Abstract
The brain-derived neurotrophic factor (BDNF) is involved in the plasticity and development of the central nervous system. Thereby the protein synthesis is highly related to neuronal activity, and its signaling pathways are associated with several substances like, e.g. glucocorticoids, which seem to be able to activate BDNF-Tropomyosin receptor kinase B (TrkB). While there is evidence that acute physical stress can result in advantageous physiological outcomes like an enhanced BDNF level, outcome parameters in response to psychosocial stress are primarily focused on psychological parameters. The existing literature pointing on the impact of acute psychosocial stress on physiological parameters is controversial and differs depending on the species, the stressor used, and the study methodology. It was hypothesized that an acute standardized psychosocial stressor would increase the BDNF level and therefore show beneficial physiological outcome parameters through psychosocial stress. The serum BDNF levels of 32 healthy young males (M = 24.31 years of age, SD = 3.35), who performed the Trier Social Stress Test (TSST), were assessed and compared to a control condition. To prove the stress-generating effect of the TSST, additional cortisol levels were measured. Acute psychosocial stress significantly increased the serum BDNF- and the cortisol-level, whereby no alteration was found during the control. This study expands the rare literature focusing on the effect of an acute standardized psychosocial stressor on the BDNF level in healthy humans, including a control condition. Implications for future studies are being discussed.
Introduction
In the context of neurological processes, the neurotrophic protein brain-derived neurotrophic factor (BDNF) takes on a major role. Its synthesis is highly related to neuronal activity (Thoenen, Citation1991), and signaling pathways are associated with several biologically active isoforms that interact with receptors (Kowiański et al., Citation2018) like the BDNF-Tropomyosin receptor kinase B (TrkB) which can be activated by glucocorticoids (Jeanneteau et al., Citation2008). The neurotrophic protein is involved in brain development and the plasticity of the central nervous system (Katoh-Semba et al., Citation2007; Lu & Gottschalk, Citation2000). Enhanced neurogenesis has been shown to affect several aspects such as learning, memory, and coping with stress (Schoenfeld & Gould, Citation2012). In contrast to glucocorticoid-related TrkB-activation, the chronic presence of enhanced glucocorticoids, as in chronic stress, is associated with neuronal atrophy and reduced BDNF levels (Castrén & Rantamäki, Citation2010; Duman & Monteggia, Citation2006).
Existing literature indicates that chronic and acute stress can have impairing effects on human health, e.g. in enhanced circulating markers of inflammation or perturbed synaptic plasticity (Marsland et al., Citation2017; Pittenger & Duman, Citation2008; Shields et al., Citation2016). Besides the aspects of stress-related negative health consequences, a growing amount of evidence is focusing on beneficial responses. The concept of positive stress-related outcomes is described in the Stress-Related Growth Theory (SRGT, Park, Citation2013; Park et al., Citation1996). It proposes that individuals develop beneficial outcomes as a result of dealing with stressful or traumatic psychosocial experiences which enable them to cope better and become more resistant to future stressors (Bi et al., Citation2016; Park et al., Citation1996; Tomich & DiBlasio, Citation2020). Until now, this stress-related growth (SRG) is mainly measured in psychological outcomes/parameters such as self-concept, social relationship, or coping skills (Bi et al., Citation2016; Park, Citation2013). Physiological outcomes, such as an increased BDNF-level, are primarily identified in response to acute physical stress. Thereby the beneficial effect seems to depend on the intensity of the stressor (Rojas Vega et al., Citation2006; Szuhany et al., Citation2015; Zimmer et al., Citation2015). It may be possible that the rising amount of cortisol during physical stress (Wahl et al., Citation2013) and its ability to support neuronal activity (Jeanneteau & Chao, Citation2013), and with that an enhanced BDNF expression (Thoenen, Citation1991), takes a prominent role.
Up to now, less is known about the advantageous physiological outcomes in response to psychosocial stress. Existing investigations differ depending on the species, the stressor used, the induction time, and the time of blood sampling (Fanous et al., Citation2010; Linz et al., Citation2019; Murakami et al., Citation2005; Rage et al., Citation2002; Rasmusson et al., Citation2002; Sharma et al., Citation2017). In animal models, studies show an increased BDNF protein expression and mRNA after social defeat stress (Fanous et al., Citation2010) and immobilization (Rage et al., Citation2002). However, Murakami et al. (Citation2005) and Rasmusson et al. (Citation2002) identified a BDNF mRNA decrease in rats after acute stress. Thereby the authors used restraints (Murakami et al., Citation2005) and electrical stress via footshocks (Rasmusson et al., Citation2002). Even though the applicability of animal studies onto humans is limited (Zimmer et al., Citation2015), existing animal models also strengthen the link between stress and BDNF (Fanous et al., Citation2010; Rage et al., Citation2002). In human models, acute social stress (timed math and cold pressor test) led to a reduced brain-derived neurotropic factor in subjects identified as social drinkers (Sharma et al., Citation2017). In contrast, investigations using a standardized psychosocial stressor (Trier Social Stresstest–TSST) in healthy participants were able to identify increased serum BDNF levels (Linz et al., Citation2019; Meng et al., Citation2011). Although human investigations provide the connection between psychosocial stress and BDNF (Linz et al., Citation2019; Meng et al., Citation2011), the current research has limitations such as the lack of control condition or the chosen blood sampling. Based on the available literature we recommend examinations with a control condition, close-meshed sampling, and a baseline measurement shortly before the used stressor without potential confounding factors, to get a clear link between acute psychosocial stress and positive physiological outcomes like BDNF.
