Abstract
Patients with atopy were found to exhibit blunted cortisol responses to acute stress stimuli. The aim of this study was to test the hypothesis that cumulative cortisol concentrations in the hair of patients with atopy are lower than in healthy subjects when related to their perceived stress experience. The sample consisted of 31 participants. The most proximal 3 cm of hair (as close to the scalp as possible), reflecting the cumulative cortisol secretion during the previous 3 months, was used for the analysis. Only in 20 subjects (9 patients with atopy and 11 healthy controls), there was a sufficient amount of hair for precise analysis using a new methodology. The results showed lower hair cortisol concentrations in patients with atopy compared to those in controls. The perceived stress scores in patients with atopy and healthy controls were not statistically different. The cortisol concentration/perceived stress score ratios were lower in patients with atopy compared to those in controls. No statistically significant correlation between hair cortisol and long-term experienced stress assessed via perceived stress scale was observed. In conclusion, the cumulative cortisol secretion in the hair of atopic patients is lower than would be expected according to their subjective scores of perceived stress. Most importantly, the previously lower stress hormone increase found in acute stress situations and in children now was confirmed in adult patients with chronic stress load.
Introduction
There are several pieces of evidence showing that patients with atopic dermatitis, seasonal allergic rhinitis exhibit blunted cortisol response to acute stress stimuli (Buske-Kirschbaum et al., Citation2002; Hlavacova et al., Citation2017). We have recently demonstrated a reduced cortisol awakening response in atopic patients compared to that in healthy subjects. Salivary cortisol concentrations throughout the rest of the day were similar to those in controls (Rajcani et al., Citation2019). So far, similarly as in other fields of research, more attention has been given to acute challenges compared to profiling long-term real-life stress situations (Buzgoova et al., Citation2020; Tseilikman et al., Citation2020).
The importance of evaluating cortisol secretion in patients with atopy is related to a potential impact of cortisol on the immune system function throughout the course of the disease. In this respect, a long-term profile of cortisol secretion would be of value. In the last decade, the methodology of hair cortisol measurements became available. Hair cortisol concentrations reflect the cumulative secretion of cortisol during several preceding months depending on the lengths of hair collected. There are however several methodological problems with cortisol extraction resulting in inconsistencies in some of the published data. We have recently reported a validated methodology of cortisol extraction from hair (Balagova & Jezova, Citation2018).
Thus, we have to emphasize that experiments so far have been focusing on acute stressor-induced cortisol concentrations, while here the chronic cumulative stress-induced cortisol changes are in the focus. As mentioned above, cortisol response to acute stress stimuli is blunted in patients with atopy. The aim of this study was to test the hypothesis that cumulative cortisol concentrations in the hair of patients with atopy are lower than in healthy subjects. The stress perception, as an estimate of the stress load, was also examined.
Material and Methods
Subjects
The sample consisted of 31 participants, of which 14 (5 men and 9 women) were diagnosed with atopy (atopic dermatitis, allergic rhinitis) and 17 (7 men and 10 women) healthy controls. All subjects were between 18 and 29-years-old with a normal body mass index (BMI). All patients were in remission and without steroid treatment for at least 1 month and antihistamine drugs for at least 1 week. The study was approved by the Ethics Committee of the Trnava Self-Governing Region, Trnava, Slovakia in agreement with the ethical guidelines of the Declaration of Helsinki. All subjects provided written informed consent prior to participating in the study.
Measurement of hair cortisol concentrations
Hair was carefully cut with scissors as close as possible to the scalp from a posterior vertex position. The most proximal 3 cm of hair (about 50 mg), which enable to measure a cumulative value for cortisol secretion during the last 3 months, was used for analysis.
Hair cortisol was measured using a modification of the methodology which has optimized several methodological steps in hair cortisol extraction (Balagova & Jezova, Citation2018). This novel method is likely to provide reliable results with low variability of cortisol concentrations measured in the same sample.
Evaluation of long-term stress perception
Long term experienced stress was assessed via the Slovak translation of the Perceived stress scale. The scale consists of 10 items, which reflect the extent to which participants experience unpredictability, uncontrollability, and overload (Cohen et al., Citation1983).
Statistical analysis
All data were checked for normality of distribution by the Shapiro–Wilk test. As the data were not normally distributed, hair cortisol concentrations and cortisol/perceived stress ratios were analyzed by Mann–Whitney U test. The perceived stress scores, which were normally distributed, were analyzed by Student’s t-test for independent groups. Correlation analysis was performed by Spearman correlation coefficient. Results are expressed as dot plots with each dot representing individual subjects with means ± SEM. The overall level of statistical significance was defined as p < 0.05.
Results
There was no difference in sex, age, and BMI of healthy subjects and patients (). Out of 31 collected hair samples, 11 samples contained an insufficient amount of hair to obtain objectively measurable cortisol concentrations. Thus, the final number of subjects considered in the statistical analysis was 9 patients with atopy and 11 healthy controls.
Table 1. Descriptive statistics of the research sample.
