins in the cortex and hippocampus (Khan et al., Citation2017). However, in another study, the expression of anti-apoptotic factor Bcl-xL was increased in the hippocampus of LPS-treated animals (Dang et al., Citation2018). Finally, structural brain abnormalities observed in patients with inflammatory illness (Shoemaker et al., Citation2014) indicate a possible role of matrix metalloproteinases (MMPs) especially MMP-9 that is involved in the degradation of extracellular matrix and pathogenesis of neuroinflammatory diseases (Könnecke & Bechmann, Citation2013). LPS can enhance the expression of MMP-9 in the rat brain astrocytes (Yang et al., Citation2019).
Tetracycline antibiotic doxycycline (Dox) easily penetrates the blood–brain barrier and can directly affect the microglial cell activation (Santa-Cecília et al., Citation2016). Dox was shown to be protective against cell damage in the hippocampus after global cerebral ischemia by inhibiting MMP-9 activity (Lee et al., Citation2009). We hypothesized that the antibiotic would prevent LPS-induced behavioral effects via attenuation of the neuroinflammation and neurodegenerative factors in the adult rat brain. To test this hypothesis, in this study, the effects of chronic LPC injected alone or in combination with Dox, on the levels of Iba-1, BDNF, Bcl-xL, and MMP-9 in brain regions in relation to stress-induced behaviors in the elevated plus-maze (EPM) test were examined.
Methods
Adult male Wistar rats of four groups treated with saline (Sal), Dox, LPS or LPS + Dox were used. Animals (8 weeks of age) were housed singly in polycarbonate cages (27.7 × 44 × 15 cm = w × l × h) with free access to food and water. Rats were kept at a temperature of 22 °C–23 °C under natural illumination. All animal use procedures were supervised and specifically approved by the ethic committee of the Institute of Cytology and Genetics in accordance with the guidelines of the Ministry of Public Health of Russia (supplement to order N 267 of June 19, 2003) and the European Council Directive (86/609/EEC). All efforts were made to minimize animal suffering and to reduce the number of animals used.
LPS from Escherichia coli serotype 055:B5 (Sigma-Aldrich Corp., St Louis, MO, USA), was injected intraperitoneally (i.p.) at a dose of 0.5 mg/kg every 2 days for a total of 7 injections, according to a published protocol (Dang et al., Citation2018), which effectively provoked neuroinflammation. Dox (Sigma-Aldrich Corp., St Louis, MO, USA) was administered daily at a dose of 25 mg/kg, i.p. throughout the period of LPS treatment. Saline in Sal and LPS groups was administered once daily and half of the Sal animals – twice a day, to mimic the Dox and LPS + Dox group manipulation. At the beginning of the experiment, animal weights were as follows: Sal – 185.3 ± 8.2 (6), Dox – 184.8 ± 3.6 (6), LPS – 182.6 ± 9.3 (8), LPS + Dox – 180.8 ± 10.9 (6).
During the experiment, all animals were weighed daily. Twenty-four hours after the last injection, rats were tested in the EPM. Two days after the test, animals were quickly decapitated, adrenals and spleen were weighed and brain tissue samples (prefrontal cortex, hippocampus, amygdala, hypothalamus, striatum, midbrain, and brainstem) were collected for protein determination by Western blot.
EPM test
The EPM test (Pellow et al., Citation1985; Walf & Frye, Citation2007) was performed between 2:00 and 4:00 p.m. The maze elevated 65 cm above the floor was consisted of two opposite open arms (45 × 10 cm2) and two opposite enclosed arms (45 × 10 × 40 cm3). All four arms were connected to a 10 × 10 cm2 center square. Before the next rat was introduced, the maze was cleaned with a water-wet sponge and then dried. The behavior of the animals was recorded on video for later analysis. Each rat was placed in the center of the maze facing an open arm. During the 5-min test, standard measures such as percentage of entries into the open arms, number of rears, fecal boli were quantified. Arm entry defined when all four paws were on the arm. In addition, the numbers of rearing (rising on the hind limbs) in the closed arms, and the number of defecation in the test were also analyzed. The distance traveled in the EPM was determined by transforming the number of 10-cm squares crossed by the animals on the open and closed arms into the distance run.
