Abstract
The main and accessory olfactory bulbs (MOB and AOB) are unique in that they produce new neurons throughout adulthood. Despite the recent knowledge about the involvement of postnatally generated cells in several aspects of olfaction, the functional role of these neurons is still not sufficiently understood. The function of newly generated olfactory bulb neurons is primarily investigated in relation to activities related to smell. Stress-induced activation of new olfactory neurons has not yet been studied. Thus, our work was aimed to investigate whether a stressful event, such as maternal separation (MS) can induce Fos expression in postnatally-born neurons in the MOB and AOB. Rat pups were exposed to single maternal separation (SMS) for 2 h at the postnatal days: P7, P14, and P21. Quantification of immunohistochemically labeled Fos + cells revealed that exposure to SMS in different age stages during the first postnatal month stimulates activity in cells of individual MOB/AOB layers in an age-dependent manner. In order to find out whether newly generated cells in the MOB/AOB could express Fos protein as a response to SMS, newborn rats were administrated with the marker of proliferation, bromodeoxyuridine (BrdU) at P0, and three weeks later (at P21) colocalization of Fos and BrdU in the neurons of the MOB and AOB was assessed. Quantitative analysis of BrdU/Fos double-labeled cells showed that Fos is expressed only in a small number of postnatally generated cells within the MOB/AOB. Our results indicate that postnatally generated MOB/AOB neurons are less sensitive to stress caused by MS than preexisting ones.
Our results showed that single maternal separation (SMS) is a stressful event that in age-dependent manner stimulates cellular activity in the main and accessory olfactory bulb (AOB) – the structures dedicated to odor information processing. The low level of Fos expression in newborn neurons of the main and accessory bulb indicates that postnatally generated cells are less sensitive to neonatal stress than preexisting neurons.
LAY SUMMARY
Introduction
The early life period is especially important in forming the individual, and research has shown that adverse experience during this stage can affect the formation of neuronal networks and exert long-lasting effects on neural function (Roque et al., Citation2014). Models of early life stress are usually associated with disruption of mother-pup interactions by maternal separation (MS) which has been reported to induce long-term effects on the neuroendocrine system and behavior (Biagini et al., Citation1998; Marais et al., Citation2008; Plotsky et al., Citation2005). Moreover, recent studies revealed the effect of MS on processes of postnatal neurogenesis in the olfactory neurogenic region of the rodent brain (Feng et al., Citation2014; Lievajova et al., Citation2011).
To study the effect of early life stress, many different paradigms of MS are used that differ in the duration of separation and in the period of lifetime when the separation is performed (Arborelius & Eklund, Citation2007; Caldji et al., Citation2000; Carrera et al., Citation2009). The most frequently used MS model is repeated separations of the pups from the mother during the first three postnatal weeks. Another important model of MS is single separation of pups from the mother (SMS). In contrast to the repeated separation of pups from the mother, which is a chronic form of stress, SMS is an aversive stimulus to the pup that activates the HPA axis response and provides an important tool to study the acute effects of stress and the development of the stress response systems. Increased corticosterone level due to SMS can activate some populations of neurons in the brain (Enthoven et al., Citation2008; Schmidt et al., Citation2004; Stanton et al., Citation1988).
The activation of different brain regions after MS has been measured by Fos expression, an immediate early gene (IEG) product. Analysis revealed that the manner of Fos expression changes developmentally and on dependence of the type of MS (Horii-Hayashi et al., Citation2013; Nishi et al., Citation2013). The examination of Fos expression has been also used in studies addressing in vivo responses of newly generated interneurons in the main olfactory bulb (MOB) (Livneh et al., Citation2014; Magavi et al., Citation2005). Olfactory stimuli are in general processed by two systems. The main olfactory system receives common olfactory stimuli and processes information necessary for maintaining life. Accessory or vomeronasal system, including the accessory olfactory bulb (AOB), mediates innate response elicited by pheromones (Brennan & Zufall, Citation2006), while both systems are anatomically and functionally interrelated (Trinh & Storm, Citation2003). OB activation during stress has been rarely studied, nevertheless, olfactory cortical areas connected with OB neurons are known to control stress response (Kondoh et al., Citation2016). It has been shown, that for example an exposure to the odor of predator can induce autonomic, endocrine, and fear behavior stress reactions (Takahashi, Citation2014).
The MOB and AOB are unique in that they produce new neurons throughout adulthood. In rodents, neuronal precursors originating in the subventricular zone migrate into the MOB and AOB and become functional interneurons (Lois & Alvarez-Buylla, Citation1994; Nunez-Parra et al., Citation2011; Oboti et al., Citation2009; Petreanu & Alvarez-Buylla, Citation2002). Integration and survival of adult-born cells within the MOB/AOB can be affected by exogenous stimuli (odor enriched environment, sexually relevant odors, socio-sexual relevant signals) (Larsen et al., Citation2008; Oboti et al., Citation2009; Portillo et al., Citation2012; Rochefort & Lledo, Citation2005). It was also shown that adult-born olfactory neurons express IEGs in response to odors in vivo (Carlen et al., Citation2002; Huang & Bittman, Citation2002). Stress-induced activation of new olfactory neurons has not yet been studied. Thus, our work was aimed at investigating whether a stressful event, such as MS can induce Fos expression in postnatally-born neurons in the MOB and AOB. Our results showed that while Fos expression in response to SMS increases with age in the MOB and AOB, new-born neurons contribute relatively little to this response. These data could help to understand the role of early life olfactory neurogenesis in stress response.
