Abstract
Regulation of stress reactivity is a fundamental priority of all organisms. Stress responses are critical for survival, yet can also cause physical and psychological damage. This review provides a synopsis of brain mechanisms designed to control physiological responses to stress, focusing primarily on glucocorticoid secretion via the hypothalamo-pituitary-adrenocortical (HPA) axis. The literature provides strong support for multi-faceted control of HPA axis responses, involving both direct and indirect actions at paraventricular nucleus (PVN) corticotropin releasing hormone neurons driving the secretory cascade. The PVN is directly excited by afferents from brainstem and hypothalamic circuits, likely relaying information on homeostatic challenge. Amygdala subnuclei drive HPA axis responses indirectly via disinhibition, mediated by GABAergic relays onto PVN-projecting neurons in the hypothalamus and bed nucleus of the stria terminalis (BST). Inhibition of stressor-evoked HPA axis responses is mediated by an elaborate network of glucocorticoid receptor (GR)-containing circuits, providing a distributed negative feedback signal that inhibits PVN neurons. Prefrontal and hippocampal neurons play a major role in HPA axis inhibition, again mediated by hypothalamic and BST GABAergic relays to the PVN. The complexity of the regulatory process suggests that information on stressors is integrated across functional disparate brain circuits prior to accessing the PVN, with regions such as the BST in prime position to relay contextual information provided by these sources into appropriate HPA activation. Dysregulation of the HPA in disease is likely a product of inappropriate checks and balances between excitatory and inhibitory inputs ultimately impacting PVN output.
It was a great honor for the senior author to present the inaugural Richard Kvetnansky lecture at the 12th International Symposium on Catecholamines and Other Neurotransmitters in Stress, held at Smolenice Castle in the summer of 2019. Richard was a consummate scientist and a strong advocate for the field of stress biology, and his absence is keenly felt.
The goal of this review is to provide a framework for understanding neurobiological mechanisms governing glucocorticoid responses to adverse events. For the purposes of framing the review with respect to the “problem” of stress, we begin with a brief conceptual introduction. The remainder of this contribution will be directed toward discussion of the neural basis of neuroendocrine responses to stressors, which has been a prevailing emphasis of our laboratory’ for the last 30+ years.
The “Stress” Concept: Origin and Evolution
Interest in stress harkens back to the early days of experimental medicine. The concept that physiological responses drive adaptation originated with Claude Bernard’s early consideration of the “milieu interieur” in the late 1800s (Bernard, Citation1865), and was a driving factor in Walter Cannon’s original exposition of homeostasis (Cannon, Citation1939). Selye first popularized the term “stress”-borrowed from engineering- to describe the “non-specific responses of the body to any demand upon it.” He based this definition on clinical observations, where he noted a common spectrum of symptoms present in a variety of disease conditions. These clinical observations were translated into basic research leading to a landmark paper published in Nature in 1936, showing that a variety of noxious conditions presented common physiological reactions, including adrenal hypertrophy, atrophy of lymphoid organs and gastric ulceration in rats (Selye, Citation1936). The former two observations are the result of elevated glucocorticoid secretion, which will be a guiding topic of this review.
Definition of “stress” emerged some time later (Selye, Citation1956, Citation1950). The definition above specifies that “stress” is defined as a “response,” rather than a cause. Over the years this definition has become clouded, as “stress” had moved from consequence to antecedent in popular parlance (indeed as fuzzily defined, stress can be both cause and effect). In deference to Selye’s definition, we will refer to causal factors as “stressors” (rather than “stress”).
Selye also noted that pleasurable or appetitive events are able to generate physiological indices of stress. He subsequently distinguished “distress” and “eustress,” with the former referring to responses to noxious, adverse or aversive stimuli, typical of what is usually thought of as “stress.” “Eustress” essentially refers to responses generated in positive as opposed to negative contexts, and reinforces the notion that physiological responses are required to help the organism perform optimally in both conditions. The distress-eustress continuum has proven hard to reconcile and difficult for the literature to navigate (Selye, Citation1975). Indeed, a consensus paper suggests limiting discussion of stress to negative conditions, so as to minimize ambiguous definitions (Koolhaas et al., Citation2011).
The distress-eustress distinction underscores the role of both neuroendocrine and autonomic regulation in energy balance. One can argue that the major so-called “stress systems,” the hypothalamo-pituitary-adrenocortical (HPA) and sympathoadrenomedullary axes, are primarily concerned with metabolism. Indeed, both systems mobilize energy at the level of the liver (gluconeogenesis, proteolysis and lipolysis in the case of glucocorticoids, glycogenolysis and glycolysis in the case of epinephrine), in an attempt to provide resources allowing the organism to adapt to challenges, be they in a negative or positive context. Indeed, this appears to be a principle role for both systems on an hour-to-hour and day-by-day schedule: their relationship with stressors is but one aspect of their primary function.
Activation of stress effectors can occur in response or anticipation of discrete events (acute stressors) or in prolonged fashion when confronted with persistent or intermittent stress exposures (chronic stress). Responses to acute stressors are generally considered adaptive, having the goal of mobilizing resources to meet bodily needs. In contrast, chronic activation of stress effectors can trigger or exacerbate pathologies, due to prolonged systemic drive, alterations in metabolic processes and/or immune dysregulation. As discussed below, pathways controlling acute and chronic responses to stressors can differ substantially, and one should not assume that chronic stress is simply the summation of individual acute responses.
Another important concept in stress is “allostasis,” a term defined by (Sterling & Eyer, Citation1988). “Allostasis” was initially proposed to explain the process of “stability through change,” as opposed to homeostasis, defined as the constant return to balance of the internal milieu in response to environmental stimuli. They postulated that the parameters proposed to be constant by the concept of homeostasis, could, in fact, have more than one optimal point of balance, depending on the external and internal circumstances the individual faces at any given moment.
This concept was later adopted by Bruce McEwen, who extended it by defining allostatic load as the "cost of chronic exposure to fluctuating or heightened neural or neuroendocrine response resulting from repeated or chronic environmental challenge” (McEwen & Stellar, Citation1993). These concepts contributed to include the cognitive functions of the individual (i.e. the memory and emotional experience with a particular stressor) as an important component of the adaptation machinery that is required for allostatic processes (Korte et al., Citation2005).
It is important here to consider the concept of emotion, since emotions drive both generation and interpretation of stressors. Early work in the field proposes that the physical responses are the root cause of emotions (the James-Lange theory of emotion; James, Citation1994). Although subsequently challenged (e.g. by Cannon, Citation1927), the physiology and emotion connection remains strong (James, Citation1994), and indeed has found new life in the somatic marker hypothesis championed by Damasio, which posits that physiological reactions can affect decision making (Damasio, Citation1996). The key feature to consider here is that emotions almost certainly occur in the context of stressors, and importantly that the physiological reactions occurring during stressors can be encoded as part of the interpreted emotion.
Dynamics of HPA axis regulation
Activation of the HPA axis occurs in a number of contexts. Importantly, the HPA axis exhibits a marked circadian rhythm, with peak secretion generally corresponding with the onset of the active part of the day-night cycle (e.g. onset of the light cycle in diurnal animals, onset of the dark cycle in nocturnal animals; Cascio et al., Citation1987; Jacobson et al., Citation1988). The circadian rise is thought to be important for mobilizing energy to meet the energetic needs of an awake, behaving organism. Glucocorticoids also rise in anticipation of meals (Feillet et al., Citation2006). The circadian peak is guided by input from the suprachiasmatic nucleus, based on endogenous rhythmicity and/or light cues (Cascio et al., Citation1987). Note that neither circadian nor metabolic release of glucocorticoids necessarily reflect a response to “stressors” per se, but rather fulfill the metabolic function of the of HPA axis (putting the “gluco(se)” in “glucocorticoid” through glyconeogenesis, promoting release of free fatty acids and amino acids by lipolysis and proteolysis, respectively).
Activation by stressors co-opts this important metabolic system to introduce glucocorticoids in order to meet external or internal challenges, real or perceived (Myers et al., Citation2014b). The important consideration here is that the HPA axis is NOT a canonical “stress system,” but rather a bodily system recruited by stressors. Consequently, all measures of HPA activation need to be weighed with respect to both stressor-elicited secretion and endogenous release patterns. The most striking need is consideration of circadian timing, which is critical to appreciating the meaning of baseline glucocorticoid changes in the context of stress and disease. Indeed, the current gold standard for measurement of human HPA axis reactivity is the cortisol awakening response (CAR), timed across a narrow window corresponding to awakening rather than clock time (Federenko et al., Citation2004; Stalder et al., Citation2016). Unfortunately, a sizable literature on human stress biology emerged prior to appreciation of the significance of the CAR, and we have not even begun to tackle the significance of anything resembling the CAR in animal models. Moreover, these concerns call the value of single-point assessment of HPA axis hormones into question, particularly during the active phase of the circadian cycle.
