Abstract
This study explored the associations between specific profiles of biological dysregulation and mental health outcomes in a national, community sample of healthy adults in the United States. A latent class analysis of data from the Midlife Development in the United States study (n = 1,757) was conducted to determine classes of biological dysregulation. Multinomial logistic regressions of class membership were employed to determine associations with measures related to depression, including whether or not individuals had sought treatment, Center for Epidemiological Studies Depression Scale, and both the generalized distress and anhedonia subscales of the Mood and Anxiety Symptoms Questionnaire. Four classes of dysregulation emerged: baseline/low dysregulation, metabolic and inflammatory dysregulation, parasympathetic dysregulation, and SAM pathway dysregulation. Individuals who met the criteria for depression measures were more likely to be in the metabolic and immune dysregulation and parasympathetic dysregulation groups as compared to the baseline group. The results suggest that mental health outcomes, such as depression, are differentially associated with specific profiles of biological dysregulation. A more nuanced approach to profiles of dysregulation could better inform treatment decisions.
Higher levels of allostatic load, which represents the cumulative wear and tear of exposure to stress, are associated with increased rates of depression and anhedonia. Specifically, parasympathetic dysregulation and immunometabolic dysregulation are associated with negative mental health outcomes
Lay summary
Introduction
Allostatic load – the cumulative, biological wear and tear from long-term exposure to stress – is associated with a wide range of physical and cognitive diseases (McEwen et al., Citation2012). Higher levels of allostatic load, as operationalized by a range of biomarkers, have been associated with cardiovascular disease (Juster & Lupien, Citation2012; Mattei et al., Citation2010; Rosmond & Bjorntorp, Citation2000; Santacroce & Crandell, Citation2014), Type 2 Diabetes Mellitus (T2DM) (Crews, Citation2007; Mattei et al., Citation2010), rheumatoid arthritis (Straub & Cutolo, Citation2001; Wilder & Elenkov, Citation1999), as well as cognitive and memory decline (Lucassen et al., Citation2017; Seeman et al., Citation2001). Recently, there has been an increased focus on the relationship between allostatic load and mental health. Examples include studies that have found associations between higher allostatic load and schizophrenia (Berger, Juster, et al., Citation2018; Berger, Lavoie, et al., Citation2018; Nugent et al., Citation2015; Savransky et al., Citation2018), bipolar disorder (Brietzke et al., Citation2011; Kapczinski et al., Citation2008; Vieta et al., Citation2013), major depressive disorder (Juster et al., Citation2011; Kobrosly et al., Citation2013, Citation2014), and anhedonia (Berger et al., Citation2019).
There is also a large body of evidence connecting individual biomarkers to mental health outcomes (e.g. Dowlati et al., Citation2010; Gill et al., Citation2008; Haapakoski et al., Citation2015; Kyrou et al., Citation2017; Maes et al., Citation1999; Pervanidou et al., Citation2007). These studies have shed light on the biological underpinnings of mental health disorders. For example, hyperactivity of the HPA axis, and the resultant hypercortisolemia is one proposed biological pathway for causing major depressive disorder (MDD) via hippocampal atrophy (McEwen, Citation2003). 20–80% of individuals experiencing depression have some form of HPA axis hyperactivity and a half to three-quarters of depressed patients have elevated cortisol levels (Pace & Miller, Citation2009; Stetler and Miller, Citation2011). Elevated cortisol levels in individuals experiencing depression may be the result of a malfunction in the negative feedback loop that allows the hippocampus to regulate cortisol (Bowers & Yehuda, Citation2017; Pariante & Miller, Citation2001).
Alternatively, the cytokine hypothesis argues that some forms of depression are the result of inflammatory processes triggered by environmental stressors (Bob et al., Citation2010). Recent meta-analyses found evidence that blood IL-6 levels were elevated in patients with major depressive disorder (Dowlati et al., Citation2010; Haapakoski et al., Citation2015).
Anhedonia, which represents a reduced or diminished desire to seek out or respond to pleasureful stimuli, is associated with multiple mood and personality disorders (American Psychiatric Association, Citation2013; Cho et al., Citation2019). Anhedonia is common among individuals with MDD, and treatments for MDD (e.g. SSRIs) are typically not effective in treating anhedonia, which may exacerbate depressive symptoms (Atherton et al., Citation2015; Lamontagne et al., Citation2018; Price et al., Citation2009). Anhedonia’s etiology remains unclear, yet research suggests that dopamine as well as glucocorticoids, such as cortisol, play a primary role in the disorder (Krugel et al., Citation2009; Lamontagne et al., Citation2018).
The goal of this study is to assess if individual biological systems or a series of biomarkers across systems are the drivers of the association between allostatic load and MDD-related outcomes. By exploring the underlying classes of biological dysregulation, this study bridges the gap between cumulative allostatic load research and research focused on individual biomarkers.
Methods
Sample
This study utilized data from the second wave of Midlife Development in the United States study (MIDUS). MIDUS, is a national, community sample of non-institutionalized adults in the United States. The purpose of the study is to better understand health and well-being as individuals age by studying behavioral, psychological, and social factors (Ryff et al., Citation2006, Citation2014). MIDUS is a de-identified, publicly available dataset, therefore institutional review board oversight is not required. Data for the current study includes individuals who participated in the MIDUS Biomarkers project. This subsample includes a subset of individuals who were part of the original MIDUS study as well as individuals who were part of a new, Refresher sample that joined at the second wave of the study. For this reason, and because biomarker data is only available at a single point in time, this study utilizes a cross-sectional methodology. The total sample of individuals included in the analysis is 1,757.
Analysis
Latent GOLD 5.1 (Statistical Innovations, Inc., Citation2017) was used to identify latent classes of biological dysregulation and Stata/MP 16 (StataCorp, Citation2019) was used to explore the associations between these latent classes and measures of depression and anhedonia while controlling for socio-demographic covariates. This type of latent class analysis typically employs a stepwise approach that involves the three-step process of (1) building a series latent class models and selecting the one with the best model fit to the data; (2) assigning observations to a given class based on posterior class membership probabilities; and (3) using the assigned classes as the dependent variable in a multinomial logistic regression that is regressed on a range of predictor variables while controlling for sociodemographic characteristics (Vermut, Citation2010).