Material and methods
Animals
In this study, Wistar albino rats of both sexes were divided into three experimental groups based on their age: 7-d old P7 (n = 16, 8 controls, and 8 SMS), 14-d old P14 (n = 16, 8 controls, and 8 SMS), and 21-d old P21 (n = 26, 8 controls, and 8 SMS for quantification of Fos positive cells and 5 control and 5 SMS for double labeling BrdU and Fos). Pups were housed together with their dam in the home cage from the day of birth (P0) until the day of single maternal separation (SMS). Pups for individual groups were taken from 3 to 4 different litters. Culling of litters to 8 pups was performed on the first postnatal day. They were kept at a 12 h light/dark cycle, the mothers with ad libitum access to food and water.
All experimental procedures were approved by the Ethical Committee of the Institute of Neurobiology, Biomedical Research Center, Slovak Academy of Sciences, and the State Veterinary and Food Administration of the Slovak Republic and carried out in accordance with EC Directive 86/609/EEC.
Bromodeoxyuridine administration
To label proliferating cells, the pups from the P21 age group were administered by single i.p. injection of BrdU (50 mg/kg of body weight in 0.09% saline; Sigma-Aldrich, St. Louis, MO) at the day of birth and were left to survive until P21.
Maternal separation
Pups of each age group (P7, P14, and P21) were exposed to single separation from their mother. The mothers were removed from the cage for 120 min and the pups were kept together in their home cage to avoid social isolation. The cage with pups was placed in an incubator with the nest temperature (34 °C). Age-matched control rats were kept under the same conditions except MS.
Tissue processing
Immediately after the separation, the pups were deeply anesthetized with isoflurane and i.p. administration of chloralhydrate and transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) with pH = 7.4–7.6. The brains were left in skulls and post-fixed overnight in 4% PFA in 0.1 M PB. The brains removed from skulls were cryoprotected in 30% sucrose in 0.1 M phosphate buffered saline (PBS) for 48 h after which they were sliced on the cryostat to 40 µm thick serial sagittal sections. The sections were stored in PBS at 4 °C and the next day they were processed for immunohistochemical analyses.
Fos immunohistochemistry
The brain sections were washed two times in PBS. This was followed by 30 min incubation in 0.3% H2O2 in 0.1 M PBS. Then, the sections were rinsed again in PBS and incubated in blocking solution for 2 h (3% Normal goat serum in PBS and 0.25% Triton X-100 (NGST)). The sections were then labeled with rabbit polyclonal anti-Fos primary antibody (Oncogene Res. Products, Cambridge, MA; dilution 1:10,000) for 48 h at room temperature. After 2 d, sections were washed six times in PBS and incubated for 2 h in biotinylated goat anti-rabbit secondary antibody at room temperature (Abcam, Cambridge, UK; 1:600 in 3% NGST). After washing, the sections were incubated in avidin-biotin complex for 1 h (ABC elite standard kit, Vector laboratories, Burlingame, CA). To visualize immunoreactive cells, the sections were immersed in 0.01% H2O2 and 0.05% diaminobenzidine (DAB) in PB for 1–3 min. Lastly, the sections were mounted on slides, air-dried, and cover-slipped with Entellan (Merck-Milipore, Burlington, MA).
Double labeling of Fos producing cells
In order to find whether some newly generated cells in the MOB and AOB layers can express Fos protein as a response to SMS, double labeling for Fos and BrdU was performed. Brain sections were washed three times in PBS and incubated in 2 N HCl two times for 15 min at 59 °C. Borate buffer wash, two times for 15 min, and triple PBS wash were followed by 1-h incubation in blocking solution (10% NGST). Then, the sections were incubated in the mixture of primary antibodies, rat anti-BrdU (BIO-RAD, Hercules CA, 1:500), and rabbit anti-c-Fos antibody (Oncogene Research Products, La Jolla, CA, 1:10,000) in 3% NGST for 48 h at 4 °C. After washing in PBS the sections were incubated in the mixture of secondary antibodies, anti-rabbit Alexa Fluor 594 (Abcam, Cambridge, UK, 1:200) and anti-rat Alexa Fluor 488 (Abcam, Cambridge, UK, 1:200) in 3% NGST for 90 min at dark in room temperature. At last, the sections were washed in PBS, mounted on slides, dried and cover-slipped with Fluoromount.
Quantitative and statistical analysis
The number of Fos + cells was examined in seven MOB and four AOB layers of both, experimental and control rats in all age groups (P7, P14, and P21). The images of individual sections were taken using digital camera (Olympus DP72) connected to a light microscope (Olympus BX51) using 20x magnification. Fos + cells were counted manually using ImageJ 1.51 Cell counter on 8–10 sections per animal in a region of interest (ROI) of 200 × 200 µm and 100 × 100 µm placed over each layer of the MOB and the AOB, respectively. To compare total activity of the MOB and the AOB after SMS between ages, Fos + cells were counted in the bulbs regardless of the layers.
Newly generated cells (BrdU+) and double-labeled cells (Fos + and BrdU+) were evaluated in the MOB and AOB layers (as above) of P21 rats. Images obtained with fluorescence microscope were merged in ImageJ version 1.51 software (Bethesda, Maryland, USA).