Activation of the HPA axis is mediated by a discrete population of neuroendocrine corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the hypothalamus (PVN; Antoni, Citation1986; Herman et al., Citation2016). These neurons produce other neuropeptides (e.g. arginine vasopressin (AVP) that enhance the efficacy of down-stream CRH action (Gillies et al., Citation1982). Hypophysiotrophic PVN CRH neurons project to the external lamina of the median eminence, whereby CRH and co-stored peptides/transmitters are released into portal veins and subsequently access anterior pituitary corticotropes to drive adrenocorticotropic hormone (ACTH) release. ACTH then travels to the adrenal cortex via the systemic circulation, driving synthesis and secretion of glucocorticoids and completing what is effectively a three-step amplification process (fg/ml CRH to pg/ml ACTH to ng/ml glucocorticoids; Herman et al., Citation2016). It is important to note that the amplification process is subject to adjustment at both the pituitary and adrenal. For example, glucocorticoid feedback (below) can block pituitary release of ACTH (Keller-Wood & Dallman, Citation1984), and sympathetic activation can enhance adrenal glucocorticoid production (Jasper & Engeland, Citation1997; Ulrich-Lai et al., Citation2006), respectively. Local effects at both the pituitary and adrenal are powerful modifiers of HPA output, and are unfortunately incompletely understood.
Glucocorticoids signal via interaction with two primary receptors, the mineralocorticoid receptor and glucocorticoid receptor (MR and GR; de Kloet et al., Citation1998). These receptors act via any of several mechanisms: as ligand activated transcription factors to drive or inhibit gene transcription; as intranuclear inhibitors of other transcription factors; or as membrane receptors providing for rapid glucocorticoid signaling (de Kloet et al., Citation2018; Oitzl et al., Citation1997). The array of signaling mechanisms provides for HPA axis influence on target systems in the time-frame of minutes (membrane) to days or even weeks (transcription; de Kloet et al., Citation2008). The MR is extensively bound by relative low levels of glucocorticoids, with nuclear-signaling receptors saturated at low circadian levels of glucocorticoids and leading some to suggest that it is important in rhythmic actions of hormone (Bradbury et al., Citation1991; De Kloet & Reul, Citation1987). Recent evidence suggests that the affinity of the membrane-bound receptor may be substantially lower, allowing it to operate at higher, stress levels of glucocorticoid secretion (Joëls, Citation2006). The GR is traditionally thought to govern the metabolic and stress actions of glucocorticoids, as this receptor is extensively occupied only at the circadian peak or following stress activation of hormone release (Reul & de Kloet, Citation1985). There is some evidence to suggest that the two receptors heterodimerize in the cell nucleus, which may affect which genes are expressed or inhibited (Trapp et al., Citation1994), although this has been debated of late (Pooley et al., Citation2020). Of note, RNAseq studies indicate that despite the high homology present in the DNA binding domain of MR and GR, occupation of the respective receptors produces vastly different patterns of gene transcription (Van Weert et al., Citation2017).
Glucocorticoid receptors are widely (but not ubiquitously) expressed in the central nervous system (Ahima & Harlan, Citation1990; Herman, Citation1993), and have physiological actions in nearly every region tested, ranging from the brainstem to prefrontal cortex. Similarly, MR is also expressed in a variety of regions (Ahima et al., Citation1991), including several where colocalization with GR is documented (e.g. hippocampus; de Kloet et al., Citation1998) or highly likely.
It is important to note that corticosteroids are released from the adrenal in a pulsatile manner, with a frequency of roughly 3 hours, entrained in accordance with pulsatile pituitary ACTH release (Sarabdjitsingh et al., Citation2012; Windle et al., Citation2013). The net impact of a stressor on corticosteroid release is dependent on pulsatility, as imposition on the rising phase of a pulse will enhance net secretion of steroid, whereas the opposite is true on the falling phase (Lightman et al., Citation2020). Pulsatile release is critical for appropriate glucocorticoid signaling, as blocking pulsatile release by constant delivery of glucocorticoids impairs GR nuclear translocation and thereby modifies glucocorticoid actions on transcription (Russell et al., Citation2015; Sarabdjitsingh et al., Citation2010).
Feedback Control of the HPA Axis
Activation of the HPA axis is limited by glucocorticoid negative feedback inhibition at several levels, ranging from peripheral tissues (e.g. fat) to brain (de Kloet & Herman, Citation2018; Myers et al., Citation2012). Negative feedback is best thought of as a multifaceted process, with glucocorticoid signals communicated largely by GR binding in target cells. Similarly, GR in pituitary corticotropes reduce expression of ACTH, limiting the capacity of the brain to drive the HPA axis, and as we shall see below, GR acts in the brain (e.g. hypothalamus, hippocampus, prefrontal cortex, etc.) to limit activation of HPA axis responses to stressors (Ding et al., Citation2019; Herman et al., Citation2016; Keller-Wood & Dallman, Citation1984; Levin et al., Citation1988). Finally, glucocorticoids can also regulate the HPA axis via peripheral mechanisms: for example, our group has shown that deletion of GR in adipocytes inhibits the HPA axis, likely through actions of end-products generated by GR action (e.g. free fatty acids generated by lipolysis; de Kloet & Herman, Citation2018).
Glucocorticoid feedback acts across multiple time domains via multiple mechanisms (Keller-Wood & Dallman, Citation1984). Traditional genomic effects occur at relatively long latencies (hours) and can initiate transcriptional and functional changes that persist beyond the period of active glucocorticoid secretion. Transcriptional actions can involve direct DNA binding to either increase (glucocorticoid response elements, GREs) or decrease (negative or nGREs) gene transcription, and perhaps are the most commonly studied. Ultimate transcriptional actions of GR (or MR) will depend on interactions with nuclear coactivators (e.g. Src) that control histone acetylation/deacetylation and ultimately associations with the transcriptional machinery (Lachize et al., Citation2009; Meijer et al., Citation2005). Additional evidence supports the ability of GR to bind other nuclear transcription factors (e.g. AP1, NFkB complexes) and modulate their transcriptional actions, providing for non-GRE dependent effects on gene expression (McKay & Cidlowski, Citation1998; Yang-Yen et al., Citation1990).
A wealth of data dating back to early studies by Dallman indicates the ability of glucocorticoids to inhibit HPA axis drive within minutes, so-called “fast feedback” (Dallman & Yates, Citation1969). In vitro electrophysiological studies from the Tasker group found that PVN neurons are inhibited by glucocorticoids at the level of the membrane, accomplished via G-protein dependent release of endocannabinoids and nitric oxide, which inhibit glutamatergic and enhance GABAergic synaptic currents, respectively, in the PVN (Di et al., Citation2003). Our group subsequently verified endocannabinoid-mediated membrane fast feedback inhibition in vivo as well (Evanson et al., Citation2010). Glucocorticoid fast feedback is blocked by PVN deletion of GR in vitro and in vivo, indicating that actions are likely mediated by membrane-associated GRs (Nahar et al., Citation2015; Solomon et al., Citation2015). While immunohistochemical studies support the existence of GR (or GR-like) molecules at or near the cell membrane (Jafari et al., Citation2012; Johnson et al., Citation2005), the exact nature of the signaling process is unknown. Rapid GR action is also observed at the level of the corticotropes, where fast feedback appears to be mediated by annexin 1 A signaling (Buckingham et al., Citation2003).
Recent data suggest that GR may also signal via ligand-independent mechanisms. Use of non-membrane-permeant GR ligands (dexamethasone-bovine serum albumin conjugate, DEX-BSA) have revealed the ability of membrane-bound GR to elicit Akt mediated nuclear translocation of unliganded GR. Nuclear GR translocated as a result of DEX-BSA treatment does not appear to directly drive transcription via binding to consensus glucocorticoid response elements (GREs) and promotes transcription of genes distinct those regulated by DEX alone, implying interaction with alternative transcription mechanisms (Rainville et al., Citation2019). The data indicate further complexities associated with cellular GR signaling as a result of membrane GR binding.
