Abstract
Chronic stress can predispose vulnerable individuals to mood disorders, including depression. Glutamate, one of the key participants in this process, may exert both pathological and therapeutic psycho-emotional effects. However, the role of expression of genes encoding proteins that provide glutamatergic signal is still unclear. In this study, we attempted to distinguish changes in expression of glutamatergic genes associated with stress-induced anhedonia, a core symptom of depression, from those related to other stress-related effects. For this, expression of genes was compared between rats after a short-term stress, which did not yet cause depressive-like symptoms, and animals exposed chronically to different stressors that produce anhedonia-like responses. The changes in gene expression induced by chronic restraint or forced swimming concomitantly with anhedonia development demonstrated similar for both stressors patterns. Main features of the expression patterns include the decrease in mRNA levels for AMPA and NMDA subunits in the midbrain and hippocampus that is consistent with the hypothesis that “monoamine (serotonin)-Glutamate/GABA long neural circuit” involved in mood regulation. The decrease in expression of these subunits in the midbrain may attenuate glutamatergic drive on the serotonergic neurons promoting a shift of excitation/inhibition balance between glutamate and GABA in the forebrain regions resulting in anhedonia. In general, changes in expression of multiple genes involved in glutamatergic neurotransmission in the forebrain and brainstem regions suggest that stress-induced anhedonia may result from the network dysfunction of this neurotransmitter system.
1. Introduction
Clinical and experimental data indicate the involvement of glutamatergic neurotransmission in the regulation of mood and its disturbances. Glutamate was shown to be important in both the pathogenesis and therapy of stress-induced depression (Sanacora et al., Citation2012). Coordinated activity of neural networks, which components are localized in various interconnected brain structures (prefrontal cortex, hippocampus, amygdala, monoaminergic nuclei of the midbrain and brainstem and some others) underlie psycho-emotional state (Belzung et al., Citation2014; Biselli et al., Citation2019; Gong & He, Citation2015; Lener et al., Citation2017). Recently, the concept of depression as a “network” pathology (“circuitopathy”) was emerging (Duman et al., Citation2019; Yun et al., Citation2018). This concept suggests that violation of the coordinated activity of the neural network involved in the regulation of the psycho-emotional state may be the main factor provoking the pathology. Most of the existing connections between neurons of brain structures and within individual areas implicated in the mood regulation are glutamatergic. However, in this regard, the role of expression of genes encoding proteins that provide neurotransmitter signaling is still unclear. Investigations of glutamatergic gene expression during induction of a depressive-like state by stress are often focused on one or two brain structures, mainly the prefrontal cortex and the hippocampus. It is necessary to study stress-induced changes in expression of these genes in other brain structures involved in mood regulation for evaluation of a “circuitopathy” nature of depression. Since brain glutamate has been implicated in many functions, including behavior, movement, sensation, memory and cognition, a definite problem is the separation of changes responsible for the psycho-emotional status from those associated with other physiological functions, for example, such as regulation of neuroendocrine and behavioral stress responses (Myers et al., Citation2017). In an attempt to solve this problem, we used two approaches. The first was to compare changes in expression of glutamatergic genes between rats exposed to a short-term stress, which did not cause depressive-like symptoms, and animals exposed chronically to different stressors that produce anhedonia-like responses. The second approach concerns the differences in chosen chronic stressors that activated or, in contrast, restrained locomotion of the animals. These comparisons may provide insights into the association of changes in glutamatergic genes expression withanhedonia, rather than with stress per se or with specific characteristics of the stressor. Therefore, the aim of the present study was to evaluate the expression of eleven glutamatergic genes encoding proteins involved in the exchange, transport and reception of glutamate in brain structures related to depression and mood regulation after a short-term (for 2 days) forced swim (FS) exposure and after FS or restraint (RS) stress for 14 days. According to the above criteria, stress-induced expression pattern of glutamate signaling genes associated with anhedonia consisted of a decreased expression of grin1, grin2b, gria2, gria3 - in the hippocampus; grin1, grin2b, gria3 – in the midbrain; grik2 in the brainstem, and in an increased grm4 expression in the brainstem.
2. Materials and methods
2.1. Animals and experimental procedures
All animal-use procedures were supervised and specifically approved by the Ethical committee of the Institute of Cytology and Genetics in accordance with the guidelines of the Ministry of Public Health of Russia (supplement to order N 267 of 19 June 2003) and the European Council Directive (86/609/EEC). All efforts were made to minimize animal suffering and to reduce the number of animals used.C
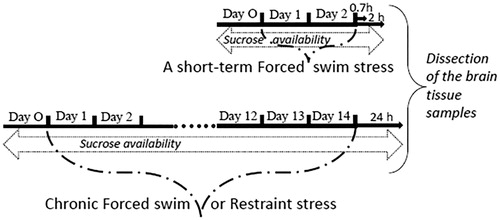
Adult males of Wistar rats (initially weighing 245–270 g) used in the study were housed singly in polycarbonate cages (27.7 × 44 × 15 cm = w × l × h) with free access to food and water. Animals were kept at a temperature of 22–23° under natural illumination. Animals were exposed to a short-term forced swim (FS) stress or chronic FS or restraint stress (RS) ().