The results were expressed as mean value ± SEM. Data were analyzed by unpaired Student’s t-test using GraphPad Prism version 6.0 software (GraphPad Inc., La Jolla, CA). p Values less than .05 were considered as statistically significant.
Results
Qualitative, light microscopic analyzes showed different density of Fos + cells in both olfactory bulbs of control rats and rats after SMS of all age groups as well as noticeable differences between age stages (). The quantification of Fos expressing cells was performed on the sections processed by immunohistochemistry and visualized with DAB.
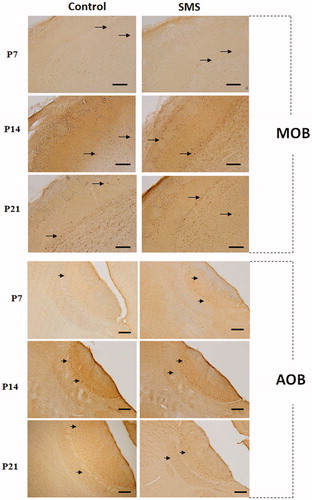
Figure 1. Fos immunoreactivity in the main olfactory bulb (MOB) and in the accessory olfactory bulb (AOB). Representative photomicrographs of parasagittal sections of the MOB and AOB of control rats (left column) and rats exposed to single maternal separation (SMS) (right column) of different ages showing the distribution of Fos positive cells (arrows) within the MOB and AOB. Increased Fos positivity in the MOB and AOB after SMS can be seen. Scale bar 200 µm.
Fos expression in the main olfactory bulb
Quantitative analysis of Fos expression was assessed in seven concentric layers of the MOB: the olfactory nerve layer (ONL), glomerular layer (GL), external plexiform layer (EPL), mitral cell layer (ML), internal plexiform layer (IPL), granule cell layer (GrL), and ventricular subependymal layer (VL). The results of quantitative analysis are summarized in .
Table 1. The number of Fos positive cells in the main olfactory bulb and accessory olfactory bulb layers in control rats and rats after the exposure to single maternal separation (SMS).
In the MOB of P7 control rats, only a sporadic occurrence of Fos + cells was detected. They were present in almost all MOB layers, except the ONL and the EPL. Quantitative analysis showed the highest number of Fos + cells in the GrL (). The exposure of P7 animals to SMS caused a significant increase of Fos + cells throughout all MOB layers and induced production of Fos protein also in cells within the EPL ().
Unlike the P7 control rats, in P14 control animals, the expression of Fos protein was observed in each of the analyzed bulbar layers. The highest number of Fos + cells was detected within the GrL and ML (). The exposure of P14 rats to SMS resulted in a slight, but not significant increase of Fos production in cells of individual MOB layers. This increase was the most prominent in the mitral and GrLs, however, without statistical significance.
The developmental increase of Fos production continued in P21 rats as well. In control animals, noticeably high density of Fos + cells was observed within the GL, ML, GrL, and ventricular VL. Small amount of labeled cells was scattered in the remaining bulbar layers (). Quantitative analysis after SMS showed significant increase of Fos + cells within each layer. This increase was the most prominent in the GrL, ventricular VL, and IPL ().
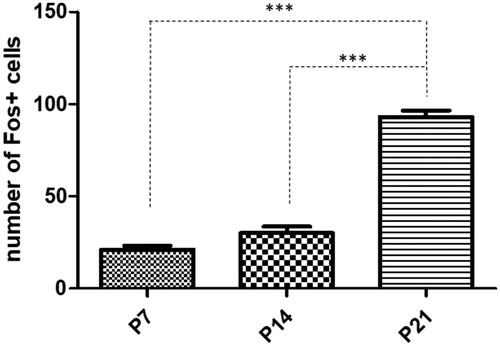
Comparison of Fos activity in the MOB following SMS between age groups revealed that the effect of SMS on Fos activity was most pronounced in P21 rats. In this age group the number of Fos positive cells was significanly higher than in P7 or P14 pups ().
Figure 2. Quantitative analysis of Fos positive cells in the main olfactory bulb in rats exposed to single maternal separation (SMS). Graph shows the number of Fos positive cells in the P7 (22.83 ± 1.13), P14 (30.00 ± 3.53), and P21 (93.10 ± 3.49) animals after SMS. Data are shown as mean ±SEM. Asterisks (***) indicate significant difference, p < .001.
Fos expression in the accessory olfactory bulb
Fos immunoreactivity in the AOB of control and experimental rats was evaluated in four layers: the GL, ML, nerve layer, and granule layer ().
In the AOB of P7 and P14 control rats, Fos + cells were detected only in the ML. The other AOB layers were without the presence of labeled cells. Exposure to the SMS resulted in a significant increase of Fos + cells in ML of both, P7 and P14 animals. Moreover, in P14 animals the SMS induced Fos production in all three other layers of the AOB, i.e. in glomerular, nerve, and GrLs.
In the AOB of P21 control rats, Fos positive cells were distributed in all four layers and the highest density of these cells was detected within the ML. Quantification of labeled cells revealed their significant increase in all observed layers after the SMS, especially in the mitral and GrL ().