Hypothalamic Mechanisms of HPA Axis Regulation
In general, glucocorticoid feedback directly or indirectly inhibits PVN neurons driving the neuroendocrine cascade. Electrophysiological studies show that neurons in the PVN are inhibited, as indicated by a reduction in mEPSC frequency and enhanced mIPSC frequency during the rapid negative feedback of the HPA axis (Di et al., Citation2003; Joëls, Citation2006). Lesion and implant studies suggest strong hypothalamic involvement in feedback, e.g. local implants of glucocorticoid pellets in the region of the PVN can attenuate corticosterone and ACTH responses to stressors (corticosterone; Feldman et al., Citation1992). In addition, local dexamethasone implants inhibit enhanced CRH and AVP immunoreactivity (Kovacs & Mezey, Citation1987) and mRNA expression driven by adrenalectomy (Sawchenko, Citation1987). Conversely, conditional knockdown of GR in PVN (and SON) neurons (using Sim1-driven expression of Cre recombinase to delete exon 1 C-2 or exon3) enhanced both basal and stress-induced ACTH and corticosterone release in male mice (Laryea et al., Citation2013). Results in females are more variable, depending on the exon targeted for deletion: females with deletion of exon 1 C-2 exhibit minimal HPA axis dysfunction, whereas the impact of exon 3 deletion is equivalent to males, suggesting a potential sex difference in the importance of GR (Laryea et al., Citation2013; Solomon et al., Citation2015). As noted above, Sim1-mediated deletion of GR blocks rapid inhibitory effects of glucocorticoids on PVN neurons (Nahar et al., Citation2015), suggesting that fast feedback actions may be linked to GR in or near the cell membrane. Overall, actions at the PVN are clearly glucocorticoid negative feedback effects.
Drive of the HPA axis may also be inhibited by input from PVN-projecting hypothalamic neurons. A large proportion of hypothalamic neurons are GABAergic, and more than 50% of synaptic inputs on PVN neurons are inhibitory, suggesting a prominent role for local inhibition on HPA axis regulation (Decavel & Van Den Pol, Citation1990). Indeed, the PVN is surrounded by a shell of GABAergic neurons. These local circuit neurons are in the projection fields of afferents from regions such as the hippocampus, lateral septum and raphe nuclei (Herman et al., Citation2002). Blockade of ionotropic glutamate receptors in this peri-PVN region increases corticosterone responses to stress (Ziegler & Herman, Citation2000), suggesting that these local neurons may be involved in mediating trans-synaptic inhibition by relaying extrahypothalamic input ().
Figure 1. Neural mechanisms of acute stress inhibition. As noted, the CRH containing region of the medial parvocellular paraventricular nucleus (PVN) receives substantial inhibitory input from hypothalamic (medial preoptic nucleus (mPOA), dorsomedial nucleus (DMH), periPVN zone and medial forebrain structures (bed nucleus of the stria terminalis, BST). The regions receive excitatory inputs from forebrain structures such as the IL infralimbic (IL) and prelimbic (PL) cortices and the ventral subiculum (vSUB), which are thought to mediate trans-synaptic inhibition of HPA axis stress responses. Upstream limbic pathways may also limit drive of the PVN by way of local, intranuclear inhibition of HPA axis excitatory circuits, e.g. the nucleus of the solitary track (NTS) and/or posterior hypothalamus (PH). Open red circles and red lines: inhibitory (e.g. GABAergic) neurons/connections; closed green circles and green lines: excitatory (e.g. glutamatergic) neurons and connections. Figure modified from Herman et al. (Citation2005), with permission, and were created with Biorender.com.
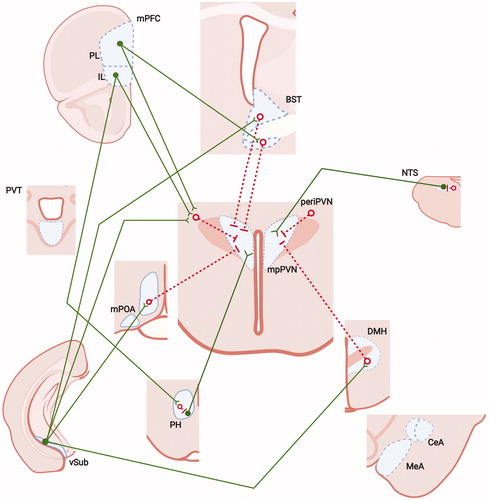
Several nuclei within the hypothalamus act to modulate HPA axis drive, including the medial preoptic area (mPOA), dorsomedial hypothalamic (DMH) and posterior hypothalamic (PH) nuclei (). Lesion studies suggest that the medial preoptic area plays a role in inhibition of the HPA axis following stress exposure. This hypothalamic region is rich in gonadal steroid receptors, and is thought to mediate the inhibitory effects of testosterone on HPA axis reactivity (Viau & Meaney, Citation1996). The DMH sends both glutamatergic and GABAergic inputs to the PVN (Bailey & Dimicco, Citation2001; Roland & Sawchenko, Citation1993), and indeed, its role in HPA axis integration appears to vary by subregion: inhibition of the ventromedial component reduces corticosterone responses to stressors, whereas dorsolateral DMH activation drives ACTH release (Bailey & Dimicco, Citation2001; Herman et al., Citation2003). GABAergic neurons in the ventromedial DMH are Fos-activated by acute stress and project to the PVN (Cullinan et al., Citation2008), suggesting a local circuit capable of stress inhibition.
Recent work from our group and that of Campeau note a strong stress-regulatory PVN input from the posterior hypothalamic area (PH; Myers et al., Citation2016; Nyhuis et al., Citation2016). This region is particularly interesting given its close link to drive of autonomic responses and defensive behaviors. Targeted inactivation and activation studies (using muscimol and bicuculline, respectively) indicate that the output of PH (in particular its rostral component) activates ACTH secretion, corticosterone release and PVN Fos expression elicited by acute noise or restraint, and drives aggressive and threat avoidance behaviors (Myers et al., Citation2016; Nyhuis et al., Citation2016). These effects appear to be mediated by direct excitatory projections to PVN CRH neurons, likely to be glutamatergic and/or peptidergic in phenotype. The effects of PH inactivation appear to inhibit habituated HPA axis responses to noise and restraint, suggesting temporally distinct actions on HPA axis regulation. Anatomical data indicate that stress-activated neurons in the infralimbic cortex (IL) and lateral septum send projections to the PH, with the IL preferentially targeting resident GABA neurons that likely convey intra-nuclear inhibition to PH output neurons (Myers et al., Citation2016; Nyhuis et al., Citation2016; ).
Figure 2. Neural mechanisms of acute stress excitation. Data suggest PVN neurons can be driven by neurons communicating homeostatic challenge, including the nucleus of the solitary tract (NTS), among others. The PVN also has numerous connections with hypothalamic nuclei and subcortical telencephalic structures, including excitatory (PH, anterior BST) and inhibitory (POA, DMH, periPVN, anteroventral BST, posterior BST) inputs. Inhibitory input to the PVN provides a substantial inhibitory tone, which can be disrupted by inhibition from upstream sites such as the medial and central amygdaloid nuclei (MeA, CeA), providing a mechanism for trans-synaptic disinhibition from the limbic forebrain. There is also some evidence suggesting that some cortical regions, such as the infralimbic region (IL) of the medial prefrontal cortex (PL), may also provide trans-synaptic excitation, perhaps via relays in the brainstem. There is less evidence for excitatory input from other forebrain stress circuits, such as the ventral subiculum (vSUB), prelimbic division of the mPFC or paraventricular thalamus (PVT). Input from limbic regions may also access the PVN by interaction with local interneurons in the PVN surround (periPVN). See legend for abbreviations and symbol definitions). Figure modified from Herman et al. (Citation2005), with permission, and were created with using Biorender.com.
