Abstract
During pregnancy, uterus undergoes the environment adaptation as part of a program of development. In the world, one in four people worldwide suffer from mental illness, especially pregnant women. β-Adrenergic receptor (β-AR) is an important regulator that converts environmental stimuli into intracellular signals in mice uterus. CD-1 (ICR) mice undergone restraint stress, which was a case in model to simulate the psychological stress. The plasma and implantation sites in uterus were obtained and examined. PCR analysis demonstrated that β2-AR expression levels in embryo day (E) 3, 5 and 7 were kept at a significantly higher level (p < 0.05) under restraint stress and higher than β1-AR and β3-AR in different gestation ages. The β2-AR protein levels were obviously increased (p < 0.05) due to the markedly elevated norepinephrine (NE) concentration (p < 0.05). In our previous study, restraint stress can induce the apoptosis and inflammation. Also, the matrix metalloprotein-9 (MMP-9) was decreased significantly (p < 0.05) under restraint stress. Meanwhile, Caspase3, p-NF-κB p65 and p-ERK1/2 were obviously increased (p < 0.05) in the work. In vitro studies showed that the p-ERK1/2 and Caspase-3 levels were raised (p < 0.05) after β2-AR was activated. However, they were decreased when PKA was blocked. The protein levels of Caspase-3 were reduced when ERK and NF-κB were blocked (p < 0.05). In conclusion, the β2-AR/cAMP/PKA pathway promoted apoptosis and affected the development of the uterus through the ERK and NF-κB signaling pathway. The findings of this study may provide evidence for female reproduction under psychological stress.
Introduction
One in four people worldwide experience the effects of mental health disorders, including posttraumatic stress disorder (PTSD), obsessive–compulsive disorder (OCD), schizophrenia and depression (Battle, Citation2013; Holmes et al., Citation2014). In particular, pregnant women are susceptible to mental illness such as depression and anxiety in adverse environments (Marquesim et al., Citation2016). Most of the studies on psychological stress during pregnancy involved offspring, focusing on preterm birth, pregnancy loss, fetal growth restriction, and low birthweight (Dunkel Schetter & Tanner, Citation2012) but not the uterus diseases. Liu et al. found that in early pregnancy, maternal uterine antioxidant capacity is decreased under restraint stress (Liu et al., Citation2014). Norepinephrine (NE) is a marker of stress development. Increased circulation of NE, found in the diseased heart as a result of sympathetic nervous system overactivation, is responsible for cell death and oxidative stress (de Lima-Seolin et al., Citation2019). NE and EPI stimulate α (α1A, α1B, α1D, α2A, α2B, and α2C) and β (β1, β2, and β3)-adrenergic receptors (ARs) with differing affinities (Madden, Citation2003). Additionally, Chen et al. found that the mRNA expression of β2-AR is much higher than that of other subtypes in the uterus of E4 mice (Chen et al., Citation2011).
β2-AR activates cAMP (cyclic adenosine monophosphate), a second messenger, which activates cAMP-dependent protein kinase A (PKA) (Bruzzone et al., Citation2014). Meanwhile, the cAMP/PKA pathway induces apoptosis in cardiac myocytes (Hasegawa et al., Citation2001). In our previous studies, restraint stress was shown to disturb the adaptive reconstruction of the endometrium during implantation in maternal mice (Liu, et al., Citation2015) and to be associated with an imbalance between cell proliferation and apoptosis (Liu et al., Citation2014, Citation2015). β2-AR was shown to activate pro-survival kinases and attenuate mitochondrial dysfunction during oxidative stress (Fajardo et al., Citation2011). However, a study showed that the activation of β2-AR is detrimental to cardiac and skeletal muscles through the induction of apoptosis (Burniston et al., Citation2005).
Chronic intermittent hypoxia (CIH) can markedly increase the β2-AR/β1-AR ratio and the phosphorylation of ERK1/2 (extracellular regulated protein kinases 1/2) and p38 (Micova et al., Citation2016). Additionally, the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, including JNK and ERK, promotes cell apoptosis (by promoting proteins including Caspase-9 and Caspase-3) (Qian et al., Citation2015). Activated Caspase-3 can induce mitochondrial apoptosis in cells. The release of cytochrome c may depend on a reduction in matrix metalloproteinase 9 (MMP-9) (Zhang et al., Citation2013). Meanwhile, oxidative stress can inhibit the expression of MMP-9 to prevent the progression of colon cancer (Zhang et al., Citation2020). In our previous study, oxidative stress induced by restraint stress was shown to lead to immune dysfunction and apoptosis in uterus (Liu et al., Citation2014). Also, there is a study that shows that oxidative stress might increase the expression of proinflammatory through the NF-κB pathway (Liu et al., Citation2018).
All in all, the mechanism by which restraint stress-induced oxidative stress disturbs the balance of the uterine microenvironment remains unclear. Thus, we hypothesize that β2-AR plays an important role in the proliferation/apoptosis disorders and imbalance between oxidants/antioxidants caused by restraint stress during early pregnancy.
Materials and Methods
Animal treatments
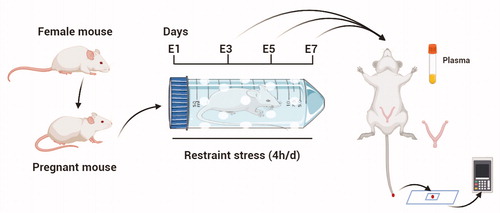
A total of 250 female and 80 male ICR mice (8 weeks of age; Vital River Laboratory Animal Technology Co. Ltd., Beijing, China) were housed under conventional conditions (at a relative humidity of 50 ± 10%, a temperature of 21 ± 1 °C) on a regular 10-h dark: 14-h light cycle (with lights on at 7:00 A.M.). First, the mice were maintained in a room for a week to allow them to adapt to the environment. Estrous mice were placed with a sexually experienced male at night, and females with vaginal sperm plugs the following morning were considered to be at E1 (Embryonic 1d) (). Female mice were individually placed into ventilated transparent plastic centrifuge tubes (they were able to breathe freely but unable to escape) to limit their movements for 4 h (from 8:00 A.M. to 12:00 P.M.) on E1. Because of the limitation of the restraint tubes, the stressed mice temporarily had no access to water or food. The control pregnant females were left untreated in their home cages, from which water and food were also temporarily removed for 4 h. During night, mice's food and water intake increase, so deprivation of food and water during the day will not cause stress to mice (Yokoyama et al., Citation2013). At the end of the restraint stress, the mice were returned to their home cages and allowed access to food and water ad libitum. The method was used by citing the reference (Liu et al., Citation2014). Restraint stress was initiated on E1 and continued until the mice were sacrificed on E3 (Embryonic day 3), E5 (Embryonic day 5) and E7 (Embryonic day 7) (). The weights of the mice were measured and recorded before and after restraint stress every day. All animal procedures were approved by the China Agricultural University Institutional Animal Care and Use Committee (AW18079102-3-2).
Figure 1. Schematic representation of the animal model used in this study. Estrous mice were placed with a sexually experienced male at night, and females with vaginal sperm plugs the following morning were considered to be at E1 (Embryonic day 1). Restraint stress was initiated on E1 and continued until the mice were sacrificed on E3 (Embryonic day 3), E5 (Embryonic day 5) and E7 (Embryonic day 7). The plasma and uterus were obtained. There were 200 mice in this study. At least 7 mice were used for the test in each group.
Plasma and tissue preparations
A total of 200 pregnant female mice were anesthetized by 2% pentobarbital (4 mL/kg body weight) on E3, E5 and E7 immediately following restraint stress. Blood glucose levels in the tail blood were measured by a Roche blood glucose meter (GC14906201, ACCU-CHEK Active, Roche, Germany) before sacrifice. Plasma samples were collected to detect NE. Some of the implantation sites of uterine specimens, excluding the embryos, were rapidly dissected and stored at −80 °C for protein analysis and mRNA analysis. Some of the uterine specimens were rapidly obtained and stored at 4% paraformaldehyde for morphological analysis.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted with TRIzol reagent (CW0580, CoWin Biotech Co., Inc., Beijing, China) according to the manufacturer's protocol. The concentration and purity of RNAwas measured with a NanoPhotometer (P330, Implen, Germany). The concentration of RNA was 1500–1800 ng/μL. Then, 2 μg of total RNA were mixed with reverse transcriptase and other reagents from the cDNA was synthesized using GoScript™ Reverse Transcription System (A5001, Promega Biotech Co., Ltd., USA), and the cDNA was diluted 10 times to conduct polymerase chain reactions. Amplification was conducted by mixing 1 μL cDNA, 5 μL 2 × Go Taq Green Master Mix (M7123, Promega, USA), 0.2 μL forward primer, 0.2 μL reverse primer, and 3.6 μL nuclease-free water. Bench Top 100 bp DNA Ladder (G8291, Promega, WI, USA) was used to determine the size of the DNA. The PCR steps were performed as follows: 95 °C for 5 min; 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s; and 72 °C for 10 min. Primers were synthesized by Invitrogen Trading of Shanghai. The following RT-PCR primers were used in these assays: β1-AR (forward:5′-CCTTTCGCTACCAGAGTTTGC-3′; reverse: 5′-CACTTGGGGTCGTTGTAGCAG-3′), β2-AR (forward: 5′-TCACTCAGGAACGGGACGAAG-3′; reverse: 5′-CAGCACACGCCAAGGAGATTATG-3′), β3-AR (forward: 5′-CACGCCGAGACTACAGACC-3′; reverse: 5′ -CAACCAGTTTCGCCCAAG-3′), β-actin (forward: 5′-TGCTGTCCCTGTATGCCTCTG-3′; reverse: 5′-TTGATGTCACG CACGATTTCC-3′). Water served as the negative control. Then, electrophoresis in 2% agarose gels pre-stained with ethidium bromide was performed with the PCR products, and the image were captured by a gel document system (BIO-BEST 200E, SIM, USA) followed by maximum OD (optical density) analysis via Gel-Pro Analyzer 4.5 (Media Cybernetics, Rockville, MD, USA). Relative β-AR gene expression was obtained by normalizing the results to β-actin. At least three tissue samples were included in each group. Each sample was repeated in triplicate.