For a short-term FS, animals were forced to swim for 2 days, 15 min at the first day and 5 min at the second day according to a published protocol (Porsolt et al., Citation1978). For chronic FS, animals were exposed to the forced swim once daily (15 min) for 14 consecutive days. For chronic RS, rats were placed in restraint plastic cages once daily (1 h) for 14 consecutive days. Groups of a short-term FS, chronic FS and RS consisted of 10 animals and were processed simultaneously with 5 unstressed animals for a short-term FS group and 10 unstressed animals for chronic FS and RS.
Sucrose preference was evaluated to control the development of anhedonia, a key symptom of depression. One day before starting, and throughout stress sessions, all rats had free access to two bottles (one with tap water and the other with 1% sucrose solution) in their home cages. The positions of the bottles were switched each day. Volume intake was estimated by weighing bottles each morning. Sucrose preference (percentage) was calculated as the volume of sucrose solution consumed devided by the total volume of fluid intake (sucrose + water). Body weights of the rats were measured each day before the stress procedures. Animals were sacrificed at time points 0.7 or 2 h after a short-term FS simultaneously with unstressed animals. Time points 0.7 h and 2 h were selected in a time frame within which a significant effect of short-term treatments on glutamatergic gene expression has been reported (Caldeira et al., Citation2007a,Citationb). Rats exposed to chronic FS or RS were decapitated 24 hours after the last stress exposure simultaneously with unstressed animals. Adrenals of the chronically stressed and unstressed rats were dissected and weighted.
Following decapitation, the brains were quickly removed, and the prefrontal cortex (Cort.), hippocampus (Hip.), amygdala (Amy.), midbrain (Mid.), and brainstem (Bst.) were rapidly isolated on ice, using the rat brain atlas coordinates (Paxinos & Watson, Citation1998), and immediately frozen in liquid nitrogen. The prefrontal cortex sample included a tissue section 1.5 mm thick cut from the between hemispheres surface approximately from AP (anterior-posterior) +4.5 to +2.2, L (lateral) 0–1.5 and DV (dorsal-ventral) 2 to 4.5 mm from the bregma. Hippocampal (AP +0.8 to −5.2, L 1-6 and DV 2-7 mm) and amygdala (AP −1.4 to −3.6, L 2-6 and DV 6.3–9 mm) samples were dissected from the brain. As previously described (Shishkina et al., Citation2007), the midbrain sample included the block of tissue from the rostral border of the superior colliculus to the rostral border of the pons to approximately −8.7 mm bregma. The brainstem that was caudal to the midbrain region included medulla oblongata.
2.2. Analysis of messenger RNAs for glutamate genes
Expression of genes was analyzed in brain structure samples of 5-6 animals from each stress or control groups. Total cellular RNA was isolated using a single-step acidic phenol extraction as was described previously (Shishkina et al., Citation2012). Only RNA samples with 260/280 ratio between 1.8 and 2.1 were used for subsequent qRT-PCR analysis. The genes for analysis were selected based on their assumed role in major depressive disorder or in depressive-like symptoms in animals (de Sousa et al., Citation2017). The TaqMan® assay-based real-time PCR for genes was performed using TaqMan® Gene Expression Assays (glul: Rn01483107_m1; grik4: Rn00561331_m1; grik2: Rn00570853_m1; gria3: Rn00583547_m1; gria2: Rn00568514_m1; grin2b: Rn00680474_m1; grin1: Rn01436034_m1; grm7: Rn00667503_m1; grm4: Rn01428450_m1; glt1: Rn00691548_m1; vglut1: Rn01462431_m1; beta-actin: Rn00667869_m1; Applied Biosystems, Foster City, CA) and the ABI Prism 7000 Sequence Detection system (Applied Biosystems). All reactions were carried out in duplicate on cDNA samples in 96-well optical plates according to the manufacturer’s protocol in 25 μl of 1× TaqMan® Universal PCR Master Mix (Applied Biosystems). The real-time PCR consisted of one cycle of 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles each of 95 °C for 15 s and 60 °C for 1 min. The comparative ΔΔCT method was used to calculate mRNA expression relative to the beta-actin as an endogenous control according to the manufacturer’s manual (Applied Biosystems).
2.3. Statistics
The effects of chronic RS or FS exposures on body weight gain and sucrose preference were analyzed using one-way ANOVA for repeated measures. Adrenal weights, immobility duration in the FS and the mRNA data were analyzed using a one-way ANOVA with time after a short-term stress (unstressed, 0.7 h, and 2 h after FS) or type of the chronic stress (unstressed, RS and FS) as a factor, followed by Bonfferoni’s post hoc test. Planned t-test comparisons were conducted to examine the effect of each stressor. The results were considered significant at a probability level less than 0.05.