This study aimed to examine the BDNF level following an acute standardized psychosocial stress test compared to a control condition and continuous sampling in healthy human subjects. Further, we aimed to investigate the relationship between acute psychosocial stress and beneficial physiological outcome parameters which may be seen as an expansion of the SRGT. Against the background of controversial literature, it was hypothesized that acute psychosocial stress, would result in an increased BDNF level and therefore a positive physiological outcome parameter compared to the resting condition.
Methods
Study protocol
The present study was approved by the local Ethical Committee (ethnical commitment number: 129/2015) and was conducted in accordance with the Declaration of Helsinki. Thirty-two male participants were recruited via an online announcement on the homepage of the German Sport University Cologne (DSHS). They read and signed a written informed consent form in order to participate in the present randomized controlled trial. A short medical history check via telephone was used to identify exclusion criteria by asking for mental disorders, acute or chronic somatic disease, intake of psychotropic drugs, vaccinations of any kind during the previous two weeks, exhausting physical exercise during the past 24 hours, being younger than 18 or older than 28, and smoking more than ten cigarettes per day. All subjects were asked to fill out several questionnaires to assess further psychological and physical impairment as possible exclusion criteria. Details are reported in the “Measurements” section. All participants performed an ergometer test on the upright bike with additional spirometry to determine the individual VO2max.
After a successful preliminary investigation including a medical history check, n = 32 participants were randomly assigned and tested in a cross within design: control and exposure to stress group. During the intervention, the subjects had to perform the Trier Social Stress Test (Kirschbaum et al., Citation1993) as an acute standardized psychosocial stressor. In the control condition, the subjects had to rest in a lying position without any external stimulus during the examination. To exclude sequence effects, the interval between intervention and control condition took a minimum of seven days. All the investigations were carried out between 13:00 and 17:00; two hours prior to testing, caloric intake was prohibited, and the two conditions were implemented mostly on consecutive days. Room temperature and humidity were held constant during the examinations. Only male subjects were included to avoid the effects of the menstrual cycle on cortisol (Kudielka et al., Citation2007; McEwen, Citation2017) as well as on BDNF (Carbone & Handa, Citation2013; Pluchino et al., Citation2013).
An intravenous catheter was used for each subject to obtain blood samples before and after the stressor. Between the application of the catheter and the first blood sample, the subjects rested for 60 minutes in the horizontal position without any external stimuli. In total, three BDNF blood samples were taken: one close to the stressor (pre = 1 min before stress induction) (T0), and two after (post) stress induction (+1min (T1); +5min (T2)). Upon request, subjects were told that they have to participate in a mental load situation and that they’ll get further information in close proximity to the test. Explicit TSST instructions were given after T0 to minimize possible anticipatory stress effects. There was a 15-minute interval between the T0 and T1. During the control condition, the participants remained in a horizontal position for 60 minutes without any external stimulus. After waiting, the two blood samples were taken (+1min (T1); +5min (T2))–according to the stress induction protocol. To verify the stress-generating effect of the TSST, cortisol levels were measured before (T0) and after stressor ((T1), (T2), +10min(T3)).
Eight ml of venous blood were collected for every sample by a Monovette-system (Sarstedt AG & Co., Nümbrecht, Germany) after flushing the Monovette-system with approx. 1 ml of a sodium chloride solution and a waiting period of one minute between flush and blood-taking. The flush was used to minimize a platelets build-up in the catheter which might influence the measurements of the BDNF (Fujimura et al., Citation2002).
A power analysis using “G*Power” v.3.1.9.2 (by Franz Faul, University of Kiel, Germany) showed that expecting an effect size of Cohen’s f = 0.30 and using an ANOVA for repeated measures as a statistical test for proving within-between interactions with two tests, a significance level of α = 0.05, and a power of 80% showed that a total sample size of min. n = 18 subjects was needed.
Psychosocial Stress–Trier Social Stress Test (TSST)
The TSST is a frequently used standardized procedure for inducing high levels of acute psychosocial stress in laboratory settings (Kudielka et al., Citation2007). The stressor is a social-evaluative situation which includes a 5-min preparation period, a 5-min mock job interview, and a subsequent 5-min arithmetic task in front of a two-person panel. The interview is sound- and video- recorded (Kirschbaum et al., Citation1993). The subjects were told to try to convince the panel that they were the perfect person for the potential job. The two-person panel was introduced as being trained in understanding nonverbal behavior and were told not to stimulate the subjects positively, e.g. by nodding in a friendly way. The approx. 15 min. of testing were performed in a standing upright position.
Measurements
Preliminary evaluation–physical aspects
Body mass index (BMI) was calculated by using the subject’s weight and height and is a simple way for assessing overweight. While humans are classified as overweight at a BMI of 25.0 or more, the healthy BMI range is 18.5 to 24.9 (BMI = kg/m2) (Cole et al., Citation1995).