Western blotting
To estimate the protein levels by Western blot analysis, brain tissue samples were homogenized in lysis buffer containing 150 mM NaCl, 50 mM Tris, 1% Triton X-100, and protease inhibitors (2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml pepstatin, and 2 μg/ml aprotinin). Protein samples (50 μg) were fractionated by electrophoresis in 12% polyacrylamide gel with sodium dodecyl sulfate in a Mini-Protean 3 Dodeca Cell (Bio-Rad, Hercules, CA, USA) and transferred onto 0.45-μm nitrocellulose membrane with a Trans-Blot system (Bio-Rad). The proteins were stained with primary antibodies: rabbit monoclonal antibodies Iba-1 (dilution 1:500; EPR16589, ab178847, Abcam, Cambridge, MA), MMP-9 (dilution 1:500; EP1254, ab76003, Abcam, Cambridge, MA) and rabbit polyclonal antibodies BDNF (dilution 1:500; N-20, sc-546, Santa Cruz Biotechnology, Dallas, TX, USA), Bcl-xS/L (dilution 1:500; S-18, sc-634, Santa Cruz Biotechnology, Dallas, TX, USA) and beta-actin (dilution 1:20000; I-19, sc-1616, Santa Cruz Biotechnology, Dallas, TX, USA). Secondary anti-rabbit IgG (Bio-Rad, Hercules, CA, USA) were used at 1:1000 dilutions for Iba-1, MMP-9, BDNF, and Bcl-xL staining and 1:10,000 dilution for beta-actin staining. The blots were developed with a SuperSignalTM West Femto Maximum Sensitivity Substrate chemiluminescence kit (Life Technologies, Carlsbad, CA, USA) and quantified after scanning with a ChemidocTM Touch Imaging System (Bio-Rad, Hercules, CA, USA) using the Scion Image 4.0.3.2 program (Scion Corporation, Chicago, IL, USA). The amounts of Iba-1, MMP-9, BDNF and Bcl-xL proteins were expressed in arbitrary units relatively to the amounts of beta-actin in the same sample.
Statistical analysis
Statistical analysis was performed by two-way ANOVA (factors LPS and Dox) followed by a Tukey’s multiple comparison post-hoc test. The body weight gain was analyzed by two-way ANOVA for repeated measures. Student’s t test was used for the direct comparisons of the two independent groups. Pearson’s correlation analysis was used to evaluate relationships between parameters. The results were considered significant at a probability level of less than 0.05.
Results
Effects of LPS or/and Dox on body weight gain, spleen, and adrenal weights
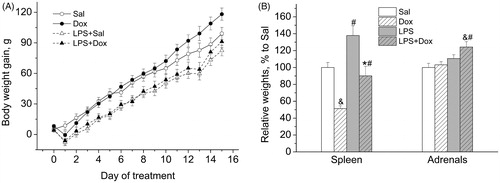
There was a decreasing effect of LPS on the body weight gain [main effect of LPS: F(1,22) = 18.225, p < 0.001] (). This effect was not influenced by the co-treatment with Dox [main effect of Dox: F(1,22) = 1.021, NS; main effect of LPS × Dox interaction: F(1,22) = 0.070, NS]. LPS increased the relative weights of spleen (mg/g BW) () [main effect of LPS, two-way ANOVA: F(1,22) = 16.293, p < 0.001], and Dox decreased this LPS-induced increase in the weights of spleen [main effect of Dox: F(1,22) = 25.775, p < 0.001]. There was a significant increasing effect of LPS on the relative weights of adrenals (mg/100 g BW) [main effect of LPS: F(1,22) = 14.744, p < 0.001].