3. Results
3.1. Stress effects on body and adrenal weights, sucrose preference and immobility time during forced swimming
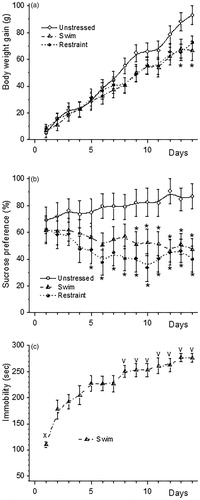
The increase in body weight of animals of both chronically stressed groups slowed down with the stress duration (; ANOVA for repeated measures: interaction Day * Stress F(26, 351) = 3.037, p < 0.000). FS and RS increased relative adrenal weights compared to unstressed animals (19.59 ± 0.85, 18.36 ± 0.60 and 15.85 ± 1.23 mg/100 g BW respectively; effect of stress F(2, 12) = 4.289, p < 0.039). Repeated measures ANOVA showed that rats from the chronically stressed groups had significantly lower sucrose preference than unstressed rats (; effect of stress: F(2, 27) = 5.819, p < 0.00792; interaction Day * Stress F(26, 351) = 1.614, p < 0.031). Immobility duration increased with the days of swimming (; F(13, 126) = 4.99, p < 0.000). On the second day of a FS, it was one and a half times higher compared to the first day (p < 0.002). However, sucrose consumption by these animals was not different from that in unstressed rats at this day (61.05 ± 11.28% and 55.36 ± 9.78% sucrose solution of the total fluid intake, respectively p > 0.5). FS for more than 8 days caused a further increase in the duration of immobility compared with the second day of swimming.
3.2. Stress effects on glutamatergic gene expression in the rat brain
In the amygdala, expression of glul (F(2, 16) = 5.475, p < 0.015) and glt1 (F(2, 13) = 4.377, p < 0.035) increased both 0.7 and 2 h after swimming. Increase in mRNA levels of grik4 (F(2, 10) = 4.420, p < 0.042) in the cortex, gria3 (F(2, 14) = 5.430, p < 0.018) in the hippocampus, grin2b (F (2, 14) = 4.191, p < 0.037), grin1 (p < 0.033 vs control), grm4 (F(2, 12) = 5.844, p < 0.017) in the amygdala and gria2 (F(2, 13) = 4.7817, p < 0.028) in the brainstem were significant only at 0.7 h after swimming. The decrease in mRNA levels of grm7 (F(2, 13) = 4.437, p < 0.034) in the hippocampus, glul (F(2, 12) = 7.402, p < 0.008) in the midbrain, glul (p < 0.023 vs control) and grin1 (F(2, 16) = 3.872, p < 0.042) in the brainstem reached significant level at 2 h after a short-term FS. Seven of the thirteen changes in glutamate genes expression induced by a short-term FS occurred in the amygdala.
Table 1. Stress-induced alterations in glutamate gene mRNA expression in the rat brain.
Chronic exposures to stressors altered mRNA levels of ten of eleven genes in one (). Among affected genes six genes were in the hippocampus, five in the brainstem, four genes in the midbrain, one gene in the amygdala, and no changes in the cortex. Chronic RS appeared to be more efficient in modulating glutamatergic genes expression then FS. The decreases in mRNA levels of grik2 (p < 0.05 vs control) and grm7 (p < 0.035 vs control) in the hippocampus, and glt1 (p < 0.05 vs control) in the brainstem as well the increase of the grik2 (F(2, 15) = 4.542, p < 0.029) expression in the amygdala were found only after chronic RS. An increase in the glul mRNA expression (F(2, 12) = 5.081, p < 0.025) in the brainstem was unique for the chronic FS.
Changes in the expressions of six glutamatergic genes in three brain structures were were in similar direction after both chronic stressors (). Most of these changes were the decrease in expression of genes encoding subunits of the ionotropic receptors gria3 (F(2,9) = 54.544, p < 0.001), gria2 (F(2,11) = 9.1226, p < 0.005), grin2b (F(2,12) = 5.0593, p < 0.025) and grin1 (both stressors p < 0.05 vs control) in the hippocampus; gria3 (F(2,12) = 17.752, p < 0.001), grin2b (F(2, 14) = 9.645, p < 0.002) and grin1(F(2, 13) = 11.232, p < 0.001) in the midbrain and grik2 (F(2, 13) = 5.572, p < 0.017) in the brainstem. In contrast to the decreased expression of subunits of the ionotropic receptor, mRNA levels for the grm4 metabotropic glutamate receptor increased (F(2, 11) = 9.240, p < 0.004) in the brainstem after both chronic stressors.