The waist-to-hip ratio is calculated as waist measurement divided by hip measurement and is an indicator for several health aspects. Normal weight is indicated by values < 0.90 for men (Molarius et al., Citation1999). An ergometer test on an upright bike was performed with additional spirometry to determine the individual maximum rate of oxygen consumption (VO2max). The step-test protocol used (Hollmann-Venrath) starts at 30 W and increases automatically by 40 W every three minutes until volitional exhaustion is reached (Hollmann et al., Citation1956). Hereby, the participants cycled between 60 and 80 revolutions per minute (rpm).
Preliminary evaluation–questionnaires
(1) The Symptom-Check-List-90-R consists of 90 items with a five-point rating scale from 0-“Not at all” to 4-“Extremly” (SCL-90-R; Derogatis, Citation1977). Cronbach’s alpha = 0.920. (2) Beck Depression Inventory consisting of 21 symptoms rated for intensity from 0 to 3 with question-dependent answers (BDI; Beck & Beamesderfer, Citation1974). Cronbach’s alpha = 0.613. (3) Perceived Stress Scale consisting of 14 items rated on a 5-point Likert scale from 0-“Never” to 4-“Very often” (PSS; Cohen et al., Citation1983). Cronbach’s alpha = 0.368. And (4) the State-Trait Anxiety Inventory Form X2 consisting of 20 items with a 4-point rating scale from 0-“Almost never” to 3-“Almost always” (STAI-X2; Laux et al., Citation1981). Cronbach’s alpha = 0.617. (5) Self-created socio-demographic questionnaire with the categories “Personal details,” “Ethnicity,” “Family status,” and “Education and Job-status.” (6) Self-created short medical history check.
Blood-analytics
After remaining for a minimum of 10 min at room temperature for the deactivation of coagulation factors, the blood samples were centrifuged for 10 min at 3400 rpm and 20 °C (Rotanta 460 R, Hettich Zentrifugen, Tuttlingen, Germany). The serum was analyzed by ELISA (Human Free BDNF Quantikine ELISA Kit–R&D Systems Europe, Ltd. Abingdon, United Kingdom). Due to BDNF gets released rapidly from platelets into serum at room temperature (Gejl et al., Citation2019), the authors suggest an overall blood BDNF measurement, consisting of plasma and platelets BDNF in this analysis.
Statistical Analyses
Data analyses were carried out using SPSS v. 24 (SPSS Inc., Chicago, IL, USA). Outliers were identified as values under and above 1.5 times the interquartile range (Walfish, Citation2006). In total, 7 sample points reached these criteria and were replaced by using multiple imputations with 10 data points.
The data were tested for normal distribution. Baseline values before the stressor/waiting period were compared with a t-test. An analysis of variance (ANOVA) for repeated measures with post-hoc testing (Bonferroni) was performed to analyze time-dependent changes while time was used as the within-subject factor. An ANCOVA was performed to analyze the test order and the VO2max as a covariate. Pearson’s partial correlation was carried out to specify the interrelationship between BDNF and the VO2max as well as BDNF and cortisol. Statistical significance was considered to be p < .05. Imputations did not affect the level of significance.
Results
Sample
Thirty-two healthy young men (age: M = 26.34, SD = 3.46) were recruited. Detailed subject characteristics are shown in .
Table 1. Characteristics of the study sample (N = 32, male 100%).
Cortisol
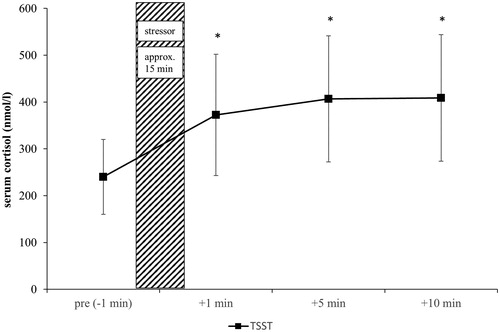
The TSST led to a highly significant increase over time (F(1.21, 37.47) = 79.68, p < .001, ηp2 = 0.72) with confirmation via post hoc analysis from pre to +1 min, +5 min and +10 min (all ps < 0.001) and, by that, proved its stress-generating effect ().
Figure 1. Cortisol timesheet of 32 healthy, male subjects related to the use of a psychosocial stressor. *Significant vs. baseline (pre stressor). Shown are the mean values with standard errors. Time information is related to the use of stress. Coarse hatching: stress induction; black squares: TSST.
BDNF
No significant difference was found while comparing the baseline-values (TSST pre vs. control +1min) (t(31) = 1.19, p > .05). While the TSST led to a highly significant increase in BDNF over time (F(1.88, 58.35) = 10.58, p < .001, ηp2 = 0.25) with confirmation via post hoc analysis from pre to +1 min (p < .001) (from pre to +5 min, p = .20), BDNF during control condition showed no significant increase over time (F(2.15, 66.59) = 2.56, p = .08) (from pre to +1 min, p = .73; from pre to +5 min, p = .75). Using the test/condition order as a covariate to measure possible sequence effects, showed no significant effect (F(1,30) = 0.01, p = .99).