The latent class analysis fits the data to a series of models ranging from one to six classes. Model fit statistics used to assess the models included Bayesian Information Criterion (BIC), Akaike’s Information Criterion (AIC), Consistent Akaike’s Information Criterion (CAIC), Log-Likelihood (LL), and bootstrapped likelihood-ratio chi-square statistic (L2). The bootstrapping approach provides an advantage over the initial chi-square statistic in that it allows for relaxing the assumption that the L2 value follows the chi-square distribution. For this approach, the most parsimonious model, that is, the model with the fewest latent classes, with a p-value greater than 0.05 is considered the best model for the data (Vermunt & Magidson, Citation2005, Citation2016a, Citation2016b).
A supplemental analysis regressed a cumulative, multisystem allostatic load measure of biological dysregulation on the same mental health variables for comparison purposes and to better understand how latent profiles of biological dysregulation differ from a common cumulative measure of allostatic load. This was completed via a multivariate linear regression that controlled for the same sociodemographic variables as the multinomial logistic regression models. A detailed explanation of how the cumulative, multisystem allostatic load score was calculated can be found elsewhere (see Carbone, Citation2020). The basic approach was to create an average score across each biological system based on biomarkers dichotomized into high-risk quartiles of the sample distribution and then add the systems-level scores to create a cumulative allostatic load measure. This approach has become more common in allostatic load research in recent years (e.g. Chen et al., Citation2012; Friedman et al., Citation2015; Ong et al., Citation2017; Priest et al., Citation2015; Schwartz, Citation2017). The scores were log-transformed to ease interpretation of the results in comparison to the study’s main findings.
Measures
Biomarkers of allostatic load
One limitation of the existing literature is the operationalization of allostatic load into a single score that represents dysregulation across systems. One approach is to simply sum scores across biomarkers to create a cumulative risk score (e.g. Allsworth et al., Citation2005; Bellatorre et al., Citation2011; Duru et al., Citation2012; Frei et al., Citation2015; Morrison et al., Citation2013). Another approach is to calculate allostatic load scores individually across the seven biological systems impacted by stress and then sum the systems-level scores to create an overall allostatic load score (e.g. Chen et al., Citation2012; Friedman et al., Citation2015; Ong et al., Citation2017; Priest et al., Citation2015; Schwartz, Citation2017). The challenge with both these approaches is that it eliminates the ability to understand variance in allostatic load by each biological system or across multiple biomarkers or systems. The complexity of the body’s responses to stress suggests that a more nuanced approach that better identifies the underlying profiles of dysregulation is warranted. By employing latent class analysis, which is a newly emerging approach in allostatic load research (e.g. Forrester et al., Citation2019), the nuanced relationships between biomarkers across biological systems can be better explicated through the identification of underlying classes of biological dysregulation.
Twenty-two biomarkers, representing seven biological systems, were included in the analysis. The biomarkers and their relevant biological systems are cortisol and D-HEAS (HPA axis); epinephrine, norepinephrine, and dopamine (sympathetic nervous system); systolic blood pressure and diastolic blood pressure (cardiovascular system); the standard deviation of heart cycle length variability (SDRR), root mean squared successive differences of the beat-to-beat interval (RMSSD), low-frequency heart rate variability (LFHRV), and high-frequency heart rate variability (HFHRV) (parasympathetic nervous system); IL-6, fibrinogen, C-reactive protein, E-selectin, and intercellular adhesion molecule 1 (ICAM-1) (inflammatory system); HDL cholesterol, LDL cholesterol, and triglycerides (lipid metabolism); HbA1c, fasting glucose, and insulin resistance (HOMA) (glucose metabolism). Biomarkers were dichotomized into high-risk quartiles based on sample distributions (1 = high risk, 0 = low risk). The approach for dichotomizing biomarkers is based on an algorithm (Seeman et al., Citation1997) that has been used extensively in the literature (see Beckie, Citation2012 for a review). For most biomarkers, those in the highest 25% of the sample distribution were categorized as high risk. For HDL cholesterol and the parasympathetic nervous system biomarkers, the lowest 25% of the sample distribution was categorized as high risk. Cortisol and DHEA-S, were split so that both the lowest and highest 12.5% were categorized as high risk. This is an approach that has been growing in popularity in recent years in an attempt to capture both hypercotisolemia and hypocortisolemia (Bellingrath et al., Citation2009; Hellhammer et al., Citation2004; Juster et al., Citation2013).
Mental health measures
Four different measures of mental health were employed in this analysis. Each measure assessed a specific aspect or dimension related to, but not necessarily exclusive to, depression. The first was a self-reported response (1 = yes, 0 = no) to whether or not the respondent had been treated for depression, anxiety, or another emotional disorder within the past 12 months. The second was whether or not the respondent met the criteria for depression based on the Center for Epidemiological Studies Depression Inventory (CES-D) (1 = yes, 0 = no). The third and fourth were based on two subscales from the Mood and Anxiety Symptom Questionnaire (MASQ). One was the Generalized Distress: Depression subscale (MASQ-GDD) and the other was the Anhedonia subscale (MASQ-A) (1 = yes, 0 = no for each subscale). These four different measures were employed to assess the association between latent class membership and different aspects of depression and mental health, with the goal of providing a more holistic picture of these relationships.
Given that the subscales of the MASQ do not have established clinical cutoffs, the literature was reviewed to determine the best cutoffs for this analysis. From a psychometric perspective, the most desirable cutoff is the one with the highest and most balanced measures of sensitivity and specificity. Utilizing this approach, a cutoff value of 25 was used for the MASQ-GDD (Schalet et al., Citation2014) and a cutoff of 21 for the MASQ-A (Bredemeier et al., Citation2010). While multiple clinical cutoffs ranging from 16 to 22 have been proposed for the CES-D, a meta-analysis found that 20 was likely the best cutoff value, so it was utilized in this study (Vilagut et al., Citation2016).