Figure 3. The changes in mRNA levels of the glutamate gene in the hippocampus (a), midbrain (b) and the brainstem (c) that changed in the same direction under both types of chronic stressors. The decreases in mRNA levels for the ionotropic receptors subunits caused by stressors were 25–50% of levels in unstressed rats. Only mRNA level for grm4 metabotropic receptor showed up to two-fold increase after chronic FS in the brainstem. *p < 0.05 compared to Unstressed.

4. Discussion
Short-term FS did not induce the core symptom of depression, anhedonia. Nevertheless, this FS caused changes in the expression of one-to-five glutamatergic genes from eleven genes investigated in each brain region. The largest number of these changes occurred in the amygdala that is in consonance with multiple data on the important contribution of glutamatergic neurotransmission in amygdala to behavioral and autonomic stress responses (Wilson et al., Citation2015). Rare changes in expression of glutamatergic genes in other structures are consistent with published data on the absence of significant short-term stress effects on the expression of many of these genes in the brain. One- or two-days FS did not change gria1 or gria2 expression in the mice hippocampus (Freudenberg, Citation2019). The enhance in glutamatergic transmission in the prefrontal cortex found in some studies after acute stress may be related to the increased surface expression of NMDA and AMPA receptors subunits (Yuen et al., Citation2009).
The pattern of glutamatergic gene expression in the brain after chronic stress was different from that after the short-term stress. The decreases in hippocampal grik2, brainstem gria3 and glt1 and an increase in amygdala grik2 gene expression unique for the chronic RS as well as an increase of the glul mRNA level in the brainstem only after chronic FS were not obligatory components of anhedonia. There was only one gene grm7 that changed in the same brain structure, hippocampus, and in the same direction after the short-term FS and chronic RS. This GRM7 is a candidate gene for major depressive disorder (de Sousa et al., Citation2017). However, similar alterations in its expression after the short-term stress, that did not induce anhedonia, and after chronic RS, but not FS, both of which induced this symptom, suggest that grm7 and other genes with stressor-specific expression changes (RS: hippocampal grik2, brainstem gria3 and glt1, amygdala grik2; FS: brainstem glul), may be involved in mediating some stressor-specific responses, but less important for the development of anhedonia.
The alterations in glutamatergic gene expression that were common for both chronic stressors and absent after a short-term FS may contribute to the mechanisms of anhedonia. Four of these changes were the decreases in mRNA levels for AMPA and NMDA receptors subunits in the hippocampus. Reported changes in gene expression for subunits of AMPA and NMDA glutamatergic receptors in this brain structure after prolonged stress are contradictory. Some authors found decreases (Duric et al., Citation2013; Liu et al., Citation2018; Mozhui et al., Citation2010; Yu et al., Citation2016), while others reported increases (Costa-Nunes et al., Citation2020; McWhirt et al., Citation2019; Nasca et al. Citation2017) or no changes (Costa-Nunes et al., Citation2020; Duric et al., Citation2013; Mozhui et al., Citation2010; McWhirt et al., Citation2019; Nasca et al., Citation2017). The effects of stressors on expression of the same subunit for AMPA or NMDA receptor in the hippocampus varied between studies and depended on the animal's genotype as well as on the duration of stress application. Some of these alterations may have behavioral manifestations. Upregulated mRNA and protein levels of glutamatergic receptor gria3 subunit were associated with anti-depressive-like behavior of rats (Liu et al., Citation2018). Stress-induced decreases in mRNA levels for AMPA and NMDA receptors subunits were found in the midbrain. These changes may occur in dopaminergic, GABAergic and serotonergic neurons, which present in the neuronal population of this structure (Kirby et al., Citation2007; Nair-Roberts et al., Citation2008). In the brainstem, both chronic RS and FS increased mRNA levels for grm4 metabotropic glutamate receptor and decreased mRNA level for grik2 kainate glutamate receptor subunit. Patients with major depression exhibited elevated expression level of the GRM4 receptor gene in locus coeruleus neurons compared to healthy individuals (Chandley et al., Citation2014). The expression of GRIK2 subunit in astrocytes of depressed patients was also higher than in controls (Nagy et al., Citation2015). However, patients in these studies died by suicide and changes in gene expression may relate to suicide, rather than to disease (Zhao et al., Citation2018). Stress and its hormones modulate glutamatergic gene expression through multiple mechanisms, such as an activation of transcription factors (Wu & Donohoe, Citation2019), miRNAs (Liu et al., Citation2018), epigenetic mechanism of gene regulation (Nasca et al., Citation2017), transcriptional and non-genomic effects of glucocorticoids, retraction of dendrites and loss of synapses (McEwen & Akil, Citation2020) and excitotoxic cell death (Lanshakov et al., Citation2016).