A highly significant Pearson’s correlation between the BDNF-values (+1) and VO2max was found (r = 0.570, p = .001). Using the VO2max as a covariate revealed a non-significant trend (F(1.86, 55.88) = 2.89, p = .067). No significant Pearson’s correlations between BDNF and cortisol were measured: Pre/-1min: r = 0.178, p = 0.33; +1min: r = 0.205, p = .26; +5min: r = 0.285, p = .11) ().
Figure 2. BDNF timesheet of 32 healthy, male subjects related to the use of a psychosocial stressor and control condition. *Significant vs. baseline (pre stressor). Shown are the mean values with standard errors. Time information is related to the use of stress. Coarse hatching: stress induction/waiting period; black squares: TSST; gray triangles: control condition.
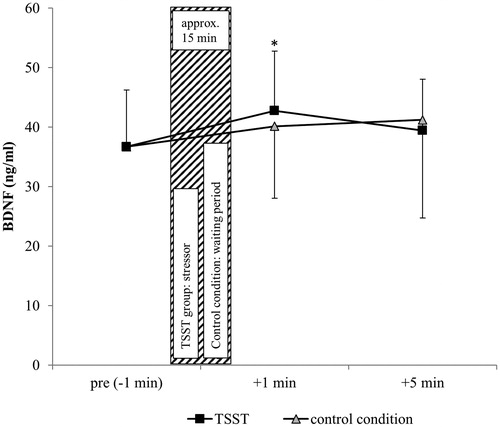
Analyzing within-subject differences in the BDNF course, significant differences were measured only in the control condition (p = .049). Detailed results concerning BDNF statistics are displayed in .
Table 2. Statistics of the within-subject differences in BDNF (N = 32).
Discussion
The present study was able to show significantly increased BDNF levels as a result of an acute standardized psychosocial stressor in healthy humans. By using a controlled trial, close-meshed sampling, and a baseline measurement shortly before stressor, we were able to show that, next to acute physical stress, even acute psychosocial stress can generate positive physiological outcomes. Against the background of existing BDNF-stress-literature (Linz et al., Citation2019; Meng et al., Citation2011; Sharma et al., Citation2017), this analysis is the first using a control condition as well as a baseline sample close to stressor. Since the findings regarding the SRGT were mainly based on psychological outcome parameter, the present results might be seen as an expansion of the theory.
Even though previous investigations provide evidence for a stress-induced increase of BDNF (Linz et al., Citation2019; Meng et al., Citation2011; Sharma et al., Citation2017) several limitations exist. Meng et al. (Citation2011) used a small sample size of ten healthy males with no additional control condition (resting condition without stress). By this, the results might be affected by outliers and the reported evidence by the absence of a controlled approach. Linz et al. (Citation2019) currently reported a well-powered investigation providing the psychosocial stress-related increase of BDNF in healthy humans. Because of their chosen sample points, some confounding factors are still not clear. The authors only used three sample points throughout an examination period of 110 minutes: 50 min pre-stressor, 15 min, and 60 min post-stressor. Due to the rare sampling, the BDNF course does not get reflected comprehensively, and a more frequented sampling would allow a better understanding of the BDNF course. With the chosen blood sample close to arrival rather than close to the stressor, potential stress during or shortly before arrival (e.g. physical activity by running or climbing the stairs or emotional stress e.g. by phone calls), as well as potential confounding factors in the 50 min after baseline sampling, cannot be ruled out. Like Meng et al. (Citation2011), also Linz and colleagues did not use an additional control condition (resting/no stress). Previous, as well as our results, provide evidence for an acute psychosocial stress-induced BDNF increase in healthy humans. By analyzing within-subject differences in the BDNF course, significant effects were found only in the control condition. Since there is evidence for a diurnal rhythm in BDNF (Begliuomini et al., Citation2008), it might be possible that BDNF differs even in a short period of time when no stressor is applicated. Existing literature indicates a diurnal rhythm in BDNF by providing BDNF values at different times of the day (Begliuomini et al., Citation2008; Piccinni et al., Citation2008) but without any course measurement. It remains unclear if the within-subject differences in the control condition, measured in this study, is a physiological course or subject-dependend effect. Further investigations might focus on the BDNF-dynamic of healthy humans in a short period of time to provide a better understanding BDNF variation.