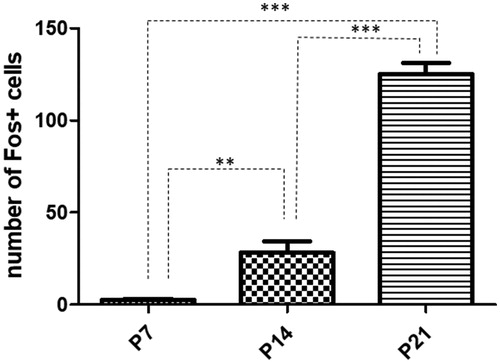
Comparison of Fos activity in the AOB between age groups following SMS showed a tendency toward increase, but this increase had a different course than in the MOB. The highest number of Fos positive cells was also recorded in P21 animals, but the number of these cells was already significantly higher at P14 compared to P7 ().
Figure 3. Quantitative analysis of Fos positive cells in the accessory olfactory bulb in rats exposed to single maternal separation (SMS). Graph shows the number of Fos positive cells in the P7 (2.610 ± 0.61), P14 (28.24 ± 6.17), and P21 (125.3 ± 5.94) animals after SMS. Data are shown as mean ± SEM. Asterisks indicate significant difference, **p < .01 and ***p < .001.
Fos expression in newly generated cells
To reveal whether SMS can induce Fos expression in newborn cells of the MOB and AOB, double immunofluorescent labeling for Fos and BrdU was performed (). The proliferation marker – BrdU was administered to P0 control and experimental animals and the animals survived until P21. Three weeks after BrdU administration, proliferating cells of different density were detectable in both analyzed structures. BrdU + cells were found scattered throughout all MOB/AOB layers (). In the MOB, BrdU labeled cells were distributed predominantly in the GrL and in the AOB mainly the granule and glomerular cell layers (). In general, the density of BrdU + cells in the AOB layers was lower than in the MOB layers, which is consistent with the results of Oboti et al. (Citation2009).
Figure 4. Fos expression in newborn cells in the main olfactory bulb (MOB) and accessory olfactory bulb (AOB) after single maternal separation (SMS). Images showing double-labeled sections for BrdU (A, D) and Fos (B, E) in the MOB and AOB of P21 animals after SMS. Arrows indicate colocalization of BrdU and Fos in the MOB (C) in the EPL, ML and Gr as well as AOB (F) in the GrL and NL. A′, B′, C′, D′, E′, F′: Magnifications of the boxed areas in A, B, C, D, E, F, respectively. Scale bar 200 µm. ONL: olfactory nerve layer; GL: glomerular layer; EPL: external plexiform layer; ML: mitral cell layer; IPL: internal plexiform layer; GrL: granule cell layer; VL: ventricular subependymal layer; NL: nerve layer.
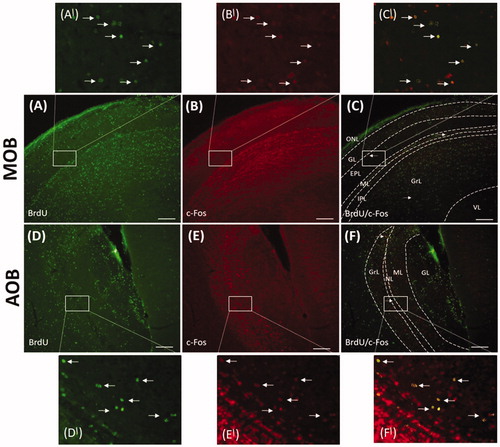
The number of newborn neurons producing Fos protein (Fos/BrdU positive cells) was evaluated 3 weeks after BrdU administration (). The MOB of control animals contained only a small number of newborn BrdU+/Fos + cells scattered throughout all bulbar layers except the glomerular and ONL (). Population of these double-labeled cells represented not even 2% of all Fos producing cells and approximately 0.49% of all BrdU + cells (an average value taken from all assessed layers). SMS caused only a minimal increase in the number of BrdU+/Fos + cells in the above-mentioned layers of the MOB and also appearance of BrdU+/Fos + cells in the GL (). In the AOB, BrdU+/Fos + cells were present in all layers except GL (, ) of both, control and separated animals. Double labeled cells represented not even 1% of all Fos + cells and 1.03% of all BrdU + cells (an average value taken from all assessed layers) in the AOB of control animals. As in the MOB, we observed only minimal increase in the number of Brdu+/Fos + cells after SMS.
Table 2. The number of newborn Fos + cells (BrdU and Fos immunopositive) in the main olfactory bulb and accessory olfactory bulb of P21 control animals and animals after SMS.
Taken together, this suggests that in the MOB/AOB of control as well as SMS rats, Fos is expressed in preexisting neurons rather than in postnatally generated cells.
Discussion
We demonstrated here that single exposure of rat pups to short-term SMS in different age stages during the first postnatal month stimulates activity in cells of individual MOB/AOB layers in an age-dependent manner. Analysis of Fos production in postnatally generated cells of the MOB/AOB has shown that only a small portion of these cells respond to stress induced by SMS.
Fos expression in the MOB and AOB
In the MOB, expression of Fos protein is regulated both during development (Klintsova et al., Citation1995; Smeyne et al., Citation1992) and postnatally by odor stimuli (Guthrie & Gall, Citation1995). Our finding obtained in control neonatal rats has shown that the number of Fos positive cells in individual layers of the MOB increases with development. This is in agreement with the results of previous studies demonstrating that the appearance of Fos expression is highly correlated with the MOB maturation during development (Klintsova et al., Citation1995; Smeyne et al., Citation1992).