We did not find any changes in mRNA levels for glutamatergic genes in the prefrontal cortex after both chronic stress that induced anhedonia. Reported data on the expression of glutamate genes in the cortex of humans and animals are ambiguous. Some authors reported no change in cortical grin1 mRNA expression after chronic stress (Duric et al., Citation2013; Shepard & Coutellier, Citation2018). In contrast, several other studies showed downregulation of the cortical expression of protein encoded by grin1 by chronic stress (Lee & Goto, Citation2011; Wei et al., Citation2014; Yuen et al., Citation2012). Moreover, chronic RS increased grin1 expression in the prefrontal cortex of male rats and this effect depended on a combination of chronic and acute stressors as well as resting period between them (Moench et al., Citation2020). Expression of GRIA2 mRNA was up-regulated in the cortex of patients with major depressive disorder (Teyssier et al., Citation2011). Another study showed increased GRIN2B mRNA levels in the parietal cortex of such patients, but lower levels were observed in their dorsolateral prefrontal cortex (Dean et al., Citation2016). Reduced grin1 expression in the medial prefrontal cortex was associated with a depression-like behavior of mice in the FS test (Montalvo-Ortiz et al., Citation2016). Impressive strain differences in stress-induce gene expression patterns in the ventromedial prefrontal cortex, hippocampus and amygdala of C57BL/6J and DBA/2J mice were found after 10-day RS (Mozhui et al., Citation2010). This stress altered the expression of two to five hundreds genes in each structure with lower number in the cortex and highest in the amygdala. However, only 2–10 of these genes in each structure changed their expression in the same direction in both strains. The authors suggested instead of a common molecular “stress network” activated to different degrees in each strain mobilization by C57BL/6J and DBA/2J mice of mostly autonomous gene networks. Having in mind genetic predisposition to suicidal behavior (Andriessen & Videtic-Paska, Citation2015) and data of Mozhui et al. (Citation2010), it looks natural that gene expression changes of synaptic proteins, including glutamatergic genes, were in opposite direction in the anterior cingulate cortex of patients with major depression in dependence on whether they had committed suicide or not (Zhao et al., Citation2018).
The discussion of the available information about effects of stress assosiated with the development of depression on the expression of glutamatergic genes in the brain demonstrates wide spectrum of changes that does not fit into a single pattern. The discrepancy in the experimental results usually thought to be related to the quality of stressor used for inducing a depressive-like state, the genotype of the creature responding to stress, as well as methods for assessing changes in gene expression. Nevertheless, in the present study, within similar genotypes, duration of stress application and method for assessing mRNA levels, a common for different chronic stress pattern of glutamate gene expression associated with anhedonia has been revealed. However, to what extent the patterns obtained under specific experimental conditions can characterize the changes associated with the depressive state as such. Multidirectional stress-induced alterations in the expression of the same genes in the same brain structures seem to be insufficient reason to assume uniqueness of the mechanisms of a depressive state in each study. An evaluation of changes in more proximate to psycho-emotional regulation brain function, such as connectivity based on the alterations in gene expression, may be a pathway, if not overcoming, then at least understand the source of variability of data between studies. Any alterations in glutamatergic gene expression under chronic stress certainly indicate some changes in the activity of glutamatergic neurotransmission that mediate most of the connections between neurons of brain structures and within individual areas implicated in the mood regulation. Numerous results of recent studies implicate glutamate in concert with GABA and monoaminergic systems in the regulation of mood via proposed “monoamine (serotonin)-glutamate/GABA long neural circuit” (Li, Citation2020). According to this hypothesis, activities of the midbrain serotonin neurons are modulated by the projections from glutamatergic neurons of the prefrontal cortex and by nearby GABA interneurons. In their turn, serotoninergic projections into the forebrain structures influence excitation/inhibition balance of glutamate pyramidal neurons and GABA interneurons. This balance resulted in pro- or antidepressive state. Forward and feedback interconnections within this long neural circuit that depend on genotype, stressor type and duration of its action as well as habituation and adaptation to it may result in common behavioral phenotype despite differences in changes in the levels of mRNAs for glutamate genes. Chronic stress-induced changes in the expression of these genes in our study seem to fit the above “circuit” hypothesis. The decreases in the AMPA and NMDA receptor subunits expression in the midbrain may lead to attenuation of glutamatergic drive onto serotonergic neurons promoting a shift of excitation/inhibition balance between glutamate and GABA in the forebrain regions resulting in anhedonia. The decrease in mRNA was accompanied by a long-lasting reduction in synaptic AMPA receptor number, consistent with reduced synaptic efficacy (Grooms et al., Citation2006). The levels of AMPA as well as NMDA receptor subunits expression correlated with the receptor’s activity (Caldeira et al., Citation2007a,Citationb). The decreases in the expression of ionotropic glutamate receptors subunits in the hippocampus of chronically stressed animals will further enhance “inhibition” and thus support anhedonia-like animals' responses to stress.