Measurement of plasma NE
The total plasma NE concentrations were detected by using a mouse NE competitive ELISA (enzyme-linked immunosorbent assay) Kit (CEA907Ge, Uscn Life Science Inc., Wuhan, China). All tests were performed according to the kit’s instructions. The limit of detection for the assay ranged from 6.17 to 5000 pg/mL. Ten plasma samples were included in each group. Each sample was tested in triplicate.
Western blotting
Whole-tissue lysates were prepared from tissue, and the protein concentrations were measured using BCA protein assay reagent (CW0014S, CoWin Biotech Co., Inc., Beijing, China). These proteins were separated by 10%–15% SDS-PAGE, and then electro transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk/0.5% Tween-20 in TBS (TBST) for 2 h at room temperature. The membranes were incubated with anti-MMP-9 antibody (1:500, ABclonal Biotech Co., Ltd, Cambridge, MA, USA), anti-Cleaved-Caspase-3 antibody (1:1000; ABclonal Biotech Co., Ltd, Cambridge, MA, USA), anti-β-actin antibody (1:3000; CW0096, CoWin Biotech Co., Inc., Beijing, China), anti-phospho-ERK1/2 antibody (1:1000; ABclonal Biotech Co., Ltd, Cambridge, MA, USA), anti-ERK1/2 antibody (1:1000; total ERK1/2; ABclonal Biotech Co., Ltd, Cambridge, MA, USA) , anti-phospho-RELA-S536 antibody (1:1000; p-NF-κB p65; ABclonal Biotech Co., Ltd, Cambridge, MA, USA), and anti-ADRB2 antibody (1:1000; β2-AR; ABclonal Biotech Co., Ltd, Cambridge, MA, USA) overnight at 4 °C. Then, the membranes were washed in TBST and incubated with horseradish peroxidase-conjugated donkey anti-mouse IgG (1:5000; CoWin Biotech Co., Inc., Beijing, China) (for MMP-9 and β-actin) or goat anti-rabbit IgG (1:5000; CoWin Biotech Co., Inc., Beijing, China) (for total ERK1/2, p-ERK1/2, p-NF-κB p65, Caspase-3 and β2-AR) for 2 h at room temperature. Immunoblotting was visualized with an ECL Western Blot Kit (1:1; CW0049M, CoWin Biotech Co., Inc., Beijing, China). The bands on the blots were scanned and analyzed by ImageJ (National Institutes of Health, Bethesda, MD, USA). The protein levels were expressed as the IOD of each band, which was normalized to the IOD of the corresponding β-actin band. At least seven tissue samples were included in each group. Each sample was repeated in triplicate.
Immunohistochemistry
The uterus with implantation sites were routinely processed to paraffin sections for immunohistochemistry. 5 μm nonconsecutive transverse sections (the section interval was 25 μm) were mounted onto 10% polylysine-coated slides. Sections were immunohistochemically stained for β2-AR (1:200, ABclonal Biotech Co., Ltd, Cambridge, MA, USA). Briefly, all sections (5 μm) were routinely dewaxed in xylene and rehydrated through graded concentrations of ethanol. All slides were subjected to antigen retrieval by means of a 0.01 M sodium citrate-hydrochloric acid buffer. Tissue sections were washed three times with phosphate-buffered saline (PBS, pH 7.0) for 5 min each time. Endogenous peroxidase activity was blocked with 3% H2O2 for 30 min. Non-specific staining was blocked with 5% normal goat serum for 30 min. The sections were incubated at 4 °C with anti-β2-AR antibody overnight. The next morning, the slides were washed with PBS and incubated sequentially with 1:400 biotin-conjugated goat anti-rabbit IgG (Beijing CoWin Biotech Co., Ltd, Beijing, China) for 2 h. The slides were washed with PBS and incubated sequentially with 1: 400 horseradish peroxidase (HRP)-streptavidin (Beijing CoWin Biotech Co., Ltd, Beijing, China) for 2 h. The immunoreactivity was revealed by diaminobenzidine (DAB) treatment, and nuclei were counterstained with hematoxylin for 30 s. The sections incubated with PBS instead of primary antibody served as negative controls. Images were acquired using an upright DP72 microscope (Olympus, Japan).
Primary culture of endometrium stromal cells (ESCs) and drug treatment
Primary mouse uterine stromal cells from E5 pregnant mouse uterus in control group were isolated as described in a related study (Liu et al., Citation2015). Briefly, uterine tissue from E5 mice was removed from the fat tissue, cut longitudinally, and washed with Hanks’ balanced salt solution (HBSS, containing 100 U/mL penicillin and 100 μg/mL streptomycin). Tissues were cut into small pieces (2-3 mm) and then digested in 0.25% trypsin-0.02% EDTA for 8 min at 37 °C. The tube was shaken gently to remove the endometrial epithelial cells, and the supernatant was discarded. The digested pieces were washed twice in HBSS and incubated in HBSS containing 0.1% collagenase for 40 min at 37 °C, and then the sample was shaken until the supernatant became turbid. At the end of digestion, the reaction was terminated with Dulbecco’s modified Eagle’s medium-F12 medium (DMEM-F12; HyClone, Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum (FBS), and the sample was centrifuged at 450 × g for 10 min. The cell pellet was passed through a nylon tissue sieve (200 nylon mesh per 2.5 cm) and washed twice with serum-free DMEM-F12 medium before the initiation of primary culture. The cell concentration was adjusted, and 2 × 105 cells/well were seeded in 24-well cell plates. The cell plates were placed in a 5% CO2 cell culture incubator at 37 °C, and the cells were cultured for 1 h. The non-adhered cells were discarded, and the cells were further cultured for 24 h.
The supernatant of ESCs that were adherent for 24 h was discarded and replaced with serum-free basal DMEM-F12. After starvation for 6 h, complete DMEM-F12 containing different concentrations of drugs was added, and culture was continued for 24 h. ISO (DL-isoproterenol hydrochloride; a nonselective agonist of β2-AR; I5627, Sigma, USA; 0.1 μM, 1 μM, 10 μM, 50 μM or 100 μM), Butox (Butoxamine hydrochloride; a selective blocker of β2-AR; B1385, Sigma, USA; 0.1 μM, 1 μM, 10 μM or 100 μM), FSK (Forskolin; a cAMP agonist; 66575-29-9, Tocris Bioscience, MN, USA; 1 μM, 10 μM, or 100 μM), H-89 (a selective inhibitor of PKA; #9844, Cell Signaling Technology, MA, USA; 1 μM, 10 μM, or 100 μM), PD98059 (a selective inhibitor of ERK; 167869-21-8, Tocris Bioscience, MN, USA; 1 μM, 10 μM, or 100 μM), PDTC (Pyrrolidinedithiocarbamate ammonium; a specific blocker of NF-κB; 5108-96-3, Tocris Bioscience, MN, USA; 1 μM, 10 μM, or 100 μM) and 0.01% DMSO (Dimethyl sulfoxide; 0231, Amresco, Ohio, USA) were added to the cells. FSK, H-89, PD98059 and PDTC were dissolved in DMSO. After the completion of culture, 5 mg/mL MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; 0793, Amresco, Ohio, USA) was added to each well to a final concentration of approximately 0.025 mg/μL on a clean bench. The cells were cultured in a 37 °C, 5% CO2 cell incubator for 4 h, 10% SDS was added to each well to a final concentration of 5%, the cells were placed in a 37 °C incubator for 2 h to better dissolve the formazan, and the OD value was measured at 570 nm using a microplate reader.
After the optimal drug concentration was determined, cells were cultured with different drug combinations. The groups were named cells (control group), cells + 0.01% DMSO (vehicle group), cells + ISO, cells + Butox + ISO, cells + FSK + ISO, cells + Butox + ISO + FSK, cells + H-89 + ISO + FSK, cells + PD98059 + ISO + FSK and cells + PDTC + ISO + FSK.
Cell proliferation was measured by the MTT assay, and the results were expressed as the stimulation index (test group OD/control group OD). After culturing for 24 h, the cells were collected with protein lysis buffer and subjected to ultrasonic vibration. The supernatant was collected by centrifugation at 14,000 rpm for 10 min, and the protein levels of β2-AR, p-ERK1/2 and Caspase-3 were detected.