Covariates
The regression models controlled for a number of demographic variables. The following variables were included in each model: age (continuous), sex (female, male), race (Caucasian, black and/or African American, other), the highest level of educational attainment (high school diploma or less, some college or associates degree, bachelor’s degree, graduate degree), employment status (employed, retired, homemaker, unemployed and searching for a job or temporarily laid off, other), and marital status (married, divorced or separated, never married, other). Each model also controlled for prescription medications that represented health conditions and diseases that are specifically linked to biomarkers included in the analysis. Separate, dichotomous variables (yes, no) were included for self-reported use of prescription medications for three conditions: hypertension, high cholesterol, and diabetes. Finally, a dichotomous (yes, no) variable was included based on self-reported use of prescription medication to treat anxiety or depression.
Results
Latent class analysis
The fit statistics for the latent classes are displayed in . Based on the diminishing improvement in fit statistics when moving from four to five classes, as well as the fact that the four-class model is the most parsimonious model with a not statistically significant bootstrapped chi-square value, the four-class model was selected as the one that best fits this data.
Table 1. Fit statistics for latent classes of biological dysregulation.
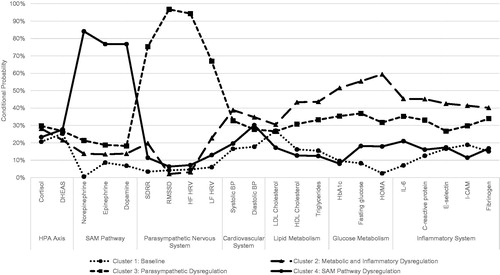
displays the four latent classes of biological dysregulation. The x-axis lists all 22 biomarkers included in the analysis and groups them by biological system, while the y-axis displays the percentage of observations that fall in the high-risk quartile of the sample distribution for a given biomarker (i.e. conditional probability). shows that individuals in Cluster 1 experience relatively low levels of dysregulation – ranging from less than 3% of the sample experiencing HOMA dysregulation to a high of 27% of the sample experiencing LDL cholesterol dysregulation – therefore this cluster is identified as the baseline group. Cluster 2 represents metabolic and inflammatory dysregulation due to the high number of observations within this cluster that demonstrate dysregulation in variables within both lipid and glucose metabolism systems as well as within the inflammatory system. As compared to the baseline group, the metabolic and inflammatory group has much higher levels of dysregulation, ranging from 30.49% of individuals experiencing dysregulation in LDL cholesterol to 59.43% of individuals with a dysregulation in HOMA levels. Cluster 3 is labeled as parasympathetic systems dysregulation. Individuals in this group are much more likely to experience dysregulation in SDRR (75.33%), RMSSD (96.75%), HFHRV (94.32%), and LFHRV (66.95%), while also experiencing relatively low levels of dysregulation across other biomarkers. The final group, cluster 4, is comprised of individuals with high sympathomedullary pathway (SAM pathway) dysregulation. This includes individuals with a dysregulation in norepinephrine (84.10%), epinephrine (76.78%), and dopamine (76.77%).
Descriptive statistics
The profile of sociodemographic characteristics for each latent class of biological dysregulation is displayed in . Chi-square and ANOVA tests showed that there were statistically significant differences across the four groups of biological dysregulation for all of the covariates (ps < 0.05–0.001).
Table 2. Sample descriptive statistics stratified by latent class.
The baseline group, which contains 34.95% of the sample (n = 614) was predominantly female (58.96%), majority Caucasian (84.20%), and had a mean age of 49.64. The majority of individuals in this group were employed (62.21%) and married (67.92%). In terms of education, the largest category was individuals with a bachelor’s degree (28.66%), followed by those with some college or associate’s degree (27.20%), and individuals with a graduate degree (21.66%). A relatively small proportion of individuals in this group were prescribed medications for anxiety or depression (9.28%), hypertension (15.47%), high cholesterol (16.78%), or diabetes (1.30%).
The metabolic and inflammatory dysregulation group, which made up 22.88% of the sample (n = 402), had a higher number of females (52.74%) than males and had a mean age of 52.90. This group also had a lower percentage of Caucasians (63.43%) and the greatest proportion of African Americans (30.85%) of all the groups. Roughly half of the members of these groups were married (55.22%) but more also had a higher proportion of individuals who were either separated/divorced or never married (21.64 and 18.91%, respectively). The largest education category for the metabolic and inflammatory dysregulation group was some college or an associate’s degree (35.82%) followed by a high school diploma or less (29.35%). Nearly half of the individuals in this group worked full time (49.00%), while more than one-fifth were retired (20.65%). This group had the highest percentage of individuals on prescription medication for hypertension (42.79%) and the second-highest percentage of individuals on prescription medication for high cholesterol (30.10%). 15.42% reported taking a prescription for anxiety or depression, while one-fifth were on prescription diabetes medication (21.64%).
Individuals in the parasympathetic dysregulation group, 22.65% of the sample (n = 398), were split between males and females (50.25 and 49.75%, respectively) and were the oldest group on average with a mean age of 57.70. This group had a high proportion of Caucasians (83.92%) and the lowest percentage of African Americans (10.55%). This group had the lowest percentage of employed individuals (45.98%) and the highest percentage of retired individuals (28.39%). Two-thirds of individuals in this group were married (67.84%). Individuals with some college or an associate’s degree were the largest educational category (29.65%) followed by those with a bachelor’s degree (27.14%). This group had the highest proportion of individuals on prescription medication for high cholesterol (35.68%) with a similar proportion taking medication for hypertension (35.43%). 18.59% were on medication for anxiety or depression, while 14.32% were on diabetes medication.
The final group was SAM pathway dysregulation and it consisted of 19.52% of the full sample (n = 343). This group was majority female (58.89%) with a mean age of 50.16. It was primarily Caucasian (69.39%) but had the second-highest percentage of African Americans (18.95). The group had a majority of individuals who were married (57.73%) followed by those who were never married (20.12%). 58.31% of individuals in this group were employed with nearly one-third having some college or an associate’s degree (29.37%) followed by those with a bachelor’s degree (26.86%). Nearly one-quarter of individuals in this group were taking prescription medication for hypertension (23.32%), with a slightly smaller proportion taking medication for high cholesterol (19.53%), and fewer individuals taking medication for anxiety or depression (10.50%) or diabetes (4.66%).
Multinomial logistic regression
Treatment
The results of the multinomial logistic regression models are displayed in . Adjusting for age, sex, race, educational attainment, employment status, marital status, and prescription medications, there was not a statistically significantly greater risk for those who had been treated for depression, anxiety, or another emotional disorder in the past 12 months of being in any of the three classes of biological dysregulation as compared to being in the baseline group.