4.1. Limitations
Brain structures studied in the present work are only a part of a complex neuro-circuit of depression. The role of glutamatergic gene expression in the functioning of other structures involved in mood regulation needs further investigation. Assessment of mRNA level in a block of tissue inherently lacks cell-type specificity. Thus, particular cell-type of the brain structure that changes the expression of glutamate genes in response to chronic stress needs further clarification.
5. Conclusion
The changes in glutamatergic gene expression induced by chronic restraint or forced swimming concomitantly with a depressive-like state demonstrated similar for both stressors patterns. Main features of the patters: the decreases of mRNA levels for AMPA and NMDA subunits in the midbrain and hippocampus consistent with the hypothesis (Li, Citation2020) that “monoamine (serotonin)-Glutamate/GABA long neural circuit” involved in mood regulation. The decreases of these subunits expression in the midbrain may attenuate glutamatergic drive on the serotonergic neurons promoting a shift of excitation/inhibition balance between glutamate and GABA in the forebrain regions resulting in a depressive-like state.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andriessen, K., & Videtic-Paska, A. (2015). Genetic vulnerability as a distal risk factor for suicidal behaviour: Historical perspective and current knowledge. Zdravstveno Varstvo, 54(3), 238–251. https://doi.org/10.1515/sjph-2015-0026
- Belzung, C., Turiault, M., & Griebel, G. (2014). Optogenetics to study the circuits of fear- and depression-like behaviors: A critical analysis. Pharmacology, Biochemistry, and Behavior, 122, 144–157. https://doi.org/10.1016/j.pbb.2014.04.002
- Biselli, T., Lange, S. S., Sablottny, L., Steffen, J., & Walther, A. (2019). Optogenetic and chemogenetic insights into the neurocircuitry of depression-like behaviour: A systematic review. European Journal of Neuroscience. https://doi.org/10.1111/ejn.14603
- Caldeira, M. V. 1., Melo, C. V., Pereira, D. B., Carvalho, R. F., Carvalho, A. L., & Duarte, C. B. (2007b). BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Molecular and Cellular Neurosciences, 35(2), 208–219. https://doi.org/10.1016/j.mcn.2007.02.019
- Caldeira, M. V., Melo, C. V., Pereira, D. B., Carvalho, R., Correia, S. S., Backos, D. S., Carvalho, A. L., Esteban, J. A., & Duarte, C. B. (2007a). Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. The Journal of Biological Chemistry, 282(17), 12619–12628. https://doi.org/10.1074/jbc.M700607200
- Chandley, M. J., Szebeni, A., Szebeni, K., Crawford, J. D., Stockmeier, C. A., Turecki, G., Kostrzewa, R. M., Gregory ., & Ordway, G. A. (2014). Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. The International Journal of Neuropsychopharmacology, 17(10), 1569–1578. https://doi.org/10.1017/S1461145714000662
- Costa-Nunes, J. P., Gorlova, A., Pavlov, D., Cespuglio, R., Gorovaya, A., Proshin, A., Umriukhin, A., Ponomarev, E. D., Kalueff, A. V., Strekalova, T., & Schroeter, C. A. (2020). Ultrasound stress compromises the correlates of emotional-like states and brain AMPAR expression in mice: Effects of antioxidant and anti-inflammatory herbal treatment. Stress, 23(4), 415–481. https://doi.org/10.1080/10253890.2019.1709435
- de Sousa, R. T., Loch, A. A., Carvalho, A. F., Brunoni, A. R., Haddad, M. R., Henter, I. D., Zarate, C. A., & Machado-Vieira, R. (2017). Genetic studies on the tripartite glutamate synapse in the pathophysiology and therapeutics of mood disorders. Neuropsychopharmacology, 42(4), 787–800. https://doi.org/10.1038/npp.2016.149
- Dean, B., Gibbons, A. S., Boer, S., Uezato, A., Meador-Woodruff, J., Scarr, E., & McCullumsmith, R. E. (2016). Changes in cortical N-methyl-D-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Australian and New Zealand Journal of Psychiatry, 50(3), 275–283. https://doi.org/10.1177/0004867415586601
- Duman, R. S., Sanacora, G., & Krystal, J. H. (2019). Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron, 102(1), 75–90. https://doi.org/10.1016/j.neuron.2019.03.013
- Duric, V., Banasr, M., Stockmeier, C. A., Simen, A. A., Newton, S. S., Overholser, J. C., Jurjus, G. J., Dieter, L., & Duman, R. S. (2013). Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. The International Journal of Neuropsychopharmacology, 16(1), 69–82. https://doi.org/10.1017/S1461145712000016
- Freudenberg, F. (2019). Quantitative analysis of Gria1, Gria2, Dlg1 and Dlg4 expression levels in hippocampus following forced swim stress in mice. Scientific Reports, 9(1), 14060. https://doi.org/10.1038/s41598-019-50689-w