Statistical analysis
The data were analyzed with SPSS 18.0 (SPSS Inc., Chicago, IL) as described in a previous study (Olejarz et al., Citation2014). To analyze the effects of restraint stress and gestational age on the experimental data, two-way analysis of variance (ANOVA) followed by Duncan’s post hoc test was performed. The difference between control and stress mice at the same gestational age was evaluated by independent sample t test. The data are expressed as the mean ± SD. *p < 0.05 and #p < 0.01 were used to denote the significance compared with the corresponding control groups. Different uppercase letters represent significant differences in the control group among E3, E5, and E7 (p < 0.05), and different lowercase letters represent significant differences in the stressed group among E3, E5 and E7 (p < 0.05). To analyze the effect of the drugs on ESCs, one-way ANOVA with LSD post hoc test was performed.
Results
Effects of restraint stress on body weight, blood glucose and NE in early pregnant mice
Compared with that of the E3 stress group, weight reduction increased 13.99% (E3 to E5, p = 0.030) and then returned to the E3 levels (). However, two-way analysis of weight loss before and after stress showed that changes in gestational age did not affect weight reduction (F (2,153) = 0.852, p = 0.429). After restraint stress treatment, the reduction in body weight was significantly increased (F (1,153) = 189.269, p < 0.001). Compared with that of the control mice, the weight reduction of E3, E5 and E7 pregnant mice increased by 82.65% (independent sample t test, p < 0.001), 120% (independent sample t test, p < 0.001) and 60.39% (independent sample t test, p < 0.001), respectively, after restraint stress. Therefore, restraint stress aroused the reduction of early pregnancy mice body weight.
Figure 2. Effects of restraint stress on reductions in weight and the concentrations of NE and blood glucose in the plasma of pregnant mice. After restraint stress treatment, body weight reduction was significantly enhanced. Restraint stress treatment caused an increase NE levels and blood glucose levels in plasma. The uppercase letters represent differences in the control group among E3, E5 and E7, and the lowercase letters represent differences in the stress group among E3, E5 and E7 (p < 0.05). *p < 0.05 and #p < 0.01 are used to denote significance compared with the corresponding control groups.
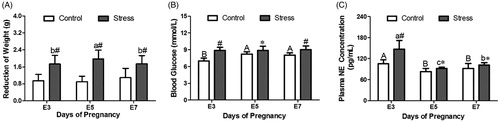
In general, external stress signals activate the HPA (hypothalamus-pituitary-adrenal gland) axis in the body to release the corticosterone (CORT). Also, stress can activate the SAS axis (Sympathetic-Adrenal medulla System axis), which release EPI and NE. In addition, stress increases blood glucose levels in the body. EPI and NE convert external stimuli to biological signals in the body. After restraint stress, the blood glucose levels of pregnant mice increased to some extent. Two-way analysis of blood glucose levels showed that changes in gestational age (F (2,43) = 5.074, p = 0.011) and restraint stress treatment (F (1,43) = 48.415, p < 0.001) caused blood glucose levels increased. Compared with those of the control group, the blood glucose levels of E3, E5 and E7 pregnant mice increased by 26.97% (independent sample t test, p < 0.001), 7.93% (independent sample t test, p = 0.032) and 12.51% (independent sample t test, p = 0.003), respectively, after restraint stress. Compared with those of the E3 control group, the blood glucose levels increased 17.48% (E3 to E5, p = 0.001) and 15.03% (E3 to E7, p < 0.001) with increasing gestation age (). All in all, restraint stress made the blood glucose increase during early pregnancy.
Restraint stress treatment (F (1,43) = 28.485, p < 0.001) affected the plasma NE levels. Compared with those of the control group of the same gestational age, the NE levels of plasma on E3, E5 and E7 increased by 39.49% (independent sample t test, p = 0.002), 11.31% (independent sample t test, p = 0.029) and 11.03% (independent sample t test, p = 0.049), respectively, after restraint stress. Change in gestational age (F (2,43) = 33.759, p < 0.001) also was involved in the change of plasma NE levels. Compared with that of the E3 control group, the NE content decreased by 21.29% (E3 to E5, p = 0.002) and 12.66% (E3 to E7, p = 0.039) as gestational age increased. In the stress group, the trend of NE content in the plasma was consistent with the trend of that in the control group, which was reduced by 37.19% (E3 to E5, p = 0.004) and 30.48% (E3 to E7, p = 0.009) compared with that of the E3 stress group (). Therefore, restraint stress led to the increase of blood glucose and NE, the SAS axis was activated after restraint stress.
Effect of restraint stress on β2-AR levels in early pregnant mice
Although studies have shown that the expression of β2-AR in the uterus is significantly higher than that of β1-AR and β3-AR, the changes in the expression of the three receptors after restraint stress are unclear. As a receptor for NE on target organs, β-AR plays a role as a bridge for the transmission of stress signals. In this study, RT-PCR was used to detect β-AR mRNA levels in the uterus of pregnant mice. Statistical analysis of mRNA expression levels showed that the changes in the expression level of β2-AR mRNA were greater than those of β1-AR mRNA and β3-AR mRNA after restraint stress ().
Figure 3. The relative expression of uterine β1-AR, β2-AR and β3-AR mRNA in pregnant mice. Only β2-AR showed a significant increase after restraint stress during early pregnancy. The uppercase letters represent differences in the control group, and the lowercase letters represent differences in the stress group (p < 0.05). *p < 0.05 and #p < 0.01 are used to denote significance compared with the corresponding control groups.
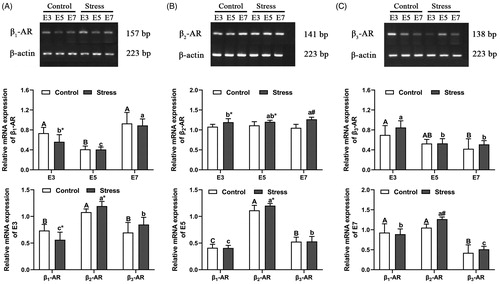
The content of β1-AR mRNA was affected by gestation age (F (2, 48) = 63.956, p < 0.001). Compared with that in the E3 control group, the β1-AR mRNA level decreased by 44.02% (E3 to E5, p < 0.001) and increased by 26.36% (E3 to E7, p = 0.106) as the gestational age increased. The trend of β1-AR mRNA content in the stressed group was consistent with the trend in the control group. Compared with that in the E3 stressed group, the β1-AR mRNA content decreased by 27.40% (E3 to E5, p = 0.036) and increased by 58.36% (E3 to E7, p < 0.001) (). The levels of β1-AR mRNA may not be affected by restraint stress (F (1, 48) = 4.027, p = 0.050429). However, compared with control mice of the same age, the β1-AR mRNA content only in E3 pregnant mice decreased by 23.61% (independent sample t test, p = 0.012) after restraint stress, but not the E5 and E7 (). Therefore, the β1-AR mRNA only was affected by the gestation age but not the restraint stress. The expression of β2-AR mRNA was increased in uterine tissue after restraint stress treatment (F (1, 36) = 39.749, p < 0.001). In addition, β2-AR mRNA content was not affected by gestational age (F (2, 36) = 0.424, p = 0.658). Compared with that in the control mice of the same age, the β2-AR mRNA content increased by 10.60% (independent sample t test, p = 0.010), 7.85% (independent sample t test, p = 0.035) and 20.24% (independent sample t test, p < 0.001) after restraint stress in mice of the three gestational ages (E3, E5 and E7), respectively. Compared with stress group, the β2-AR mRNA content was not significant in E5 but increased 5.86% in E7 (E3 to E7, p = 0.046) (). Therefore, restraint stress induced the increased of β2-AR during early pregnancy. The content of β3-AR mRNA was affected by gestational age (F (2, 31) = 16.291, p < 0.001) but not restraint stress (F (1, 31) = 2.546, p = 0.121). Compared with that in the E3 control group, the β3-AR mRNA content decreased by 24.61% (E3 to E5, p = 0.100) and 39.63% (E3 to E7, p = 0.009) with increasing gestational age. The trend of β3-AR mRNA content in the stress group was consistent with the trend in the control group. Compared with that in the E3 stress group, the β3-AR mRNA content decreased by 37.57% (E3 to E5, p < 0.001) and 39.81% (E3 to E7, p < 0.001) (). In the three β-ARs, the expression of β2-AR mRNA was most obvious and was affected by restraint stress in three ages ().
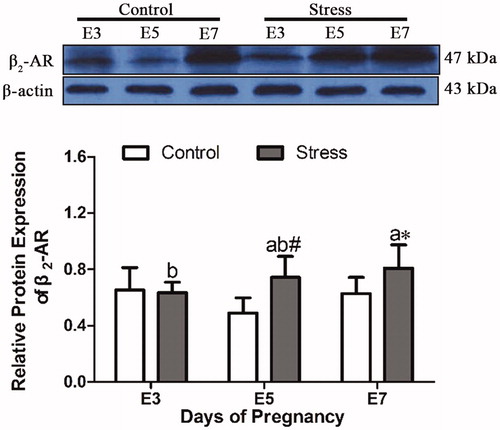
Next, to observe whether restraint stress affects the receptor protein level, the β2-AR protein was detected by western blotting. After restraint stress treatment, the expression of β2-AR protein in uterine tissue was increased (F (1, 28) = 9.064, p = 0.005), but the content of β2-AR protein was not affected by gestational age (F (2, 28) = 1.656, p = 0.209). Compared with that in control mice of the same age, the β2-AR protein content increased by 52.30% (independent sample t test, p = 0.003) and 28.77% (independent sample t test, p = 0.042) at E5 and E7 after restraint stress ().