Figure 1. (A) Repeated LPS administration reduced body weight gain. (B) LPS increased the relative weights of spleen and adrenals. Co-treatment with Dox prevented the effect of LPS on spleen, but not on the adrenals. Data are presented as Mean ± SEM. &p < 0.05 vs Sal, #p < 0.05 vs. corresponding group without LPS, *p < 0.05 vs. LPS.
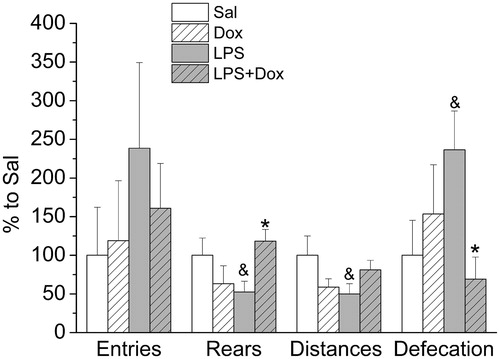
Effects of LPS or/and Dox on behaviors in the EPM test
Treatments with LPS and Dox, alone or together, had no effects on the number of entries in the open arms, a classical measure of anxiety-like behavior in the EPM test (). However, the significant effects of both drugs on locomotor activity make it difficult to interpret the test results as anxiety-like behavior. After LPS, there was a significant decrease in the number of rears and the distance traveled on the arms. Co-treatment with Dox prevented decreases in both parameters. This reflects interactions between LPS and Dox [for rears: F(1,22) = 7.583, p < 0.05; distance: F(1,22) = 5.495, p < 0.05]. Numbers of defecation, an additional index of anxiety-related behavior, were significantly increased in LPS-treated animals and this increase was prevented by co-treatment with Dox [interaction between LPS and Dox: F(1,22) = 4.912, p < 0.05].
Effects of LPS or/and Dox on Iba-1, BDNF and Bcl-xL protein levels in brain regions in relation to behaviors in the EPM
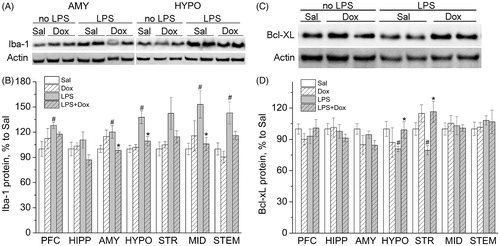
Evaluation of microglial activation in LPS or/and Dox treated animals showed () that Iba-1 protein levels were significantly increased by endotoxin in six of the seven brain regions investigated. Two-way ANOVA revealed a significant increasing effect of LPS on Iba-1 in the prefrontal cortex [F(1,18) = 4.637, p < 0.05], striatum [F(1,21) = 4.472, p < 0.05] and brainstem [F(1,21) = 11.468, p < 0.01]. A significant interaction between LPS and Dox was observed in the amygdala [F(1,22) = 8.219, p < 0.01] and the midbrain [F(1,21) = 5.214, p < 0.05]. In the hypothalamus, significant effects of both LPS [F(1,22) = 16.391, p < 0.001] and Dox [F(1,22) = 5.580, p < 0.05] as well as their interaction [F(1,22) = 7.454, p < 0.05] were found. There were no effects in the hippocampus. Dox alone did not influence Iba-1, but attenuated LPS-induced increase in Iba-1 levels in some brain regions. Post hoc analysis revealed a decrease in Iba-1 levels in rats treated in addition to LPS with Dox in the amygdala (p < 0.05), hypothalamus (p < 0.01), and the midbrain (p < 0.05).