In this study, the stressful event for induction of Fos expression was single short-duration separation of rat pups from their dams. Although, MS is not a typical model of odor stimulation, the involvement of the olfactory system in mediation of the separation-evoked effects has been suggested formerly (Hofer, Citation1975). In our previous study, we have found increased Fos expression following SMS in neurons of the anterior olfactory nucleus (Fabianová et al., Citation2018) – an olfactory cortical structure that plays a significant role in olfactory signal processing (Illig, Citation2005). In the present work, we have shown SMS induced Fos expression in the MOB, and in the AOB – the structures dedicated to odor information processing and where expression of Fos protein is usually evoked by odor stimulation (Kay, Citation2011).
The separation of rat pups from the dams on P7, P14, and P21 resulted in a marked increase of the number of Fos positive cells in each layer of the MOB. This suggests that the response of individual anatomical elements of the MOB to the stress induced by MS is coordinated. We suppose that the stress reaction in pups is caused by the lack of mother odor. It is consistent with the finding that adverse olfactory signals can induce a stress response. Indeed, it was revealed that olfactory bulb neurons are directly connected to the olfactory cortical areas, which in turn activate hypothalamic CRH (corticotropin-releasing hormone) neurons that induce the stress hormone response (Kondoh et al., Citation2016). In addition, several studies indicate that the olfactory bulb receives signals from brain areas related to stress processing, such as amygdala (Zurawicki, Citation2010) or hypothalamus (Schneider et al., Citation2020). Therefore, we cannot rule out the possibility that central inputs had role in activation of OB neurons following SMS.
Besides examination within the MOB, we also analyzed the impact of SMS on Fos production in the AOB. Whilst in the AOB of P7 and P14 control rats the Fos positive cells were detected only within the ML, in P21 control rats Fos positive cells were distributed in all four layers of the AOB. The increase of Fos expression after SMS was region specific: in P7 rats Fos production was restricted only to the ML; however, in P14 rats separation induced earlier maturation of Fos producing cells in all AOB layers. According to these findings, probably only the AOB principal neurons (mitral cells) are capable of Fos protein production right after birth, suggesting that cells forming other AOB layers acquire the complete prerequisites necessary for the Fos signal transduction cascade only later in development. SMS on P21 caused a significant increase in the number of Fos positive cells in all observed layers of the AOB. On the basis of our results, we can assume, that later in the development (around P21) besides mitral cells also the periglomerular and the granule cells forming the glomerular and granular AOB layers, respectively, are capable of Fos protein production and we suppose that these cells retain the ability to produce Fos throughout adulthood. This finding is in accordance with the findings of Matsuoka et al. (Citation1999), who observed Fos positive cells in all AOB layers in adult male mice after exposure to soiled bedding of females. It is interesting that while in P14 control rats the Fos immunopositivity was detected only in the mitral cells layer, MS launched the production of Fos protein in all AOB layers. This finding indicates that stress due do MS can induce Fos production in cells, which do not produce this protein under normal conditions (in the calm state).
Comparison of Fos activity following SMS in the MOB/AOB between age groups revealed that the response to SMS has an increasing tendency with increasing age in both structures. Differences in Fos positivity between age groups could be related to increased responsiveness/higher sensitivity of rats with age to stress caused by MS/lack of mother odor.
Low Fos expression in the AOB of P7 rats can be associated with later maturation of this structure. While the maturation of synapses between olfactory sensory neurons and MOB occurs during embryonic development, AOB synapses mature by the end of the first postnatal week (Suárez et al., Citation2012).
The main and accessory olfactory system are described as functionally different, with the olfactory epithelium detecting volatile odorants and the vomeronasal organ detecting nonvolatile pheromones through direct physical contact with the source (Mori et al., Citation2000). Our results, indicating that the AOB can be activated not only by pheromone-like chemo-signal, but also by the absence of maternal odor caused by separation from the mother, suggest that these two systems might not be as functionally distinct as was originally thought.
Fos expression in newly generated cells
Neurogenesis of the local circuit neurons of the MOB and into a lesser extent of the AOB is not completed at the end of the embryonic period, rather continuing throughout life (Lois & Alvarez-Buylla, Citation1994; Oboti et al., Citation2009). Postnatal olfactory interneurons derive from neuronal stem cells located in the subventricular zone of the lateral ventricles (Doetsch et al., Citation2002) from where they migrate a long distance to the target structures – the MOB and AOB. Here, the neuroblasts differentiate into different types of interneurons, mainly the granule and periglomerular cells (Belluzzi et al., Citation2003; Carleton et al., Citation2003, Oboti et al., Citation2009; Luskin, Citation1993). Despite the recent knowledge about involvement of postnatal neurogenesis in several aspects of olfactory function such as, olfactory perceptual learning (Moreno et al., Citation2009), odor discrimination (Belnoue et al., Citation2011), and short-term and long-term odor memory (Breton-Provencher et al., Citation2009), the functional role of newly generated cells is still not sufficiently understood. Interestingly, the results of studies investigating the function of newborn neurons suggest that adult-born neurons differ from neurons generated during development in several properties (reviewed in Ming & Song, Citation2011).