Figure 4. Effects of restraint stress on the relative protein expression of β2-AR in the uterus of pregnant mice. After restraint stress treatment, the expression of β2-AR protein in uterine tissue was increased, and the content of β2-AR protein was not affected by gestational age. The uppercase letters represent differences in the control group among E3, E5 and E7, and the lowercase letters represent differences in the stress group among E3, E5 and E7 (p < 0.05). *p < 0.05 and #p < 0.01 are used to denote significance compared with the corresponding control groups.
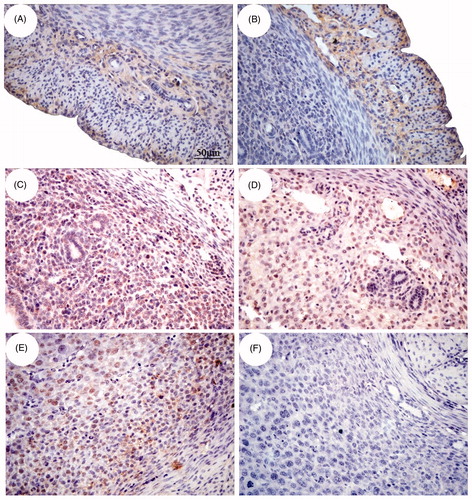
To study the signaling pathway downstream of β2-AR, we determined the immunohistochemical localization of β2-AR to select cell types for subsequent experiments. β2-AR immunohistochemical staining showed that β2-AR positive cells (yellow) were not only expressed in the longitudinal muscle layer and vascular layer of the uterus but also in the endometrium. At E3 and E5, β2-AR expression was found in glandular epithelial cells, luminal epithelial cells and uterine stromal cells. At E7, a large amount of β2-AR was located in decidual cells (). Therefore, the stromal cells were used to study the mechanism.
Figure 5. Immunohistochemical localization of β2-AR in the uterus of pregnant mice. The position of β2-AR in the uterus changes with the age of pregnancy. The expression of β2-AR-positive cells in the myometrium (A), vascular layer (B) and part of endometrium (C) on E3. Moreover, at E5 (D) and E7 (E), β2-AR expression was found in glandular epithelial cells, luminal epithelial cells and endometrium stromal cells. F shows β2-AR-negative cells. Bar = 50 μm.
Activation of the apoptosis pathway by restraint stress
To investigate whether the microenvironment of the uterus in early pregnancy is disordered, uterus cell apoptosis and oxidative stress-related proteins were examined. We found that the levels of apoptosis and antioxidants in the uterus were disrupted by restraint stress.
MMP-9 plays an important role in mouse embryo implantation and prevents the release of cytochrome c from blocking the occurrence of apoptosis. If its expression is disturbed, the microenvironment of the uterus is destroyed. MMP-9 protein expression in the uterus was affected by gestational age (F (2, 18) = 52.718, p < 0.001) and restraint stress treatment (F (1, 18) = 93.987, p < 0.001). Compared with that in the uterus of control mice of the same age, the MMP-9 protein content in the uterus of E3, E5 and E7 pregnant mice decreased by 39.44% (independent sample t test, p = 0.028), 63.45% (independent sample t test, p < 0.001) and 24.67% (independent sample t test, p = 0.004), respectively, after restraint stress. Compared with that in the E3 control group, the MMP-9 protein content increased by 181.50% (E3 to E5, p < 0.001) and 90.85% (E3 to E7, p < 0.001) as gestational age increased. Compared with that in the E3 stress group, the MMP-9 protein content increased by 70.10% (E3 to E5, p = 0.014) and 137.46% (E3 to E7, p < 0.001) (). Caspase-3 is an effector of apoptosis, and changes in its expression affect the microenvironment of the uterus, disturbing the balance of cell proliferation and apoptosis in the uterus. Caspase-3 protein content in uterine tissue was not affected by gestational age (F (2, 53) = 2.971, p = 0.060) and was only affected by restraint stress treatment (F (1, 53) = 26.682, p < 0.001). Compared with that in the uterus of control mice of the same age, the Caspase-3 protein content in the uterus of E3, E5 and E7 pregnant mice was increased by 44.69% (E3, independent sample t test, p = 0.012), 42.92% (E5, independent sample t test, p < 0.001) and 30.10% (E7, independent sample t test, p = 0.019), respectively, after restraint stress ().
Figure 6. Effects of restraint stress on the relative protein levels of MMP-9, Caspase-3, p-ERK1/2 and p-NF-κB p65 in the uterus of pregnant mice. MMP-9 protein expression in the uterus was affected by gestational age and restraint stress treatment. (B) Caspase-3 protein content in uterine tissue was not affected by gestational age and was only affected by restraint stress treatment. (C) The level of p-ERK1/2 protein in uterine tissue was not affected by gestational age and was only affected by restraint stress treatment. (D) The p-NF-κB p65 protein content in uterine tissue was affected by gestational age and restraint stress treatment. The uppercase letters represent differences in the control group amongE3, E5 and E7, and the lowercase letters represent differences in the stressed group among E3, E5 and E7 (p < 0.05). *p < 0.05 and #p < 0.01 are used to denote significance compared with the corresponding control groups.
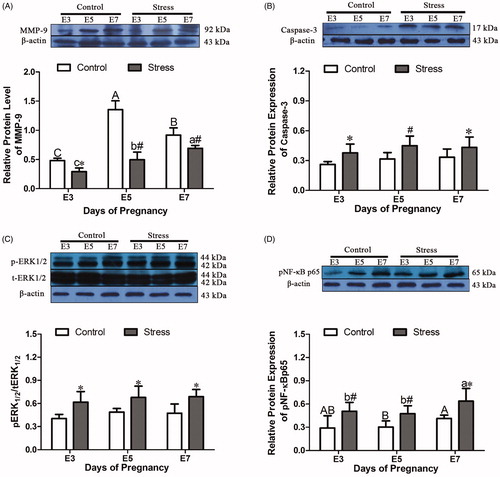
Oxidative stress during pregnancy can trigger disorder of the immune microenvironment of the uterus, and oxidative stress can induce the activation of NF-κB by activating ERK, thereby promoting inflammation. Additionally, the activation of ERK and NF-κB can promote the occurrence of cell apoptosis. The level of p-ERK1/2 protein in uterine tissue was not affected by gestational age (F (2, 18) = 1.055, p = 0.369) and was only affected by restraint stress treatment (F (1, 18) = 19.922, p < 0.001). Compared with that in the uterus of control mice of the same age, the p-ERK1/2 level in the uterus of E3, E5 and E7 pregnant mice was increased by 52.30% (independent sample t test, p = 0.025), 38.85% (independent sample t test, p = 0.049) and 44.84% (independent sample t test, p = 0.030), respectively, after restraint stress (). The p-NF-κB p65 protein content in uterine tissue was affected by gestational age (F (2, 52) = 6.758, p = 0.002) and restraint stress treatment (F (1, 52) = 42.177, p < 0.001). Compared with that in the uterus of control mice of the same age, the p-NF-κB p65 protein content in the uterus of E3, E5 and E7 pregnant mice was increased by 74.47% (independent sample t test, p = 0.001), 56.12% (independent sample t test, p = 0.001) and 54.38% (independent sample t test, p = 0.019), respectively, after restraint stress. In the control group, the trend of p-NF-κB p65 protein may not be affected with the change of ages. However, compared with that in the E3 stress group, the p-NF-κB p65 protein content increased by 25.64% (E3 to E7, p = 0.039) ().
Effect of β2-AR activation on the proliferative activity of ESCs
The E3, E5 and E7 were the pre-implantation, implantation and post-implantation period, respectively. Implantation was the most important period during pregnancy. Therefore, the uterus of E5 mice were used to study the mechanism. In subsequent studies, protein was extracted from ESCs for western blotting detection. The result showed that β2-AR was present in ESCs, which provided a reliable theoretical basis for adding drug to cells in vitro ().
Figure 7. Effects of different drugs at different concentrations on the stimulation index of endometrial stromal cells (ESCs) from E5 pregnant mice. (A) β2-AR was expressed in primary endometrial stromal cells (ESCs). (B) ISO inhibited cell activity, and the suitable drug concentration was 10 μM. (C) FSK synergized with ISO to inhibit the proliferation of cells, and the suitable drug concentration was 1 μM. (D, E, F, G) Butox, H89, PD98059 and PDTC reversed the decrease in cell activity caused by ISO, and the suitable concentration for all drugs was 1 μM. (H) The activation of β2-AR inhibited the proliferation of ESCs mainly by activating the downstream ERK/MAPK signal through the cAMP/PKA pathway and inducing NF-κB signaling, thereby initiating apoptosis. *p < 0.05 and **p < 0.01 are used to denote significance compared with the control group, +p < 0.05 and ++p < 0.01 denote significance compared with the ISO-treated group.