Figure 3. (A) Representative blots of proteins from amygdala and hypothalamus. (B) Iba-1 protein levels were significantly increased by LPS in 6 from 7 brain regions investigated as compared with saline. Dox alone did not influence Iba-1, but attenuated its increase induced by LPS in some brain regions. (C) Representative blots of proteins from the hypothalamus. (D) Compared with saline, Bcl-xL protein levels were significantly decreased after LPS in the hypothalamus and striatum. Dox alone did not influence Bcl-xL, but prevented LPS-induced decrease in Bcl-xL levels in both brain regions. Data are presented as Mean ± SEM. #p < 0.05 vs. corresponding group without LPS, *p < 0.05 vs. LPS. PFC: prefrontal cortex; HIPP: hippocampus; AMY: amygdala: HYPO: hypothalamus; STR: striatum; MID: midbrain; STEM: brainstem.
Correlation analysis performed in all experimental groups revealed correlations between Iba-1 protein levels and values of plus-maze behaviors. Significant (p < 0.05) negative correlations were found between Iba-1 levels in the amygdala (r = −0.44), hypothalamus (r = −0.55), striatum (r = −0.56), midbrain (r = −0.59) and the number of rears. There were significant (p < 0.05) positive correlations between Iba-1 levels in the amygdala (r = 0.52) and hypothalamus (r = 0.55), and the number of defecation.
No effects of LPS or Dox or their interaction were obtained for BDNF protein levels (data not shown).
The levels of anti-apoptotic protein Bcl-xL were significantly decreased after LPS in the striatum, whereas co-treatment with Dox prevented this effect of LPS [main effect of Dox: F(1,21) = 13.075, p < 0.01] (). Although the interaction between LPS and Dox for hypothalamic Bcl-xL was not statistically significant [F(1,22) = 3.486, p = 0.075], t-test showed similar differences in this structure also: Sal vs. LPS – t(12) = 2.787; p < 0.05; LPS vs. LPS + Dox – t(12) = −2.436; p < 0.05.
Effects of LPS or/and Dox on MMP-9 protein levels in the amygdala in relation to behaviors in the EPM
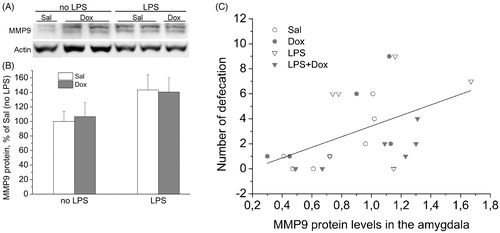
Analysis of the levels of MMP-9 protein revealed significant up-regulating effects of LPS on MMP-9 in the amygdala [main effect of LPS: F(1,21) = 4.387, p < 0.05] (). LPS-induced elevation in MMP-9 levels was not prevented by co-treatment with Dox. There was a significant positive correlation between amygdala protein levels of MMP-9 and the number of defecation in the EPM test ().
Figure 4. (A) Representative blot of MMP-9 in the protein samples of the amygdala. (B) Western blotting analysis of MMP-9 showed an increasing effect of LPS in the amygdala. Data are presented as Mean ± SEM. (C) Positive correlation (r = 0.497, p < 0.05) of the amygdala MMP-9 protein levels with the numbers of defecations in the EPM. Correlations between parameters for separate groups: Sal – r = 0.82, n = 6; Dox – r = 0.65, n = 6; LPS – r = 0.28, n = 8; LPS + Dox – r = 0.80, n = 6.