- Gong, Q., & He, Y. (2015). He 2. Depression, neuroimaging and connectomics: A selective overview. Biological Psychiatry, 77(3), 223–235. https://doi.org/10.1016/j.biopsych.2014.08.009
- Grooms, S. Y. 1., Noh, K. M., Regis, R., Bassell, G. J., Bryan, M. K., Carroll, R. C., & Zukin, R. S. (2006). Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons. Journal of Neuroscience, 26(32), 8339–8351. https://doi.org/10.1523/JNEUROSCI.0472-06.2006
- Kirby, L. G., Pan, Y.-Z., Freeman-Daniels, E., Rani, S., Nunan, J. D., Akanwa, A., & Beck, S. G. (2007). Cellular effects of swim stress in the dorsal raphe nucleus (published correction appears in Psychoneuroendocrinology. 2007 Sep-Nov;32(8-10):1167). Psychoneuroendocrinology, 32(6), 712–723. https://doi.org/10.1016/j.psyneuen.2007.05.001
- Lanshakov, D. A., Sukhareva, E. V., Kalinina, T. S., & Dygalo, N. N. (2016). Dexamethasone-induced acute excitotoxic cell death in the developing brain. Neurobiology of Disease, 91, 1–9. https://doi.org/10.1016/j.nbd.2016.02.009
- Lee, Y. A., & Goto, Y. (2011). Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure-prefrontal cortex interaction. The European Journal of Neuroscience, 34(3), 426–436. https://doi.org/10.1111/j.1460-9568.2011.07750.x
- Lener, M. S., Niciu, M. J., Ballard, E. D., Park, M., Park, L. T., Nugent, A. C., & Zarate, C. A. (2017). Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biological Psychiatry, 81(10), 886–897. https://doi.org/10.1016/j.biopsych.2016.05.005
- Li, Y. F. (2020). A hypothesis of monoamine (5-HT)-glutamate/GABA long neural circuit: Aiming for fast-onset antidepressant discovery. Pharmacology & Therapeutics, 208, 107494. https://doi.org/10.1016/j.pharmthera.2020.107494
- Liu, Q., Sun, N. N., Wu, Z. Z., Fan, D. H., & Cao, M. Q. (2018). Chaihu-Shugan-San exerts an antidepressive effect by downregulating miR-124 and releasing inhibition of the MAPK14 and Gria3 signaling pathways. Neural Regeneration Research, 13(5), 837–845. https://doi.org/10.4103/1673-5374.232478
- McEwen, B. S., & Akil, H. (2020). Revisiting the stress concept: Implications for affective disorders. The Journal of Neuroscience, 40(1), 12–21. https://doi.org/10.1523/JNEUROSCI.0733-19.2019
- McWhirt, J., Sathyanesan, M., Sampath, D., & Newton, S. S. (2019). Effects of restraint stress on the regulation of hippocampal glutamate receptor and inflammation genes in female C57BL/6 and BALB/c mice. Neurobiology of Stress, 10, 100169. https://doi.org/10.1016/j.ynstr.2019.100169
- Moench, K. M., Breach, M. R., & Wellman, C. L. (2020). Prior stress followed by a novel stress challenge results in sex-specific deficits in behavioral flexibility and changes in gene expression in rat medial prefrontal cortex. Hormones and Behavior, 117, 104615. https://doi.org/10.1016/j.yhbeh.2019.104615
- Montalvo-Ortiz, J. L., Bordner, K. A., Carlyle, B. C., Gelernter, J., Simen, A. A., & Kaufman, J. (2016). The role of genes involved in stress, neural plasticity, and brain circuitry in depressive phenotypes: Convergent findings in a mouse model of neglect. Behavioural Brain Research, 315, 71–74. https://doi.org/10.1016/j.bbr.2016.08.010
- Mozhui, K., Karlsson, R.-M., Kash, T. L., Ihne, J., Norcross, M., Patel, S., Farrell, M. R., Hill, E. E., Graybeal, C., Martin, K. P., Camp, M., Fitzgerald, P. J., Ciobanu, D. C., Sprengel, R., Mishina, M., Wellman, C. L., Winder, D. G., Williams, R. W., & Holmes, A. (2010). Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. The Journal of Neuroscience, 30(15), 5357–5367. https://doi.org/10.1523/JNEUROSCI.5017-09.2010
- Myers, B., Scheimann, J. R., Franco-Villanueva, A., & Herman, J. P. (2017). Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience and Biobehavioral Reviews, 74(Pt B), 366–375. https://doi.org/10.1016/j.neubiorev.2016.05.011
- Nagy, C., Suderman, M., Yang, J., Szyf, M., Mechawar, N., Ernst, C., & Turecki, G. (2015). Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Molecular Psychiatry, 20(3), 320–328. https://doi.org/10.1038/mp.2014.21
- Nair-Roberts, R. G., Chatelain-Badie, S. D., Benson, E., White-Cooper, H., Bolam, J. P., & Ungless, M. A. (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience, 152(4), 1024–1031. https://doi.org/10.1016/j.neuroscience.2008.01.046
- Nasca, C., Bigio, B., Zelli, D., Angelis, P., Lau, T., Okamoto, M., Soya, H., Ni, J., Brichta, L., Greengard, P., Neve, R. L., Lee, F. S., & McEwen, B. S. (2017). Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron, 96(2), 402–413.e5. https://doi.org/10.1016/j.neuron.2017.09.020
- Paxinos, G., & Watson, C. (1998). The rat brain in stereotaxic coordinates. Academic Press.