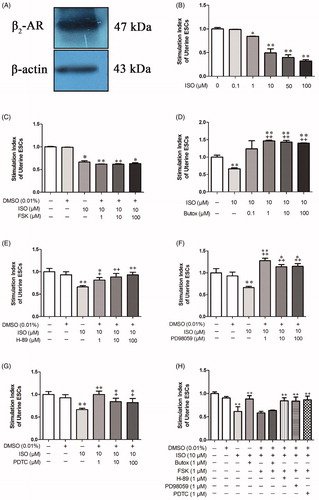
To further investigate the β2-AR signaling pathway, appropriate concentrations of ISO, FSK, butoxamine, H-89, PD98059 and PDTC were determined based on their effect on cell viability. When the ISO were 10 μM, 50 μM and 100 μM, the cell proliferation activity was extremely significantly reduced (p < 0.01) (). At the basic of ISO, the FSK leaded proliferation activity decreased obviously on the concentration range of 1 μM−100 μM (p < 0.05) (). Meanwhile, compare with the ISO group, the stimulation index was increased obviously (p < 0.01) when the Butox (1 μM, 10 μM and 100 μM) was added at the basic of ISO (p < 0.01) (). H89 increased significantly stimulation index compare with the ISO group (p < 0.05) when the concentrations of H89 were 1 μM, 10 μM and 100 μM (). When the PD98059 were 1 μM, 10 μM and 100 μM, the cell proliferation activity was extremely increased obviously compare with ISO group (p < 0.01) (). At the basic of ISO, the PDTC led to proliferation activity increased obviously on the concentration range of 1 μM (p < 0.001), 10 μM and 100 μM (p < 0.05) (). Considering the toxic effect of drugs on cells, the concentrations of ISO and FSK were determined as 10 μM and 1 μM, which decreased 50.72% and 37.38% compare with control group. Both concentration of butoxamine, H-89, PD98059 and PDTC were determined as 1 μM, respectively, which increased significantly 120%, 22.55%, 92.19% and 50.73% compare with ISO group.
As an organic solvent, DMSO was used to dissolve FSK, H-89, PD98059 and PDTC in this study. Therefore, a DMSO group was established to determine whether 0.01% DMSO in cell suspension affects cell proliferation. Compared with that of the blank control group, cell proliferation of a cell suspension was not affected after incubation in 0.01% DMSO for 24 h (p = 0.106). This result indicated that dissolving the drug in medium containing 0.01% DMSO did not interfere with the effect of the drugs on the cells ().
Compared with that of control cells, proliferation was significantly reduced by 38.60% (p < 0.001) in cells treated with 10 μM ISO in vitro. However, compared with ISO, the addition of the β2-AR blocker butoxamine reversed the antiproliferative effect of ISO, resulting in a significant increase (43.95%, p < 0.001) in cell proliferation. It was again demonstrated that β2-AR plays a major role in the proliferation of ESCs (). In the drug concentration exploration test, it was found that FSK and ISO showed a synergistic antiproliferative effect. After adding the β2-AR blocker Butoxamine, and adding FSK and ISO to the cells for 24 h. Compared with that of the control group, proliferative activity was still reduced by 36.36% (p < 0.001), and the difference was not significant compared with the ISO-treated group (p = 0.720). However, compared with ISO, the addition of the PKA inhibitor H-89 significantly increased cell proliferative activity by 37.55% (p < 0.001). This result indicated that the activation of β2-AR inhibited the proliferation of ESCs mainly through the cAMP/PKA pathway (). The MEK inhibitor PD98059 specifically blocks the activation of ERK. Compared with ISO, the addition of the MEK inhibitor PD98059 significantly increased cell proliferation by 36.31% (p = 0.001). Compared with ISO, the addition of PDTC (a specific blocker of NF-κB) increased the cell proliferation by 40.80% (p < 0.001). This result indicated that the activation of β2-AR inhibited the proliferation of ESCs mainly by activating downstream ERK/MAPK signaling through the cAMP/PKA pathway and inducing NF-κB activation, thereby initiating apoptosis ().
Effect of β2-AR activation on intracellular signal changes in endometrial stromal cells (ESCs) from pregnant mice
ESCs treated with different drugs were collected, and the expression of p-ERK1/2 in the cells was detected by the WB method. The DMSO-treated group exhibited no significant change in the expression of p-ERK1/2 compared with that of the blank control group. The addition of the β2-AR agonist ISO in vitro significantly increased the expression of p-ERK1/2 by 37.84% (p = 0.005) compared with that in the group. It indicated that ISO was involved in the activation of the p-ERK1/2 protein, which is involved in cell proliferation and apoptosis. However, after the addition of the adenylyl cyclase (AC) agonist FSK, the expression of p-ERK1/2 in the cells was significantly increased by 58.03% (p < 0.001) compared with that in the control group, and the difference was not significant compared with the ISO-treated group. The addition of the PKA inhibitor H-89 reversed the expression of p-ERK1/2. Compared with that in the ISO-treated group, the expression of p-ERK1/2 was significantly reduced by 21.83% (p = 0.007). However, after treatment with the β2-AR blocker butoxamine for 30 min, FSK and ISO were added to the cells, and the cells were cultured for 24 h. Compared with that in the control group, the expression of p-ERK1/2 was significantly increased by 54.71% (p < 0.001). The difference was not significant compared with the ISO-treated group (p = 0.133). This result indicated that the stimulation of β2-AR inhibited cell proliferation and induced apoptosis of ESCs mainly through the cAMP/PKA pathway. The MEK inhibitor PD98059 specifically blocked the activation of ERK. Compared with ISO, the addition of PD98059 significantly reduced the expression of p-ERK1/2 in cells by 70.08% (p < 0.001). The expression of p-ERK1/2 was similar with the control when the PDTC was added. Compared with ISO, the addition of PDTC significantly decreased the expression of p-ERK1/2 in the cells by 61.04% (p < 0.001) ().
Figure 8. Effects of β2-AR/cAMP/PKA signaling on the relative protein levels of Caspase-3 and ERK1/2 in uterine ESCs from E5 pregnant mice. The activation of β2-AR promoted the apoptosis of ESCs mainly by activating downstream ERK/MAPK signaling through the cAMP/PKA pathway and inducing NF-κB activation, thereby initiating apoptosis. 1–9: (1) cells, (2) cells + 0.01% DMSO, (3) cells + ISO, (4) cells + butoxamine + ISO, (5) cells + FSK + ISO, (6) cells + butoxamine + ISO + FSK, (7) cells + H-89 + ISO + FSK, (8) cells + PD98059+ ISO + FSK, (9) cells + PDTC + ISO + FSK. *p < 0.05 is used to denote significance compared with the control group, +p < 0.05 and ++p < 0.01 denote significance compared with the ISO-treated group.
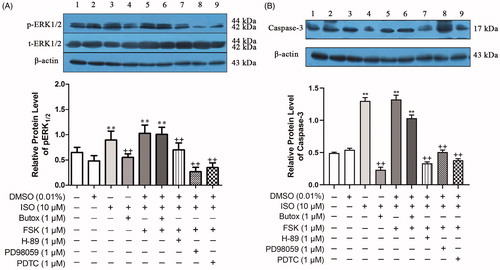
Caspase-3, a member of the Caspase family, plays an important role in the activation of apoptosis, which links mitochondria inside the cell with the external death receptor. Uterine ESCs treated with different drugs were collected, and the expression of Caspase-3 in the cells was detected by the WB method. The DMSO-treated group exhibited no significant change in the expression of Caspase-3 compared with that in the control group (p = 0.434). The addition of the β2-AR agonist ISO in vitro significantly increased the expression of Caspase-3, and the expression of Caspase-3 was significantly increased by 163.58% (p < 0.001) compared with that in the control group. These results indicated that ISO induced the activation of Caspase-3 protein during ESC apoptosis. Also, after the addition of the AC agonist FSK, there was no significant change in the expression of Caspase-3 in the cells compared with that in the ISO-treated group (p = 0.684). The addition of the PKA inhibitor H-89 reversed the expression of Caspase-3 induced by ISO and FSK. Compared with that in the ISO-treated group, the expression of Caspase-3 was significantly reduced by 74.27% (p < 0.001). After the cells were treated with the β2-AR blocker butoxamine for 30 min, ISO was applied to the cells for 24 h. Compared with that in the ISO-treated group, the expression of Caspase-3 was significantly decreased by 81.97% (p < 0.001). The process of β2-AR-induced apoptosis in ESCs was mainly achieved through the cAMP/PKA pathway. The MEK inhibitor PD98059 specifically blocked the activation of ERK. Compared with ISO, the addition of the MEK inhibitor PD98059 significantly reduced the expression of Caspase-3 in cells by 60.90% (p < 0.001). The cells were treated with PDTC for 24 h, and then ISO and FSK were added; the expression of Caspase-3 did not increase in ISO + FSK + PDTC group compared with ISO-treated cells. The addition of PDTC significantly decreased the expression of Caspase-3 in the cells by 70.59% (p < 0.001). In conclusion, the activation of β2-AR promoted the apoptosis of ESCs mainly by activating downstream ERK/MAPK signaling through the cAMP/PKA pathway and inducing NF-κB activation, thereby initiating apoptosis ().
Discussion
Restraint stress disturbs the developmental and endocrine environment in pregnant mice
A study of schizophrenia and PTSD demonstrated that the corticotropin-releasing factor (CRF) and NE systems, both of which are activated by stress, are the most widely implicated systems in stress-induced psychopathology (Rajbhandari et al., Citation2015). In our work, we found that NE levels decreased as the gestational age increased (). After restraint stress, NE concentrations at E3, E5 and E7 were significantly increased. The activation of noradrenergic (NE) receptors increases the risk of pregnancy-induced hypertension (Newport et al., Citation2016). Additionally, gluconeogenesis is usually enhanced by glucocorticoids and then increases blood glucose levels (Fagerholm et al., Citation2008). In our previous study, CORT was increased in early pregnancy under restraint stress (Liu et al., Citation2014). In this work, blood glucose levels were increased under the restraint stress at three gestational ages (), suggesting a high risk for gestational diabetes. Restraint stress results in a decrease in body weight due to a massive consumption of energy and a continuous reduction in body temperature (Jeong et al., Citation2013). Therefore, diabetes may gradually reduce body weight and maintain normal functions of the body (Komorita et al., Citation2018). In our study, the reduction in body weight was increased early after restraint stress (). Furthermore, body weight decreases, possibly causing embryonic growth retardation (Liu et al., Citation2014).
