Abstract
Post-traumatic stress disorder (PTSD) is a debilitating psychiatric condition with a wide range of behavioral disturbances and serious consequences for both patient and society. One of the main reasons for unsuccessful therapies is insufficient knowledge about its underlying pathomechanism. In the search for centrally signaling molecules that might be relevant to the development of PTSD we focus here on arginine vasopressin (AVP). So far AVP has not been strongly implicated in PTSD, but different lines of evidence suggest a possible impact of its signaling in all clusters of PTSD symptomatology. More specifically, in laboratory rodents, AVP agonists affect behavior in a PTSD-like manner, while significant reduction of AVP signaling in the brain e.g. in AVP-deficient Brattleboro rats, ameliorated defined behavioral parameters that can be linked to PTSD symptoms. Different animal models of PTSD also show alterations in the AVP signaling in distinct brain areas. However, pharmacological treatment targeting central AVP receptors via systemic routes is hampered by possible side effects that are linked to the peripheral action of AVP as a hormone. Indeed, the V1a receptor, the most common receptor subtype in the brain, is implicated in vasoconstriction. Thus, systemic treatment with V1a receptor antagonists would be implicated in hypotonia. This implies that novel treatment concepts are needed to target AVP receptors not only at brain level but also in distinct brain areas, to offer alternative treatments for PTSD.
Post-traumatic stress disorder in humans
“Post-traumatic stress disorder” (PTSD) is defined by criteria written down in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) of the Americal Psychiatrical Association. For a positive diagnosis, the subject should be exposed to or be a witness of “death, threatened death, actual or threatened serious injury, or actual or threatened sexual violence” and to develop at least one intrusion, one avoidance, and two cognitive symptoms (American Psychiatric Association, Citation2013; ). The symptoms should last for at least one month, should create distress or functional impairment, and should not result from medication, substance use or other illness. Full diagnostic criteria are not met until at least six months after the trauma(s), although the onset of symptoms may occur immediately. The estimated prevalence of PTSD is approximately 5–16% (Miao et al., Citation2018). Thus, it is a relatively common disorder, having serious consequences both for the patient and for society.
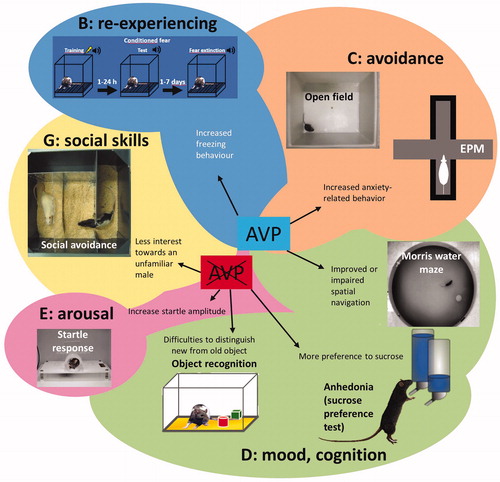
Figure 1. Schematic drawing illustrating the behavioral tests used in studies of post-traumatic stress disorder (PTSD) clusters and effects of arginine vasopressin (AVP) in these studies. EPM: Elevated Plus-Maze; AVP: central AVP presence/excess by synthetic AVP; AVP crossed out: missing/reduced or blockade of central AVP signaling.
A key component of PTSD vulnerability, development and treatment seems to involve the regulation of the endocrine stress axis: the hypothalamic-pituitary adrenocortical (HPA) axis (Morris et al., Citation2016; Yehuda, Citation2002). In particular, glucocorticoids, in addition to other signals of the axis, including adrenocorticotropic hormone (ACTH), may play a prominent role in modulating the brain signaling finally causing PTSD. Arginine vasopressin (AVP) is an important co-stimulator of the HPA axis (Rotzinger et al., Citation2010; ) modulating glucocorticoid release synergistically with corticotrophin-releasing hormone (CRH; Engelmann et al., Citation2004; Rotzinger et al., Citation2010; Zelena et al., Citation2015). Therefore, its contribution to PTSD might be suggested.
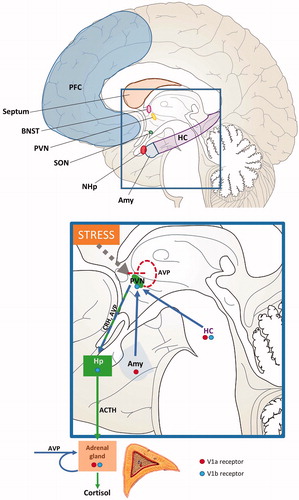
Figure 2. Human brain areas known to be involved in the generation of post-traumatic stress disorder (PTSD) and to contain arginine vasopressin (AVP) fibers and/or AVP receptors, and contribution of central and peripheral AVP to the regulation of the HPA-axis. The rectangle is shown in a higher resolution to illustrate the AVP contribution to HPA axis regulation. The thick dotted line with arrow ending shows the perception of stress by the individual, which is known to affect neuronal activity in the PVN. The arrows indicate the stimulatory action of central and peripheral AVP . The dotted line with straight ending indicates the suggested auto-inhibitory action of AVP of PVN origin within the PVN. ACTH: adrenocorticotropic hormone; Amy: amygdala; BNST: bed nucleus of the stria terminalis; CRH: corticotropin-releasing hormone; HC: hippocampus; Hp: hypophysis; NHp: neurohypophysis; PFC: prefrontal cortex; PVN: nucleus paraventricularis; SON: supraoptic nucleus.
Animal models of PTSD
Since PTSD is defined as a human disorder, non-human animal species cannot, by definition, have PTSD. However, rodents that experience severe stress can develop long-lasting behavioral abnormalities that resemble defined symptoms of PTSD in humans. Therefore, rodents have been used, with certain limitations, as animal models of PTSD. Using animal models may help to better understand the pathophysiology of PTSD, since invasive research methods are not applicable in humans. In particular, animal models may allow access to the neurobiological basis of the vulnerability to PTSD-inducing traumatic events and predict the treatment response in PTSD patients. The final goal is to contribute to a more accurate, personalized approach to treat PTSD in humans (Torok et al., Citation2019).
There are several paradigms that are used to mimic PTSD-like symptoms in rodents (; Torok et al., Citation2019). The most prevalent, using stressors to mimic the traumatic component inducing PTSD (Cluster A) in laboratory rodents, are single prolonged stress (SPS), electric foot-shock (FS) and predator odor exposure (Torok et al., Citation2019). These experimental interventions, as measured by plasma levels of ACTH and corticosterone (the cortisol equivalent in rodents), are severe stressors. Interestingly, the stressor immobilization, although inducing high ACTH and corticosterone levels (Jezova et al., Citation1999), failed to produce fear-like memories in rats (Daviu et al., Citation2012).
Several behavioral tests are thought to measure key symptoms resembling PTSD. The most widely examined is freezing in a trauma context, but behavior suggestive of anxiety such as pathologically increased object burying (Mikics et al., Citation2008a; Poulos et al., Citation2014), depression-like behavior such as increased immobility in the forced swim test (FST), cognitive impairment in the active avoidance (e.g. shuttle-box) test (Shanks and Anisman, Citation1988) and aggression (Mikics et al., Citation2008b) can also be monitored (for further details, see Torok et al., Citation2019). Some severe stressors have been reported to induce changes lasting days to weeks in the above mentioned tests (Daviu et al., Citation2012) thereby fulfilling the criterion of durability. AVP signaling at brain level as neurotransmitter and/or neuromodulator contributes to the control of many of the above-mentioned behaviors in mammals, which might have high relevance to the complex symptomatology of PTSD.
Brain areas where AVP might contribute to PTSD symptoms
In PTSD, three main brain areas were implicated: the prefrontal cortex (PFC), the hippocampus, and the basolateral amygdala (BLA; Torok et al., Citation2019). These brain areas are not rich in AVP-producing cells but contain vasopressin receptors (AVPR) at high density. This suggests, that AVP, either directly innervating these areas as a neurotransmitter or released in remote areas such as the hypothalamus and traveling via the extracellular space as a neuromodulator, is able to influence behavior that is also relevant to PTSD.
In any case, a possible impact of AVP in these areas is supported by the observation that the density of V1aR [the most widespread AVPR in the central nervous system (CNS)] in the PFC was linked to changes in social behavior. For example, fatherhood enhanced V1aR density in marmosets (Kozorovitskiy et al., Citation2006), while in voles the promiscuous ones had higher number of V1aR (Smeltzer et al., Citation2006). In chimpanzees, based upon magnetic resonance imaging (MRI), the PFC activity could be linked to V1aR single nucleotide polymorphismus (SNP) and social cognition (Roper et al., Citation2011). In humans, near-infrared spectroscopy of the PFC demonstrated SNP- and sex-specific activation of V1aR in response to a smiling child video stimulus (Nishitani et al., Citation2017). In this context it was suggested that intranasal AVP administration affects PFC-amygdala connectivity during social stimuli (Brunnlieb et al., Citation2013).
In the BLA the role of AVP seems to be related to stress and stressor-related behavior. For example, injecting an AVP agonist into the BLA of mice increased depressive-like behavior (FST, tail suspension; Kim et al., Citation2016), and there was a tendency toward a higher stressor exposure-induced (FST) c-Fos activation in the BLA of AVP-deficient rats (Balazsfi et al., Citation2015). The amygdala also express the V1bR (Corbani et al., Citation2018) that was suggested to serve as autoreceptor on AVPergic cells (Corbani et al., Citation2018).