Table 3. Multinomial logistic regression results.
CES-D
Individuals who met the CES-D criteria for depression were at 80% greater risk of being in the metabolic and inflammatory dysregulation group as compared to the baseline group than those who did not meet the CES-D threshold criteria (ARR = 1.80, 95% CI: 1.13, 2.88). Those who met the CES-D criteria had 71% greater risk of being in the parasympathetic dysregulation group as opposed to the baseline group (ARR = 1.71, 95% CI: 1.04, 2.82). There was not a statistically significant difference between the SAM pathway dysregulation group and the baseline group.
MASQ-GDD
Individuals who met the MASQ-GDD subscale criteria had a 78% greater risk (ARR = 1.78, 95% CI: 1.08, 2.96) of being in the metabolic and inflammatory dysregulation group as compared to the baseline group. MASQ-GDD was not associated with a higher risk of membership in either the parasympathetic dysregulation or the SAM pathway dysregulation groups.
MASQ-anhedonia
With respect to anhedonia, individuals who met the MASQ criteria for this subscale had nearly twice the risk of being in the metabolic and inflammatory dysregulation group (ARR = 1.95, 95% CI: 1.01, 3.75) than in the baseline group. Neither parasympathetic dysregulation nor SAM pathway dysregulation was statistically significantly different from the baseline group.
Supplemental analysis
A supplemental analysis was completed utilizing a more traditional allostatic load measure of cumulative, systems-level dysregulation. Results of a series of multivariate linear regression models showed that seeking treatment for anxiety, depression, or another emotional disorder was not associated with cumulative allostatic load (p = 0.50). Individuals who met the criteria for the CES-D has 13% higher cumulative allostatic load (B = 0.129, p < 0.01), while those who met the MASQ general distress subscale and anhedonia subscale criteria had 14% and 21% higher cumulative allostatic load scores, respectively, than those who did not meet the criteria for each subscale (MASQ GDD B = 0.144, p < 0.01; MASQ anhedonia B = 0.212, p < 0.01).
Discussion
This study sought to both identify underlying classes of biological dysregulation and to assess the degree to which membership in classes of dysregulation was associated with depression-related mental health outcomes. The findings suggest that individuals who met the criteria of the CES-D were at a higher risk, as compared to those who did not, of being in the metabolic and inflammatory dysregulation group and the parasympathetic dysregulation group as opposed to being in the baseline group. In addition, those who met the criteria for the MASQ anhedonia subscale and the MASQ general distress depression subscale were at a greater risk of experiencing metabolic and inflammatory dysregulation.
It is important to note that these findings may generalize beyond depression to other mental health disorders. Anhedonia is not a symptom that is exclusive to depression. It is experienced by individuals afflicted by a number of disorders including – but not limited to – schizophrenia, social anxiety, and posttraumatic stress disorder (Shankman et al., Citation2014). Therefore, these findings should also be considered in light of their implications for emotional disorders more broadly.
The use of latent class analysis adds to previous research on cumulative biological risk, as it disaggregates the association between specific biomarkers and systems and mental health outcomes. While the findings from the multivariate linear regression models for cumulative, multisystem dysregulation completed in the supplemental analysis aligned with the multinomial logistic regression results, that model is not able to identify which biological systems are driving the association. A such, these findings align with new research that has emerged in recent years that focuses on the important roles of systems-level and cross-systems biological dysregulation. Examples of this emerging research include the growth in the utilization of Polyvagal Theory and the developing field of immunometabolism.
Porges (Citation1995, Citation2001) introduced Polyvagal Theory as a means of explaining the relationship between parasympathetic nervous system functioning and behavior. Specifically, respiratory sinus arrhythmia (i.e. high-frequency heart rate variability) is utilized as a measure of parasympathetic nervous system functioning, often referred to as vagal tone (Porges, Citation1995, Citation2001). While certain fundamental components and assumptions of Polyvagal Theory as they relate to human evolution have been disputed (see Grossman & Taylor, Citation2007), parasympathetic nervous system functioning as operationalized by measures of heart rate variability (HRV) continues to be an area of intense and diverse research. In a systematic review and meta-analysis of 150 case-control and treatment studies, Alvares et al. (Citation2016) found that HRV among individuals with psychiatric disorders was statistically significantly lower than controls without psychiatric disorders. In addition, while each of the other subgroups (mood disorder, anxiety disorder, and substance dependence) were statistically significantly different from the control group, individuals with psychotic disorders had the largest effect size (Hedges g = −0.952, 95% CI −1.105, −0.800, p < 0.00). Brown et al. (Citation2018) completed two meta-analyses, one of clinical trials and the other of community studies, to assess the association between HRV and depression among older adults and found that decreased HRV was associated with increased levels of depression. Specifically, low-frequency HRV, but not high-frequency HRV, was associated with depression. In all, the current state of research suggests that a more nuanced conceptualization and operationalization of parasympathetic nervous system functioning may be necessary to better understand this system’s role in mental health outcomes.
The past decade has seen increased recognition of the interplay between metabolic and immune function. This has led to a growth in biomedical literature focused on what is now known as immunometabolism (Mathis & Shoelson, Citation2011; Murray et al., Citation2015). Research in this area suggests a complex, recursive relationship and interaction between the immune and metabolic systems. This two-way relationship creates complicated interactions and positive reinforcing loops, such as when inflammation from multiple sources (e.g. cancer, diet, infection, or injury) leads to changes in systems-level metabolic functions, which then trigger cellular level changes in the metabolic functioning of immune cells, in turn resulting in modifications to immune system functioning (Buck et al., Citation2017).
Given that immunometabolic research is relatively new, much additional work is needed to explicate the complex relationships between metabolic and immune function at both the cellular and whole-body levels (Buck et al., Citation2017; Man et al., Citation2017; O’Neill et al., Citation2016). The results of this study suggest that allostatic load researchers should further explore the immunometabolic perspective in their research. As immunologists and metabolic researchers continue to consider how these two biological systems interact and result in biological dysregulation and disease, allostatic load researchers should consider how environmental stressors influence metabolic and immune processes as well as interactions between these systems.