Restraint stress disturbs adaptive changes in proliferation and apoptosis in the uterus of pregnant mice
Increasing evidence has indicated that excessive reactive oxygen species (ROS) result in caspase activation (Droge, Citation2002; Lassen et al., Citation2013). Restraint stress was shown to disrupt the oxidative/antioxidative balance (Liu et al., Citation2014) and the proliferation/apoptosis balance (Liu et al., Citation2015) in our previous studies (). In this study, the protein levels of Caspase-3 were significantly increased under restraint stress during early pregnancy at three gestational ages (). The above mentioned findings suggest that an increase in Caspase-3 is harmful for embryo implantation (Liu et al., Citation2014, Citation2015). Decreased expression of MMP-9 results in cytochrome c release from mitochondria into the cytosol to induce cell apoptosis (Dai et al., Citation2013). In our study, the protein levels of MMP-9 were obviously decreased under restraint stress during early pregnancy at the three gestational ages (). Meanwhile, under control conditions, we found that the protein levels of MMP-9 were higher on E5 than on the other two gestational days (). We speculate that MMP-9 is not only involved in apoptosis but is also important for implantation. MMP-9 is a major contributor to normal implantation and plays a critical role in embryonic trophoblast development (Chen et al., Citation2020a). A study showed that pregnant MMP-9-null mice exhibit clinical symptoms of preeclampsia (Plaks et al., Citation2013). All in all, restraint stress may decrease the expression of MMP-9, which promotes the release of cytochrome c and then induces apoptosis ().
Figure 9. A schematic diagram of the β2-AR/cAMP/PKA pathway under restraint stress. Restraint stress disturbs the balance between antioxidation and oxidation and then promotes an increase in Caspase-3, which leads to an imbalance between apoptosis and proliferation. ERK is activated by the β2-AR/cAMP/PKA pathway, promoting apoptosis. Combined with our previous results, our findings suggest that ERK may induce inflammation by activating NF-κB p65.
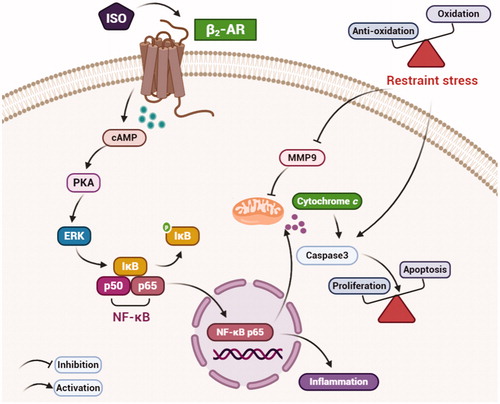
In our previous study, restraint stress was shown to cause local immune regulation disorder in the uterus (Liu, et al., Citation2014). Additionally, a study showed that CD4+ T cells mediate oxidative stress to cause hypertension during preeclampsia (Wallace et al., Citation2014). In our study, the protein levels of p-NF-κB p65 were increased under restraint stress during early pregnancy on E3, E5 and E7 (). p-NF-κB p65 may promote inflammation, which is consistent with our previous studies. The release of TNF-α (tumor necrosis factor-alpha) is affected by increases of NF-κB and results in endothelial cell dysfunction, leading to preeclampsia (Kim et al., Citation2017). In addition, a study showed that fenvalerate activates the ERK/NF-κB pathway, inducing apoptosis in the liver (Qiu et al., Citation2019). Thus, we speculated that ERK1/2 may be involved in restraint stress. In our study, the protein levels of ERK1/2 were detected. The results showed that the protein expression of ERK1/2 was increased significantly under restraint stress during early pregnancy (). ERK1/2 participates in depression in offspring caused by prenatal stress (Guan et al., Citation2013). In summary, uterine development may be affected by ERK1/2/NF-κB under restraint stress during early pregnancy ().
β2-AR plays an important role in the effect of restraint stress on the uterine microenvironment in pregnant mice
Stress activates the HPA and the sympathetic-adrenal medullary system (SAS), which induce the release of glucocorticoids from the adrenal cortex and the catecholamines EPI and NE from the adrenal medulla and sympathetic nerve termini (Kolmus et al., Citation2015). In our study, only β2-AR was significantly increased under restraint stress (). Additionally, β2-AR is a G protein-coupled receptor. It promotes or inhibits the expression of cAMP by combining with Gs or Gi (Philipp & Hein, Citation2004). β-AR-mediated oxidative stress has detrimental effects (Zhang et al., Citation2005).
Therefore, our study used β2-AR as a starting point to study signaling pathways. In this work, we also measured the protein levels of β2-AR, which were obviously increased on E5 and E7 under restraint stress. Meanwhile, β2-AR is only expressed in cells incubated with embryonic culture media from embryos (Bruzzone et al., Citation2005). Our work was consistent with this related study. Next, we determined the immunohistochemical localization of β2-AR in the uterus. β2-AR was expressed not only in the longitudinal muscle and the vascular layers of the uterus but also in the endometrium (glandular epithelial cells, luminal epithelial cells and endometrium stromal cells) in E3 and E5. On E7, a large amount of β2-AR was present in decidual cells. Therefore, ESCs were used to explore the β2-AR signaling pathway.
The cAMP/PKA pathway mediates the effects of β2-AR on the uterus of pregnant mice
It was reported that β2-AR increased in normal implantation compared with non-pregnancy mice (Bruzzone et al., Citation2005), which was related with secretory products of embryo, implying that β2-AR plays an significant role in the intrauterine microenvironment. However, implantation abnormalities are influenced by environmental factors such as stress-induced sympathetic activation, which was related with abnormal higher of β2-AR (Chen et al., Citation2011). When NE‐stimulated apoptosis was mimicked by both ISO and FSK, its effect can be inhibited by H89, an inhibitor of PKA, suggesting that this process involves PKA (Singh et al., Citation2001). As an intracellular second messenger, cAMP activates the major downstream protein PKA. Meanwhile, PKA is involved in the mechanism of β2-AR/cAMP-induced apoptosis. β2-AR activation results in embryonic proliferation inhibition and pluripotent stem cell cycle arrest. This effect selectively depends on β2-AR signaling, which involves the regulation of AKT (protein kinase B), ERK and Cyclin E (Sun et al., Citation2015). In our study, the stimulation index of uterus ESCs was significantly decreased when β2-AR was activated by ISO. There was no change in the ISO + FSK-treated group compared with the ISO-treated group (). It demonstrated that β2-AR-cAMP was activated to regulate downstream signals (). A previous study also proved that excessive NE can activate β2-AR/cAMP in rat peritoneal macrophages (Zhuang et al., Citation2016). Next, Butox was added before ISO, the stimulation index of uterus ESCs was obviously increased compared with that in the ISO-treated group, and the ISO + FSK-treated group exhibited no change compared with the ISO-treated group. Furthermore, the activation of β2-AR/cAMP was explained. Similarly, Caspase-3 was significantly activated in the ISO-treated group, and the change observed in the ISO + FSK-treated group was the same as that observed in the ISO-treated group. However, compared to ISO, Butox inhibited Caspase-3, but there was no difference compared to ISO + FSK (). The direct activation of PKA by cAMP or expression of the PKA catalytic subunit leads to the activation of Caspase-8 and the induction of endothelial cell apoptosis (Kim et al., Citation2002). In addition, a study showed that a cAMP agonist and PKA induce neuronal apoptosis in mice with Alzheimer's disease (AD) and type 2 diabetes (T2D) and that H-89 treatment reverses apoptosis (Li et al., Citation2018). However, a study reported AKAP121/PKA as a new molecular target that stabilizes mitochondrial networks and mitochondrial function and elicits antioxidant responses by phosphorylating Drp1 (Zhang et al., Citation2019). Meanwhile, in our study, compared with ISO, H-89 also prevented apoptosis and increase the stimulation index. It was further demonstrated that in ESCs, the inhibition of proliferation and apoptosis by β2-AR activation was achieved through the cAMP/PKA pathway.
Meanwhile, p-ERK1/2 was obviously increased in the ISO-treated group and the ISO + FSK-treated group. However, Butox prevented the increased expression of p-ERK1/2. A PKA inhibitor weakened the p-ERK1/2 levels. These results demonstrated that ERK was involved in apoptosis and was regulated by PKA (). However, in prostate cancer cells, PAGE4 (prostate-associated gene 4) promotes cell survival by regulating MAPK, JNK (c-Jun N-terminal kinase) and c-jun and increasing the phosphorylation of ERK1/2 (Lv et al., Citation2019). During preeclampsia, inositol second messengers are highly expressed and promote significant MAPK/ERK phosphorylation (D'Oria et al., Citation2017). Additionally, a study indicated that β2-AR activation induces microglial PHOX activation and dopaminergic (DA) neurotoxicity and that the ERK-dependent/PKA-independent pathway is involved in this process (Qian et al., Citation2009). Therefore, PKA-induced apoptosis may occur via ERK phosphorylation. This mechanism needs to be further studied.