In the hippocampus, the primary target for studies investigating learning and memory, both V1aR (Chappell et al., Citation2016) and V1bR (Cilz et al., Citation2019; Paban et al., Citation1999; Roper et al., Citation2011) are present. Despite previous studies showing that CA2 signaling via V1bR was involved in social aggression (Leroy et al., Citation2018), a recent study reported that removal of the N-methyl-d-aspartate (NMDA) receptor subtype from V1bR-positive cells failed to affect social behavior and memory in male mice (Williams Avram et al., Citation2019). Interestingly, it was reported that systemic administration of a V1aR antagonist (SR49059) was effective in influencing spatial memory in the Morris water maze (MWM; Yang et al., Citation2017), whereas local retrodialysis of a peptidergic V1aR antagonist into the mediolateral septum failed to do so (Engelmann et al., Citation1992). In relation to cognition, the CA2 region of hippocampus of rodents also contains V1bR (Cilz et al., Citation2019; Paban et al., Citation1999; Roper et al., Citation2011) and their activation induces synaptic potentiation, which is mechanistically similar to the process of long-term potentiation (Yayou et al., Citation2008) and modulates synaptic strength or plasticity (Pagani et al., Citation2015; Yayou et al., Citation2008). Further studies have yet to reveal the relevance of local AVP signaling via V1bR. At present, most of the behavioral alterations monitored are linked to central AVP signaling via the V1aR subtype (An & Tai, Citation2014).
Hippocampus, cell proliferation and PTSD
One of the core symptoms of PTSD is the patient’s impaired extinction of the traumatic event, which is often interpreted as reduced flexibility. In US veterans lower CA1 volumes were associated with PTSD measured by MRI (Chen et al., 2018). One possible explanation could be a reduced neuroneogenesis in these patients. In mammals, adult neurogenesis, besides being important for forming new memories, is thought also to play a role in memory extinction (Kikuchi et al., Citation2008). The main area of adult neurogenesis [as measured by incorporation of 5-bromo-2′-deoxyuridine (BrdU)] is the hippocampus (Kikuchi et al., Citation2008). A correlation between stress and (reduced) neurogenesis has already been reported in several rodent stress-models (Boldrini et al., Citation2013; Lau et al., Citation2016). In mice, water-immersion restraint stress (Tamaki et al., Citation2008), and in rats inescapable FS (Kikuchi et al., Citation2008; both tests are used to model PTSD symptoms in animals, Torok et al., Citation2019) caused reduced neurogenesis in the hippocampus. In rodent PTSD models, the normalization of behavior by treadmill (Kim & Seo, Citation2013) and rosmarinic acid (Nie et al., Citation2014) in rats, and by theanine in mice (Takarada et al., Citation2015) was accompanied by restoration of cell proliferation. Interestingly, predator odor, despite inducing PTSD-like behavioral symptoms, was not able to induce any changes in hippocampal cell proliferation 2 h and 4 weeks after stressor exposure in rats (Lau et al., Citation2016).
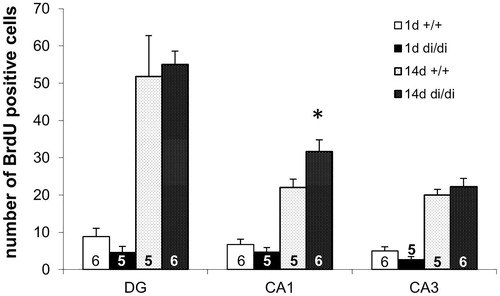
AVP is able to promote cell proliferation in peripheral organs including heart (Chen et al., Citation2017) and gut (Miro et al., Citation2014). Treatment with a V1R antagonist resulted in an effect similar to that seen in V1bR knockout (KO) mice: the normalization of the adrenalectomy-induced decrease in BrdU incorporation in the anterior pituitary of the adults rats (Subburaju & Aguilera, Citation2007). In our experiments we found a higher number of BrdU positive cells in all three hippocampal regions examined (CA1, CA3, and dentate gyrus) at day 14 (representing survival) than at day 1 (representing proliferation) after BrdU injection in adult male Brattleboro rats (). However, the AVP-deficiency resulted in enhanced survival of newly born neurones only in the CA1 region. Indeed, the level in the CA1 of a different marker of neurogenesis, brain-derived neurotropic factor (BDNF), correlated with PTSD-like behavior in Sprague–Dawley rats (Kozlovsky et al., Citation2007). Together these data suggest a modulatory role of AVP in balancing the neuroneogenesis in the mammalian hippocampus mainly in the CA1 region, which may contribute the effects seen in PTSD symptomatology.
Figure 3. Number of BrdU immunopositive cells in the hippocampus of in adult male Brattleboro rats (means + SEM). The number of 5-bromo-2′-deoxyuridine (BrdU) positive cells in all hippocampal subareas was higher at 14 days than at 1 day after BrdU administration. In the CA1 region the number of BrdU positive neurons was significantly higher in vasopressin-deficient (di/di) males compared to non-AVP-deficit controls (+/+). DG: dentate gyrus; CA: cornu ammonis of hippocampus; the number in or above the columns show the number of animals in the group; *p < 0.05; U-test.
Animal models with altered AVP signaling and their behavioral alterations linked to PTSD-like symptomatology
Genetic models
We will investigate here the consequences of the absence of either AVP as signal or AVPR as signal receivers to shed light on its behavioral and endocrine function in the context of PTSD.
The AVP-deficient Brattleboro rat – a model for the missing AVP signal
The Brattleboro rat was the first and still is an up-to-date mutant rodent model for neuroendocrine research (Zelena & Engelmann, Citation2018). Homozygous Brattleboro (di/di) rats lack the ability to synthesize central AVP due to a spontaneous point mutation (Schmale & Richter, Citation1984). This makes them suitable for studying the role AVP plays in HPA axis regulation and generally in selected brain functions linked to human PTSD. It should be noted, however, that in the Brattleboro rat some compensatory mechanisms may mask some effects of AVP (Zelena et al., Citation2009b).
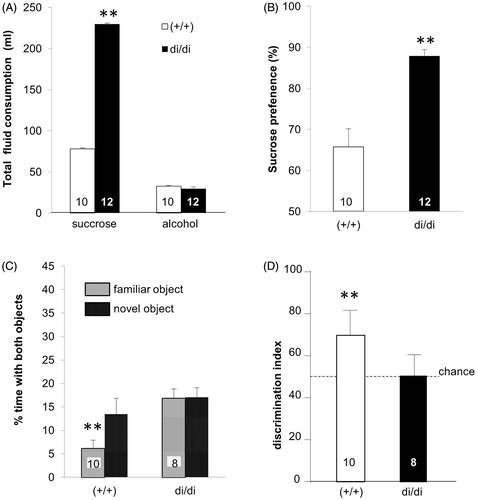
Based on the role that AVP seems to play in both the generation of emotion-like behavior and the control of HPA axis activity, one might expect that the Brattleboro rat’s congenital AVP-deficiency would also affect the outcome in tests that are thought to correspond to PTSD-like symptoms. When Brattleboro rats were exposed to a condition of fear (for details, see Zelena et al., Citation2016) male di/di animals emitted ultrasound calls (in the 22 kHz range; thought to reflect distress) with a significant delay and for a shorter duration than controls (Brattleboro rats with intact AVP alleles = +/+; ). Moreover, when these animals were confronted with the place of the trauma (context) 24 h later, the time spent freezing was lower in di/di rats compared to +/+ controls (). Since AVP was implicated in pain sensitivity (Koshimizu & Tsujimoto, Citation2009), we used thermal pain and could, at least for this parameter, exclude the mentioned behavioral alterations being due to reduced pain perception (, inset). In line with diminished HPA axis response to specific stressors (Fodor et al., Citation2013, Citation2016b; Zelena et al., Citation2009a) AVP deficiency was associated with reduced anxiety- and depressive-like behavior not only in males (Mlynarik et al., Citation2007) and females (Fodor et al., Citation2016b), but also in lactating mothers (Fodor et al., Citation2012). In addition, an increased sucrose preference (reflecting diminished anhedonia, a core symptom of depression), was detectable in di/di rats (). In contrast, an impaired (not enhanced) memory performance was detected in male, but not female, di/di animals (Fodor et al., Citation2016b). In that context, both social memory (Paul et al., Citation2016; Schatz et al., Citation2018) and object recognition were found to be impaired (; Demeter et al., Citation2016; Varga et al., Citation2014). Although memory decline is usually considered to be detrimental, in the case of PTSD the loss of memory for the traumatic event might be beneficial and may contribute to a reduced symptomatology (Kida, Citation2019).
Figure 4. Measures obtained in conditioned fear testing in adult male Brattleboro rats (means + SEM). (A) shows the duration of ultrasonic calls emitted by vasopressin-deficient (di/di) males compared to non-AVP-deficit controls (+/+) during the trauma (shocked) or without foot-shock (control). The inset in (A) shows the similar temperature pain sensitivity of animals of the two genotypes as indicated by the hot-plate test. (B) shows the duration of freezing when re-exposed to the apparatus 24 h after conditioning or control treatment. (C) shows the plasma concentration for adrenocorticotropic hormone (ACTH) and (D) that for corticosterone collected after the end of the re-exposure session.*p < 0.05, t-test.
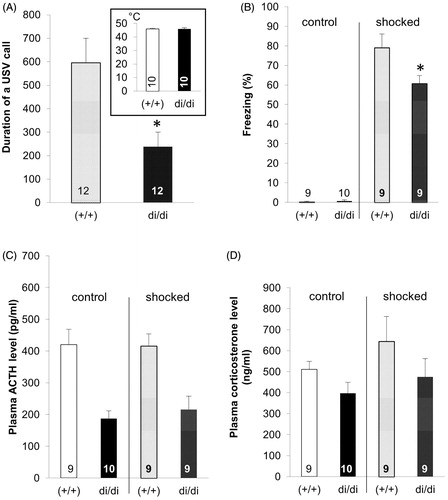
Figure 5. Performance of adult male Brattleboro rat sucrose preference and short-term recognition memory tests (means + SEM). (A) illustrates that di/di drank significantly more succrose than +/+ controls, but no difference in alcohol consumption was seen, suggesting that the sucrose preference was not due to enhanced total fluid consumption. (B) Shows the sucrose preference expressed as percentage. (C) shows the fraction of the totel observation time spent by the animals with familiar or novel objects in the choice session 30 min after learning. (D) shows the recognition index (time spend with the novel object/(time spend with the familiar + novel object) × 100). Controls (+/+) showed in both parameters intact recognition, as indicated by the fact that they investigated the novel over the familiar object (C: paired t-test) and showed a recognition index significantly higher than chance level (= 50; D: one sample t-test versus 50). **p < 0.001.