The function of newly generated olfactory bulb neurons is, of course, studied primarily in relation to activities related to smell. The observed response of neurons in the MOB/AOB to SMS led us to investigate whether postnatally generated cells are involved in the response to this stressful stimulus. For this aim, newborn rats were administrated with the marker of proliferation – BrdU, and three weeks later (at P21), colocalization of Fos and BrdU in the neurons of the MOB and AOB was assessed. In line with previous results (reviewed in Lledo & Saghatelyan, Citation2005) we have observed that dividing cells that had incorporated BrdU on the day of birth reached the MOB/AOB within 21 d of BrdU injection. Double labeling for BrdU and for the mature neuronal marker NeuN has shown that vast majority of BrdU positive cells differentiated into neurons (data not shown). Quantitative analysis of BrdU/Fos double-labeled cells showed that Fos is expressed only in a small number of postnatally generated cells within the MOB/AOB. This result confirms previous observations regarding the functional difference between postnatally generated cells and preexisting ones (Belluzzi et al., Citation2003; Magavi et al., Citation2005; Saghatelyan et al., Citation2005). However, these studies have demonstrated that adult-born neurons show a stronger response to exogenous stimuli than those prenatally generated. In a similar experimental paradigm to ours, Magavi et al. (Citation2005) used IEGs to assess the response of newborn olfactory granule neurons to odorants, and showed that adult-born neurons have a greater population IEG response to novel odors than mature, preexisting neurons. Contrary to these results, we have observed low level of Fos expression in newborn neurons of the MOB and AOB of rats exposed to SMS.
Together, our results indicate that postnatally generated olfactory MOB/AOB neurons are less sensitive to stress caused by MS than preexisting ones. Another explanation for the small percentage of newborn Fos positive cells in proportion to the total number of Fos producing cells might be that newborn cells although differentiated are not fully mature three weeks after their generation. Given that 21-d old neurons are not Fos-reactive to stressors, subsequent work (for example, retroviral labeling followed by electrophysiological analysis) will be required to determine if new neurons are functionally mature.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Monika Závodská
Monika Závodská, Dr. rer. nat., PhD. is a young researcher at the Department of Regenerative Medicine and Cell Therapy of the INb BMC SAS, Slovakia. She is experienced in morphological analysis of the CNS and in quantitative and image analysis.
Kamila Fabianová
Kamila Fabianová, Dr. rer. nat., PhD. is a young researcher at the Department of Regenerative Medicine and Cell Therapy of the INb BMC SAS, Slovakia. Her research is focused on the effect of environmental factors on postnatal neurogenesis in the olfactory neurogenic region and corresponding behavioral changes.
Marcela Martončíková
Marcela Martončíková, Dr. rer. nat., PhD. is senior researcher at the Department of Regenerative Medicine and Cell Therapy of the INb BMC SAS, Slovakia. She is an expert in the field of postnatal neurogenesis and developmental studies in the olfactory system.
Adam Raček
Adam Raček, DVM., PhD. is a postdoctoral fellow at the Department of Anatomy, Histology and Physiology, University of Veterinary Medicine and Pharmacy in Košice. He finished his PhD in the Neuroscience. His research is oriented on adult neurogenesis in relation to the pathogenesis of neurodegenerative diseases.
Enikő Račeková
Enikő Račeková, Dr rer. nat., PhD. is the Head of the Laboratory of Neuromorphology and Developmental Neurobiology of the INb BMC, SAS in Košice, Slovakia. Her research team focuses on investigation the processes of postnatal neurogenesis in the rat olfactory system under physiological and pathological conditions.
References
- Arborelius, L., & Eklund, M. B. (2007). Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience, 145(2), 738–750. https://doi.org/10.1016/j.neuroscience.2006.12.007
- Belluzzi, O., Benedusi, M., Ackman, J., & LoTurco, J. J. (2003). Electrophysiological differentiation of new neurons in the olfactory bulb. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(32), 10411–10418. https://doi.org/10.1523/JNEUROSCI.23-32-10411.2003
- Belnoue, L., Grosjean, N., Abrous, D. N., & Koehl, M. (2011). A critical time window for the recruitment of bulbar newborn neurons by olfactory discrimination learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(3), 1010–1016. https://doi.org/10.1523/JNEUROSCI.3941-10.2011
- Biagini, G., Pich, E. M., Carani, C., Marrama, P., & Agnati, L. F. (1998). Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. International Journal of Developmental Neuroscience, 16(3-4), 187–197. https://doi.org/10.1016/S0736-5748(98)00019-7
- Brennan, P. A., & Zufall, F. (2006). Pheromonal communication in vertebrates. Nature, 444(7117), 308–315. https://doi.org/10.1038/nature05404