Discussion
Investigations of the effects of LPS on microglial activation were mainly focused on the hippocampus and prefrontal cortex. In our study, the protein levels of Iba-1, the microglia activation marker, were determined after LPS in seven brain regions and in most of them, a significant increase in Iba-1 was observed. The greatest responses to LPS were found in the midbrain, brainstem, striatum, and hypothalamus and in a lesser extent, in the amygdala and prefrontal cortex. Surprisingly, in our experimental conditions, there was no significant increase in the levels of Iba-1 in the hippocampus, although expression of the pro-inflammatory cytokine interleukin-1 beta demonstrated a significant increase in this structure after LPS in our (unpublished data) and other (Dang et al., Citation2018) studies. The presence of an increase in the levels of Iba-1 or pro-inflammatory cytokines is sufficient to confirm the activation of neuroinflammation (Hoogland et al., Citation2015). However, published neuroinflammatory responses to repeated LPS vary between studies. For example, the absence of any signs of neuroinflammatory activation was observed after injections of LPS for 26 days (Tiwari et al., Citation2016). Differences in the LPS effects may be due to revealed interaction between the dose of endotoxin and duration of its administration, as well as the time elapsed after exposure to endotoxin and sample collection for analysis (Lopes, Citation2016). The importance of consideration of the dose and timing is supported by numerous data. Thus, Tiwari et al. (Citation2016) collected their brain samples with no inflammatory effects at 48 h after the last administration of LPS at a dose of 0.125 mg/kg. LPS at a dose of 0.25 mg/kg/day for 7 days significantly increased the expression of Iba-1 in the cortex and hippocampus (Khan et al., Citation2017). After a dose of 1.2 mg/kg/day for 14 days, increased expression of Iba-1 protein in the rostral ventrolateral medulla was lasted for at least 14 days (Wu et al., Citation2012). In our study, increased levels of Iba-1 were observed at 72 h after the last injection of LPS at a dose of 0.5 mg/kg.
LPS-induced microglial cell activation was attenuated by Dox. The mechanisms of this effect involve the inhibition of p38 MAP kinase and NF-kB-dependent signaling pathways (Santa-Cecília et al., Citation2016). Dox, as indicated by the decrease of spleen weights, had effects at the systemic level also. Earlier, a significant decrease in proinflammatory cytokines in the blood and inducible nitric oxide synthase activity in the spleen of LPS-exposed mice after Dox were shown (Milano et al., Citation1997).
In line with previous studies (Dang et al., Citation2018, Gong et al., Citation2019), LPS administration produced sickness behavior evidenced by reduced body weight gain, decreased locomotor activity, and increased anxiety. In our study, the elevation in anxiety-like behavior in LPS-treated rats was suggested on the more fecal boli produced by these animals in the EPM test. Numerous data indicate that defecation in the EPM and other behavioral tests is an important indicator of emotionality (Archer, Citation1973; Clinton et al., Citation2014). In a review of Archer (Citation1973), defecation was linked to a higher fear state. We did not find marked differences between groups using such classical measure of anxiety-like behavior as the number of entries in the open arms. This parameter is dependent on locomotor activity which was affected by both drugs. The changes in indicators of locomotion and anxiety-like behavior, a decrease in the number of rears and increase in the number of defecation, induced by LPS were attenuated by co-treatment with Dox, supporting the relation of the LPS behavioral effects to activation of neuroinflammation. A significant positive correlation between Iba-1 levels in the amygdala and the numbers of defecation could evidence for the interrelation between increased inflammatory marker and anxiety-like behavior. Dox alone injected for 2 weeks did not affect behaviors in the EPM test whereas previously it was shown that the consumption of Dox with drinking water for 4 days can temporarily induce an anxiogenic-like phenotype (Shishkina et al., Citation2018).
Mechanisms of the behavioral effects of LPS may include the attenuation of the protective function in the brain. Adaptive activation of brain systems involved in cell-protection in response to negative stimuli may mitigate its adverse effects. For example, an increased expression of anti-apoptotic factors Bcl-xL was observed in the hippocampus of rats treated with LPS for 2 weeks together with the elevation of pro-inflammatory cytokines (Dang et al., Citation2018). An increase in this anti-apoptotic protein expression associated with an attenuation of the behavioral symptoms of mood disorders (Shishkina et al., Citation2010). In our study, treatment with LPS caused pro-apoptotic changes in the brain, as demonstrated by the decreases in the levels of Bcl-xL in the hypothalamus and striatum. These effects of LPS were prevented by Dox.
We did not find any changes in BDNF levels in the brain region investigated after LPS or/and Dox. No effect of LPS on BDNF in the dentate gyrus was also observed after LPS injected at a dose of 0.1 mg/kg for 5 days (Shaw et al., Citation2001).