While allostatic load research to date has explored the role of immune system dysregulation as it relates to mental health, such as the application of cytokine theory as previously discussed (Bob et al., Citation2010), combining immune and metabolic dysregulation may result in a clearer picture of common comorbidities as they relate to MDD. Specifically, obesity, diabetes, and metabolic syndrome are common MDD comorbidities (Anderson et al., Citation2001; Dunbar et al., Citation2008; Olvera et al., Citation2015). While the causality and directionality of the relationship between mental health and metabolic disorders is complex and likely reciprocal, a better understanding of the immunometabolic processes through the allostatic load theoretical lens may allow researchers and clinicians to identify the best targets for interventions that are most likely to break this reciprocal cycle.
These findings reinforce the need to integrate an understanding of the biological processes that are associated with the etiology of mental health disorders. By considering the unique underlying biological systems associated with specific mental health disorders, specific and targeted interventions can be identified and implemented to affect these systems.
Limitations
The findings of this study must be considered in light of its limitations. First, latent class analysis is an exploratory technique and the findings of any analysis should be used primarily to guide future research. Second, like most studies of allostatic load, the operationalization of high-risk biomarkers is based on the sample distribution, therefore the findings are sample-specific and may not generalize to a larger population. Third, the measure of treatment is a triple-barreled question, as it asks about treatment for depression, anxiety, or another emotional disorder. If these disorders were disaggregated into multiple questions, it is possible that significant associations between individual disorders and specific classes of dysregulation may emerge. Finally, individuals in two of the latent classes of biological dysregulation are statistically significantly different from those in the baseline group on a number of variables, yet the ARRs and their corresponding confidence intervals show that there is not much distinction between the two biologically dysregulated groups. One potential reason for this is the relatively high level of metabolic and immune dysregulation in the parasympathetic dysregulation group as compared to the baseline group. While this dysregulation does not rise to the level seen in the immune and metabolic dysregulation group, it does differ from the baseline group. Future research should explore this issue and better differentiate the role of each type of dysregulation as well as how they interact.
Conclusion
The findings of this study have important implications for future research related to parasympathetic nervous system dysregulation, immunometabolic dysregulation, and mental health outcomes. Parasympathetic nervous system dysregulation and immunometabolic dysregulation continue to be developing fields of research that can be integrated into the understanding of the biological etiology of mental health disorders such as MDD. This approach can aid researchers in developing effective treatments to reduce the prevalence of these disorders. Allostatic load theory can be employed as the conceptual framework for integrating these perspectives and ensuring that a holistic biological perspective is maintained by researchers as they work to address issues of mental health.
Acknowledgments
The author would like to thank Stephen Edward McMillin, Julie Birkenmaier, Michael G. Vaughn, Jin Huang, and Travis Loux for their feedback and suggestions for this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Jason T. Carbone
Jason T. Carbone, MSW, PhD., is an assistant professor in the School of Social Work at Wayne State University.
References
- Allsworth, J. E., Weitzen, S., & Boardman, L. A. (2005). Early age at menarche and allostatic load: Data from the Third National Health and Nutrition Examination Survey. Annals of Epidemiology, 15(6), 438–444. https://doi.org/10.1016/j.annepidem.2004.12.010
- Alvares, G. A., Quintana, D. S., Hickie, I. B., & Guastella, A. J. (2016). Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. Journal of Psychiatry & Neuroscience, 41(2), 89–104. https://doi.org/10.1503/jpn.140217
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing.
- Anderson, R. J., Freedland, K. E., Clouse, R. E., & Lustman, P. J. (2001). The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care, 24(6), 1069–1078. https://doi.org/10.2337/diacare.24.6.1069
- Atherton, B. D., Nevels, R. M., & Moore, M. T. (2015). Predicting symptoms of depression from social anhedonia and emotion regulation. The Journal of Nervous and Mental Disease, 203(3), 170–174. https://doi.org/10.1097/NMD.0000000000000262
- Beckie, T. M. (2012). A systematic review of allostatic load, health, and health disparities. Biological Research for Nursing, 14(4), 311–346. https://doi.org/10.1177/1099800412455688
- Bellatorre, A., Finch, B. K., Do, D., Bird, C. E., & Beck, A. N. (2011). Contextual predictors of cumulative biological risk: Segregation and allostatic load. Social Science Quarterly, 92, 1338–1362. https://doi.org/10.1111/j.1540-6237.2011.00821.x
- Bellingrath, S., Weigl, T., & Kudielka, B. M. (2009). Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers: Original research report. Stress, 12(1), 37–48. https://doi.org/10.1080/10253890802042041
- Berger, M., Juster, R. P., Westphal, S., Amminger, G. P., Bogerts, B., Schiltz, K., Bahn, S., Steiner, J., & Sarnyai, Z. (2018). Allostatic load is associated with psychotic symptoms and decreases with antipsychotic treatment in patients with schizophrenia and first-episode psychosis. Psychoneuroendocrinology, 90, 35–42. https://doi.org/10.1016/j.psyneuen.2018.02.001
- Berger, M., Lavoie, S., McGorry, P. D., Nelson, B., Markulev, C., Yuen, H. P., Schaefer, M., Sarnyai, Z., & Amminger, G. P. (2018). Relationship between allostatic load and clinical outcomes in youth at ultra-high risk for psychosis in the NEURAPRO study. Schizophrenia Research. Advance online publication. https://doi.org/10.1016/j.schres.2018.10.002
- Berger, M., Taylor, S., Harriss, L., Campbell, S., Thompson, F., Jones, S., Sushames, A., Amminger, P., Sarnyai, Z., & McDermott, R. (2019). Hair cortisol, allostatic load, and depressive symptoms in Australian Aboriginal and Torres Strait Islander people. Stress, 22(3), 312–320. https://doi.org/10.1080/10253890.2019.1572745
- Bob, P., Raboch, J., Maes, M., Susta, M., Pavlat, J., Jasova, D., Vevera, J., Uhrova, J., Benakova, H., & Zima, T. (2010). Depression, traumatic stress and interleukin-6. Journal of Affective Disorders, 120(1–3), 231–234. https://doi.org/10.1016/j.jad.2009.03.017
- Bowers, M. E., & Yehuda, R. (2017). Neuroendocrinology of posttraumatic stress disorder: Focus on the HPA Axis. In G. Fink (Ed.), Stress: Neuroendocrinology and neurobiology, handbook of stress (Vol. 2, pp. 165–172). Academic Press.