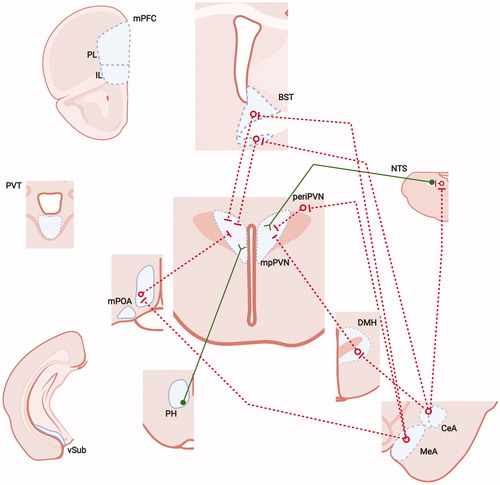
The hypothalamus also plays a major role in homeostatic regulation, being largely responsible for coordination of metabolism, thermoregulation, fluid/electrolyte balance and sleep. Disruption of any of the above processes can activate the HPA axis, consistent with the need to provide energy to meet current or potential emergencies. For example, negative energy balance promotes activation of melanocortin neurons in the arcuate nucleus that may in turn activate CRH neurons and result in increased ACTH and corticosterone release (Bell et al., Citation2000; Liu et al., Citation2007).
Top-down regulation by cortical and limbic region
Hippocampus
Glucocorticoid signaling in the hippocampus is perhaps a prime example of distant regulatory processes controlling the PVN. Lesion, stimulation and steroid infusion studies were used in the 1970s to 1990s to support a preeminent role of the hippocampus in feedback inhibition of the HPA axis (Herman et al., Citation2003; Jacobson & Sapolsky, Citation1991). This hypothesis was further supported by rich expression of GR as well as MR in hippocampal neurons (Herman et al., Citation1989; Van Eekelen et al., Citation1988). Lesion studies (including our own) indicate that extensive hippocampal damage, fornix sections or ibotenic acid lesions of the dorsal and ventral hippocampus increase PVN CRH mRNA expression and enhance corticosterone responses to stress (see summary in Herman et al., Citation2003; Radley & Sawchenko, Citation2011). These results were not replicated in all studies (Bradbury et al., Citation1993; Coover et al., Citation1971; Tuvnes et al., Citation2003), perhaps related to stimulus-dependent hippocampal control of HPA axis responses. For example, hippocampal damage causes hypersecretion of corticosterone when exposed to stimuli connected with novel environments/situations (e.g. restraint, open field exposure, novelty), but not to interoceptive cues (e.g. ether inhalation, hypoxia; Herman et al., Citation1998; Mueller et al., Citation2004; Radley & Sawchenko, Citation2011). Together, these data argue against a generalized or obligate role for the hippocampus in HPA axis negative feedback.
The ventral hippocampus is implicated in inhibition of the HPA axis, via relays to cortical output neurons in the ventral subiculum (SUBv). Neurons in this region are stress-sensitive and project to a number of sub-cortical regions that in turn project to the PVN, but not the PVN proper, including the bed nucleus of the stria terminalis (BST), the medial preoptic area, and dorsomedial and posterior hypothalamic nuclei (Cullinan et al., Citation1995, Citation1993; Myers et al., Citation2016; ). Importantly, PVN-projecting neurons are apposed by terminals of SUBv projection neurons, suggestive of synaptic relay. Moreover, the vast majority of PVN inputs from the BST and preoptic area are GABAergic in nature, implying a mechanism for trans-synaptic inhibition of the HPA axis (Cullinan et al., Citation1993). The nature of these connections indicates that inhibitory effects of the hippocampus on the HPA axis require an intermediary neuron.
Finally, the temporal dynamics of ventral subiculum regulation of HPA axis function are worthy of comment. Specific damage to this region (as opposed to whole hippocampal lesion) results in an enhanced or prolonged peak in corticosterone release (Herman et al., Citation1998, Citation1995), rather than delayed shut-off reported following large hippocampal lesions (Sapolsky et al., Citation1984). The impact of SUBv lesions appears to enhance responsiveness to stressors rather than return to baseline. Thus, actions of the SUBv likely trigger trans-synaptic PVN inhibition, functioning to shut down the corticosterone response more quickly.
Prefrontal cortex
Lesion studies indicate that the infralimbic (IL) and prelimbic (PL) divisions of the medial prefrontal cortex function as modulators of HPA axis output (Note that some of the earlier studies do not parse medial prefrontal subregions, in which case the region will be referred to as IL/PL or the ventromedial prefrontal cortex (vmPFC). Damage to or inactivation of the IL/PL and PL increase corticosterone and ACTH responses to restraint or novelty but not hypoxia or ether, suggesting that like the hippocampus, these regions integrate stimuli relevant to anticipated threat rather than physical challenge (Diorio et al., Citation1993; Figueiredo et al., Citation2003). However, closer examination of the literature reveals a more nuanced role in HPA integration: in response to repeated challenge, damage to the right (but not left) vmPFC reduces corticosterone secretion (Sullivan & Gratton, Citation1999), suggesting an excitatory (and lateralized!) role for this region with repeated homotypic stress.
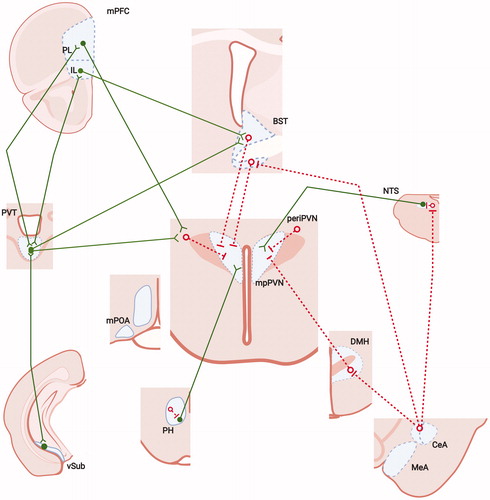
The IL and PL express both GR and MR in abundance (Ahima et al., Citation1991; Ahima & Harlan, Citation1990; Herman, Citation1993) and GR-containing subpopulations of neurons are activated under conditions of acute stress (Ostrander et al., Citation2003). vmPFC GR is down-regulated under conditions of chronic stress (Mizoguchi et al., Citation2003), and chronic stress drives increased IL and PL deltaFosB expression (Flak et al., Citation2012), consistent with a putative role in temporal processing of repeated stressors. (i.e. chronic stress; ). Corticosterone implants into the vmPFC inhibit HPA axis responses to restraint but not ether (Diorio et al., Citation1993), suggesting that the vmPFC uses glucocorticoid signals to modify output relevant to anticipatory stimuli. We have gone on to demonstrate regional specificity of GR signaling via viral vector-based knockdown of GR in the IL and PL. Our data indicate that GR knockdown in both regions increases HPA responsiveness to acute restraint (McKlveen et al., Citation2013). More recently we have used a recently-developed GR flox rat in conjunction with a CaMKII-driven Cre recombinase to confirm that specific deletion of GR in PL projection neurons enhances acute stress reactivity (Scheimann et al., Citation2019). Importantly, knockdown of GR in the IL (but not PL) was sufficient to enhance HPA axis reactivity (as well as passive coping behavior) in the context of chronic stress, indicative of a role in processing stress chronicity (McKlveen et al., Citation2013). These data are recapitulated by knockdown of glutamate vesicular packaging in IL neurons (Myers et al., Citation2017), attesting to a role for the IL in control of HPA axis responses to repeated challenge ().
Figure 3. Neural mechanisms controlling chronic stress regulation of the HPA axis. Pathways responsible for drive of the HPA axis under chronic stress are not as well understood as those mediating acute response. There is strong evidence that the PVT, which is not involved in acute stress excitation or inhibition, is required for both stress habituation and stress facilitation, suggesting a role in communicating stress chronicity. Importantly, the PVT has extensive reciprocal projections to the IL, PL and vSUB, as well as projections to the area of the BST. Neuronal activation studies indicate the existence of a small network of structures that are differentially activated by chronic unpredictable stress (relative to restraint), including the IL, PL, PH and NTS. The NTS itself appears to contribute to chronic stress-related HPA drive via peptidergic neurons. Importantly, the PH and NTS are both connected with the IL, and both mediate acute stress excitation, suggesting a possible integrated circuit mediating chronic stress drive. Finally, chronic stress increases tone of CRH-expressing stress circuitry in the CeA, suggesting that CRH systems may be recruited by chronic stress and participate in HPA axis hyperdrive. See legend for abbreviations and symbol definitions). Figure modified from Herman et al. (Citation2005), with permission, and were created with Biorender.com.
In all cases, IL and PL manipulations fail to alter baseline HPA axis activity and, unlike the hippocampus, do not appear to affect long-term shut-off. Primary actions are evident as potentiation or prolongation of peak corticosterone activation. As was the case for the hippocampus, the IL and PL have few if any direct projections to the PVN, showing strong projections to many of the same structures innervated by the SUBv, most notably the BST (Radley et al., Citation2009).