Even though glucocorticoids, such as the stress hormone cortisol, can have impairing effects on the neurogenesis (Schoenfeld & Gould, Citation2012) and the BDNF metabolism (Linz et al., Citation2019; Murakami et al., Citation2005) and although the stressor used showed its cortisol/stress-generating effect, the brain-derived neurotrophic factor increased. Thereby no significant correlation between BDNF and the glucocorticoid was found. While existing literature indicates a functional interaction of these two parameters (Jeanneteau & Chao, Citation2013; Numakawa et al., Citation2017) the underlying dynamic is still not clear. By the fact that BDNF-receptors can be activated by glucocorticoids, the impairment to the BDNF metabolism seems to be related to the duration of the exposure time (Jeanneteau & Chao, Citation2013). While long-lasting enhanced cortisol-levels as in chronic stress and in a psychological disorder like depression, are in line with reduced BDNF levels (Pittenger & Duman, Citation2008; Schaaf et al., Citation2000), repeated/interrupted acute stress showed enhanced BDNF protein and mRNA in several brain regions of rats 2 h and 28 days after social defeat stress (Fanous et al., Citation2010). Cortisol, on the other hand, decreases when it comes to repeated application of psychosocial stress in humans (Petrowski et al., Citation2012). The underlying mechanisms are still not cleared and might be connected to the BDNF metabolism. As glucocorticoids can have destructive effects on the nervous system related to the exposure time (Jeanneteau & Chao, Citation2013), possible habituation and neuroprotective effects via BDNF increase (Jeanneteau et al., Citation2008) and cortisol decrease after repeated stress are conceivable. However, it is discussable if this short- to medium-term growth has any sustained growth effects. It might be possible that the short-term BDNF increase, measured in this study, is a kind of cortisol-counterpart generated to protect the nervous system from the upcoming glucocorticoids and their possible negative impact on the cell proliferation and differentiation (Anacker et al., Citation2013; Numakawa et al., Citation2017). This raises the question of the needed stressor-characteristics which separates a BDNF increase, and thereby the protective effect, from a BDNF decrease like in psychological disorders (Numakawa et al., Citation2017; Pittenger & Duman, Citation2008). The measured serum BDNF level vanished within minutes after stress induction ended, which is in line with Rojas Vega et al. (Citation2006). Without having examined the following cascades related to the BDNF increase, it remains speculative if this short-term BDNF increase is enough to generate beneficial effects known for BDNF (Numakawa et al., Citation2017). If repeated stress leads to a cortisol decrease, as shown by Petrowski et al. (Citation2012), and a BDNF increase which provides more than protective pathways, short-lasting and repeated stress induction might be used for neurogenesis and neuronal plasticity. Besides, our stress induction was performed just once, daily life stress might be at multiple times and might result in multiple short-term BDNF increases. If applicable, this mechanism could be seen as an aspect of stress-related growth, defined as positive outcomes (in this case: cell proliferation/differentiation) resulting from stress (Bi et al., Citation2016).
Since it is not possible and may even not useful to eliminate all forms of daily life stress, the link to resilience training and its goal of increasing regulatory flexibility regarding stress and therefore increasing mental and physical health (Bennett et al., Citation2018), seems to be of interest. If repeated acute stress, physically or psychosocial (Petrowski et al., Citation2012; Wahl et al., Citation2013), leads to positive regulatory mechanisms/stress-related growth, current resilience training concepts may pay attention to this. Also, the need for regeneration phases after acute psychosocial stress to generate stress-related growth but avoid chronic stress conditions becomes more relevant.
The use of other stress inductions, such as the dexamethasone-corticotrophin-releasing-hormone (DEX-CRH) test with an injection of the human corticotrophin-releasing hormone (CRH) (Kirschbaum et al., Citation1992; Petrowski et al., Citation2012), or a cold pressor test (CPT–brief injection) as well as a socially evaluated cold pressor test (SECPT) (Schwabe et al., Citation2008), increase the cortisol amount and may have heterogeneous effects on the BDNF metabolisms. This raises the question of what kind of stress and stress intensity is needed to generate stress-related growth in healthy as well as, e.g. subjects with chronic stress. Follow-up investigations might compare different stress stimuli regarding their growth potential.
The present examination showed a highly significant and medium-high correlation between the fitness level and the BDNF-values. Based on Selye (Citation1981) and his concept of stress, the cross-stressor-adaptation-(CSA) hypothesis was set up. It implies that physical activity might be seen as a stressor leading to appropriate adaptation processes. Accordingly, the stress reactivity of athletic individuals should also be less pronounced in nonphysical stressors (Kobasa et al., Citation1982; Sothmann et al., Citation1996). While high-intensity interval training results in a significantly increased BDNF-level (Zimmer et al., Citation2015), it may be possible that there is a link to the CSA-hypothesis: physically fit individuals may benefit not only by having a better stress reactivity in nonphysical stress conditions, they may also profit from generating more BDNF than unfit individuals.
However, the present study has some limitations. The authors were able to report the measured effects only for a small number of young, healthy Caucasian men. As McEwen (Citation2017) reported differences depending on the sex in neural remodeling after chronic stress and Carbone and Handa (Citation2013) as well as Pluchino et al. (Citation2013), pointed out gonadal steroid-dependent BDNF differences, the transfer of these differences to young, healthy (Caucasian) women might be of interest as well. To ensure the replicability of this effect, further studies should recruit an adequately-sized (Naegelin et al., Citation2018) and more diverse sample size in general, like Linz et al. (Citation2019) but pay attention to methodological aspects listed in this manuscript. Next, the present study was able to show the effects solely related to psychosocial stress. Other kinds of stress, which also increase the cortisol level, might have similar effects to the BDNF level but are more suitable for daily use.
Even though we used a close-meshed sampling after stressor, an enlarged assessment with more than three sample points would have been beneficial to enable a better view of the BDNF dynamics. While having the advantage of a control condition in a lying position, this study provides the BDNF course without any external stimulus. Against the background that physical activity (Zimmer et al., Citation2015) can influence the BDNF level, a control condition with an activity level comparable to the TSST condition might be useful to verify the stress-related BDNF increase and exclude a possible increase through more physical demand.