- Bredemeier, K., Spielberg, J. M., Silton, R. L., Berenbaum, H., Heller, W., & Miller, G. A. (2010). Screening for depressive disorders using the Mood and Anxiety Symptoms Questionnaire Anhedonic Depression Scale: A receiver-operating characteristic analysis. Psychological Assessment, 22(3), 702–710. https://doi.org/10.1037/a0019915
- Brietzke, E., Kapczinski, F., Grassi-Oliveira, R., Grande, I., Vieta, E., & McIntyre, R. S. (2011). Insulin dysfunction and allostatic load in bipolar disorder. Expert Review of Neurotherapeutics, 11(7), 1017–1028. https://doi.org/10.1586/ern.10.185
- Brown, L., Karmakar, C., Gray, R., Jindal, R., Lim, T., & Bryant, C. (2018). Heart rate variability alterations in late life depression: A meta-analysis. Journal of Affective Disorders, 235, 456–466. https://doi.org/10.1016/j.jad.2018.04.071
- Buck, M. D., Sowell, R. T., Kaech, S. M., & Pearce, E. L. (2017). Metabolic instruction of immunity. Cell, 169(4), 570–586. https://doi.org/10.1016/j.cell.2017.04.004
- Carbone, J. T. (2020). Neighborhood perceptions and allostatic load: Evidence from Midlife in the United States study. Health & Place, 61, 102263. https://doi.org/10.1016/j.healthplace.2019.102263
- Chen, E., Miller, G. E., Lachman, M. E., Gruenewald, T. L., & Seeman, T. E. (2012). Protective factors for adults from low-childhood socioeconomic circumstances: The benefits of shift-and-persist for allostatic load. Psychosomatic Medicine, 74(2), 178–186. https://doi.org/10.1097/PSY.0b013e31824206fd
- Cho, J., Stone, M. D., & Leventhal, A. M. (2019). Anhedonia as a phenotypic marker of familial transmission of polysubstance use trajectories across midadolescence. Psychology of Addictive Behaviors: Behaviors, 33(1), 15–25. https://doi.org/10.1037/adb0000427
- Crews, D. E. (2007). Composite estimates of physiological stress, age, and diabetes in American Samoans. American Journal of Physical Anthropology, 133(3), 1028–1034. https://doi.org/10.1002/ajpa.20612
- Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., & Lanctôt, K. L. (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. https://doi.org/10.1016/j.biopsych.2009.09.033
- Dunbar, J. A., Reddy, P., Davis-Lameloise, N., Philpot, B., Laatikainen, T., Kilkkinen, A., Bunker, S. J., Best, J. D., Vartiainen, E., Lo, S. K., & Janus, E. D. (2008). Depression: An important comorbidity with metabolic syndrome in a general population. Diabetes Care, 31(12), 2368–2373. https://doi.org/10.2337/dc08-0175
- Duru, O. K., Harawa, N. T., Kermah, D., & Norris, K. C. (2012). Allostatic load burden and racial disparities in mortality. Journal of the National Medical Association, 104(1–2), 89–95. https://doi.org/10.1016/S0027-9684(15)30120-6
- Forrester, S. N., Leoutsakos, J., Gallo, J. J., Thorpe, R. J., & Seeman, T. E. (2019). Association between allostatic load and health behaviours: A latent class approach. Journal of Epidemiology and Community Health, 73(4), 340–345. https://doi.org/10.1136/jech-2018-211289
- Frei, R., Haile, S. R., Mutsch, M., & Rohrmann, S. (2015). Relationship of serum vitamin D concentrations and allostatic load as a measure of cumulative biological risk among the US population: A cross-sectional study. PLoS One, 10(10), e0139217. https://doi.org/10.1371/journal.pone.0139217
- Friedman, E. M., Karlamangla, A. S., Gruenewald, T. L., Koretz, B., & Seeman, T. E. (2015). Early life adversity and adult biological risk profiles. Psychosomatic Medicine, 77(2), 176–185. https://doi.org/10.1097/PSY.0000000000000147
- Gill, J., Vythilingam, M., & Page, G. G. (2008). Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. Journal of Traumatic Stress, 21(6), 530–539. https://doi.org/10.1002/jts.20372
- Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. https://doi.org/10.1016/j.biopsycho.2005.11.014
- Haapakoski, R., Mathieu, J., Ebmeier, K. P., Alenius, H., & Kivimäki, M. (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity, 49, 206–215. https://doi.org/10.1016/j.bbi.2015.06.001
- Hellhammer, J., Schlotz, W., Stone, A. A., Pirke, K. M., & Hellhammer, D. (2004). Allostatic load, perceived stress, and health: A prospective study in two age groups. Annals of the New York Academy of Sciences, 1032, 8–13. https://doi.org/10.1196/annals.1314.002
- Juster, R. P., & Lupien, S. (2012). A sex- and gender-based analysis of allostatic load and physical complaints. Gender Medicine, 9(6), 511–523. https://doi.org/10.1016/j.genm.2012.10.008
- Juster, R.-P., Marin, M.-F., Sindi, S., Nair, N. P. V., Ng, Y. K., Pruessner, J. C., & Lupien, S. J. (2011). Allostatic load associations to acute, 3-year and 6-year prospective depressive symptoms in healthy older adults. Physiology & Behavior, 104(2), 360–364. https://doi.org/10.1016/j.physbeh.2011.02.027
- Juster, R.-P., Moskowitz, D. S., Lavoie, J., & D’Antono, B. (2013). Sex-specific interaction effects of age, occupational status, and workplace stress on psychiatric symptoms and allostatic load among healthy Montreal workers. Stress, 16(6), 616–629. https://doi.org/10.3109/10253890.2013.835395
- Kapczinski, F., Vieta, E., Andreazza, A. C., Frey, B. N., Gomes, F. A., Tramontina, J., Kauer-Sant’anna, M., Grassi-Oliveira, R., & Post, R. M. (2008). Allostatic load in bipolar disorder: Implications for pathophysiology and treatment. Neuroscience and Biobehavioral Reviews, 32(4), 675–692. https://doi.org/10.1016/j.neubiorev.2007.10.005
- Kobrosly, R. W., Seplaki, C. L., Cory-Slechta, D. A., Moynihan, J., & van Wijngaarden, E. (2013). Multisystem physiological dysfunction is associated with depressive symptoms in a population-based sample of older adults. International Journal of Geriatric Psychiatry, 28(7), 718–727. https://doi.org/10.1002/gps.3878
- Kobrosly, R. W., van Wijngaarden, E., Seplaki, C. L., Cory-Slechta, D. A., & Moynihan, J. (2014). Depressive symptoms are associated with allostatic load among community-dwelling older adults. Physiology & Behavior, 123, 223–230. https://doi.org/10.1016/j.physbeh.2013.10.014
- Krugel, L. K., Biele, G., Mohr, P. N., Li, S. C., & Heekeren, H. R. (2009). Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proceedings of the National Academy of Sciences of the United States of America, 106(42), 17951–17956. https://doi.org/10.1073/pnas.0905191106
- Kyrou, I., Randeva, H. S., & Tsigos, C. (2017). Stress, insulin resistance, and type 2 diabetes. In G. Fink (Ed.), Stress: Neuroendocrinology and neurobiology, handbook of stress (Vol. 2, pp. 351–358.) Academic Press.