Statistical analysis by Mann-Whitney U-test showed significantly lower hair cortisol concentrations in patients with atopy (U = 9.0, z = −3.08, p < 0.01) compared to those in controls ().
Figure 1. (A) Hair cortisol concentrations and (B) hair cortisol concentration/perceived stress score ratios in the group of atopic patients and the group of healthy controls. Results are expressed as dot plots with each dot representing individual subject with means ± SEM represented by horizontal lines. Statistical significance as revealed by Mann–Whitney U test: *p < 0.05, ** p < 0.01.
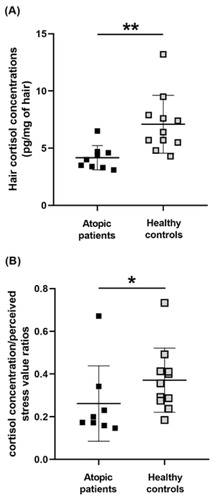
No statistically significant correlation between hair cortisol and experienced stress assessed via perceived stress scale was observed. The comparison of perceived stress scores recorded in patients with atopy and in healthy controls by the Student’s t-test did not show any statistical difference. To evaluate the chronic stress load and hair cortisol in further detail, the hair cortisol/perceived stress ratios were calculated. Statistical analysis by Mann–Whitney U test showed significantly lower cortisol concentration/perceived stress score ratios (U = 17.0 z = −2.23, p < 0.05) in patients with atopy compared to those in controls ().
Discussion
This study revealed significantly lower cumulative cortisol secretion as measured in hair in patients with atopy compared to that in healthy subjects. The degree of stress perception was not different between the patients and healthy controls. However, the cortisol concentration/perceived stress score ratios were lower in patients with atopy compared to those in controls indicating a lower cumulative cortisol secretion than would be expected according to the perceived stress.
In agreement with our hypothesis, hair cortisol concentrations in patients with atopy were found to be lower than in healthy controls. To our knowledge, no other study on adult patients was described in the literature available. The present results are however consistent with data obtained in children (Priftis et al., Citation2006, Citation2008). Kamps et al. (Citation2014) reported lower hair cortisol concentrations in prepubertal children with asthma compared to healthy children using a similar sample size. Hair cortisol concentrations were also lower in a larger group of adolescent patients with asthma recently reported by Baan et al. (Citation2020). It is however difficult to understand the age of the patients and controls as the authors state the upper age limit as 21 years in the abstract and 18 years in the text.
The present finding on lower cortisol concentration in hair is in agreement with previous studies on the blunted salivary cortisol response to acute stressors (Buske-Kirschbaum et al., Citation2002; Hlavacova et al., Citation2017) and reduced cortisol awakening response in atopic patients (Rajcani et al., Citation2019). It may be suggested that low cumulative cortisol concentration in patients is a result of repeated blunted cortisol response to acute stressors and to awakening.
The patients could have experienced a different number of stress situations throughout the last 3 months before hair collection. It is conceivable but not very likely because the perceived stress scores of patients were not different from those in controls. A community-based study (Wells et al., Citation2014) showed that hair cortisol concentrations increased with higher perceived stress. As this was not the case in the present sample, we have calculated the hair cortisol concentration/perceived stress score ratios, which were significantly lower in patients compared to controls.
The present data also underline the importance of methodological aspects of cortisol extraction from hair. There are many studies in the literature in which the authors did not put enough attention to methodological details. We have settled the minimal amount of hair that needs to be extracted for precise analysis by enzyme-linked immunosorbent assay (Balagova & Jezova, Citation2018). As the result, we had to exclude numerous subjects from the evaluation.
Next to methodological aspects, the obtained findings have a potential impact on the immune system function of patients with atopy. Since the present patients with atopy were in remission and without glucocorticoid treatment, the present results motivate the performance of further clinical studies throughout the course of the disease.
In conclusion, lower hair cortisol concentrations in atopic patients might be the result of repeated blunted cortisol secretion in response to acute real-life stress stimuli and to awakening during the last three months before hair collection. The cumulative cortisol secretion in atopic patients is lower than would be expected according to the perceived stress. Most importantly, the previously lower stress hormone increase found in acute stress situations and in children now was confirmed in adult patients with chronic stress load.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
P. Solarikova
Petra Solarikova, PhD. is young researcher at the Department of Psychology, Faculty of Arts, Comenius University in Bratislava, in the field of general, experimental and clinical psychology. Her research is focused on psychological and neuroendocrine aspects of stress and stress reduction interventions.
L. Karailievova
Lucia Karailievova Dr rer. nat., PhD is a postdoctoral fellow at the Laboratory of Pharmacological Neuroendocrinology which is part of the Department of Endocrine Regulations and Psychopharmacology of the IEE BMC SAS, Slovakia. She has finished her PDd in animal physiology at the Faculty of Natural Sciences of Comenius University. Her research is focused on behavioural and neuroendocrine factors in stress in animal and human studies.
J. Rajcani
Jakub Rajcani, Mgr. PhD., is a postdoctoral fellow at the Laboratory of psychophysiology in the Department of Psychology, Faculty of Arts, Comenius University in Bratislava, Slovakia. He finished his PhD in clinical psychology, at the Comenius University in Bratislava, Slovakia. His research is oriented on the cognitive, emotional and neuroendocrine aspects of stress in humans.