Different studies suggested a reduced prepulse inhibition in di/di animals, a test outcome that is sometimes interpreted as reflecting a schizophrenic-like symptomatology (Demeter et al., Citation2016; Feifel & Priebe, Citation2001). However, the startle response, reflecting hyperarousal, was not significantly altered in AVP-deficient compared to control rats (Fodor et al., Citation2016a; Schatz et al., Citation2018). Similarly, the trauma remainder-induced ACTH and corticosterone peak was also not altered by AVP-deficiency (). Male, but not female, di/di rats showed impaired social avoidance (Fodor et al., Citation2016a, Citation2016b), while lactating mothers, but not sexually experienced males, showed impaired aggression toward a male intruder (Fodor et al., Citation2014).
Taken together, the results reported here indicate that di/di animals show a behavioral profile that could be interpreted as a diminished symptomatic in defined clusters of PTSD-like behavior (). If this could be validated in humans, then treatment with AVP antagonists might offer a way to alleviate the symptoms in at least some PTSD subjects.
Table 1. Behavioral profile of AVP-deficient male Brattleboro rats (di/di), and V1b and V1a KO mice associated with human PTSD clusters.
V1bR knockout mice – a model for a missing AVP receptor subtype
The V1bR is lacking in the congenital V1bR KO mouse, thereby mimicking the missing AVP signaling of di/di rats on the HPA axis activity at the level of the pituitary (Roper et al., Citation2011). Although the V1bR KO mouse could be suitable for monitoring traumatic stress response, it has, to the best of our knowledge, not been employed in addressing this question. Reviewing the available data in the literature, we noticed that V1bR KO mice failed to show alterations in anxiety- and depression-like behavior. The only sign of “exaggerated emotion” was a more pronounced startle response (Egashira et al., Citation2009). No learning and memory disturbance were detected in the MWM test, while in relation to social skills, social impairment was present.
As stated above, AVP is an important regulator of HPA axis activity, where it was suggested to exert its effects via V1bRs on the anterior lobe of the pituitary. Interestingly, and similar to the results from di/di rats (Zelena et al., Citation2009a), resting ACTH and corticosterone levels were indistinguishable between V1bR KO and wild-type (WT) animals (Lolait et al., Citation2007). Also, no differences between V1bR KO and WT were detected after application of restraint or of a hypoglycemic stressor. In the case of repeated restraint levels of ACTH, but not of corticosterone, were found to be lower in V1b KO mice (Lolait et al., Citation2007). This implies that, from the view of searching for an animal model for PTSD, V1b KO mice require further testing and/or indeed may not be suitable. One reason might be that AVP signaling via other receptor subtype(s) is essential to be able to detect alterations similar to those seen in the Brattleboro rat.
The V1aR KO mouse – the second model for a missing AVP receptor subtype
At first sight, one might assume that congenital V1aR KO mice would not be viable, due to the prominent role peripheral V1aR plays in vasoconstriction that may result in deadly hypotonia. However, there is evidence that the peripheral V1aR, although being important in life-threatening situations of massive blood loss and shock, is of minor relevance under resting, physiological conditions (Zelena, Citation2012), which might explain the viability of the V1a KO animals.
Along the PTSD cluster V1aR KO mice exhibit reduced anxiety (on the elevated plus maze, EPM), but not depressive-like behavior or impairment in spatial learning. Similarly to V1bR, V1aR KO showed also enhanced startle response and reduced social skills (Egashira et al., Citation2007).
Although it is known that glucocorticoids influence the V1aR density in the brain (Watters et al., Citation1996), no information is available on whether, or how, the lack of signaling via V1aR affects the regulation of the HPA axis. According to the available sparse information V1aR KO mice may provide only limited access, if any, to modeling PTSD in animals.
The V1aR and V1bR double knockout
The available literature characterizes V1aR + V1bR double KO mice as showing reduced anxiety-like behavior in both the open field and EPM tests, which seems to be more pronounced that the effect of knocking out either receptor alone (Shimizu et al., Citation2018). Although the double KO mice did not show an altered olfactory perception, they showed more sexual behavior than WT counter pairs. Interestingly, V1aR + V1bR double KO mice were resistant to jet-lag simulations, suggesting that targeting both receptors and therefore AVP signaling simultaneously might be suitable for the treatment of circadian rhythm misalignment, such as occurs in shift work (Yamaguchi et al., Citation2013). It is noteworthy that circadian rhythm dysregulation was also implicated in development of PTSD (Dayan et al., Citation2016). Further studies are required to investigate this double KO mouse for its suitability as animal model for PTSD.
Rodent breeding lines with altered AVP signaling
The spontaneous and engeneered mutants described above showed in the first place an altered AVP signaling and were then tested for its consequences. The alternative appoach is to select and breed animals on the basis of their behavioral performance and then look for possible alteration(s) in AVP signaling. In that way another side effect is targeted: individual variability. This variability is partly due to the well-known gene-environment interaction (Daskalakis et al., Citation2013; Koolhaas et al., Citation2010), which can be reduced in animal studies by selective breeding. The base of the selection of the animals for breeding can be the emotional and behavioral variability or the coping strategies of the animals. Subsequently, we will discuss a few examples of selective breeding lines that were linked to an altered AVP signaling and their potential relevance for studying the origin of PTSD symptomatology in animals.
High anxiety behavior and low anxiety behavior rats and mice
High-anxiety behavior (HAB) and low-anxiety behavior (LAB) rodents were selectively bred with animals differing in their behavior on the EPM (Landgraf & Wigger, Citation2002). Interestingly, LAB animals show a behavior on the EPM similar to di/di animals, which is paralleled by reduced AVP expression and release in the PVN (Wigger et al., Citation2004). Blocking AVP signaling via the V1bR, especially within the PVN (Wigger et al., Citation2004), decreased anxiety- and depression-like behavior and HPA responsivity to stress in HAB animals. This suggested that affective disorders might be related to excessive AVP signaling in this part of the hypothalamus and, consequently, that targeting the PVN with AVPR antagonists might be an interesting tool for a therapeuthic intervention (Surget & Belzung, Citation2008).
Considering PTSD-like symptoms, HAB rats showed significant deficits in their ability to extinguish a learned fear response (Muigg et al., Citation2008; Veenema & Neumann, Citation2007). Thus, they could represent a psychopathologically relevant animal model of impaired fear memory and extinction (Muigg et al., Citation2008; Ponder et al., Citation2007). HAB rats performed well in less aversive learning tests, but poorly in challenging situations (Ponder et al., Citation2007). They were more susceptible to stress exposure and preferred more passive coping strategies (Veenema & Neumann, Citation2007). HAB rats are characterized by a hyperdrive of the HPA axis (Landgraf & Wigger, Citation2002; Surget & Belzung, Citation2008; Veenema & Neumann, Citation2007), which correlated with the expression of AVP in PVN. AVP release-patterns within the septum were dependent upon the level of aggression displayed by the individual, but locally released AVP did not seem to be directly involved in the regulation of aggression. It rather modulated non-aggressive social and anxiety-related behaviors (Beiderbeck et al., Citation2007).
Taken together, HAB-LAB rats and mice may help to characterize the relationship between trait anxiety and resistance to extinction of fear memory in relation to PTSD (Landgraf & Wigger, Citation2002; Muigg et al., Citation2008).
Short and long attack latency mice
The short (SAL) and long attack latency (LAL) mice were selected based upon their attack latencies in a resident-intruder test reflecting their coping style as either active (SAL) or passive (LAL; Benus et al., Citation1991). Their different coping strategies are also detectable in the defensive burying test (Sluyter et al., Citation1996) and in the higher immobility of LAL mice in FST (Veenema et al., Citation2003).
Concerning the intracerebral AVPergic fiber network, SAL mice are characterized by a lower AVP-immunoreactivity in the bed nucleus of stria terminalis (BNST) and a reduced number of AVPergic fibers in the lateral septum, when compared with LAL mice (Veenema & Neumann, Citation2007). These findings are comparable to those in di/di and LAB animals. Moreover, in HPA axis responsivity to nonsocial stressors SAL mice are also similar to LAB rats. On the other hand, SAL animals had attenuated HPA response to social stress exposure (resident-intruder, sensory contact) in contrast to LAB rats (Veenema et al., Citation2004).
Interestingly, animals of this strain were not considered for testing in the context of animal models for PTSD, although there is a significant correlation between dominant, competitive aggression and active coping (Sluyter et al., Citation1996), and humans prone to active coping strategies appear to be more resilient to psychiatric disorders, including PTSD.
BALB/c mice
The BALB/c mouse strain is characterized by highly anxious behavior and was widely used as a rodent model for investigating stress-related mental diseases (An et al., Citation2017; An & Tai, Citation2014). Animals of this strain show reduced locomotor activity paralleled by “exaggerated emotional response” behavior (as measured by stress-sensitivity and anxiety), including acquired fear-like behavior and fear-related conditions (An & Tai, Citation2014; Brinks et al., Citation2008; Roper et al., Citation2011). In a conditioned fear paradigm, BALB/c mice showed non-discriminative generalized fear reactions (Brinks et al., Citation2008). Further studies are required, to test to what extent these behavioral characteristics suggest PTSD and whether they are caused by alteration in the promoter region of the of V1bR gene, resulting in an enhanced receptor number (Roper et al., Citation2011).
Pharmacological interventions modulating AVP signaling
There are no systematic animal studies available reporting AVP agonist/antagonist treatment effects in behavioral tests corresponding to all clusters of PTSD. Only sparse information is available, with different tests in different strains and various drugs. From the findings described above, one would expect that treatment with an AVPR antagonist that diminishes the stress-response, especially a centrally acting V1aR antagonist and probably also a V1bR antagonist, might ameliorate PTSD-like symptoms. In contrast, administration of respective agonists might model the development of symptoms. Here we briefly summarize the presently incoherent information.