- Lamontagne, S. J., Melendez, S. I., & Olmstead, M. C. (2018). Investigating dopamine and glucocorticoid systems as underlying mechanisms of anhedonia. Psychopharmacology, 235(11), 3103–3113. https://doi.org/10.1007/s00213-018-5007-4
- Lucassen, P. J., Korosi, A., Krugers, H. J., & Oomen, C. A. (2017). Early life stress- and sex-dependent effects on hippocampal neurogenesis. in Fink, G. (Ed.) Stress: Neuroendocrinology and Neurobiology, Handbook of Stress., Volume 2 (pp. 135–146), Academic Press.
- Maes, M., Lin, A., Delmeire, L., Van Gastel, A., Kenis, G., De Jongh, R., & Bosmans, E. (1999). Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic. Biological Psychiatry, 45(7), 833–839. https://doi.org/10.1016/S0006-3223(98)00131-0
- Man, K., Kutyavin, V. I., & Chawla, A. (2017). Tissue immunometabolism: Development, physiology, and pathobiology. Cell Metabolism, 25(1), 11–26. https://doi.org/10.1016/j.cmet.2016.08.016
- Mathis, D., & Shoelson, S. E. (2011). Immunometabolism: An emerging frontier. Nature Reviews. Immunology, 11(2), 81–83. https://doi.org/10.1038/nri2922
- Mattei, J., Demissie, S., Falcon, L. M., Ordovas, J. M., & Tucker, K. (2010). Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Social Science & Medicine, 70(12), 1988–1996. https://doi.org/10.1016/j.socscimed.2010.02.024
- McEwen, B. S. (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. https://doi.org/10.1016/S0006-3223(03)00177-X
- McEwen, B. S., Nasveld, P., Palmer, M., & Anderson, R. (2012). Allostatic Load: A review of the literature. Department of Veterans’ Affairs.
- Morrison, S., Shenassa, E. D., Mendola, P., Wu, T., & Schoendorf, K. (2013). Allostatic load may not be associated with chronic stress in pregnant women, NHANES 1999-2006. Annals of Epidemiology, 23(5), 294–297. https://doi.org/10.1016/j.annepidem.2013.03.006
- Murray, P. J., Rathmell, J., & Pearce, E. (2015). SnapShot: Immunometabolism. Cell Metabolism, 22(1), 190–190.e1. https://doi.org/10.1016/j.cmet.2015.06.014
- Nugent, K. L., Chiappelli, J., Rowland, L. M., & Hong, L. E. (2015). Cumulative stress pathophysiology in schizophrenia as indexed by allostatic load. Psychoneuroendocrinology, 60, 120–129. https://doi.org/10.1016/j.psyneuen.2015.06.009
- Olvera, R. L., Williamson, D. E., Fisher-Hoch, S. P., Vatcheva, K. P., & McCormick, J. B. (2015). Depression, obesity, and metabolic syndrome: Prevalence and risks of comorbidity in a population-based representative sample of Mexican Americans. The Journal of Clinical Psychiatry, 76(10), e1300–e1305. https://doi.org/10.4088/JCP.14m09118
- Ong, A. D., Williams, D. R., Nwizu, U., & Gruenewald, T. L. (2017). Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cultural Diversity and Ethnic Minority Psychology, 23(1), 27–35. https://doi.org/10.1037/cdp0000087
- O’Neill, L. A. J., Kishton, R. J., & Rathmell, J. (2016). A guide to immunometabolism for immunologists. Nature Reviews Immunology, 16(9), 553–565. https://doi.org/10.1038/nri.2016.70
- Pace, T. W. W., & Miller, A. H. (2009). Cytokines and glucocorticoid receptor signaling. Annals of the New York Academy of Sciences, 1179, 86–105. https://doi.org/10.1111/j.1749-6632.2009.04984.x
- Pariante, C. M., & Miller, A. H. (2001). Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biological Psychiatry, 49(5), 391–404. https://doi.org/10.1016/S0006-3223(00)01088-X
- Pervanidou, P., Kolaitis, G., Charitaki, S., Margeli, A., Ferentinos, S., Bakoula, C., Lazaropoulou, C., Papassotiriou, I., Tsiantis, J., & Chrousos, G. P. (2007). Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology, 32(8–10), 991–999. https://doi.org/10.1016/j.psyneuen.2007.07.001
- Porges, S. W. (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology, 32(4), 301–318. https://doi.org/10.1111/j.1469-8986.1995.tb01213.x
- Porges, S. W. (2001). The Polyvagal Theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology: Psychophysiology, 42(2), 123–146. https://doi.org/10.1016/S0167-8760(01)00162-3
- Price, J., Cole, V., & Goodwin, G. M. (2009). Emotional side-effects of selective serotonin reuptake inhibitors: Qualitative study. British Journal of Psychiatry, 195(3), 211–217. https://doi.org/10.1192/bjp.bp.108.051110
- Priest, J. B., Woods, S. B., Maier, C. A., Parker, E. O., Benoit, J. A., & Roush, T. R. (2015). The biobehavioral family model: Close relationships and allostatic load. Social Science & Medicine, 142, 232–240. https://doi.org/10.1016/j.socscimed.2015.08.026
- Rosmond, R., & Bjorntorp, P. (2000). The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of Internal Medicine, 247(2), 188–197. https://doi.org/10.1046/j.1365-2796.2000.00603.x