Although we tried to minimize anticipatory pre-stress effects during the TSST group with a communication model which showed reduced baseline cortisol level close to the stressor (Petrowski et al., Citation2012), it remains speculative if scientific investigations without any anticipatory stress in general (and concomitant changes in parameters) are possible.
There are other implications for future studies: enlarged sample size and sampling in combination with the VO2max/the fitness level as a covariate should be used to verify the link between the BDNF and physical fitness. Therefore, the authors recommend an objective method, such as the VO2max via spirometry, for measuring the activity level. Also, the focus on other growth factors, such as the vascular endothelial growth factor (VEGF) or the insulin-like growth factor 1 (IGF-1) in the same human sample group, should expand the effects on physiological parameters for further SRGT- and CSA-hypothesis-testing.
Geolocation Information
Europe–Germany–Cologne.
Authors' contribution
K. Petrowski and R. Hermann designed the study, supervised the data collection and worked out the statistis while A. Schaller, W. Bloch and C. Albus assisted and advised. D. Lay carried out the medical examinations and W. Bloch did the blood analytics. All the authors interpreted the data and contributed to the intellectual content.
Informed consent
Informed consent was obtained from the individual participants included in the study.
Health and safety
The authors confirm that all mandatory laboratory health and safety procedures have been complied with the course of conducting any experimental work reported in this paper.
Financial support
This research did not receive any specific grant from funding agencies of the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors would like to thank all the participants in the study. A special thanks goes to Ms. Elisabeth Orrison for proofreading this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anacker, C., Cattaneo, A., Musaelyan, K., Zunszain, P. A., Horowitz, M., Molteni, R., Luoni, A., Calabrese, F., Tansey, K., Gennarelli, M., Thuret, S., Price, J., Uher, R., Riva, M. A., & Pariante, C. M. (2013). Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, 110(21), 8708–8713. https://doi.org/10.1073/pnas.1300886110
- Beck, A., & Beamesderfer, A. (1974). Assessment of depression: The depression inventory. In P. Pichot & R. Oliver-Martin (Eds.), Psychological Measurements in Psychopharmacology (pp. 151–169). https://doi.org/10.1159/000395074
- Begliuomini, S., Lenzi, E., Ninni, F., Casarosa, E., Merlini, S., Pluchino, N., Valentino, V., Luisi, S., Luisi, M., & Genazzani, A. R. (2008). Plasma brain-derived neurotrophic factor daily variations in men: Correlation with cortisol circadian rhythm. Journal of Endocrinology, 197(2), 429–435. https://doi.org/10.1677/JOE-07-0376
- Bennett, J. M., Rohleder, N., & Sturmberg, J. P. (2018). Biopsychosocial approach to understanding resilience: Stress habituation and where to intervene. Journal of Evaluation in Clinical Practice, 24(6), 1339–1346. https://doi.org/10.1111/jep.13052
- Bi, X., Proulx, J., & Aldwin, C. M. (2016). Stress-related growth. In Encyclopedia of Mental Health (2nd ed., pp. 244–248). https://doi.org/10.1016/B978-0-12-397045-9.00052-5
- Carbone, D. L., & Handa, R. J. (2013). Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience, 239, 295–303. https://doi.org/10.1016/j.neuroscience.2012.10.073
- Castrén, E., & Rantamäki, T. (2010). The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental Neurobiology, 70(5), 289–297. https://doi.org/10.1002/dneu.20758
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Cole, T. J., Freeman, J. V., & Preece, M. A. (1995). Body mass index reference curves for the UK, 1990. Archives of Disease in Childhood, 73(1), 25–29. https://doi.org/10.1136/adc.73.1.25
- Derogatis, L. (1977). Administration, scoring, and procedures manual for the SCL-90-R. Clinical Psychometrics Research.