Earlier studies reported that intracerebroventricular administration of AVP enhanced the avoidance of a FS-associated arm in a T-maze test in rats (Kumar & Karanth, Citation1995). At first sight this seems to be in contrast to our hypothesis based on the findings in AVP-deficient Brattleboro animals. However, it should be noted that this type of systemic central administration is likely to mask the opposite effects that can be seen in FS behavior when endogenous AVP signaling is blocked either in the septum or medial amygdala (MeA; Ebner et al., Citation1999, Citation2002).
In mice, intranasal administration of the AVP fragment 4–8 (lacking the peripheral effects and suggested to reach brain targets directly) improved working (measured on Y-maze) and spatial memory (measured by MWM) in a genetic model of Alzheimer’s disorder (Zhang et al., Citation2019). Moreover, during passive avoidance training AVP significantly enhanced retention time (enhanced learning), which was effectively blocked by pretreatment with a V1aR antagonist (Boccia et al., Citation1998).
The V1bR is primarily responsible for the impact of AVP on HPA axis activity. Therefore, targeting this AVPR was of particular interest for companies developing pharmacological tools treating psychiatric disorders linked to HPA axis dysregulation (Sanofi compound: SSR149415, Griebel et al., Citation2002; GlaxoSmithKline, Smethurst et al., Citation2011; AbbVie: ABT-436, Katz et al., Citation2016). Animal studies with peripheral administration of some of these antagonist were very promising because they diminished anxiety- and depressive-like behavior (most intensively studied was SSR149415: Griebel et al., Citation2002; Hodgson et al., Citation2007; Iijima & Chaki, Citation2007) In hamsters, the pharmacological blockade of V1bR reduced both social investigation and aggressive behavior (Blanchard et al., Citation2005). In rats, the V1bR antagonist A-988315 reduced FS-induced memory impairment in an object-discrimination task, and this was paralleled by a blunted corticosterone peak (Barsegyan et al., Citation2015). Also, treatment with a V1bR antagonist reversed the stressor-induced suppression of adult neurogenesis (Alonso et al., Citation2004). However, the same drug applied in clinical studies to patients suffering from major depression failed to confirm the animal observations (Griebel et al., Citation2012). Recently, the new ABT compound showed a moderate beneficial effect in a small sample (phase 1 clinical trial; Katz et al., Citation2017). The inconclusive outcome of clinical studies contributed to the decline in the development of new non-peptide agonists and antagonists targeting AVPR for clinical use.
As already mentioned, the ineffectiveness of drugs targeting the V1bR might be partly due to the fact that within the CNS the V1aR is considered to play a key role in relaying the AVP signal that contributes to the generation of the appropriate behavioral response (e.g. anxiolytic-like effect of a V1aR antagonist, Bleickardt et al., Citation2009). Some attempts were made to develop V1aR antagonists for clinical application (Mittapalli et al., Citation2010). Presently, V1aR agonists, rather than antagonists, are the focus of drug development because of the involvement of this receptor subtype in the control of vasoconstriction (Zelena, Citation2012). Such V1aR agonists are widely used for shock prevention and treatment in the clinic, suggesting that systemically applied and therefore peripherally acting V1a antagonists might not be a good choice, because of their expected serious effects on blood pressure. Further attempts are required to design centrally acting antagonists that can be used to target PTSD.
Changes in the AVPergic system in animal models for PTSD
Manipulation of intracerebral AVP-signaling may affect the subjective interpretation of the trauma (Csikota et al., Citation2016; Zelena et al., Citation2015), but trauma itself may lead to changes in the AVPergic system, contributing to long lasting PTSD-like consequences.
Single prolonged stress (SPS)
SPS consists of consecutive exposure to three stressors (i.e. restraint, FST and ether inhalation) within several hours, often followed by re-exposure to the stressors or to FS-induced fear testing (Torok et al., Citation2019). This procedure can induce long-lasting behavioral and neurological changes similar to human PTSD, in which the patients suffer the consequences of exposure to combined stressors (Tanaka et al., Citation2018; Torok et al., Citation2019). Exposure to SPS failed to alter plasma AVP, but induced an accumulation of AVP in the SON and altered the morphology of SON neurons: it was reported that in rats during the first week after trauma the size of AVP neurons, including the soma and dendrites, extended gradually in the SON, while the AVP mRNA expression was significantly reduced (Yoshii et al., Citation2008). Moreover, severe SPS in rats resulted in a short-term (1 day after SPS) reduction of AVP mRNA in the PVN, while in a long-term manner (10 days after SPS) an enhancement was detectable (Mironova & Rybnikova, Citation2010).
Foot-shock-induced trauma
FS in rats and mice is widely used to model trauma, showing symptoms similar to those essential for PTSD diagnosis (Daviu et al., Citation2012; Torok et al., Citation2019).
In rats FS exposure was shown to increase AVP levels in plasma (Onaka et al., Citation1986) and cerebrospinal fluid (Laczi et al., Citation1984), and the expression of AVP was also increased even in the suprachiasmatic nucleus, the centrum of circadian rhythm (Handa et al., Citation2007). Fourteen days after FS, the AVP immunreactivity was also enhanced in the external zone of the median eminence, known to contain the hypopyseotropic regulatory nerve endings (comming from the parvocellular part of the PVN; van Dijken et al., Citation1993). However, intermittent FS reduced the plasma level and SON content of AVP, paralleled by an increased intranuclear release (Onaka et al., Citation2003), and contextual reminders (putting the animals back into the traumatic environment) also decreased AVP plasma levels gradually (Onaka et al., Citation1986).
If AVP was administered intracerebroventricularly it was shown to increase FS-induced fear memories in a T-maze paradigm (Kumar & Karanth, Citation1995). Moreover, both post-training (1 min) or pretest (5 min) intraperitoneal injection of lysine vasopressin facilitated memory recall if measured 24 h after FS (Izquierdo et al., Citation1988). In accordance, our results in AVP deficient rats revealed significantly less freezing compared to shocked +/+ animals (). This suggests that the absence of central AVP signaling may promote resilience to this type of trauma.
Predator odor exposure
Predator odor (mostly urine or its components from cat, fox or ferret, but also exposure to the fur of the predator) is considered an unpleasant stimulus, to which the experimental animal is exposed in a single session (Adamec et al., Citation1998; Dopfel et al., Citation2019; Torok et al., Citation2019). Under these conditions, predator odor exposure can model trauma, causing anxiety similar to that associated with PTSD (Adamec et al., Citation1998; Dopfel et al., Citation2019).
It was shown that the MeA plays important role in acute or enduring behavioral responses to predator odor exposure (Bowen et al., Citation2014). Hypomethylation – often accompanied by enhanced gene expression – of the AVP promoter in the MeA was associated with active coping to cat fur in adult rats (Bowen et al., Citation2014). Moreover, male mice chronically infected with Toxoplasma gondii showed reduced innate fear of predators; this was associated with AVP promoter hypomethylation in their MeA (Tong et al., Citation2019). These data suggest that local AVP signaling might have contributed to the modulation of the fear response.
Human data for AVP in PTSD
Changes of central AVP signaling is considered to contribute to a variety of neuropsychiatric disorders, especially those that are characterized by anxiety, irregular fear and altered neuroendocrine stress responsiveness and abnormal social behavior or aggression, including PTSD (Reijnen et al., Citation2017; Zelena, Citation2012). Different lines of investigation also indicate changes in AVP signaling in PTSD patients. For example, some case-reports associate adolescent secondary enuresis with PTSD, where the contribution AVP cannot be excluded (Akan et al., Citation2015). In one study an association was found between avoidance symptoms and plasma AVP levels in PTSD patients (de Kloet et al., Citation2008). Other authors reported that the development of PTSD symptoms in policemen and -women failed to affect their resting saliva AVP levels (Frijling et al., Citation2015). It is of note that plasma/salivary AVP does not seem to be a useful susceptibility biomarker, as its level might not reflect the development of PTSD symptoms over time (Reijnen et al., Citation2017) and it does not allow conclusions about the intracerebral release and signaling, even if originating from the same neuronal population (Engelmann et al., Citation2004). Indeed, central rather than peripheral, AVP signaling might be responsible for the behavioral alterations and by extrapolation, to the PTSD-like status (Mironova et al., Citation2015; Mironova & Rybnikova, Citation2010). One explanation cites the fact that AVP circulating in the blood is not able to penetrate the blood–brain barrier which separates its central from its peripheral signaling compartment (Reijnen et al., Citation2017).
However, intranasal AVP administration is suggested to reach brain targets directly. It improved social cognition and increased humans’ willingness to cooperate, which is an important social skill (Brunnlieb et al., Citation2016; Caldwell & Albers, Citation2016; Marshall, Citation2013). Moreover, intranasal AVP administration reduced the amygdala activation (measured by blood oxygen level, BOLD, in MRI) to angry faces (emotion) and this was antagonized by a V1aR antagonist (Lee et al., Citation2013). In male participants the effect of intranasal AVP administration on PFC BOLD level depended on their emotional state, suggesting a rather complex role of AVP in divergent behaviors (Feng et al., Citation2015). In young men similar treatment enhanced amygdala activation as well as amygdala-PFC connectivity to emotional (Zink et al., Citation2010) and social challenges measured by MRI (Akan et al., Citation2015). It has to be mentioned, however, that the targets and the method of detecting the passage of intranasally applied nonapeptides, such as AVP, are not without doubt, as is the detection of some of the reported effects (Leng & Ludwig, Citation2016; Ludwig et al., Citation2013).