Finally, it appears that IL/PL GR enhances activation of cortical projection neuron output, which would be roughly consistent with drive of trans-synaptic inhibition. GR activation can enhance glutamatergic transmission in layer V PL pyramidal neurons after acute stress. This effect is thought to be mediated by increased expression of post synaptic AMPA and NMDA receptors (Joëls et al., Citation2012; Yuen et al., Citation2011, Citation2009). Similar effects on glutamatergic signaling following stress and subsequent GR activation are also seen in the hippocampus (Karst & Joëls, Citation2005). Further work in PL suggests that GR binding inhibits GABAergic interneurons, thereby affording activation of cortical outflow (Hill et al., Citation2011). Recent data from our group demonstrates that chronic stress enhances inhibitory input onto IL projection neurons, an observation that is correlated with reduced GR expression in cortical interneurons (McKlveen et al., Citation2016).
Amygdala
In contrast to the hippocampus and vmPFC, the HPA axis appears to be activated by input from the amygdala, mediated primarily by the medial (MeA) and central (CeA) nuclei (Ulrich-Lai & Herman, Citation2009). The MeA and CeA lack substantial connectivity with the PVN, and appear to modulate HPA axis function by interactions with intermediary neurons in the same nuclei receiving input from the hippocampus and vmPFC, e.g. the BST, DMH, PH and mPOA (Myers et al., Citation2016, Citation2014a; ). As the majority of MeA and CeA projections are GABAergic (Swanson & Petrovich, Citation1998), it is thought that activation of the HPA axis by these structures is mediated by disinhibition, i.e. blockade of tonic PVN inhibition via direct GABAergic projections in these regions. Whereas there is overlap of limbic innervation to all subcortical regions targeted by descending HPA axis-regulatory regions, the relative weighting of projections differs across the various subcortical targets (Herman et al., Citation2003; Ulrich-Lai & Herman, Citation2009).
The MeA and CeA appear to have complementary roles in mediating different stressor modalities. Lesions of the MeA diminish stressor responses to psychogenic (restraint) but not systemic (interleukin-1 beta) stimuli, whereas CeA damage impairs responses to systemic (IL-1beta) but not psychogenic (restraint) stimuli (Dayas et al., Citation2001, Citation1999). Stressor selectivity is also evident in patterns of cellular activation in the two nuclei, with the MeA evincing strong Fos activation by psychogenic stress such as restraint, the CeA by immune stimuli, hypovolemia and pain (Dayas et al., Citation2001).
Complementary roles of MeA and CeA afferents are commensurate with the very different biological processes controlled by the two regions. The MeA is in receipt of considerable olfactory input and is thought to be critical in control of social behavior and aggression (Chen et al., Citation2019; Haller, Citation2018; Sah et al., Citation2003). The CeA receives information from the thoracic and abdominal viscera and is an important regulator of autonomic nervous system responses (Viltart et al., Citation2006; Browning and Travagli, Citation2014). Both regions receive excitatory input from the basolateral amygdala (BLA; Sah et al., Citation2003), which is in receipt of a variety of multimodal sensory input and suggests it may play a “gate-keeper” function in driving MeA and CeA neurons controlling HPA axis stressor responses. Indeed, the BLA acts as a critical node in many of the behaviors associated with stress, including fear and anxiety (Janak and Tye, Citation2015). The exact role of the BLA in the control of the HPA axis has been difficult to pin down, possibly due to intrinsic heterogeneity of neurons responsive to positive or negative valence.
In contrast to the hippocampus and PFC, chronic glucocorticoids and stressor exposure stimulate dendritic plasticity in the BLA (McEwen et al., Citation2016; Vyas et al., Citation2002). Increased plasticity (in the form of dendritic hypertrophy and greater synaptic connectivity) here is thought to promote, rather than oppose, stress responsivity, especially to chronic stressors (Ashokan et al., Citation2016; Mitra et al., Citation2005). These effects have been linked to increased GR translocation and activation of downstream pathways in the BLA (Novaes et al., Citation2017). Moreover, in contrast with actions in the PVN, glucocorticoids promote CRH synthesis in the CeA, which is linked to chronic stress-related pathology (Dallman et al., Citation2003; ). The molecular mechanism underlying positive regulation of CRH is linked to tissue-specific alternative splicing of the GR binding partner Src, which dictates the directionality of GR transcription in this region (Lachize et al., Citation2009; Zalachoras et al., Citation2013).
Thalamus
The posterior paraventricular nucleus of the thalamus (PVT) appears to be selectively involved in control of HPA axis responses to chronic rather than acute stress. Lesions of this region block sensitization of the HPA axis by chronic cold exposure, and prohibit habituation of HPA axis responses to homotypic stress (Bhatnagar et al., Citation2002; Bhatnagar & Dallman, Citation1998). Moreover, habituation of HPA axis responses can be blocked by chronic inhibition of both GR and MR in this region, suggesting that glucocorticoid signals are essential to this process (Jaferi et al., Citation2003). The PVT has extensive projections to amygdala, prefrontal and ventral subiculum regions involved in HPA axis regulation (above), and in turn receives input from each region (Li & Kirouac, Citation2012; Vertes et al., Citation2015). Given the strong data supporting effects of the PVT on HPA drive by chronic stress, it is possible that these reciprocal projections may play a role in tuning the overall responsiveness of the HPA axis to repeated or prolonged stress (Hsu et al., Citation2014), perhaps via coordinated action across excitatory and inhibitory stress nodes.
Hindbrain mechanisms: nucleus of the solitary tract (NTS)
Physiological adversity reliably drives activation of the HPA axis, likely as a mechanism to generate energy resources to meet the challenge. Homeostatic perturbations are relayed in part by neurons resident in the hindbrain, which are responsible for relaying interceptive sensory information to the PVN. The NTS plays a particularly important role in this process, as it receives ascending vagal information from the thoracic and abdominal viscera (and perhaps immune system) and heavily innervates the PVN (Myers et al., Citation2017). The NTS sends heavy catecholaminergic (noradrenergic (NE) and adrenergic (E; Cunningham et al., Citation1990; Cunningham & Sawchenko, Citation1988) and non-catecholaminergic (for example, glucagon-like peptide-1 (GLP-1; Ghosal et al., Citation2013) projections to the PVN, preferentially targeting the CRH-containing medial parvocellular subdivision. Importantly, catecholaminergic NTS neurons do no co-express GLP-1 (Maniscalco & Rinaman, Citation2017), indicating that these cell populations are distinct and raising the possibility of differential actions on the HPA axis (see below).
Damage to ascending NTS pathways thought to contain PVN-projecting axons (6-hydroxydopamine lesions of ventral noradrenergic bundle/NTS or saporin-conjugated anti-DBH infusion directly into the NTS) cause reductions in ACTH and corticosterone release and/or PVN Fos induction to homeostatic perturbations (e.g. ether, cytokine injection, glucose deprivation) but not stressors of a more psychogenic nature (restraint, footshock, swim; Bundzikova-Osacka et al., Citation2015; Flak et al., Citation2014; Gaillet et al., Citation1993, Citation1991; Ritter et al., Citation2003), suggesting stressor specificity. Notably this specificity does not extend to NTS GLP-1 neurons, which modulate responses to psychogenic as well as physical stressors (Ghosal et al., Citation2017). Overall, the literature indicates that direct NTS projections to the PVN stimulate HPA axis responses to acute stressors () in a neuronal cell type-specific manner.
The NTS also mediates HPA axis responses to chronic stress exposure. Chronic variable stress (CVS) promotes drive of TH mRNA and protein expression in NTS catecholaminergic neurons, but markedly reduces expression of the GLP-1 precursor preproglucagon (GCG; Zhang et al., Citation2010). Despite presumptive increases in biosynthesis, lesions of ascending NTS NE and E neurons do not attenuate enhanced HPA axis drive following CVS (Flak et al., Citation2014). In contrast, intracerebroventricular supplementation of GLP-1 in rat enhances HPA axis drive following CVS. In contrast, infusion of a GLP-1 receptor antagonists of selective deletion of the GLP-1 receptor in the PVN blocks CVS potentiation of HPA axis stress responses (Tauchi et al., Citation2008). Thus, GLP-1 but not catecholamine neurons appear to be required for chronic stress-induced HPA axis sensitization ().