- Breton-Provencher, V., Lemasson, M., Peralta, M. R., & Saghatelyan, A. (2009). Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(48), 15245–15257. https://doi.org/10.1523/JNEUROSCI.3606-09.2009
- Caldji, C., Diorio, J., & Meaney, M. J. (2000). Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry, 48(12), 1164–1174. https://doi.org/10.1016/s0006-3223(00)01084-2
- Carlen, M., Cassidy, R. M., Brismar, H., Smith, G. A., Enquist, L. W., & Frisen, J. (2002). Functional integration of adult-born neurons. Current Biology, 12(7), 606–608. https://doi.org/10.1016/s0960-9822(02)00771-6
- Carleton, A., Petreanu, L. T., Lansford, R., Alvarez-Buylla, A., & Lledo, P. M. (2003). Becoming a new neuron in the adult olfactory bulb. Nature Neuroscience, 6(5), 507–518. https://doi.org/10.1038/nn1048
- Carrera, O., Cerrato, M., Sanchez, A., & Gutierrez, E. (2009). Long maternal separation has protective effects in rats exposed to activity-based anorexia. Developmental Psychobiology, 51(8), 616–624. https://doi.org/10.1002/dev.20396
- Doetsch, F., Petreanu, L., Caille, I., Garcia-Verdugo, J. M., & Alvarez-Buylla, A. (2002). EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron, 36(6), 1021–1034. https://doi.org/10.1016/s0896-6273(02)01133-9
- Enthoven, L., Oitzl, M. S., Koning, N., van der Mark, M., & de Kloet, E. R. (2008). Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology, 149(12), 6366–6377. https://doi.org/10.1210/en.2008-0238
- Fabianová, K., Závodská, M., Raček, A., Angelidis, A., Martončíková, M., & Račeková, E. (2018). Analysis of Fos expression in the rat olfactory neurogenic region following single exposure to maternal separation during different neonatal stages. General Physiology and Biophysics, 37(3), 275–283. https://doi.org/10.4149/gpb_2017056
- Feng, M., Sheng, G., Li, Z., Wang, J., Ren, K., Jin, X., & Jiang, K. (2014). Postnatal maternal separation enhances tonic GABA current of cortical layer 5 pyramidal neurons in juvenile rats and promotes genesis of GABAergic neurons in neocortical molecular layer and subventricular zone in adult rats. Behavioural Brain Research, 260, 74–82. https://doi.org/10.1016/j.bbr.2013.11.040
- Guthrie, K. M., & Gall, C. M. (1995). Functional mapping of odor-activated neurons in the olfactory bulb. Chemical Senses, 20(2), 271–282. https://doi.org/10.1093/chemse/20.2.271
- Hofer, M. A. (1975). Studies on how early maternal separation produces behavioral change in young rats. Psychosomatic Medicine, 37(3), 245–264. https://doi.org/10.1097/00006842-197505000-00003
- Horii-Hayashi, N., Sasagawa, T., Matsunaga, W., Matsusue, Y., Azuma, C., & Nishi, M. (2013). Developmental changes in desensitisation of c-Fos expression induced by repeated maternal separation in pre-weaned mice. Journal of Neuroendocrinology, 25(2), 158–167. https://doi.org/10.1111/j.1365-2826.2012.02377.x
- Huang, L., & Bittman, E. L. (2002). Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Hormones and Behavior, 41(3), 343–350. https://doi.org/10.1006/hbeh.2002.1767
- Illig, K. R. (2005). Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. The Journal of Comparative Neurology, 488(2), 224–231. https://doi.org/10.1002/cne.20595
- Kay, L. M. (2011). Olfactory coding: Random scents make sense. Current Biology, 21, 928–929. https://doi.org/10.1016/j.cub.2011.10.008
- Klintsova, A. Y., Philpot, B. D., & Brunjes, P. C. (1995). Fos protein immunoreactivity in the developing olfactory bulbs of normal and naris-occluded rats. Brain Research Developmental Brain Research, 86(1–2), 114–122. https://doi.org/10.1016/0165-3806(95)00015-6
- Kondoh, K., Lu, Z., Ye, X., Olson, D. P., Lowell, B. B., & Buck, L. B. (2016). A specific area of the olfactory cortex involved in stress hormone responses to predator odors. Nature, 532(7597), 103–106. https://doi.org/10.1038/nature17156
- Larsen, C. M., Kokay, I. C., & Grattan, D. R. (2008). Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Hormones and Behavior, 53(4), 509–517. https://doi.org/10.1016/j.yhbeh.2007.11.020
- Lievajova, K., Blasko, J., Martoncikova, M., Cigankova, V., & Racekova, E. (2011). Delayed maturation and altered proliferation within the rat rostral migratory stream following maternal deprivation. European Journal of Histochemistry, 55(4), e33. https://doi.org/10.4081/ejh.2011.e33
- Livneh, Y., Adam, Y., & Mizrahi, A. (2014). Odor processing by adult-born neurons. Neuron, 81(5), 1097–1110. https://doi.org/10.1016/j.neuron.2014.01.007
- Lledo, P. M., & Saghatelyan, A. (2005). Integrating new neurons into the adult olfactory bulb: Joining the network, life-death decisions, and the effects of sensory experience. Trends in Neurosciences, 28(5), 248–254. https://doi.org/10.1016/j.tins.2005.03.005
- Lois, C., & Alvarez-Buylla, A. (1994). Long-distance neuronal migration in the adult mammalian brain. Science, 264(5162), 1145–1148. https://doi.org/10.1126/science.8178174
- Luskin, M. B. (1993). Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron, 11(1), 173–189. https://doi.org/10.1016/0896-6273(93)90281-u
- Magavi, S. S., Mitchell, B. D., Szentirmai, O., Carter, B. S., & Macklis, J. D. (2005). Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(46), 10729–10739. https://doi.org/10.1523/JNEUROSCI.2250-05.2005