Conclusion
In the present paper, we aimed to investigate the role AVP may play in the development of PTSD-like symptoms, which are paralleled by the alterations in the activity of the endocrine stress axis. The data presented here suggest that at the brain level in laboratory rodents reduced AVP signaling might be beneficial as many PTSD-like symptoms were milder or even missing in the absence of AVP or under AVPR antagonist treatment. In contrast, AVP administration produced many PTSD-like behavioral effects in different behavioral tasks. Interestingly, knocking out the V1bR or V1aR, the essential elements for AVP signaling, as well as antagonizing these receptors with pharmacological tools, influenced only a few of these behaviors. On one hand this suggests that the presence/manipulation of both receptors is required for producing the full behavioral effects. It is of note that an increased AVP signaling in different brain areas seems to result in opposite behavioral responses. Thus, a treatment that affects simultaneously two brain areas, in which AVPergic signaling controls the behavioral response in an opposite manner (e.g. septum and MeA, Ebner et al., Citation1999, Citation2002) may mask the importance of AVP in a defined brain area. On the other hand, and against that background, technical limitations, including systemic versus local effects caused by the treatment, may still hinder a full understanding of the fine-tuning caused by central AVP signaling to cope with challenging events that can turn into PTSD vulnerability or even resilience. If this review helps to get closer to efficient therapeutic interventions aimed at normalizing the behavioral and endocrine stress response under pathological conditions, we, the authors, are fully satisfied.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Eszter Sipos
Eszter Sipos is a biologist and now a senior scientist at the Institute of Experimental Medicine, Budapest, Hungary.
Bibiána Török
Bibiána Török was educated as a biologist and presently is a PhD student at Semmelweis University working at Institute of Experimental Medicine, Budapest Hungary.
István Barna
István Barna is a biologist and retired from the Institute of Experimental Medicine, Budapest Hungary.
Mario Engelmann
Mario Engelmann studied biology and chemistry in Leipzig, Germany, and is now senior scientist and group leader at the Otto-von-Guericke-University in Magdeburg, Germany.
Dóra Zelena
Dóra Zelena was educated as a medical doctor and at present is a scientific advisor at the Institute of Experimental Medicine, Budapest, Hungary as well as full professor at Pécs University, Physiology Department, Pécs, Hungary.
References
- Adamec, R., Kent, P., Anisman, H., Shallow, T., & Merali, Z. (1998). Neural plasticity, neuropeptides and anxiety in animals – Implications for understanding and treating affective disorder following traumatic stress in humans. Neuroscience & Biobehavioral Reviews, 23(2), 301–318. https://doi.org/10.1016/S0149-7634(98)00032-3
- Akan, S., Urkmez, A., Yildirim, C., Sahin, A., Yuksel, O. H., & Verit, A. (2015). Late-onset secondary nocturnal enuresis in adolescents associated with post-traumatic stress disorder developed after a traffic accident. Archivio Italiano di Urologia, Andrologia : Organo Ufficiale [di] Societa Italiana di Ecografia Urologica e Nefrologica, 87(3), 250–251. https://doi.org/10.4081/aiua.2015.3.250
- Alonso, R., Griebel, G., Pavone, G., Stemmelin, J., Le Fur, G., & Soubrie, P. (2004). Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Molecular Psychiatry, 9(3), 278–286, 24. https://doi.org/10.1038/sj.mp.4001464
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Association.
- An, D., Chen, W., Yu, D. Q., Wang, S. W., Yu, W. Z., Xu, H., Wang, D. M., Zhao, D., Sun, Y. P., Wu, J. C., Tang, Y. Y., & Yin, S. M. (2017). Effects of social isolation, re-socialization and age on cognitive and aggressive behaviors of Kunming mice and BALB/c mice. Animal Science Journal = Nihon Chikusan Gakkaiho, 88(5), 798–806. https://doi.org/10.1111/asj.12688
- An, X. L., & Tai, F. D. (2014). AVP and Glu systems interact to regulate levels of anxiety in BALB/cJ mice. Dong Wu Xue Yan Jiu = Zoological Research, 35(4), 319–325. https://doi.org/10.13918/j.issn.2095-8137.2014.4.319
- Balazsfi, D., Pinter, O., Klausz, B., Kovacs, K. B., Fodor, A., Torok, B., Engelmann, M., & Zelena, D. (2015). Restoration of peripheral V2 receptor vasopressin signaling fails to correct behavioral changes in Brattleboro rats. Psychoneuroendocrinology, 51, 11–23. https://doi.org/10.1016/j.psyneuen.2014.09.011
- Barsegyan, A., Atsak, P., Hornberger, W. B., Jacobson, P. B., van Gaalen, M. M., & Roozendaal, B. (2015). The Vasopressin 1b receptor antagonist A-988315 blocks stress effects on the retrieval of object-recognition memory. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 40(8), 1979–1989. https://doi.org/10.1038/npp.2015.48
- Beiderbeck, D. I., Neumann, I. D., & Veenema, A. H. (2007). Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. The European Journal of Neuroscience, 26(12), 3597–3605. https://doi.org/10.1111/j.1460-9568.2007.05974.x
- Benus, R. F., Bohus, B., Koolhaas, J. M., & van Oortmerssen, G. A. (1991). Behavioural differences between artificially selected aggressive and non-aggressive mice: response to apomorphine. Behavioural Brain Research, 43(2), 203–208. https://doi.org/10.1016/s0166-4328(05)80072-5
- Blanchard, R. J., Griebel, G., Farrokhi, C., Markham, C., Yang, M., & Blanchard, D. C. (2005). AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacology, Biochemistry, and Behavior, 80(1), 189–194. https://doi.org/10.1016/j.pbb.2004.10.024
- Bleickardt, C. J., Mullins, D. E., Macsweeney, C. P., Werner, B. J., Pond, A. J., Guzzi, M. F., Martin, F. D., Varty, G. B., & Hodgson, R. A. (2009). Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology, 202(4), 711–718. https://doi.org/10.1007/s00213-008-1354-x
- Boccia, M. M., Kopf, S. R., & Baratti, C. M. (1998). Effects of a single administration of oxytocin or vasopressin and their interactions with two selective receptor antagonists on memory storage in mice. Neurobiology of Learning and Memory, 69(2), 136–146. https://doi.org/10.1006/nlme.1997.3817
- Boldrini, M., Santiago, A. N., Hen, R., Dwork, A. J., Rosoklija, G. B., Tamir, H., Arango, V., & John Mann, J. (2013). Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology, 38(6), 1068–1077. https://doi.org/10.1038/npp.2013.5
- Bowen, M. T., Dass, S. A., Booth, J., Suraev, A., Vyas, A., & McGregor, I. S. (2014). Active coping toward predatory stress is associated with lower corticosterone and progesterone plasma levels and decreased methylation in the medial amygdala vasopressin system. Hormones and Behavior, 66(3), 561–566. https://doi.org/10.1016/j.yhbeh.2014.08.004
- Brinks, V., de Kloet, E. R., & Oitzl, M. S. (2008). Strain specific fear behaviour and glucocorticoid response to aversive events: modelling PTSD in mice. Progress in Brain Research, 167, 257–261. https://doi.org/10.1016/S0079-6123(07)67019-8
- Brunnlieb, C., Munte, T. F., Tempelmann, C., & Heldmann, M. (2013). Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Research, 1499, 29–42. https://doi.org/10.1016/j.brainres.2013.01.009
- Brunnlieb, C., Nave, G., Camerer, C. F., Schosser, S., Vogt, B., Munte, T. F., & Heldmann, M. (2016). Vasopressin increases human risky cooperative behavior. Proceedings of the National Academy of Sciences of the United States of America, 113(8), 2051–2056. https://doi.org/10.1073/pnas.1518825113
- Caldwell, H. K., & Albers, H. E. (2016). Oxytocin, vasopressin, and the motivational forces that drive social behaviors. Current Topics in Behavioral Neurosciences, 27, 51–103. https://doi.org/10.1007/7854_2015_390
- Chappell, A. R., Freeman, S. M., Lin, Y. K., LaPrairie, J. L., Inoue, K., Young, L. J., & Hayes, L. D. (2016). Distributions of oxytocin and vasopressin 1a receptors in the Taiwan vole and their role in social monogamy. Journal of Zoology (London, England : 1987), 299(2), 106–115. https://doi.org/10.1111/jzo.12332
- Chen, L. W., Sun, D., Davis, S. L., Haswell, C. C., Dennis, E. L., Swanson, C. A., Whelan, C. D., Gutman, B., Jahanshad, N., Iglesias, J. E., Thompson, P., Mid-Atlantic, M. W., Wagner, H. R., Saemann, P., LaBar, K. S., & Morey, R. A, Mid-Atlantic MIRECC Workgroup. (2018). Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depression and Anxiety, 35(11), 1018–1029. https://doi.org/10.1002/da.22833
- Chen, Y., Xu, F., Zhang, L., Wang, X., Wang, Y., Woo, A. Y., & Zhu, W. (2017). GRK2/β-arrestin mediates arginine vasopressin-induced cardiac fibroblast proliferation. Clinical and Experimental Pharmacology & Physiology, 44(2), 285–293. https://doi.org/10.1111/1440-1681.12696
- Cilz, N. I., Cymerblit-Sabba, A., & Young, W. S. (2019). Oxytocin and vasopressin in the rodent hippocampus. Genes, Brain, and Behavior, 18(1), e12535. https://doi.org/10.1111/gbb.12535
- Corbani, M., Marir, R., Trueba, M., Chafai, M., Vincent, A., Borie, A. M., Desarmenien, M. G., Ueta, Y., Tomboly, C., Olma, A., Manning, M., & Guillon, G. (2018). Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands. General and Comparative Endocrinology, 258, 15–32. https://doi.org/10.1016/j.ygcen.2017.10.011
- Csikota, P., Fodor, A., Balazsfi, D., Pinter, O., Mizukami, H., Weger, S., Heilbronn, R., Engelmann, M., & Zelena, D. (2016). Vasopressinergic control of stress-related behavior: studies in Brattleboro rats. Stress (Amsterdam, Netherlands), 19(4), 349–361. https://doi.org/10.1080/10253890.2016.1183117
- Daskalakis, N. P., Bagot, R. C., Parker, K. J., Vinkers, C. H., & de Kloet, E. R. (2013). The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38(9), 1858–1873. https://doi.org/10.1016/j.psyneuen.2013.06.008
- Daviu, N., Delgado-Morales, R., Nadal, R., & Armario, A. (2012). Not all stressors are equal: behavioral and endocrine evidence for development of contextual fear conditioning after a single session of footshocks but not of immobilization. Frontiers in Behavioral Neuroscience., 6, 69.