- Duman, R. S., & Monteggia, L. M. (2006). A neurotrophic model for stress-related mood disorders. Biological Psychiatry, 59(12), 1116–1127. https://doi.org/10.1016/j.biopsych.2006.02.013
- Fanous, S., Hammer, R. P., & Nikulina, E. M. (2010). Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience, 167(3), 598–607. https://doi.org/10.1016/j.neuroscience.2010.02.064
- Fujimura, H., Altar, C. A., Chen, R., Nakamura, T., Nakahashi, T., Kambayashi, J-i., Sun, B., & Tandon, N. N. (2002). Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thrombosis and Haemostasis, 87(4), 728–734. https://doi.org/10.1055/s-0037-1613072
- Gejl, A. K., Enevold, C., Bugge, A., Andersen, M. S., Nielsen, C. H., & Andersen, L. B. (2019). Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Scientific Reports, 9(1), 1–9. https://doi.org/10.1038/s41598-019-45976-5
- Hollmann, W., Venrath, H., & Tietz, N. (1956). Range of oxygen absorption values in spiro-ergometric studies. Zeitschrift fur Kreislaufforschung, 45(3–4), 95–99. http://www.ncbi.nlm.nih.gov/pubmed/13325696
- Jeanneteau, F., & Chao, M. V. (2013). Are BDNF and glucocorticoid activities calibrated? Neuroscience, 239, 173–195. https://doi.org/10.1016/j.neuroscience.2012.09.017
- Jeanneteau, F., Garabedian, M. J., & Chao, M. V. (2008). Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proceedings of the National Academy of Sciences of the United States of America, 105(12), 4862–4867. https://doi.org/10.1073/pnas.0709102105
- Katoh-Semba, R., Wakako, R., Komori, T., Shigemi, H., Miyazaki, N., Ito, H., Kumagai, T., Tsuzuki, M., Shigemi, K., Yoshida, F., & Nakayama, A. (2007). Age-related changes in BDNF protein levels in human serum: Differences between autism cases and normal controls. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 25(6), 367–372. https://doi.org/10.1016/j.ijdevneu.2007.07.002
- Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1-2), 76–81. https://doi.org/119004 https://doi.org/10.1159/000119004
- Kirschbaum, C., Wüst, S., Faig, H. G., & Hellhammer, D. H. (1992). Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. The Journal of Clinical Endocrinology and Metabolism, 75(6), 1526–1530. https://doi.org/10.1210/jcem.75.6.1464659
- Kobasa, S. C., Maddi, S. R., & Puccetti, M. C. (1982). Personality and exercise as buffers in the stress-illness relationship. Journal of Behavioral Medicine, 5(4), 391–404. https://doi.org/10.1007/BF00845369
- Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., & Moryś, J. (2018). BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 38(3), 579–593. https://doi.org/10.1007/s10571-017-0510-4
- Kudielka, B. M., Hellhammer, D. H., & Kirschbaum, C. (2007). Ten years of research with the Trier Social Stress Test – revisited. In Social neuroscience: Integrating biological and psychological explanations of social behavior. (pp. 56–83). Guilford Press.
- Laux, L., Glanzmann, P., Schaffner, P., & Spielberger, C. (1981). The state-trait anxiety inventory. Göttingen, Bern Hogrefe, 5(3 & 4). Retrieved from https://scholar.google.de/scholar?hl=de&q=Laux%252C+L.%252C+Glanzmann%252C+P.%252C+Schaffner%252C+P.%252C+%2526+Spielberger%252C+D.+%25281970%2529.+Das+State-Trait-Angstinventar+%2528STAI%2529.+Göttingen%253A+Beltz.&btnG=&lr=
- Linz, R., Puhlmann, L. M. C., Apostolakou, F., Mantzou, E., Papassotiriou, I., Chrousos, G. P., Engert, V., & Singer, T. (2019). Acute psychosocial stress increases serum BDNF levels: An antagonistic relation to cortisol but no group differences after mental training. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 44(10), 1797–1804. https://doi.org/10.1038/s41386-019-0391-y
- Lu, B., & Gottschalk, W. (2000). Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Progress in Brain Research, 128, 231–241. https://doi.org/10.1016/S0079-6123(00)28020-5
- Marsland, A. L., Walsh, C., Lockwood, K., & John-Henderson, N. A. (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. https://doi.org/10.1016/j.bbi.2017.01.011
- McEwen, B. S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 247054701769232. https://doi.org/10.1177/2470547017692328
- Meng, D., Wu, T. C., Rao, U., North, C. S., Xiao, H., Javors, M. A., & Adinoff, B. (2011). Serum NPY and BNDF response to a behavioral stressor in alcohol-dependent and healthy control participants. Psychopharmacology, 218(1), 59–67. https://doi.org/10.1007/s00213-011-2414-1
- Molarius, A., Seidell, J. C., Sans, S., Tuomilehto, J., & Kuulasmaa, K. (1999). Waist and hip circumferences, and waist-hip ratio in 19 populations of the WHO MONICA project. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 23(2), 116–125. https://doi.org/10.1038/sj.ijo.0800772
- Murakami, S., Imbe, H., Morikawa, Y., Kubo, C., & Senba, E. (2005). Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neuroscience Research, 53(2), 129–139. https://doi.org/10.1016/j.neures.2005.06.008
- Naegelin, Y., Dingsdale, H., Säuberli, K., Schädelin, S., Kappos, L., & Barde, Y. A. (2018). Measuring and validating the levels of brain-derived neurotrophic factor in human serum. Eneuro, 5(2), ENEURO.0419-17.2018. https://doi.org/10.1523/ENEURO.0419-17.2018