- Marais, L., van Rensburg, S. J., van Zyl, J. M., Stein, D. J., & Daniels, W. M. (2008). Maternal separation of rat pups increases the risk of developing depressive like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neuroscience Research, 61(1), 106–112. https://doi.org/10.1016/j.neures.2008.01.011
- Matsuoka, M., Yokosuka, M., Mori, Y., & Ichikawa, M. (1999). Specific expression pattern of Fos in the accessory olfactory bulb of male mice after exposure to soiled bedding of females. Neuroscience Research, 35(3), 189–195. https://doi.org/10.1016/s0168-0102(99)00082-6
- Ming, G. L., & Song, H. (2011). Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron, 70(4), 687–702. https://doi.org/10.1016/j.neuron.2011.05.001
- Moreno, M. M., Linster, C., Escanilla, O., Sacquet, J., Didier, A., & Mandairon, N. (2009). Olfactory perceptual learning requires adult neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 106(42), 17980–17985. https://doi.org/10.1073/pnas.0907063106
- Mori, K., von Campenhause, H., & Yoshihara, Y. (2000). Zonal organization of the mammalian main and accessory olfactory systems. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 355(1404), 1801–1812. https://doi.org/10.1098/rstb.2000.0736
- Nishi, M., Horii-Hayashi, N., Sasagawa, T., & Matsunaga, W. (2013). Effects of early life stress on brain activity: Implications from maternal separation model in rodents. General and Comparative Endocrinology, 181, 306–309. https://doi.org/10.1016/j.ygcen.2012.09.024
- Nunez-Parra, A., Pugh, V., & Araneda, R. C. (2011). Regulation of adult neurogenesis by behavior and age in the accessory olfactory bulb. Molecular and Cellular Neurosciences, 47(4), 274–285. https://doi.org/10.1016/j.mcn.2011.05.003
- Oboti, L., Savalli, G., Giachino, C., De Marchis, S., Panzica, G. C., Fasolo, A., & Peretto, P. (2009). Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. The European Journal of Neuroscience, 29(4), 679–692. https://doi.org/10.1111/j.1460-9568.2009.06614.x
- Petreanu, L., & Alvarez-Buylla, A. (2002). Maturation and death of adult-born olfactory bulb granule neurons: Role of olfaction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(14), 6106–6113. https://doi.org/10.1523/JNEUROSCI.22-14-06106.2002
- Plotsky, P. M., Thrivikraman, K. V., Nemeroff, C. B., Caldji, C., Sharma, S., & Meaney, M. J. (2005). Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 30(12), 2192–2204. https://doi.org/10.1038/sj.npp.1300769
- Portillo, W., Unda, N., Camacho, F. J., Sánchez, M., Corona, R., Arzate, D. M., Díaz, N. F., & Paredes, R. G. (2012). Sexual activity increases the number of newborn cells in the accessory olfactory bulb of male rats. Frontiers in Neuroanatomy, 6, 25. https://doi.org/10.3389/fnana.2012.00025
- Rochefort, C., & Lledo, P. M. (2005). Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. The European Journal of Neuroscience, 22(11), 2863–2870. https://doi.org/10.1111/j.1460-9568.2005.04486.x
- Roque, S., Mesquita, A. R., Palma, J. A., Sousa, N., & Correia-Neves, M. (2014). The behavioral and immunological impact of maternal separation: a matter of timing. Frontiers in Behavioral Neuroscience, 8, 1–10. https://doi.org/10.3389/fnbeh.2014.00192
- Saghatelyan, A., Roux, P., Migliore, M., Rochefort, C., Desmaisons, D., Charneau, P., Shepherd, G. M., & Lledo, P. M. (2005). Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron, 46(1), 103–116. https://doi.org/10.1016/j.neuron.2005.02.016
- Schmidt, M., Enthoven, L., van Woezik, J. H., Levine, S., de Kloet, E. R., & Oitzl, M. S. (2004). The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. Journal of Neuroendocrinology, 16(1), 52–57. https://doi.org/10.1111/j.1365-2826.2004.01123.x
- Schneider, N. Y., Chaudy, S., Epstein, A. L., Viollet, C., Benani, A., Pénicaud, L., Grosmaître, X., Datiche, F., & Gascuel, J. (2020). Centrifugal projections to the main olfactory bulb revealed by transsynaptic retrograde tracing in mice. The Journal of Comparative Neurology, 528(11), 1805–1819. https://doi.org/10.1002/cne.24846
- Smeyne, R. J., Curran, T., & Morgan, J. I. (1992). Temporal and spatial expression of a fos-lacZ transgene in the developing nervous system. Brain Research. Molecular Brain Research, 16(1–2), 158–162. https://doi.org/10.1016/0169-328x(92)90206-q
- Stanton, M. E., Gutierrez, Y. R., & Levine, S. (1988). Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience, 102(5), 692–700. https://doi.org/10.1037//0735-7044.102.5.692
- Suárez, R., García-González, D., & de Castro, F. (2012). Mutual influences between the main olfactory and vomeronasal systems in development and evolution. Frontiers in Neuroanatomy, 6, 50. https://doi.org/10.3389/fnana.2012.00050
- Takahashi, L. K. (2014). Olfactory systems and neural circuits that modulate predator odor fear. Frontiers in Behavioral Neuroscience, 8, 72. https://doi.org/10.3389/fnbeh.2014.00072
- Trinh, K., & Storm, D. R. (2003). Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nature Neuroscience, 6(5), 519–525. https://doi.org/10.1038/nn1039
- Zurawicki, L. (2010). Neuromarketing: Exploring the brain of the consumer (p. 22). Springer Science & Business Media.