- Dayan, J., Rauchs, G., & Guillery-Girard, B. (2016). Rhythms dysregulation: A new perspective for understanding PTSD? Journal of Physiology, Paris, 110(4 Pt B), 453–460. https://doi.org/10.1016/j.jphysparis.2017.01.004
- de Kloet, C. S., Vermetten, E., Geuze, E., Wiegant, V. M., & Westenberg, H. G. (2008). Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. Journal of Psychiatric Research, 42(3), 192–198. https://doi.org/10.1016/j.jpsychires.2006.11.009
- Demeter, K., Torok, B., Fodor, A., Varga, J., Ferenczi, S., Kovacs, K. J., Eszik, I., Szegedi, V., & Zelena, D. (2016). Possible contribution of epigenetic changes in the development of schizophrenia-like behavior in vasopressin-deficient Brattleboro rats. Behavioural Brain Research, 300, 123–134. https://doi.org/10.1016/j.bbr.2015.12.007
- Dopfel, D., Perez, P. D., Verbitsky, A., Bravo-Rivera, H., Ma, Y., Quirk, G. J., & Zhang, N. (2019). Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nature Communications, 10(1), 2372. https://doi.org/10.1038/s41467-019-09926-z
- Ebner, K., Wotjak, C. T., Holsboer, F., Landgraf, R., & Engelmann, M. (1999). Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. European Journal of Neuroscience, 11(3), 997–1002. https://doi.org/10.1046/j.1460-9568.1999.00508.x
- Ebner, K., Wotjak, C. T., Landgraf, R., & Engelmann, M. (2002). Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. European Journal of Neuroscience, 15(2), 384–388. https://doi.org/10.1046/j.0953-816x.2001.01869.x
- Egashira, N., Mishima, K., Iwasaki, K., Oishi, R., & Fujiwara, M. (2009). New topics in vasopressin receptors and approach to novel drugs: role of the vasopressin receptor in psychological and cognitive functions. Journal of Pharmacological Sciences, 109(1), 44–49. https://doi.org/10.1254/jphs.08r14fm
- Egashira, N., Tanoue, A., Matsuda, T., Koushi, E., Harada, S., Takano, Y., Tsujimoto, G., Mishima, K., Iwasaki, K., & Fujiwara, M. (2007). Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behavioural Brain Research, 178(1), 123–127. https://doi.org/10.1016/j.bbr.2006.12.009
- Engelmann, M., Bures, J., & Landgraf, R. (1992). Vasopressin administration via microdialysis into the septum interferes with the acquisition of spatial memory in rats. Neuroscience Letters, 142(1), 69–72. https://doi.org/10.1016/0304-3940(92)90622-e
- Engelmann, M., Landgraf, R., & Wotjak, C. T. (2004). The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Frontiers in Neuroendocrinology, 25(3-4), 132–149. https://doi.org/10.1016/j.yfrne.2004.09.001
- Feifel, D., & Priebe, K. (2001). Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biological Psychiatry, 50(6), 425–433. https://doi.org/10.1016/s0006-3223(01)01100-3
- Feng, C., DeMarco, A. C., Haroon, E., & Rilling, J. K. (2015). Neuroticism modulates the effects of intranasal vasopressin treatment on the neural response to positive and negative social interactions. Neuropsychologia, 73, 108–115. https://doi.org/10.1016/j.neuropsychologia.2015.05.004
- Fodor, A., Barsvari, B., Aliczki, M., Balogh, Z., Zelena, D., Goldberg, S. R., & Haller, J. (2014). The effects of vasopressin deficiency on aggression and impulsiveness in male and female rats. Psychoneuroendocrinology, 47, 141–150. https://doi.org/10.1016/j.psyneuen.2014.05.010
- Fodor, A., Klausz, B., Pinter, O., Daviu, N., Rabasa, C., Rotllant, D., Balazsfi, D., Kovacs, K. B., Nadal, R., & Zelena, D. (2012). Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Hormones and Behavior, 62(4), 539–551. https://doi.org/10.1016/j.yhbeh.2012.09.003
- Fodor, A., Klausz, B., Toth, B., & Zelena, D. (2016a). The prepulse inhibition deficit appearance is largely independent on the circadian cycle, body weight, and the gender of vasopressin deficient Brattleboro rat. Endocrine Regulations, 50(1), 16–23. https://doi.org/10.1515/enr-2016-0004
- Fodor, A., Kovacs, K. B., Balazsfi, D., Klausz, B., Pinter, O., Demeter, K., Daviu, N., Rabasa, C., Rotllant, D., Nadal, R., & Zelena, D. (2016b). Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiology & Behavior, 158, 100–111. https://doi.org/10.1016/j.physbeh.2016.02.041
- Fodor, A., Pinter, O., Domokos, A., Langnaese, K., Barna, I., Engelmann, M., & Zelena, D. (2013). Blunted HPA axis response in lactating, vasopressin-deficient Brattleboro rats. The Journal of Endocrinology, 219(2), 89–100. https://doi.org/10.1530/JOE-13-0224
- Frijling, J. L., van Zuiden, M., Nawijn, L., Koch, S. B., Neumann, I. D., Veltman, D. J., & Olff, M. (2015). Salivary oxytocin and vasopressin levels in police officers with and without post-traumatic stress disorder. Journal of Neuroendocrinology, 27(10), 743–751. https://doi.org/10.1111/jne.12300
- Griebel, G., Beeske, S., & Stahl, S. M. (2012). The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. The Journal of Clinical Psychiatry, 73(11), 1403–1411. https://doi.org/10.4088/JCP.12m07804
- Griebel, G., Simiand, J., Serradeil-Le Gal, C., Wagnon, J., Pascal, M., Scatton, B., Maffrand, J. P., & Soubrie, P. (2002). Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proceedings of the National Academy of Sciences of the United States of America, 99(9), 6370–6375. https://doi.org/10.1073/pnas.092012099
- Handa, R. J., Zoeller, R. T., & McGivern, R. F. (2007). Changes in vasoactive intestinal peptide and arginine vasopressin expression in the suprachiasmatic nucleus of the rat brain following footshock stress. Neuroscience Letters, 425(2), 99–104. https://doi.org/10.1016/j.neulet.2007.08.044
- Hodgson, R. A., Higgins, G. A., Guthrie, D. H., Lu, S. X., Pond, A. J., Mullins, D. E., Guzzi, M. F., Parker, E. M., & Varty, G. B. (2007). Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacology, Biochemistry, and Behavior, 86(3), 431–440. https://doi.org/10.1016/j.pbb.2006.12.021
- Iijima, M., & Chaki, S. (2007). An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31(3), 622–627. https://doi.org/10.1016/j.pnpbp.2006.12.008
- Izquierdo, I., Dalmaz, C., Dias, R. D., & Godoy, M. G. (1988). Memory facilitation by posttraining and pretest ACTH, epinephrine, and vasopressin administration: Two separate effects. Behavioral Neuroscience, 102(5), 803–806. https://doi.org/10.1037//0735-7044.102.5.803
- Jezova, D., Ochedalski, T., Glickman, M., Kiss, A., & Aguilera, G. (1999). Central corticotropin-releasing hormone receptors modulate hypothalamic-pituitary-adrenocortical and sympathoadrenal activity during stress. Neuroscience, 94(3), 797–802. https://doi.org/10.1016/S0306-4522(99)00333-4
- Katz, D. A., Liu, W., Locke, C., Dutta, S., & Tracy, K. A. (2016). Clinical safety and hypothalamic-pituitary-adrenal axis effects of the arginine vasopressin type 1B receptor antagonist ABT-436. Psychopharmacology, 233(1), 71–81. https://doi.org/10.1007/s00213-015-4089-5
- Katz, D. A., Locke, C., Greco, N., Liu, W., & Tracy, K. A. (2017). Hypothalamic-pituitary-adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one-week randomized Phase 1b trial. Brain and Behavior, 7(3), e00628. https://doi.org/10.1002/brb3.628
- Kida, S. (2019). Reconsolidation/destabilization, extinction and forgetting of fear memory as therapeutic targets for PTSD. Psychopharmacology, 236(1), 49–57. https://doi.org/10.1007/s00213-018-5086-2
- Kikuchi, A., Shimizu, K., Nibuya, M., Hiramoto, T., Kanda, Y., Tanaka, T., Watanabe, Y., Takahashi, Y., & Nomura, S. (2008). Relationship between post-traumatic stress disorder-like behavior and reduction of hippocampal 5-bromo-2'-deoxyuridine-positive cells after inescapable shock in rats. Psychiatry and Clinical Neurosciences, 62(6), 713–720. https://doi.org/10.1111/j.1440-1819.2008.01875.x
- Kim, B. K., & Seo, J. H. (2013). Treadmill exercise alleviates post-traumatic stress disorder-induced impairment of spatial learning memory in rats. Journal of Exercise Rehabilitation, 9(4), 413–419. https://doi.org/10.12965/jer.130058
- Kim, T. K., Lee, J. E., Kim, J. E., Park, J. Y., Choi, J., Kim, H., Lee, E. H., & Han, P. L. (2016). G9a-mediated regulation of OXT and AVP expression in the basolateral amygdala mediates stress-induced lasting behavioral depression and its reversal by exercise. Molecular Neurobiology, 53(5), 2843–2856. https://doi.org/10.1007/s12035-015-9160-z
- Koolhaas, J. M., de Boer, S. F., Coppens, C. M., & Buwalda, B. (2010). Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Frontiers in Neuroendocrinology, 31(3), 307–321. https://doi.org/10.1016/j.yfrne.2010.04.001
- Koshimizu, T. A., & Tsujimoto, G. (2009). New topics in vasopressin receptors and approach to novel drugs: Vasopressin and pain perception. Journal of Pharmacological Sciences, 109(1), 33–37. https://doi.org/10.1254/jphs.08r18fm
- Kozlovsky, N., Matar, M. A., Kaplan, Z., Kotler, M., Zohar, J., & Cohen, H. (2007). Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. The International Journal of Neuropsychopharmacology, 10(6), 741–758. https://doi.org/10.1017/S1461145707007560
- Kozorovitskiy, Y., Hughes, M., Lee, K., & Gould, E. (2006). Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nature Neuroscience, 9(9), 1094–1095. https://doi.org/10.1038/nn1753
- Kumar, K. B., & Karanth, K. S. (1995). Effects of central administration of arginine-vasopressin on aversive memory retrieval. Brain Research, 699(2), 293–296. https://doi.org/10.1016/0006-8993(95)00921-c
- Laczi, F., Gaffori, O., Fekete, M., de Kloet, E. R., & de Wied, D. (1984). Levels of arginine-vasopressin in cerebrospinal fluid during passive avoidance behavior in rats. Life Sciences, 34(24), 2385–2391. https://doi.org/10.1016/0024-3205(84)90426-0
- Landgraf, R., & Wigger, A. (2002). High vs low anxiety-related behavior rats: An animal model of extremes in trait anxiety. Behavior Genetics, 32(5), 301–314. https://doi.org/10.1023/a:1020258104318
- Lau, C., Hebert, M., Vani, M. A., Walling, S., Hayley, S., Lagace, D. C., & Blundell, J. (2016). Absence of neurogenic response following robust predator-induced stress response. Neuroscience, 339, 276–286. https://doi.org/10.1016/j.neuroscience.2016.10.001
- Lee, R. J., Coccaro, E. F., Cremers, H., McCarron, R., Lu, S. F., Brownstein, M. J., & Simon, N. G. (2013). A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: an fMRI study. Frontiers in Systems Neuroscience, 7, 100.