Therefore, we wanted to determine whether ERK promotes apoptosis. When ERK was inhibited by PD98059, the stimulation index was significantly increased and Caspase-3 was obviously decreased compared with the levels observed in the ISO-treated group. Thus, ERK activation induced apoptosis via the PKA pathway (). A study showed that oxidative stress-induced neuronal apoptosis is mainly mediated by the NF-κB, JNK, and ERK pathways (Qian et al., Citation2015). When NF-κB p65 was inhibited by PDTC, the change was the same as that observed when ERK was suppressed. Meanwhile, the expression of p-ERK1/2 did not change after NF-κB p65 was inhibited. Moreover, in our previous study, restraint stress was shown to induce inflammation during early pregnancy () (Liu et al., Citation2014).
Conclusion
All in all, in vivo tests showed that restraint stress leads to an increase in NE and blood glucose levels and reduction of body weight. The body receives exogenous stress signals, which leads to activation of β2-AR, then transmitting the signals into cells, resulting in an elevation of Caspase-3 and phosphorylated ERK and ultimately in apoptosis. Meanwhile, p-NF-κB p65 is also elevated, confirming that restraint stress induces the onset of inflammation. In vitro experiments with β2-AR being activated showed that ERK is activated by β2-AR/cAMP/PKA signaling, which then promotes apoptosis and inhibits proliferation. At the same time, ERK promotes the elevation of NF-κB p65, thereby promoting the occurrence of inflammation ().
Ethics approval and consent to participate
In my manuscript, all animal procedures were approved by the China Agricultural University Institutional Animal Care and Use Committee (AW18079102-3-2).
Consent for publication
My manuscript does not contain data from any individual person, this section is not applicable in my manuscript.
Authors' contributions
JYL, GHL and YLD designed the experiments. GHL carried out the experiments and interpreted the data. ZXW and JC checked the figures. JYL and YLD wrote the manuscript. YXC checked the manuscript.
Supplemental Material
Download JPEG Image (41.2 KB)Acknowledgements
The authors are grateful for the experimental animal platform of China Agricultural University for their support for this research. The authors acknowledge the comments of the reviewers that helped to improve the study.
Disclosure statement
The authors disclose that there is no conflict of financial interest that influenced this work.
Data availability statement
All data supporting the conclusion of this article are included in this published article.
Additional information
Funding
References
- Battle, D. E. (2013). Diagnostic and statistical manual of mental disorders (DSM). CoDAS, 25(2), 191–192. https://doi.org/10.1590/s2317-17822013000200017
- Bruzzone, M. E., Fabres, C., Benitez, D. A., Castellon, E. A., & Zegers-Hochschild, F. (2005). Influence of embryonic conditioned media upon the endometrial beta-adrenergic receptor. Reproductive Biomedicine Online, 11(1), 58–63. https://doi.org/10.1016/S1472-6483(10)61299-0
- Bruzzone, A., Sauliere, A., Finana, F., Senard, J. M., Luthy, I., & Gales, C. (2014). Dosage-dependent regulation of cell proliferation and adhesion through dual beta2-adrenergic receptor/cAMP signals. The FASEB Journal, 28(3), 1342–1354. https://doi.org/10.1096/fj.13-239285
- Burniston, J. G., Tan, L. B., & Goldspink, D. F. (2005). Beta2-Adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. Journal of Applied Physiology, (1985) 98(4), 1379–1386. https://doi.org/10.1152/japplphysiol.00642.2004
- Chen, Q., Ni, Y., Han, M., Zhou, W. J., Zhu, X. B., & Zhang, A. J. (2020a). Integrin-linked kinase improves uterine receptivity formation by activating Wnt/β-catenin signaling and up-regulating MMP-3/9 expression. American Journal of Translational Research, 12(6), 3011–3022.
- Chen, Q., Zhang, Y., Peng, H., Lei, L., Kuang, H., Zhang, L., Ning, L., Cao, Y., & Duan, E. (2011). Transient {beta}2-adrenoceptor activation confers pregnancy loss by disrupting embryo spacing at implantation. The Journal of Biological Chemistry, 286(6), 4349–4356. https://doi.org/10.1074/jbc.M110.197202
- Dai, C., Zhang, B., Liu, X., Guo, K., Ma, S., Cai, F., Yang, Y., Yao, Y., Feng, M., Bao, X., Deng, K., Jiao, Y., Wei, Z., Junji, W., Xing, B., Lian, W., & Wang, R.(2013). Pyrimethamine sensitizes pituitary adenomas cells to temozolomide through cathepsin B-dependent and caspase-dependent apoptotic pathways. International Journal of Cancer, 133(8), 1982–1993. https://doi.org/10.1002/ijc.28199
- de Lima-Seolin, B. G., Nemec-Bakk, A., Forsyth, H., Kirk, S., da Rosa Araujo, A. S., Schenkel, P. C., Bello-Klein, A., & Khaper, N. (2019). Bucindolol modulates cardiac remodeling by attenuating oxidative stress in H9c2 cardiac cells exposed to norepinephrine. Oxidative Medicine and Cellular Longevity, 2019, 6325424. https://doi.org/10.1155/2019/6325424
- D'Oria, R., Laviola, L., Giorgino, F., Unfer, V., Bettocchi, S., & Scioscia, M. (2017). PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: novel potential insights in endothelial dysfunction in preeclampsia. Pregnancy Hypertension, 10, 107–112. https://doi.org/10.1016/j.preghy.2017.07.001
- Droge, W. (2002). Free radicals in the physiological control of cell function. Physiological Reviews, 82(1), 47–95. https://doi.org/10.1152/physrev.00018.2001
- Dunkel Schetter, C., & Tanner, L. (2012). Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Current Opinion in Psychiatry, 25(2), 141–148. https://doi.org/10.1097/YCO.0b013e3283503680
- Fagerholm, V., Rokka, J., Nyman, L., Sallinen, J., Tiihonen, J., Tupala, E., Haaparanta, M., & Hietala, J. (2008). Autoradiographic characterization of alpha(2C)-adrenoceptors in the human striatum. Synapse (New York, N.Y.), 62(7), 508–515. https://doi.org/10.1002/syn.20520
- Fajardo, G., Zhao, M., Berry, G., Wong, L. J., Mochly-Rosen, D., & Bernstein, D. (2011). Beta2-adrenergic receptors mediate cardioprotection through crosstalk with mitochondrial cell death pathways. Journal of Molecular and Cellular Cardiology, 51(5), 781–789. https://doi.org/10.1016/j.yjmcc.2011.06.019
- Guan, L., Jia, N., Zhao, X., Zhang, X., Tang, G., Yang, L., Sun, H., Wang, D., Su, Q., Song, Q., Cai, D., Cai, Q., Li, H., & Zhu, Z. (2013). The involvement of ERK/CREB/Bcl-2 in depression-like behavior in prenatally stressed offspring rats. Brain Research Bulletin, 99, 1–8. https://doi.org/10.1016/j.brainresbull.2013.08.003
- Hasegawa, K., Iwai-Kanai, E., & Sasayama, S. (2001). Neurohormonal regulation of myocardial cell apoptosis during the development of heart failure. Journal of Cellular Physiology, 186(1), 11–18. https://doi.org/10.1002/1097-4652(200101)186:1<11::AID-JCP1013>3.0.CO;2-5
- Holmes, E. A., Craske, M. G., & Graybiel, A. M. (2014). Psychological treatments: A call for mental-health science. Nature, 511(7509), 287–289. https://doi.org/10.1038/511287a
- Jeong, J. Y., Lee, D. H., & Kang, S. S. (2013). Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinology and Metabolism (Seoul, Korea), 28(4), 288–296. https://doi.org/10.3803/EnM.2013.28.4.288
- Kim, S., Bakre, M., Yin, H., & Varner, J. A. (2002). Inhibition of endothelial cell survival and angiogenesis by protein kinase A. The Journal of Clinical Investigation, 110(7), 933–941. https://doi.org/10.1172/JCI14268
- Kim, J., Lee, K.-S., Kim, J.-H., Lee, D.-K., Park, M., Choi, S., Park, W., Kim, S., Choi, Y. K., Hwang, J. Y., Choe, J., Won, M.-H., Jeoung, D., Lee, H., Ryoo, S., Ha, K.-S., Kwon, Y.-G., & Kim, Y.-M. (2017). Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radical Biology & Medicine, 104, 185–198. https://doi.org/10.1016/j.freeradbiomed.2017.01.010
- Kolmus, K., Tavernier, J., & Gerlo, S. (2015). β2-Adrenergic receptors in immunity and inflammation: Stressing NF-κB. Brain, Behavior, and Immunity, 45, 297–310. https://doi.org/10.1016/j.bbi.2014.10.007