- Ryff, C., Almeida, D. M., Ayanian, J., Carr, D. S., Cleary, P. D., Coe, C., Davidson, R., Krueger, R. F., Lachman, M. E., Marks, N. F., Mroczek, D. K., Seeman, T., Seltzer, M. M., Singer, B. H., Sloan, R. P., Tun, P. A., Weinstein, M., Williams, D. (2006). Midlife in the United States (MIDUS 2), 2004-2006. https://doi.org/10.3886/ICPSR04652.v7
- Ryff, C., Almeida, D. M., Ayanian, J., Binkley, N., Carr, D. S., Coe, C., Davidson, R., Grzywacz, J., Karlamangla, A., Krueger, R., Lachman, M., Love, G., Mailick, M., Mroczek, D., Radler, B., Seeman, T., Sloan, R., Thomas, D., Weinstein, M., Williams, D. (2014). Midlife in the United States (MIDUS 3),2013-2014 (ICPSR 36346). NACDA. https://www.icpsr.umich.edu/web/NACDA/studies/36346/versions/V6
- Santacroce, S. J., & Crandell, J. B. (2014). Feasibility and preliminary findings from a pilot study of allostatic load in adolescent-young adult childhood cancer survivors and their siblings. Journal of Pediatric Oncology Nursing: Nursing, 31(3), 122–134. https://doi.org/10.1177/1043454213520190
- Savransky, A., Chiappelli, J., Fisseha, F., Wisner, K. M., Xiaoming, D., Mirmomen, S. M., Jones, A. D., Adhikari, B. M., Bruce, H. A., Rowland, L. M., & Hong, L. E. (2018). Elevated allostatic load early in the course of schizophrenia. Translational Psychiatry, 8(1), 1–7. https://doi.org/10.1038/s41398-018-0299-z
- Schwartz, J. A., (2017). Long-term physical health consequences of perceived inequality: Results from a twin comparison design. Social Science & Medicine, 187, 184–192. https://doi.org/10.1016/j.socscimed.2017.06.006
- Schalet, B. D., Cook, K. F., Choi, S. W., & Cella, D. (2014). Establishing a common metric for self-reported anxiety: Linking the MASQ, PANAS, and GAD-7 to PROMIS Anxiety. Journal of Anxiety Disorders, 28, 88–96. https://doi.org/10.1016/j.janxdis.2013.11.006
- Shankman, S. A., Katz, A. C., DeLizza, A. A., Sarapas, C., Gorka, S. M., & Campbell, M. L. (2014). The different facets of anhedonia and their associations with different psychopathologies. In M. Ritsner (Ed.), Anhedonia: A comprehensive handbook (Vol I, pp. 3–22). Sringer.
- Statistical Innovations Inc. (2017). LatentGOLD® version 5.1.0. [computer software]. https://www.statisticalinnovations.com/
- StataCorp (2019). Stata Statistical Software: Release 16. [computer software]. https://www.stata.com
- Seeman, T. E., McEwen, B. S., Rowe, J. W., & Singer, B. H. (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America, 98(8), 4770–4775. https://doi.org/10.1073/pnas.081072698
- Seeman, T. E., Singer, B. H., Rowe, J. W., Horwitz, R. I., & McEwen, B. S. (1997). Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Archives of Internal Medicine, 157(19), 2259–2268. https://doi.org/10.1001/archinte.1997.00440400111013
- Stetler, C., & Miller, G. E. (2011). Depression andhypothalamic-pituitary-adrenal activation: a quantitative summary of fourdecades of research. Psychosomatic Medicine, 73(2), 114–126.
- Straub, R. H., & Cutolo, M. (2001). Involvement of the hypothalamic--pituitary--adrenal/gonadal axis and the peripheral nervous system in rheumatoid arthritis: Viewpoint based on a systemic pathogenetic role. Arthritis & Rheumatism, 44(3), 493–507. https://doi.org/10.1002/1529-0131(200103)44:3<493::AID-ANR95>3.0.CO;2-U
- Vermunt, J. K., & Magidson, J. (2005). Latent GOLD 4.0 user’s guide. Statistical Innovations Inc.
- Vermunt, J. K., & Magidson, J. (2016a). Upgrade manual for Latent GOLD 5.1. Statistical Innovations Inc.
- Vermunt, J. K., & Magidson, J. (2016b). Technical guide for Latent GOLD 5.1: Basic, advanced, and syntax. Statistical Innovations Inc.
- Vermunt, J. K. (2010). Latent class modeling with covariates: Two improved three-step approaches. Political Analysis, 18, 450–469. https://doi.org/10.1093/pan/mpq025
- Vieta, E., Popovic, D., Rosa, A. R., Solé, B., Grande, I., Frey, B. N., Martinez-Aran, A., Sanchez-Moreno, J., Balanzá-Martínez, V., Tabarés-Seisdedos, R., & Kapczinski, F. (2013). The clinical implications of cognitive impairment and allostatic load in bipolar disorder. European Psychiatry: Psychiatry, 28(1), 21–29. https://doi.org/10.1016/j.eurpsy.2011.11.007
- Vilagut, G., Forero, C. G., Barbaglia, G., & Alonso, J. (2016). Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): A systematic review with meta-analysis. PLoS One, 11(5), e0155431. https://doi.org/10.1371/journal.pone.0155431
- Wilder, R. L., & Elenkov, I. J. (1999). Hormonal regulation of tumor necrosis factor-alpha, interleukin-12 and interleukin-10 production by activated macrophages. A disease-modifying mechanism in rheumatoid arthritis and systemic lupus erythematosus? Annals of the New York Academy of Sciences, 876, 14–31. https://doi.org/10.1111/j.1749-6632.1999.tb07619.x