I. Brezina
Igor Brezina, PhD. has been the head of Department of Psychology, Faculty of Arts at Comenius University in Bratislava for many years. He has a profound knowledge in the domain of cognitive psychology and emotionality with an accent on psychological an psychophysiological components of stress. He is an expert in the field of attention and memory. He founded the first laboratory of psychophysiology in the Slovak Republic. The scientific activity of assoc. prof. Brezina and particularly his research on the organization of attention was awarded by the American Psychological Society (with R.J. Audley, UCL, London). He is/was a member of many scientific institutions or organizations.
D. Jezova
Daniela Jezova, PharmD is the Head of the Laboratory of Pharmacological Neuroendocrinology being a part of the Department of Endocrine Regulations and Psychopharmacology of the IEE BMC SAS and a full professor of Pharmacology at the Faculty of Medicine of Comenius University, Bratislava, Slovakia. She is serving as an international expert, e.g. in the panel of European Research Council (ERC) on Endocrinology, Pathophysiology and Physiology. She has been a vice-president of the Slovak Academy of Sciences and vice-president of All European Academies. Her research team belongs to the evaluated top research teams in Slovakia. She has experience with laboratory psychosocial stress models and daily life stress investigations in healthy volunteers and patients with stress-related mental, metabolic and cardiovascular disease.
References
- Baan, E. J., van den Akker, E., Engelkes, M., de Rijke, Y. B., de Jongste, J. C., Sturkenboom, M., Verhamme, K. M., & Janssens, H. M. (2020). Hair cortisol and inhaled corticosteroid use in asthmatic children. Pediatric Pulmonology, 55(2), 316–321. https://doi.org/10.1002/ppul.24551
- Balagova, L., & Jezova, D. (2018). Importance of methodological details in the measurement of cortisol in human hair. Endocrine Regulations, 52(3), 134–138. https://doi.org/10.2478/enr-2018-0016
- Buske-Kirschbaum, A., Geiben, A., Höllig, H., Morschhäuser, E., & Hellhammer, D. (2002). Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. The Journal of Clinical Endocrinology and Metabolism, 87(9), 4245–4251. https://doi.org/10.1210/jc.2001-010872
- Buzgoova, K., Balagova, L., Marko, M., Kapsdorfer, D., Riecansky, I., & Jezova, D. (2020). Higher perceived stress is associated with lower cortisol concentrations but higher salivary interleukin-1beta in socially evaluated cold pressor test. Stress, 23(3), 248–255. https://doi.org/10.1080/10253890.2019.1660872
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Hlavacova, N., Solarikova, P., Marko, M., Brezina, I., & Jezova, D. (2017). Blunted cortisol response to psychosocial stress in atopic patients is associated with decrease in salivary alpha-amylase and aldosterone: Focus on sex and menstrual cycle phase. Psychoneuroendocrinology, 78, 31–38. https://doi.org/10.1016/j.psyneuen.2017.01.007
- Kamps, A. W., Molenmaker, M., Kemperman, R., van der Veen, B. S., Bocca, G., & Veeger, N. J. (2014). Children with asthma have significantly lower long-term cortisol levels in their scalp hair than healthy children. Acta Paediatrica, 103(9), 957–961. https://doi.org/10.1111/apa.12685
- Priftis, K. N., Papadimitriou, A., Anthracopoulos, M. B., Gatsopoulou, E., Fretzayas, A., Nicolaidou, P., & Chrousos, G. P. (2006). Adrenal function improves in asthmatic children on inhaled steroids: A longitudinal study. Neuroimmunomodulation, 13(1), 56–62. https://doi.org/10.1159/000094645
- Priftis, K. N., Papadimitriou, A., Nicolaidou, P., & Chrousos, G. P. (2008). Testing for hypothalamic-pituitary-adrenal axis suppression in asthmatic children. Pediatric allergy and immunology: Official publication of the. Pediatric Allergy and Immunology, 19(5), 466–470. https://doi.org/10.1111/j.1399-3038.2008.00726.x
- Rajcani, J., Solarikova, P., Buzgoova, K., Brezina, I., & Jezova, D. (2019). Patients with atopy exhibit reduced cortisol awakening response but not cortisol concentrations during the rest of the day. Immunologic Research, 67(2–3), 176–181. https://doi.org/10.1007/s12026-019-09076-w
- Tseilikman, V., Dremencov, E., Tseilikman, O., Pavlovicova, M., Lacinova, L., & Jezova, D. (2020). Role of glucocorticoid- and monoamine-metabolizing enzymes in stress-related psychopathological processes. Stress, 23(1), 1–12. https://doi.org/10.1080/10253890.2019.1641080
- Wells, S., Tremblay, P. F., Flynn, A., Russell, E., Kennedy, J., Rehm, J., Van Uum, S., Koren, G., & Graham, K. (2014). Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress, 17(4), 334–342. https://doi.org/10.3109/10253890.2014.930432