Catecholaminergic and GLP-1 neurons in the NTS express GR (Harfstrand et al., Citation1986; Rinaman, Citation2011), suggesting the potential for glucocorticoid regulation of output. Local implants of pellets containing the GR antagonist mifepristone increase drive of the HPA axis by an acute stressor, whereas implants of corticosterone reduce stress-induced corticosterone secretion. Moreover, local mifepristone implants increase resting corticosterone secretion following chronic stress and potentiate HPA axis sensitization following CVS (Bechtold et al., Citation2009; Ghosal et al., Citation2014), consistent with blockade of negative feedback effects on the NTS. These data are consistent with GR-mediated feedback inhibition of HPA axis responding via the NTS. Given that 1) GLP-1 neurons are differentially implicated in potentiation of responses to chronic stress, and 2) GCG mRNA expression and GLP-1 immunoreactivity are decreased by CVS exposure (Ghosal et al., Citation2017; Zhang et al., Citation2010), it is plausible that effects of GR are mediated by attenuation of GLP-1 drive to PVN neurons.
The role of the NTS in both physical vs. psychogenic stress is somewhat at odds with the notion of specific circuitry mediating two distinct classes of stressors, a hypothesis put forward by our group and Paul Sawchenko’s in the late 1990s (Herman et al., Citation1998; Li et al., Citation1996). The newer data suggest that the NTS may instead be a common conduit of stress integration of multiple modalities, with psychogenic stressor responses and chronic stress reactivity likely controlled by non-catecholaminergic neurons, perhaps via descending inputs from limbic projection regions (such as the ventromedial prefrontal cortex and central amygdaloid nuclei; Schwaber et al., Citation1982; van der Kooy et al., Citation1984).
Putting It All Together: Role of the Bed Nucleus of the Stria Terminalis
One common feature shared by all of the above regions is a connection with the BST (). The BST is known to play a role in anxiety processing (as opposed to fear), becoming important in generation of responses to stimuli that do not necessarily predict a defined outcome (Avery et al., Citation2016; Walker et al., Citation2003; a function well connected with general conceptualization of stress). Recent work highlights a role for the BST in the development and expression of contextual conditioning, implicating the BST as an interpreter of contextual information (Goode & Maren, Citation2017; Luyck et al., Citation2020), a process consistent with generating responses in anticipation of potential threat.
The BST is intimately involved in control of HPA axis responses to stressful stimuli. Early work from our group demonstrated a complex role for the BST in stress: lesions of the anteromedial divisions of the BST inhibit HPA axis responses to stress, whereas lesion of posterior nuclei enhanced HPA axis stressor reactivity (Choi et al., Citation2007). Recent work further identifies a specified role for the anterolateral region in HPA axis activation, conferring inhibition of stressor responses via PL projections (Radley et al., Citation2009; Radley & Johnson, Citation2018). Notably, the role of the anteromedial region appears to change under conditions of chronic drive, becoming stress-inhibitory rather than stress-excitatory (Choi et al., Citation2008). The mechanism underlying this switch is unclear but could be related to the complex neurotransmitter content of BST-PVN connections, which express both GABA and CRH, the latter a predominantly excitatory neuropeptide. As neuropeptides are generally secreted at more intense levels of stimulation than classical transmitters (Mains & Eipper, Citation1999), it is possible that alterations in BST drive in acute vs. chronic stress may differential favor downstream activation or inhibition of PVN neurons. It is also important to consider that chronic stress and/or corticosterone administration can reverse the chloride gradient in PVN neurons, causing GABA to be excitatory rather than inhibitory (Bains et al., Citation2015; Inoue & Bains, Citation2014).
The BST receives input from all the purported stress regulatory regions listed above. The distribution of inputs can vary somewhat by BST subregion. For example, the CeA heavily innervates the anterolateral and anteroventral regions of the BST, whereas the MeA projects heavily to posterior subnuclei (Dong et al., Citation2001). In some cases, inputs from limbic regions can innervate the same BST neurons: in fact, dual tracing studies suggest direct innervation of individual BST neurons by both PL and SUBv projectors, consistent with the ability of BST neurons to summate influences from different HPA regulatory pathways (Radley & Sawchenko, Citation2011). Connections between the IL/PL and BST are thought to mediate inhibition of HPA axis responses to acute stressors (Radley et al., Citation2009; Spencer et al., Citation2005). Moreover, the anterior BST has extensive interactions with the NTS, being a major target of both catecholaminergic and GLP-1ergic neurons (Bundzikova-Osacka et al., Citation2015; Ghosal et al., Citation2013; Maniscalco & Rinaman, Citation2017). Thus, the BST is at a crossroads for handling of information from a variety of stress effector systems.
The intrinsic organization of the BST is itself complex. Anterior subnuclei are generally associated with anxiety and emotional responses, including HPA axis activation, and appear to mediate BST potentiation of fear and drug relapse and reinstatement (Miles & Maren, Citation2019; Ressler et al., Citation2011), whereas the posterior BST subnuclei have rich connections with structures linked with agonistic and social behavior (e.g. MeA; Dong et al., Citation2001). In addition, the BST also has intrinsic interconnections across subnuclei that may differentially interface with input from prefrontal and amygdala projections (Gungor & Paré, Citation2016).
The BST is known to play a major role in processing of emotional responses to context. Prior studies indicate that the BST plays a major role in non-associative sensitization of startle (Davis et al., Citation2010; Walker & Davis, Citation1997) and in expression of contextual fear (Ali et al., Citation2012; Sullivan et al., Citation2004; Zimmerman & Maren, Citation2011).The BST is also critical for reinstatement of contextual fear responses following extinction (Goode et al., Citation2015; Waddell et al., Citation2006). Finally, activation of the BST is required for stress-related relapse of drug and alcohol self-administration (see reviews of Centanni et al., Citation2019; Goode & Maren, Citation2019). Together the data suggest that the BST is involved in processing information on the overall significance of stressors, integrating inputs from corticolimbic as well as hindbrain structures involved in determining the stimulus significance and salience. The net output of the BST then broadly affects how the organism responds to stress, adjusting behavioral, autonomic as well as HPA axis reactions in accordance with its “interpretation” of the summated neuronal input
Summary
Regulation of the HPA axis is of critical importance to the organism, given the behavioral and energetic signal carried by glucocorticoid hormones. Glucocorticoids act in a variety of body compartments and by numerous neuronal systems to achieve a net signaling level that optimizes the response of the organism to stressors. Secretion is regulated by diverse somatic and psychological signals and held in check by a diverse feedback system designed to “dial in” responses to best meet the real or perceived challenge. The checks and balances system is by no means perfect, and disruption by chronic drive or disease can move response magnitude and/or signaling capacity into either inadequate or over-reactive ranges, with potentially important consequences. In some cases, responses to stressors may be “adaptive” in nature: for example, habituation of stressor responses limits potential damage associated with glucocorticoid over-reactivity, and one can argue that sensitization of HPA axis responses to chronic stress may promote readiness of the organism to cope with a “now hostile” world. In contrast, inappropriate secretory activity resulting from developmental adversity, aging or disease may result in out-of-context hypo- or hypersecretion, generating “stress” reactions under conditions where stress responses are not beneficial (or even harmful, i.e. allostatic overload). Overall, the weight of evidence indicates that management of stress is above all a network problem, requiring integration of a substantial array of signals that link psychological responses to the internal state of the organism. Consequently, convergent inputs from regions such as the BST, hypothalamic nuclei and perhaps the NTS send sensory-processed and salience-adjusted information to the PVN, where it is integrated into a go- no go response. This response is then adjusted by neuronal and hormonal feedback signals designed to provide temporal control of the responses. It is this exquisite balance of appropriate drive and hormonal/neuronal inhibition that provides life-long efficiency of stressor responses and limits disruptions in physiology and behavior.
Acknowledgements
Submitted for the Special Issue: 12th International Symposium on Catecholamines and Other Neurotransmitters in Stress.