- Porsolt, R. D., Anton, G., Blavet, N., & Jalfre, M. (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. European Journal of Pharmacology, 47(4), 379–391. https://doi.org/10.1016/0014-2999(78)90118-8 204499
- Sanacora, G., Treccani, G., & Popoli, M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology, 62(1), 63–77. https://doi.org/10.1016/j.neuropharm.2011.07.036
- Shepard, R., & Coutellier, L. (2018). Changes in the prefrontal glutamatergic and parvalbumin systems of mice exposed to unpredictable chronic stress. Molecular Neurobiology, 55(3), 2591–2602. https://doi.org/10.1007/s12035-017-0528-0
- Shishkina, G. T., Kalinina, T. S., Berezova, I. V., & Dygalo, N. N. (2012). Stress-induced activation of the brainstem Bcl-xL gene expression in rats treated with fluoxetine: Correlations with serotonin metabolism and depressive-like behavior. Neuropharmacology, 62(1), 177–183. https://doi.org/10.1016/j.neuropharm.2011.06.016
- Shishkina, G. T., Kalinina, T. S., & Dygalo, N. N. (2007). Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience, 150(2), 404–412. ‐https://doi.org/10.1016/j.neuroscience.2007.09.017
- Teyssier, J.-R., Ragot, S., Chauvet-Gélinier, J.-C., Trojak, B., & Bonin, B. (2011). Activation of a ΔFOSB dependent gene expression pattern in the dorsolateral prefrontal cortex of patients with major depressive disorder. Journal of Affective Disorders, 133(1–2), 174–178. https://doi.org/10.1016/j.jad.2011.04.021
- Wei, J., Yuen, E. Y., Liu, W., Li, X., Zhong, P., Karatsoreos, I. N., McEwen, B. S., & Yan, Z. (2014). Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Molecular Psychiatry, 19(5), 588–598. https://doi.org/10.1038/mp.2013.83
- Wilson, M. A., Grillo, C. A., Fadel, J. R., & Reagan, L. P. (2015). Stress as a one-armed bandit: Differential effects of stress paradigms on the morphology, neurochemistry and behavior in the rodent amygdala. Neurobiology of Stress, 1, 195–208. https://doi.org/10.1016/j.ynstr.2015.06.001
- Wu, T., & Donohoe, M. E. (2019). Yy1 regulates Senp1 contributing to AMPA receptor GluR1 expression following neuronal depolarization. Journal of Biomedical Science, 26(1), 79. https://doi.org/10.1186/s12929-019-0582-1
- Yu, M., Zhang, Y., Chen, X. Y., & Zhang, T. (2016). Antidepressant-like effects and possible mechanisms of amantadine on cognitive and synaptic deficits in a rat model of chronic stress. Stress, 19(1), 104–113. https://doi.org/10.3109/10253890.2015.1108302
- Yuen, E. Y., Liu, W., Karatsoreos, I. N., Feng, J., McEwen, B. S., & Yan, Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14075–14079. https://doi.org/10.1073/pnas.0906791106
- Yuen, E. Y., Wei, J., Liu, W., Zhong, P., Li, X., & Yan, Z. (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron, 73(5), 962–977. https://doi.org/10.1016/j.neuron.2011.12.033
- Yun, S., Reynolds, R. P., Petrof, I., White, A., Rivera, P. D., Segev, A., Gibson, A. D., Suarez, M., DeSalle, M. J., Ito, N., Mukherjee, S., Richardson, D. R., Kang, C. E., Ahrens-Nicklas, R. C., Soler, I., Chetkovich, D. M., Kourrich, S., Coulter, D. A., & Eisch, A. J. (2018). Stimulation of entorhinal cortex-dentate gyrus circuitry is antidepressive. Nature Medicine, 24(5), 658–666. https://doi.org/10.1038/s41591-018-0002-1
- Zhao, J., Verwer, R. W. H., Gao, S.-F., Qi, X.-R., Lucassen, P. J., Kessels, H. W., & Swaab, D. F. (2018). Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. Journal of Psychiatric Research, 102, 261–274. https://doi.org/10.1016/j.jpsychires.2018.04.020