Group of Kim et al. (Citation2017) reported that several matrix metalloproteinases (MMPs) are upregulated under neuroinflammatory conditions and had a role of pro-inflammatory mediators in the brain. Our data show a significant up-regulating effect of LPS on MMP-9 protein in the amygdala. This LPS-induced elevation in MMP-9 levels was not prevented by co-treatment with Dox, despite that Dox is a potent inhibitor of human MMP-9 (Lee et al., Citation2009). It is possible that some other than neuroinflammation mechanisms, for example, LPS-induced increase in adrenal function may contribute to up-regulation of MMP-9 expression. The relationship between brain MMP-9 and adrenocortical activation is supported by data that chronic restraint stress enhanced MMP-9-mediated gelatinase activity in the hippocampus (van der Kooij et al., Citation2014). The increase in MMP-9 protein levels in the amygdala may play a role in lower gray matter volumes in this region in rats with chronic pulmonary inflammation induced by LPS (Chen et al., Citation2019). The animals from this model have a low level of rearing and high level of anxiety-like behavior. Lower gray matter volumes observed in some of the inflammatory markers related regions including amygdala were associated with more severe anxiety. A significant positive correlation revealed between the MMP-9 protein levels in this brain structure and the numbers of defecation in the EPM test support the link of the enzyme expression in amygdala and the marker of anxiety-like behavior.
Limitation
There are some limitations to this study. First, a single test for anxiety evaluation was used. Second, the mode of drug treatment was daily intraperitoneal 1–2 injections that could add stressful stimuli to LPS effects in animals. However, this is an unavoidable problem with any administration of drugs.
Conclusions
The novel finding of this study is that the mechanisms of anxiety-like behavior induced by LPS may include an increase in MMP-9 in the amygdala. Dox can attenuate LPS-induced emotional behavior though inhibition of microglial cell activation in several brain regions.
Disclosure statement
All authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Archer, J. (1973). Tests for emotionality in rats and mice: A review. Animal Behaviour, 21(2), 205–235. https://doi.org/10.1016/S0003-3472(73)80065-X
- Chen, J., Yan, Y., Yuan, F., Cao, J., Li, S., Eickhoff, S. B., & Zhang, J. (2019). Brain grey matter volume reduction and anxiety-like behavior in lipopolysaccharide-induced chronic pulmonary inflammation rats: A structural MRI study with histological validation. Brain, Behavior, and Immunity, 76, 182–197. https://doi.org/10.1016/j.bbi.2018.11.020
- Clinton, S. M., Watson, S. J., & Akil, H. (2014). High novelty-seeking rats are resilient to negative physiological effects of the early life stress. Stress (Amsterdam, Netherlands), 17(1), 97–107. https://doi.org/10.3109/10253890.2013.850670
- Costa-Nunes, J. P., Gorlova, A., Pavlov, D., Cespuglio, R., Gorovaya, A., Proshin, A., Umriukhin, A., Ponomarev, E. D., Kalueff, A. V., Strekalova, T., & Schroeter, C. A. (2020). Ultrasound stress compromises the correlates of emotional-like states and brain AMPAR expression in mice: Effects of antioxidant and anti-inflammatory herbal treatment. Stress (Amsterdam, Netherlands), 23(4), 481–415. https://doi.org/10.1080/10253890.2019.1709435
- Dang, R., Zhou, X., Tang, M., Xu, P., Gong, X., Liu, Y., Jiao, H., & Jiang, P. (2018). Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. European Journal of Nutrition, 57(3), 893–906. https://doi.org/10.1007/s00394-016-1373-z
- Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W., & Kelley, K. W. (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews. Neuroscience, 9(1), 46–56. https://doi.org/10.1038/nrn2297
- Golia, M. T., Poggini, S., Alboni, S., Garofalo, S., Ciano Albanese, N., Viglione, A., Ajmone-Cat, M. A., St-Pierre, A., Brunello, N., Limatola, C., Branchi, I., & Maggi, L. (2019). Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain, Behavior, and Immunity, 81, 484–494. https://doi.org/10.1016/j.bbi.2019.07.003
- Gong, X., Hu, H., Qiao, Y., Xu, P., Yang, M., Dang, R., Han, W., Guo, Y., Chen, D., & Jiang, P. (2019). The involvement of renin-angiotensin system in lipopolysaccharide-induced behavioral changes, neuroinflammation, and disturbed insulin signaling. Frontiers in Pharmacology, 10, 318. https://doi.org/10.3389/fphar.2019.00318
- Hoogland, I. C., Houbolt, C., van Westerloo, D. J., van Gool, W. A., & van de Beek, D. (2015). Systemic inflammation and microglial activation: Systematic review of animal experiments. Journal of Neuroinflammation, 12, 114. https://doi.org/10.1186/s12974-015-0332-6
- Khan, M. S., Ali, T., Abid, M. N., Jo, M. H., Khan, A., Kim, M. W., Yoon, G. H., Cheon, E. W., Rehman, S. U., & Kim, M. O. (2017). Lithium ameliorates lipopolysaccharide-induced neurotoxicity in the cortex and hippocampus of the adult rat brain. Neurochemistry International, 108, 343–354. https://doi.org/10.1016/j.neuint.2017.05.008
- Kim, J., Jeong, Y. H., Lee, E. J., Park, J. S., Seo, H., & Kim, H. S. (2017). Suppression of neuroinflammation by matrix metalloproteinase-8 inhibitor in aged normal and LRRK2 G2019S Parkinson’s disease model mice challenged with lipopolysaccharide. Biochemical and Biophysical Research Communications, 493(2), 879–886. https://doi.org/10.1016/j.bbrc.2017.09.129
- Könnecke, H., & Bechmann, I. (2013). The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clinical & Developmental Immunology, 2013, 914104. https://doi.org/10.1155/2013/914104
- Lee, H., Park, J. W., Kim, S. P., Lo, E. H., & Lee, S. R. (2009). Doxycycline inhibits matrix metalloproteinase-9 and laminin degradation after transient global cerebral ischemia. Neurobiology of Disease, 34(2), 189–198. https://doi.org/10.1016/j.nbd.2008.12.012
- Lopes, P. C. (2016). LPS and neuroinflammation: a matter of timing. Inflammopharmacology, 24(5), 291–293. https://doi.org/10.1007/s10787-016-0283-2
- Ma, M., Ren, Q., Yang, C., Zhang, J. C., Yao, W., Dong, C., Ohgi, Y., Futamura, T., & Hashimoto, K. (2017). Antidepressant effects of combination of brexpiprazole and fluoxetine on depression-like behavior and dendritic changes in mice after inflammation. Psychopharmacology, 234(4), 525–533. ). https://doi.org/10.1007/s00213-016-4483-7
- Milano, S., Arcoleo, F., D'Agostino, P., & Cillari, E. (1997). Intraperitoneal injection of tetracyclines protects mice from lethal endotoxemia downregulating inducible nitric oxide synthase in various organs and cytokine and nitrate secretion in blood. Antimicrobial Agents and Chemotherapy, 41(1), 117–121. https://doi.org/10.1128/AAC.41.1.117
- Miwa, T., Furukawa, S., Nakajima, K., Furukawa, Y., & Kohsaka, S. (1997). Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. Journal of Neuroscience Research, 50(6), 1023–1029. https://doi.org/10.1002/(SICI)1097-4547(19971215)50:6<1023::AID-JNR13>3.0.CO;2-5
- Mograbi, K. M., Suchecki, D., da Silva, S. G., Covolan, L., & Hamani, C. (2020). Chronic unpredictable restraint stress increases hippocampal pro-inflammatory cytokines and decreases motivated behavior in rats. Stress (Amsterdam, Netherlands), 23(4), 427–411. https://doi.org/10.1080/10253890.2020.1712355