- Leng, G., & Ludwig, M. (2016). Intranasal oxytocin: Myths and delusions. Biological Psychiatry, 79(3), 243–250. https://doi.org/10.1016/j.biopsych.2015.05.003
- Leroy, F., Park, J., Asok, A., Brann, D. H., Meira, T., Boyle, L. M., Buss, E. W., Kandel, E. R., & Siegelbaum, S. A. (2018). A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature, 564(7735), 213–218. https://doi.org/10.1038/s41586-018-0772-0
- Lolait, S. J., Stewart, L. Q., Jessop, D. S., Young, W. S., 3rd, & O'Carroll, A. M. (2007). The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology, 148(2), 849–856. https://doi.org/10.1210/en.2006-1309
- Ludwig, M., Tobin, V. A., Callahan, M. F., Papadaki, E., Becker, A., Engelmann, M., & Leng, G. (2013). Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats. Journal of Neuroendocrinology, 25(7), 655–667. https://doi.org/10.1111/jne.12046
- Marshall, A. D. (2013). Posttraumatic stress disorder and partner-specific social cognition: A pilot study of sex differences in the impact of arginine vasopressin. Biological Psychology, 93(2), 296–303. https://doi.org/10.1016/j.biopsycho.2013.02.014
- Miao, X. R., Chen, Q. B., Wei, K., Tao, K. M., & Lu, Z. J. (2018). Posttraumatic stress disorder: From diagnosis to prevention. Military Medical Research, 5(1), 32. https://doi.org/10.1186/s40779-018-0179-0
- Mikics, E., Baranyi, J., & Haller, J. (2008a). Rats exposed to traumatic stress bury unfamiliar objects-a novel measure of hyper-vigilance in PTSD models? Physiology & Behavior, 94(3), 341–348. https://doi.org/10.1016/j.physbeh.2008.01.023
- Mikics, E., Toth, M., Varju, P., Gereben, B., Liposits, Z., Ashaber, M., Halasz, J., Barna, I., Farkas, I., & Haller, J. (2008b). Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology, 33(9), 1198–1210. https://doi.org/10.1016/j.psyneuen.2008.06.006
- Miro, L., Perez-Bosque, A., Maijo, M., Naftalin, R. J., & Moreto, M. (2014). Vasopressin regulation of epithelial colonic proliferation and permeability is mediated by pericryptal platelet-derived growth factor A. Experimental Physiology, 99(10), 1325–1334. https://doi.org/10.1113/expphysiol.2014.080952
- Mironova, V. I., & Rybnikova, E. A. (2010). Stable modifications to the expression of neurohormones in the rat hypothalamus in a model of post-traumatic stress disorder. Neuroscience and Behavioral Physiology, 40(1), 111–115. https://doi.org/10.1007/s11055-009-9216-5
- Mironova, V. I., Rakitskaya, V. V., Pivina, S. G., & Ordyan, N. E. (2015). [Stress-induced patterns of the hypothalamic Crh and vasopressin expression in female rats in a model of posttraumatic stress disorder]. Rossiiskii Fiziologicheskii Zhurnal Imeni I.M. Sechenova, 101(12), 1355–1365.
- Mittapalli, G., Abgaryan, L., Brown, S. J., Saldanha, S. A., Volmar, C. H., Ferguson, J., Roberts, E., Hodder, P., & Rosen, H. (2010). Optimization and characterization of an antagonist for vasopressin 1a (V1a) receptor. Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US).
- Mlynarik, M., Zelena, D., Bagdy, G., Makara, G. B., & Jezova, D. (2007). Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Hormones and Behavior, 51(3), 395–405. https://doi.org/10.1016/j.yhbeh.2006.12.007
- Morris, M. C., Hellman, N., Abelson, J. L., & Rao, U. (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. https://doi.org/10.1016/j.cpr.2016.09.001
- Muigg, P., Hetzenauer, A., Hauer, G., Hauschild, M., Gaburro, S., Frank, E., Landgraf, R., & Singewald, N. (2008). Impaired extinction of learned fear in rats selectively bred for high anxiety-evidence of altered neuronal processing in prefrontal-amygdala pathways. The European Journal of Neuroscience, 28(11), 2299–2309. https://doi.org/10.1111/j.1460-9568.2008.06511.x
- Nie, H., Peng, Z., Lao, N., Wang, H., Chen, Y., Fang, Z., Hou, W., Gao, F., Li, X., Xiong, L., & Tan, Q. (2014). Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 51, 16–22. https://doi.org/10.1016/j.pnpbp.2014.01.002
- Nishitani, S., Ikematsu, K., Takamura, T., Honda, S., Yoshiura, K. I., & Shinohara, K. (2017). Genetic variants in oxytocin receptor and arginine-vasopressin receptor 1A are associated with the neural correlates of maternal and paternal affection towards their child. Hormones and Behavior, 87, 47–56. https://doi.org/10.1016/j.yhbeh.2016.09.010
- Onaka, T., Hamamura, M., & Yagi, K. (1986). Suppression of vasopressin secretion by classically conditioned stimuli in rats. The Japanese Journal of Physiology, 36(6), 1261–1266. https://doi.org/10.2170/jjphysiol.36.1261
- Onaka, T., Serino, R., & Ueta, Y. (2003). Intermittent footshock facilitates dendritic vasopressin release but suppresses vasopressin synthesis within the rat supraoptic nucleus. Journal of Neuroendocrinology, 15(7), 629–632. https://doi.org/10.1046/j.1365-2826.2003.01053.x
- Paban, V., Alescio-Lautier, B., Devigne, C., & Soumireu-Mourat, B. (1999). Fos protein expression induced by intracerebroventricular injection of vasopressin in unconditioned and conditioned mice. Brain Research, 825(1-2), 115–131. https://doi.org/10.1016/s0006-8993(99)01232-9
- Pagani, J. H., Zhao, M., Cui, Z., Avram, S. K., Caruana, D. A., Dudek, S. M., & Young, W. S. (2015). Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Molecular Psychiatry, 20(4), 490–499. https://doi.org/10.1038/mp.2014.47
- Paul, M. J., Peters, N. V., Holder, M. K., Kim, A. M., Whylings, J., Terranova, J. I., & de Vries, G. J. (2016). Atypical social development in vasopressin-deficient Brattleboro rats. eNeuro, 3(2), ENEURO.0150-15.2016. https://doi.org/10.1523/ENEURO.0150-15.2016
- Ponder, C. A., Kliethermes, C. L., Drew, M. R., Muller, J., Das, K., Risbrough, V. B., Crabbe, J. C., Gilliam, T. C., & Palmer, A. A. (2007). Selection for contextual fear conditioning affects anxiety-like behaviors and gene expression. Genes, Brain, and Behavior, 6(8), 736–749. https://doi.org/10.1111/j.1601-183X.2007.00306.x
- Poulos, A. M., Reger, M., Mehta, N., Zhuravka, I., Sterlace, S. S., Gannam, C., Hovda, D. A., Giza, C. C., & Fanselow, M. S. (2014). Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biological Psychiatry, 76(4), 306–314. https://doi.org/10.1016/j.biopsych.2013.10.007
- Reijnen, A., Geuze, E., & Vermetten, E. (2017). Individual variation in plasma oxytocin and vasopressin levels in relation to the development of combat-related PTSD in a large military cohort. Journal of Psychiatric Research, 94, 88–95. https://doi.org/10.1016/j.jpsychires.2017.06.010
- Roper, J., O'Carroll, A. M., Young, W., 3rd, & Lolait, S. (2011). The vasopressin Avpr1b receptor: molecular and pharmacological studies. Stress (Amsterdam, Netherlands), 14(1), 98–115. https://doi.org/10.3109/10253890.2010.512376
- Rotzinger, S., Lovejoy, D. A., & Tan, L. A. (2010). Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides, 31(4), 736–756. https://doi.org/10.1016/j.peptides.2009.12.015
- Schatz, K. C., Kyne, R. F., Parmeter, S. L., & Paul, M. J. (2018). Investigation of social, affective, and locomotor behavior of adolescent Brattleboro rats reveals a link between vasopressin’s actions on arousal and social behavior. Hormones and Behavior, 106, 1–9. https://doi.org/10.1016/j.yhbeh.2018.08.015
- Schmale, H., & Richter, D. (1984). Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature, 308(5961), 705–709. https://doi.org/10.1038/308705a0
- Shanks, N., & Anisman, H. (1988). Stressor-provoked behavioral changes in six strains of mice. Behavioral Neuroscience, 102(6), 894–905. https://doi.org/10.1037//0735-7044.102.6.894
- Shimizu, K., Nakamura, K., Yokosuka, M., & Kondo, Y. (2018). Modulation of male mouse sociosexual and anxiety-like behaviors by vasopressin receptors. Physiology & Behavior, 197, 37–41. https://doi.org/10.1016/j.physbeh.2018.09.016
- Sluyter, F., Korte, S. M., Bohus, B., & Van Oortmerssen, G. A. (1996). Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacology, Biochemistry, and Behavior, 54(1), 113–116. https://doi.org/10.1016/0091-3057(95)02164-7