Disclosure statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Ahima, R. S., & Harlan, R. E. (1990). Charting of Type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience, 39(3), 579–604. https://doi.org/10.1016/0306-4522(90)90244-X
- Ahima, R. S., Krozowski, Z., & Harlan, R. E. (1991). Type I corticosteroid receptor-like immunoreactivity in the rat CNS: Distribution and regulation by corticosteroids. The Journal of Comparative Neurology, 313(3), 522–538. https://doi.org/10.1002/cne.903130312
- Ali, A. E. A., Wilson, Y. M., & Murphy, M. (2012). Identification of neurons specifically activated after recall of context fear conditioning. Neurobiology of Learning and Memory, 98(2), 139–147. https://doi.org/10.1016/j.nlm.2012.07.004
- Antoni, F. A. (1986). Hypothalamic control of adrenocorticotropin secretion: Advances since the discovery of 41-residue corticotropin-releasing factor. Endocrine Reviews, 7(4), 351–378. https://doi.org/10.1210/edrv-7-4-351
- Ashokan, A., Hegde, A., & Mitra, R. (2016). Short-term environmental enrichment is sufficient to counter stress-induced anxiety and associated structural and molecular plasticity in basolateral amygdala. Psychoneuroendocrinology, 69, 189–196. https://doi.org/10.1016/j.psyneuen.2016.04.009
- Avery, S. N., Clauss, J. A., & Blackford, J. U. (2016). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(1), 126–141. https://doi.org/10.1038/npp.2015.185
- Bailey, T. W., & Dimicco, J. A. (2001). Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 280(1), R8–R15. https://doi.org/10.1152/ajpregu.2001.280.1.R8
- Bains, J. S., Cusulin, J. I. W., & Inoue, W. (2015). Stress-related synaptic plasticity in the hypothalamus. Nature Reviews Neuroscience, 16, 377–388. https://doi.org/10.1038/nrn3881
- Bechtold, A. G., Patel, G., Hochhaus, G., & Scheuer, D. A. (2009). Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 296, R1445–R1454. https://doi.org/10.1152/ajpregu.00095.2008
- Bell, M. E., Bhatnagar, S., Akana, S. F., Choi, S. J., & Dallman, M. F. (2000). Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(17), 6707–6713. https://doi.org/10.1523/JNEUROSCI.20-17-06707.2000
- Bernard, Claude (1865). Introduction à l'étude de la médecine expérimentale. Paris / Leipzig: J.-B. Baillère et fils /Jung-Treuttel
- Bhatnagar, S., & Dallman, M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience, 84(4), 1025–1039. https://doi.org/10.1016/S0306-4522(97)00577-0
- Bhatnagar, S., Huber, R., Nowak, N., & Trotter, P. (2002). Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology, 14(5), 403–410. https://doi.org/10.1046/j.0007-1331.2002.00792.x
- Bradbury, M. J., Akana, S. F., Cascio, C. S., Levin, N., Jacobson, L., & Dallman, M. F. (1991). Regulation of basal ACTH secretion by corticosterone is mediated by both type I (MR) and type II (GR) receptors in rat brain. Journal of Steroid Biochemistry and Molecular Biology, 40(1–3), 133–140. https://doi.org/10.1016/0960-0760(91)90176-6
- Bradbury, M. J., Strack, A. M., & Dallman, M. F. (1993). Lesions of the hippocampal efferent pathway (fimbria-fornix) do not alter sensitivity of adrenocorticotropin to feedback inhibition by corticosterone in rats. Neuroendocrinology, 58(4), 396–407. https://doi.org/10.1159/000126569
- Browning, K. N., & Travagli, R. A. (2014). Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Comprehensive Physiology, 4(4), 1339–1368. https://doi.org/10.1002/cphy.c130055 25428846
- Buckingham, J. C., Solito, E., John, C., Tierney, T., Taylor, A., Flower, R., Christian, H., & Morris, J. (2003). Annexin 1: A paracrine/juxtacrine mediator of glucorticoid action in the neuroendocrine system. Cell Biochemistry and Function, 21(3), 217–221. https://doi.org/10.1002/cbf.1076
- Bundzikova-Osacka, J., Ghosal, S., Packard, B. A., Ulrich-Lai, Y. M., & Herman, J. P. (2015). Role of nucleus of the solitary tract noradrenergic neurons in post-stress cardiovascular and hormonal control in male rats. Stress (Amsterdam, Netherlands), 18(2), 221–232. https://doi.org/10.3109/10253890.2015.1013531
- Cannon, W. B. (1927). The James-Lange theory of emotions: A critical examination and an alternative theory. American Journal of Psychology, 39, 106–124. https://doi.org/10.2307/1415404
- Cannon, W. B. (1939). The wisdom of the body (2nd ed.). Norton & Co.
- Cascio, C. S., Shinsako, J., & Dallman, M. F. (1987). The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Research, 423(1–2):173–178. https://doi.org/10.1016/0006-8993(87)90837-7
- Centanni, S. W., Bedse, G., Patel, S., & Winder, D. G. (2019). Driving the downward spiral: Alcohol-induced dysregulation of extended amygdala circuits and negative affect. Alcoholism: Clinical and Experimental Research, 43(10), 2000–2013. https://doi.org/10.1111/acer.14178
- Chen, P. B., Hu, R. K., Wu, Y. E., Pan, L., Huang, S., Micevych, P. E., & Hong, W. (2019). Sexually dimorphic control of parenting behavior by the medial amygdala. Cell, 176(5), 1206–1221.e18. https://doi.org/10.1016/j.cell.2019.01.024
- Choi, D. C., Evanson, N. K., Furay, A. R., Ulrich-Lai, Y. M., Ostrander, M. M., & Herman, J. P. (2008). The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology, 149(2), 818–826. https://doi.org/10.1210/en.2007-0883
- Choi, D. C., Furay, A. R., Evanson, N. K., Ostrander, M. M., Ulrich-Lai, Y. M., & Herman, J. P. (2007). Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: Implications for the integration of limbic inputs. Journal of Neuroscience, 27(8), 2025–2034. https://doi.org/10.1523/JNEUROSCI.4301-06.2007
- Coover, G. D., Goldman, L., & Levine, S. (1971). Plasma corticosterone levels during extinction of a lever-press response in hippocampectomized rats. Physiology & Behavior, 7(5), 727–732. https://doi.org/10.1016/0031-9384(71)90140-5
- Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H., & Watson, S. J. (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience, 64(2), 477–505. https://doi.org/10.1016/0306-4522(94)00355-9
- Cullinan, W. E., Herman, J. P., & Watson, S. J. (1993). Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. The Journal of Comparative Neurology, 332(1), 1–20. https://doi.org/10.1002/cne.903320102
- Cullinan, W. E., Ziegler, D. R., & Herman, J. P. (2008). Functional role of local GABAergic influences on the HPA axis. Brain Structure & Function, 213(1–2), 63–72. https://doi.org/10.1007/s00429-008-0192-2
- Cunningham, E. T., Bohn, M. C., & Sawchenko, P. E. (1990). Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. The Journal of Comparative Neurology, 292(4), 651–667. https://doi.org/10.1002/cne.902920413
- Cunningham, E. T., & Sawchenko, P. E. (1988). Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. The Journal of Comparative Neurology, 274(1), 60–76. https://doi.org/10.1002/cne.902740107
- Dallman, M. F., Pecoraro, N., Akana, S. F., La Fleur, S. E., Gomez, F., Houshyar, H., Bell, M. E., Bhatnagar, S., Laugero, K. D., & Manalo, S. (2003). Chronic stress and obesity: A new view of “comfort food. Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11696–11701. https://doi.org/10.1073/pnas.1934666100
- Dallman, M. F., & Yates, F. E. (1969). Dynamic asymmetries in the corticosteroid feedback path and distribution metabolism‐binding elements of the adrenocortical system. Annals of the New York Academy of Sciences, 156(2), 696–721. https://doi.org/10.1111/j.1749-6632.1969.tb14008.x
- Damasio, A. R. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 351(1346), 1413–1420. https://doi.org/10.1098/rstb.1996.0125
- Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–135. https://doi.org/10.1038/npp.2009.109
- Dayas, C. V., Buller, K. M., Crane, J. W., Xu, Y., & Day, T. A. (2001). Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European Journal of Neuroscience, 14(7), 1143–1152. https://doi.org/10.1046/j.0953-816x.2001.01733.x
- Dayas, C. V., Buller, K. M., & Day, T. A. (1999). Neuroendocrine responses to an emotional stressor: Evidence for involvement of the medial but not the central amygdala. European Journal of Neuroscience, 11(7), 2312–2322. https://doi.org/10.1046/j.1460-9568.1999.00645.x