- Numakawa, T., Odaka, H., & Adachi, N. (2017). Actions of brain-derived neurotrophic factor and glucocorticoid stress in neurogenesis. International Journal of Molecular Sciences, 18(11), 2312. https://doi.org/10.3390/ijms18112312
- Park, C. L. (2013). The meaning making model: A framework for understanding meaning, spirituality, and stress-related growth in health psychology. European Health Psychologist, 15(2), 40–47. https://www.researchgate.net/profile/Crystal_Park/publication/272955903_Meaning_spirituality_and_health_a_brief_introduction/links/56afa60808ae9c1968b46691.pdf
- Park, C. L., Cohen, L. H., & Murch, R. L. (1996). Assessment and prediction of stress-related growth. Journal of Personality, 64(1), 71–105. https://doi.org/10.1111/j.1467-6494.1996.tb00815.x
- Petrowski, K., Wintermann, G., & Siepmann, M. (2012). Cortisol response to repeated psychosocial stress. Applied Psychophysiology and Biofeedback, 37(2), 103–107. https://doi.org/10.1007/s10484-012-9183-4
- Petrowski, K., Wintermann, G.-B., Kirschbaum, C., & Bornstein, S. R. (2012). Dissociation between ACTH and cortisol response in DEX-CRH test in patients with panic disorder. Psychoneuroendocrinology, 37(8), 1199–1208. https://doi.org/10.1016/j.psyneuen.2011.12.013
- Piccinni, A., Marazziti, D., Del Debbio, A., Bianchi, C., Roncaglia, I., Mannari, C., Origlia, N., Catena Dell’Osso, M., Massimetti, G., Domenici, L., & Dell’Osso, L. (2008). Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: An analysis of sex differences. Chronobiology International, 25(5), 819–826. https://doi.org/10.1080/07420520802387773
- Pittenger, C., & Duman, R. S. (2008). Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 33(1), 88–109. https://doi.org/10.1038/sj.npp.1301574
- Pluchino, N., Russo, M., Santoro, A. N., Litta, P., Cela, V., & Genazzani, A. R. (2013). Steroid hormones and BDNF. Neuroscience, 239, 271–279. https://doi.org/10.1016/j.neuroscience.2013.01.025
- Rage, F., Givalois, L., Marmigère, F., Tapia-Arancibia, L., & Arancibia, S. (2002). Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neuroscience, 112(2), 309–318. https://doi.org/10.1016/S0306-4522(02)00072-6
- Rasmusson, A. M., Shi, L., & Duman, R. (2002). Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 27(2), 133–142. https://doi.org/10.1016/S0893-133X(02)00286-5
- Rojas Vega, S., Strüder, H. K., Vera Wahrmann, B., Schmidt, A., Bloch, W., & Hollmann, W. (2006). Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Research, 1121(1), 59–65. https://doi.org/10.1016/j.brainres.2006.08.105
- Schaaf, M. J. M., De Kloet, E. R., & Vreugdenhil, E. (2000). Corticosterone Effects on BDNF expression in the hippocampus implications for memory formation. Stress, 3(3), 201–208. https://doi.org/10.3109/10253890009001124
- Schoenfeld, T. J., & Gould, E. (2012). Stress, stress hormones, and adult neurogenesis. Experimental Neurology, 233(1), 12–21. https://doi.org/10.1016/j.expneurol.2011.01.008
- Schwabe, L., Haddad, L., & Schachinger, H. (2008). HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology, 33(6), 890–895. https://doi.org/10.1016/j.psyneuen.2008.03.001
- Selye, H. (1981). Geschichte und Grundzüge des Stresskonzepts. In J. Nitsch (Ed.), Stress: Theorien; Untersuchungen; Massnahmen. (pp. 163–187). Huber.
- Sharma, S., Graham, R., Rohde, R., & Ceballos, N. A. (2017). Stress-induced change in serum BDNF is related to quantitative family history of alcohol use disorder and age at first alcohol use. Pharmacology, Biochemistry, and Behavior, 153, 12–17. https://doi.org/10.1016/j.pbb.2016.12.002
- Shields, G. S., Sazma, M. A., & Yonelinas, A. P. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. https://doi.org/10.1016/j.neubiorev.2016.06.038
- Sothmann, M. S., Buckworth, J., Claytor, R. P., Cox, R. H., White-Welkley, J. E., & Dishman, R. K. (1996). Exercise training and the cross-stressor adaptation hypothesis. Exercise and Sport Sciences Reviews, 24, 267–287. https://doi.org/10.1249/00003677-199600240-00011
- Szuhany, K., Bugatti, M., & Otto, M. (2015). A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of Psychiatric Research, 60, 56–64. https://www.sciencedirect.com/science/article/pii/S0022395614002933 https://doi.org/10.1016/j.jpsychires.2014.10.003
- Thoenen, H. (1991). The changing scene of neurotrophic factors. Trends in Neurosciences, 14(5), 165–170. https://doi.org/10.1016/0166-2236(91)90097-E
- Tomich, P. L., & DiBlasio, A. M. (2020). Stress-related growth: An experimental approach to examine whether stressful events cause perceived growth. Current Psychology, 1–8. https://doi.org/10.1007/s12144-020-00721-4
- Wahl, P., Mathes, S., Köhler, K., Achtzehn, S., Bloch, W., & Mester, J. (2013). Acute metabolic, hormonal, and psychological responses to different endurance training protocols. Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones et Metabolisme, 45(11), 827–833. https://doi.org/10.1055/s-0033-1347242
- Walfish, S. (2006). A review of statistical outlier methods. Pharmaceutical Technology, 30(4), 1–5. http://www.pharmtech.com/review-statistical-outlier-methods?id=&pageID=1&sk=&date=
- Zimmer, P., Oberste, M., & Bloch, W. (2015). Einfluss von Sport auf das zentrale Nervensystem – Molekulare und zelluläre wirkmechanismen. Deutsche Zeitschrift für Sportmedizin, 2015(02), 42–49. https://doi.org/10.5960/dzsm.2015.164