- Smeltzer, M. D., Curtis, J. T., Aragona, B. J., & Wang, Z. (2006). Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neuroscience Letters, 394(2), 146–151. https://doi.org/10.1016/j.neulet.2005.10.019
- Smethurst, C. A., Borthwick, J. A., Gaines, S., Watson, S., Green, A., Schulz, M. J., Burton, G., Buson, A. A., & Arban, R. (2011). The characterization of a novel V1b antagonist lead series. Bioorganic & Medicinal Chemistry Letters, 21(1), 92–96. https://doi.org/10.1016/j.bmcl.2010.11.061
- Subburaju, S., & Aguilera, G. (2007). Vasopressin mediates mitogenic responses to adrenalectomy in the rat anterior pituitary. Endocrinology, 148(7), 3102–3110. https://doi.org/10.1210/en.2007-0103
- Surget, A., & Belzung, C. (2008). Involvement of vasopressin in affective disorders. European Journal of Pharmacology, 583(2-3), 340–349. https://doi.org/10.1016/j.ejphar.2007.11.065
- Takarada, T., Nakamichi, N., Kakuda, T., Nakazato, R., Kokubo, H., Ikeno, S., Nakamura, S., Hinoi, E., & Yoneda, Y. (2015). Daily oral intake of theanine prevents the decline of 5-bromo-2'-deoxyuridine incorporation in hippocampal dentate gyrus with concomitant alleviation of behavioral abnormalities in adult mice with severe traumatic stress. Journal of Pharmacological Sciences, 127(3), 292–297. https://doi.org/10.1016/j.jphs.2014.12.018
- Tamaki, K., Yamada, K., Nakamichi, N., Taniura, H., & Yoneda, Y. (2008). Transient suppression of progenitor cell proliferation through NMDA receptors in hippocampal dentate gyrus of mice with traumatic stress experience. Journal of Neurochemistry, 105(5), 1642–1655. https://doi.org/10.1111/j.1471-4159.2008.05253.x
- Tanaka, K. I., Yagi, T., Nanba, T., & Asanuma, M. (2018). Application of single prolonged stress induces post-traumatic stress disorder-like characteristics in mice. Acta Medica Okayama, 72(5), 479–485. https://doi.org/10.18926/AMO/56245
- Tong, W. H., Abdulai-Saiku, S., & Vyas, A. (2019). Testosterone reduces fear and causes drastic hypomethylation of arginine vasopressin promoter in medial extended amygdala of male mice. Frontiers in Behavioral Neuroscience, 13, 33. https://doi.org/10.3389/fnbeh.2019.00033
- Torok, B., Sipos, E., Pivac, N., & Zelena, D. (2019). Modelling posttraumatic stress disorders in animals. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 90, 117–133. https://doi.org/10.1016/j.pnpbp.2018.11.013
- van Dijken, H. H., de Goeij, D. C., Sutanto, W., Mos, J., de Kloet, E. R., & Tilders, F. J. (1993). Short inescapable stress produces long-lasting changes in the brain-pituitary-adrenal axis of adult male rats. Neuroendocrinology, 58(1), 57–64. https://doi.org/10.1159/000126512
- Varga, J., Klausz, B., Domokos, Á., Kálmán, S., Pákáski, M., Szűcs, S., Garab, D., Zvara, Á., Puskás, L., Kálmán, J., Tímár, J., Bagdy, G., & Zelena, D. (2014). Increase in Alzheimer’s related markers preceeds memory disturbances: studies in vasopressin-deficient Brattleboro rat. Brain Research Bulletin, 100, 6–13. https://doi.org/10.1016/j.brainresbull.2013.10.010
- Veenema, A. H., & Neumann, I. D. (2007). Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain, Behavior and Evolution, 70(4), 274–285. https://doi.org/10.1159/000105491
- Veenema, A. H., Koolhaas, J. M., & de Kloet, E. R. (2004). Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Annals of the New York Academy of Sciences, 1018, 255–265. https://doi.org/10.1196/annals.1296.030
- Veenema, A. H., Meijer, O. C., de Kloet, E. R., & Koolhaas, J. M. (2003). Genetic selection for coping style predicts stressor susceptibility. Journal of Neuroendocrinology, 15(3), 256–267. https://doi.org/10.1046/j.1365-2826.2003.00986.x
- Watters, J. J., Wilkinson, C. W., & Dorsa, D. M. (1996). Glucocorticoid regulation of vasopressin V1a receptors in rat forebrain. Brain Research. Molecular Brain Research, 38(2), 276–284. https://doi.org/10.1016/0169-328x(95)00345-s
- Wigger, A., Sanchez, M. M., Mathys, K. C., Ebner, K., Frank, E., Liu, D., Kresse, A., Neumann, I. D., Holsboer, F., Plotsky, P. M., & Landgraf, R. (2004). Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 29(1), 1–14. https://doi.org/10.1038/sj.npp.1300290
- Williams Avram, S. K., Lee, H. J., Fastman, J., Cymerblit-Sabba, A., Smith, A., Vincent, M., Song, J., Granovetter, M. C., Lee, S. H., Cilz, N. I., Stackmann, M., Chaturvedi, R., & Young, W. S. (2019). NMDA receptor in vasopressin 1b neurons is not required for short-term social memory, object memory or aggression. Frontiers in Behavioral Neuroscience, 13, 218. https://doi.org/10.3389/fnbeh.2019.00218
- Yamaguchi, Y., Suzuki, T., Mizoro, Y., Kori, H., Okada, K., Chen, Y., Fustin, J.-M., Yamazaki, F., Mizuguchi, N., Zhang, J., Dong, X., Tsujimoto, G., Okuno, Y., Doi, M., & Okamura, H. (2013). Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science (New York, N.Y.), 342(6154), 85–90. https://doi.org/10.1126/science.1238599
- Yang, C., Zhang, X., Gao, J., Wang, M., & Yang, Z. (2017). Arginine vasopressin ameliorates spatial learning impairments in chronic cerebral hypoperfusion via V1a receptor and autophagy signaling partially. Translational Psychiatry, 7(7), e1174. https://doi.org/10.1038/tp.2017.121
- Yayou, K., Sato, Y., Ito, S., & Nakamura, M. (2008). Comparison between the central effects of CRH and AVP in steers. Physiology & Behavior, 93(3), 537–545. https://doi.org/10.1016/j.physbeh.2007.10.014
- Yehuda, R. (2002). Post-traumatic stress disorder. The New England Journal of Medicine, 346(2), 108–114. https://doi.org/10.1056/NEJMra012941
- Yoshii, T., Sakamoto, H., Kawasaki, M., Ozawa, H., Ueta, Y., Onaka, T., Fukui, K., & Kawata, M. (2008). The single-prolonged stress paradigm alters both the morphology and stress response of magnocellular vasopressin neurons. Neuroscience, 156(3), 466–474. https://doi.org/10.1016/j.neuroscience.2008.07.049
- Zelena, D. (2012). Vasopressin in health and disease with a focus on affective disorders. Central Nervous System Agents in Medicinal Chemistry, 12(4), 286–303. https://doi.org/10.2174/187152412803760609
- Zelena, D., & Engelmann, M. (2018). The Brattleboro rat: the first and still up‐to‐date mutant rodent model for neuroendocrine research. In M. Ludwig & G. Levkowitz (Eds.), Model Animals in Neuroendocrinology: From Worm to Mouse to Man. Wiley & Sons.
- Zelena, D., Domokos, A., Jain, S. K., Jankord, R., & Filaretova, L. (2009a). The stimuli-specific role of vasopressin in the hypothalamus-pituitary-adrenal axis response to stress. The Journal of Endocrinology, 202(2), 263–278. https://doi.org/10.1677/JOE-09-0096
- Zelena, D., Langnaese, K., Domokos, A., Pinter, O., Landgraf, R., Makara, G. B., & Engelmann, M. (2009b). Vasopressin administration into the paraventricular nucleus normalizes plasma oxytocin and corticosterone levels in Brattleboro rats. Endocrinology, 150(6), 2791–2798. https://doi.org/10.1210/en.2008-1007
- Zelena, D., Mikics, E., Balazsfi, D., Varga, J., Klausz, B., Urban, E., Sipos, E., Biro, L., Miskolczi, C., Kovacs, K., Ferenczi, S., & Haller, J. (2016). Enduring abolishment of remote but not recent expression of conditioned fear by the blockade of calcium-permeable AMPA receptors before extinction training. Psychopharmacology, 233(11), 2065–2076. https://doi.org/10.1007/s00213-016-4255-4
- Zelena, D., Pinter, O., Balazsfi, D. G., Langnaese, K., Richter, K., Landgraf, R., Makara, G. B., & Engelmann, M. (2015). Vasopressin signaling at brain level controls stress hormone release: The vasopressin-deficient Brattleboro rat as a model. Amino Acids, 47(11), 2245–2253. https://doi.org/10.1007/s00726-015-2026-x
- Zhang, X., Zhao, F., Wang, C., Zhang, J., Bai, Y., Zhou, F., Wang, Z., Wu, M., Yang, W., Guo, J., & Qi, J. (2019). AVP(4-8) improves cognitive behaviors and hippocampal synaptic plasticity in the APP/PS1 mouse model of Alzheimer’s disease. Neuroscience Bulletin, 36, 254–262.
- Zink, C. F., Stein, J. L., Kempf, L., Hakimi, S., & Meyer-Lindenberg, A. (2010). Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 30(20), 7017–7022. https://doi.org/10.1523/JNEUROSCI.4899-09.2010