- Pellow, S., Chopin, P., File, S. E., & Briley, M. (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14(3), 149–167. https://doi.org/10.1016/0165-0270(85)90031-7
- Reichenberg, A., Yirmiya, R., Schuld, A., Kraus, T., Haack, M., Morag, A., & Pollmächer, T. (2001). Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry, 58(5), 445–452. https://doi.org/10.1001/archpsyc.58.5.445
- Sandiego, C. M., Gallezot, J. D., Pittman, B., Nabulsi, N., Lim, K., Lin, S. F., Matuskey, D., Lee, J. Y., O'Connor, K. C., Huang, Y., Carson, R. E., Hannestad, J., & Cosgrove, K. P. (2015). Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proceedings of the National Academy of Sciences of the United States of America, 112(40), 12468–12473. https://doi.org/10.1073/pnas.1511003112
- Santa-Cecília, F. V., Socias, B., Ouidja, M. O., Sepulveda-Diaz, J. E., Acuña, L., Silva, R. L., Michel, P. P., Del-Bel, E., Cunha, T. M., & Raisman-Vozari, R. (2016). Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotoxicity Research, 29(4), 447–459. https://doi.org/10.1007/s12640-015-9592-2
- Shaw, K. N., Commins, S., & O'Mara, S. M. (2001). Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behavioural Brain Research, 124(1), 47–54. https://doi.org/10.1016/S0166-4328(01)00232-7
- Shishkina, G. T., Kalinina, T. S., Berezova, I. V., Bulygina, V. V., & Dygalo, N. N. (2010). Resistance to the development of stress-induced behavioral despair in the forced swim test associated with elevated hippocampal Bcl-xl expression. Behavioural Brain Research, 213(2), 218–224. https://doi.org/10.1016/j.bbr.2010.05.003
- Shishkina, G. T., Lanshakov, D. A., Bannova, A. V., Kalinina, T. S., Agarina, N. P., & Dygalo, N. N. (2018). Doxycycline used for control of transgene expression has its own effects on behaviors and Bcl-xL in the rat hippocampus. Cellular and Molecular Neurobiology, 38(1), 281–288. https://doi.org/10.1007/s10571-017-0545-6
- Shoemaker, R. C., House, D., & Ryan, J. C. (2014). Structural brain abnormalities in patients with inflammatory illness acquired following exposure to water-damaged buildings: a volumetric MRI study using NeuroQuant®. Neurotoxicology and Teratology, 45, 18–26. https://doi.org/10.1016/j.ntt.2014.06.004
- Tiwari, V., Singh, M., Rawat, J. K., Devi, U., Yadav, R. K., Roy, S., Gautam, S., Saraf, S. A., Kumar, V., Ansari, N., Saeedan, A. S., & Kaithwas, G. (2016). Redefining the role of peripheral LPS as a neuroinflammatory agent and evaluating the role of hydrogen sulphide through metformin intervention. Inflammopharmacology, 24(5), 253–264. https://doi.org/10.1007/s10787-016-0274-3
- van der Kooij, M. A., Fantin, M., Rejmak, E., Grosse, J., Zanoletti, O., Fournier, C., Ganguly, K., Kalita, K., Kaczmarek, L., & Sandi, C. (2014). Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nature Communications, 5, 4995. https://doi.org/10.1038/ncomms5995
- Walf, A. A., & Frye, C. A. (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols, 2(2), 322–328. https://doi.org/10.1038/nprot.2007.44
- Wu, K. L., Chan, S. H., & Chan, J. Y. (2012). Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. Journal of Neuroinflammation, 9, 212. https://doi.org/10.1186/1742-2094-9-212
- Yang, C. C., Lin, C. C., Hsiao, L. D., Kuo, J. M., Tseng, H. C., & Yang, C. M. (2019). Lipopolysaccharide-induced matrix metalloproteinase-9 expression associated with cell migration in rat brain astrocytes. International Journal of Molecular Sciences, 21(1), 259. https://doi.org/10.3390/ijms21010259