- Komorita, Y., Iwase, M., Fujii, H., Ohkuma, T., Ide, H., Jodai-Kitamura, T., Sumi, A., Yoshinari, M., Nakamura, U., Kang, D., & Kitazono, T. (2018). Impact of body weight loss from maximum weight on fragility bone fractures in japanese patients with type 2 diabetes: The Fukuoka Diabetes Registry. Diabetes Care, 41(5), 1061–1067. https://doi.org/10.2337/dc17-2004
- Lassen, J. F., Botker, H. E., & Terkelsen, C. J. (2013). Timely and optimal treatment of patients with STEMI. Nature Reviews Cardiology, 10(1), 41–48. https://doi.org/10.1038/nrcardio.2012.156
- Li, H., Yang, S., Wu, J., Ji, L., Zhu, L., Cao, L., Huang, J., Jiang, Q., Wei, J., Liu, M., Mao, K., Wei, N., Xie, W., & Yang, Z. (2018). cAMP/PKA signaling pathway contributes to neuronal apoptosis via regulating IDE expression in a mixed model of type 2 diabetes and Alzheimer's disease. Journal of Cellular Biochemistry, 119(2), 1616–1626. https://doi.org/10.1002/jcb.26321
- Liu, G., Dong, Y., Wang, Z., Cao, J., & Chen, Y. (2014). Restraint stress alters immune parameters and induces oxidative stress in the mouse uterus during embryo implantation. Stress (Amsterdam, Netherlands), 17(6), 494–503. https://doi.org/10.3109/10253890.2014.966263
- Liu, G., Dong, Y., Wang, Z., Cao, J., & Chen, Y. (2015). Restraint stress delays endometrial adaptive remodeling during mouse embryo implantation. Stress, 18(6), 699–709. https://doi.org/10.3109/10253890.2015.1078305
- Liu, C.-W., Lee, T.-L., Chen, Y.-C., Liang, C.-J., Wang, S.-H., Lue, J.-H., Tsai, J.-S., Lee, S.-W., Chen, S.-H., Yang, Y.-F., Chuang, T.-Y., & Chen, Y.-L. (2018). PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Particle and Fibre Toxicology, 15(1), 4. https://doi.org/10.1186/s12989-018-0240-x
- Lv, C., Fu, S., Dong, Q., Yu, Z., Zhang, G., Kong, C., Fu, C., & Zeng, Y. (2019). PAGE4 promotes prostate cancer cells survive under oxidative stress through modulating MAPK/JNK/ERK pathway. Journal of Experimental and Clinical Cancer Research, 38(1), 24. https://doi.org/10.1186/s13046-019-1032-3
- Madden, K. S. (2003). Catecholamines, sympathetic innervation, and immunity. Brain, Behavior, and Immunity, 17(1), 5–10. https://doi.org/10.1016/S0889-1591(02)00059-4
- Marquesim, N. A. Q., Cavassini, A. C. M., Morceli, G., Magalhães, C. G., Rudge, M. V. C., Calderon, I. D. M. P., Kron, M. R., & Lima, S. A. M. (2016). Depression and anxiety in pregnant women with diabetes or mild hyperglycemia. Archives of Gynecology and Obstetrics, 293(4), 833–837. https://doi.org/10.1007/s00404-015-3838-3
- Micova, P., Hahnova, K., Hlavackova, M., Elsnicova, B., Chytilova, A., Holzerova, K., Zurmanova, J., Neckar, J., Kolar, F., Novakova, O., & Novotny, J. (2016). Chronic intermittent hypoxia affects the cytosolic phospholipase A2α/cyclooxygenase 2 pathway via β2-adrenoceptor-mediated ERK/p38 stimulation. Molecular and Cellular Biochemistry, 423(1–2), 151–163. https://doi.org/10.1007/s11010-016-2833-8
- Newport, D. J., Hostetter, A. L., Juul, S. H., Porterfield, S. M., Knight, B. T., & Stowe, Z. N. (2016). Prenatal psychostimulant and antidepressant exposure and risk of hypertensive disorders of pregnancy. The Journal of Clinical Psychiatry, 77(11), 1538–1545. https://doi.org/10.4088/JCP.15m10506
- Olejarz, W., Bryk, D., Zapolska-Downar, D., Małecki, M., Stachurska, A., & Sitkiewicz, D. (2014). Mycophenolic acid attenuates the tumour necrosis factor-α-mediated proinflammatory response in endothelial cells by blocking the MAPK/NF-κB and ROS pathways. European Journal of Clinical Investigation, 44(1), 54–64. https://doi.org/10.1111/eci.12191
- Philipp, M., & Hein, L. (2004). Adrenergic receptor knockout mice: Distinct functions of 9 receptor subtypes. Pharmacology & Therapeutics, 101(1), 65–74. https://doi.org/10.1016/j.pharmthera.2003.10.004
- Plaks, V., Rinkenberger, J., Dai, J., Flannery, M., Sund, M., Kanasaki, K., Ni, W., Kalluri, R., & Werb, Z. (2013). Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proceedings of the National Academy of Sciences of the United States of America, 110(27), 11109–11114. https://doi.org/10.1073/pnas.1309561110
- Qian, Y., Cao, L., Guan, T., Chen, L., Xin, H., Li, Y., Zheng, R., & Yu, D. (2015). Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharmaceutical Biology, 53(8), 1124–1132. https://doi.org/10.3109/13880209.2014.962057
- Qian, L., Hu, X., Zhang, D., Snyder, A., Wu, H. M., Li, Y., Wilson, B., Lu, R. B., Hong, J. S., & Flood, P. M. (2009). beta2 Adrenergic receptor activation induces microglial NADPH oxidase activation and dopaminergic neurotoxicity through an ERK-dependent/protein kinase A-independent pathway. Glia, 57(15), 1600–1609. https://doi.org/10.1002/glia.20873
- Qiu, L.-L., Wang, C., Yao, S., Li, N., Hu, Y., Yu, Y., Xia, R., Zhu, J., Ji, M., Zhang, Z., & Wang, S.-L. (2019). Fenvalerate induces oxidative hepatic lesions through an overload of intracellular calcium triggered by the ERK/IKK/NF-κB pathway. FASEB Journal, 33(2), 2782–2795. https://doi.org/10.1096/fj.201801289R
- Rajbhandari, A. K., Baldo, B. A., & Bakshi, V. P. (2015). Predator stress-induced CRF release causes enduring sensitization of basolateral amygdala norepinephrine systems that promote PTSD-like startle abnormalities. The Journal of Neuroscience , 35(42), 14270–14285. https://doi.org/10.1523/JNEUROSCI.5080-14.2015
- Singh, K., Xiao, L., Remondino, A., Sawyer, D. B., & Colucci, W. S. (2001). Adrenergic regulation of cardiac myocyte apoptosis. Journal of Cellular Physiology, 189(3), 257–265. https://doi.org/10.1002/jcp.10024
- Sun, F., Yang, X.-J., Lv, H.-Y., Tang, Y.-B., An, S.-M., Ding, X.-P., Li, W.-B., Teng, L., Shen, Y., Chen, H.-Z., & Zhu, L. (2015). β2-Adrenoreceptor-mediated proliferation inhibition of embryonic pluripotent stem cells. Journal of Cellular Physiology, 230(11), 2640–2646. https://doi.org/10.1002/jcp.24937
- Wallace, K., Cornelius, D. C., Scott, J., Heath, J., Moseley, J., Chatman, K., & LaMarca, B. (2014). CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension (Dallas, Tex: 1979), 64(5), 1151–1158. https://doi.org/10.1161/HYPERTENSIONAHA.114.03590
- Yokoyama, S., Kinoshita, K., Muroi, Y., & Ishii, T. (2013). The effects of bilateral lesions of the mesencephalic trigeminal sensory nucleus on nocturnal feeding and related behaviors in mice. Life Sciences, 93(18–19), 681–686. https://doi.org/10.1016/j.lfs.2013.09.015
- Zhang, J., Feng, J., Ma, D., Wang, F., Wang, Y., Li, C., Wang, X., Yin, X., Zhang, M., Dagda, R. K., & Zhang, Y. (2019). Neuroprotective mitochondrial remodeling by AKAP121/PKA protects HT22 cell from glutamate-induced oxidative stress. Molecular Neurobiology, 56(8), 5586–5607. https://doi.org/10.1007/s12035-018-1464-3
- Zhang, G. X., Kimura, S., Nishiyama, A., Shokoji, T., Rahman, M., Yao, L., Nagai, Y., Fujisawa, Y., Miyatake, A., & Abe, Y. (2005). Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovascular Research, 65(1), 230–238. https://doi.org/10.1016/j.cardiores.2004.08.013
- Zhang, N., Liu, C., Jin, L., Zhang, R., Wang, T., Wang, Q., Chen, J., Yang, F., Siebert, H. C., & Zheng, X. (2020). Ketogenic diet elicits antitumor properties through inducing oxidative stress, inhibiting MMP-9 expression, and rebalancing M1/M2 tumor-associated macrophage phenotype in a mouse model of colon cancer. Journal of Agricultural and Food Chemistry. 68(40):11182–11196. http://doi.org/10.1021/acs.jafc.0c04041.
- Zhang, S., Qi, Y., Xu, Y., Han, X., Peng, J., Liu, K., & Sun, C. K. (2013). Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochemistry International, 63(5), 522–532. https://doi.org/10.1016/j.neuint.2013.08.008
- Zhuang, C., Liu, D., Yang, X., Wang, H., Han, L., & Li, Y. (2016). The immunotoxicity of aluminum trichloride on rat peritoneal macrophages via β2-adrenoceptors/cAMP pathway acted by norepinephrine. Chemosphere, 149, 34–40. https://doi.org/10.1016/j.chemosphere.2016.01.084