- de Kloet, A. D., & Herman, J. P. (2018). Fat-brain connections: Adipocyte glucocorticoid control of stress and metabolism. Frontiers in Neuroendocrinology, 48, 50–57. https://doi.org/10.1016/j.yfrne.2017.10.005
- de Kloet, E. R., Karst, H., & Joëls, M. (2008). Corticosteroid hormones in the central stress response: quick-and-slow. Frontiers in Neuroendocrinology, 29(2), 268–272. https://doi.org/10.1016/j.yfrne.2007.10.002
- de Kloet, E. R., Meijer, O. C., de Nicola, A. F., de Rijk, R. H., & Joëls, M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Frontiers in Neuroendocrinology, 49, 124–145. https://doi.org/10.1016/j.yfrne.2018.02.003
- de Kloet, E. R., Vreugdenhil, E., Oitzl, M. S., & JoëLs, M. (1998). Brain corticosteroid receptor balance in health and disease 1. Endocrine Reviews, 19(3), 269–301. https://doi.org/10.1210/edrv.19.3.0331
- De Kloet, E. R., & Reul, J. M. H. M. (1987). Feedback action and tonic influence of corticosteroids on brain function: A concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology, 12(2), 83–105. https://doi.org/10.1016/0306-4530(87)90040-0
- Decavel, C., & Van Den Pol, A. N. (1990). GABA: A dominant neurotransmitter in the hypothalamus. The Journal of Comparative Neurology, 302(4), 1019–1037. https://doi.org/10.1002/cne.903020423
- Di, S., Malcher-Lopes, R., Halmos, K. C., & Tasker, J. G. (2003). Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(12), 4850–4857. https://doi.org/10.1523/JNEUROSCI.23-12-04850.2003
- Ding, J., da Silva, M. S., Lingeman, J., Chen, X., Shi, Y., Han, F., & Meijer, O. C. (2019). Late glucocorticoid receptor antagonism changes the outcome of adult life stress. Psychoneuroendocrinology, 107, 169–178. https://doi.org/10.1016/j.psyneuen.2019.05.014
- Diorio, D., Viau, V., & Meaney, M. J. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 13(9), 3839–3847. https://doi.org/10.1523/JNEUROSCI.13-09-03839.1993
- Dong, H. W., Petrovich, G. D., & Swanson, L. W. (2001). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews, 38(1–2), 192–246. https://doi.org/10.1016/S0165-0173(01)00079-0
- Evanson, N. K., Tasker, J. G., Hill, M. N., Hillard, C. J., & Herman, J. P. (2010). Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology, 151(10), 4811–4819. https://doi.org/10.1210/en.2010-0285
- Federenko, I., Wüst, S., Hellhammer, D. H., Dechoux, R., Kumsta, R., & Kirschbaum, C. (2004). Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology, 29(2), 174–184. https://doi.org/10.1016/S0306-4530(03)00021-0
- Feillet, C. A., Albrecht, U., & Challet, E. (2006). “Feeding time” for the brain: A matter of clocks. Journal of physiology, Paris, 100(5–6), 252–260. https://doi.org/10.1016/j.jphysparis.2007.05.002
- Feldman, S., Saphier, D., & Weidenfeld, J. (1992). Corticosterone implants in the paraventricular nucleus inhibit ACTH and corticosterone responses and the release of corticotropin-releasing factor following neural stimuli. Brain Research, 578(1-2), 251–255. https://doi.org/10.1016/0006-8993(92)90254-7
- Figueiredo, H. F., Bruestle, A., Bodie, B., Dolgas, C. M., & Herman, J. P. (2003). The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. European Journal of Neuroscience, 18(8), 2357–2364. https://doi.org/10.1046/j.1460-9568.2003.02932.x
- Flak, J. N., Myers, B., Solomon, M. B., Mcklveen, J. M., Krause, E. G., & Herman, J. P. (2014). Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. European Journal of Neuroscience, 39(11), 1903–1911. https://doi.org/10.1111/ejn.12587
- Flak, J. N., Solomon, M. B., Jankord, R., Krause, E. G., & Herman, J. P. (2012). Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. European Journal of Neuroscience, 36(4):2547–2555. https://doi.org/10.1111/j.1460-9568.2012.08161.x
- Gaillet, S., Alonso, G., Le Borgne, R., Barbanel, G., Malaval, F., Assenmacher, I., & Szafarczyk, A. (1993). Effects of discrete lesions in the ventral noradrenergic ascending bundle on the corticotropic stress response depend on the site of the lesion and on the plasma levels of adrenal steroids. Neuroendocrinology, 58(4), 408–419. https://doi.org/10.1159/000126570
- Gaillet, S., Lachuer, J., Malaval, F., Assenmacher, I., & Szafarczyk, A. (1991). The involvement of noradrenergic ascending pathways in the stress-induced activation of ACTH and corticosterone secretions is dependent on the nature of stressors. Experimental Brain Research, 87(1), 173–180. https://doi.org/10.1007/BF00228518
- Ghosal, S., Bundzikova-Osacka, J., Dolgas, C. M., Myers, B., & Herman, J. P. (2014). Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology, 45, 142–153. https://doi.org/10.1016/j.psyneuen.2014.03.018
- Ghosal, S., Myers, B., & Herman, J. P. (2013). Role of central glucagon-like peptide-1 in stress regulation. Physiology & Behavior, 122, 201–207. https://doi.org/10.1016/j.physbeh.2013.04.003
- Ghosal, S., Packard, A. E. B., Mahbod, P., McKlveen, J. M., Seeley, R. J., Myers, B., Ulrich-Lai, Y., Smith, E. P., D'Alessio, D. A., & Herman, J. P. (2017). Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37(1), 184–193. https://doi.org/10.1523/JNEUROSCI.1104-16.2016
- Ghosal, S., Packard, A. E. B., Mahbod, P., McKlveen, J. M., Seeley, R. J., Myers, B., Ulrich-Lai, Y., Smith, E. P., D'Alessio, D. A., & Herman, J. P. (2017). Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. The Journal of Neuroscience: The Oscience, 37(1), 184–193. https://doi.org/10.1523/JNEUROSCI.1104-16.2016
- Gillies, G. E., Linton, E. A., & Lowry, P. J. (1982). Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature , 299(5881), 355–357. https://doi.org/10.1038/299355a0
- Goode, T. D., Kim, J. J., & Maren, S. (2015). Reversible inactivation of the bed nucleus of the stria terminalis prevents reinstatement but not renewal of extinguished fear. eNeuro, 2(3), ENEURO.0037-15.2015. https://doi.org/10.1523/ENEURO.0037-15.2015
- Goode, T. D., & Maren, S. (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning & Memory (Cold Spring Harbor, N.Y.), 24(9), 480–491. https://doi.org/10.1101/lm.044206.116
- Goode, T. D., & Maren, S. (2019). Common neurocircuitry mediating drug and fear relapse in preclinical models. Psychopharmacology (Berl), 236(1), 415–437. https://doi.org/10.1007/s00213-018-5024-3
- Gungor, N. Z., & Paré, D. (2016). Functional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience, 36(31), 8038–8049. https://doi.org/10.1523/JNEUROSCI.0856-16.2016
- Haller, J. (2018). The role of central and medial amygdala in normal and abnormal aggression: A review of classical approaches. Neuroscience & Biobehavioral Reviews, 85, 34–43. https://doi.org/10.1016/j.neubiorev.2017.09.017
- Harfstrand, A., Fuxe, K., Cintra, A., Agnati, L. F., Zini, I., Wikström, A. C., Okret, S., Yu, Z. Y., Goldstein, M., & Steinbusch, H. (1986). Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proceedings of the National Academy of Sciences, 83(24), 9779–9783. https://doi.org/10.1073/pnas.83.24.9779
- Herman, J. P. (1993). Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cellular and Molecular Neurobiology, 13(4), 349–372. https://doi.org/10.1007/BF00711577
- Herman, J. P., Cullinan, W. E., Morano, M. I., Akil, H., & Watson, S. J. (1995). Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo‐pituitary‐adrenocortical axis. Journal of Neuroendocrinology, 7(6), 475–482. https://doi.org/10.1111/j.1365-2826.1995.tb00784.x
- Herman, J. P., Cullinan, W. E., Ziegler, D. R., & Tasker, J. G. (2002). Role of the paraventricular nucleus microenvironment in stress integration. The European Journal of Neuroscience, 16(3), 381–385. https://doi.org/10.1046/j.1460-9568.2002.02133.x
- Herman, J. P., Dolgas, C. M., & Carlson, S. L. (1998). Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience, 86(2), 449–459. https://doi.org/10.1016/S0306-4522(98)00055-4
- Herman, J. P., Figueiredo, H., Mueller, N. K., Ulrich-Lai, Y., Ostrander, M. M., Choi, D. C., & Cullinan, W. E. (2003). Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology, 24(3), 151–180. https://doi.org/10.1016/j.yfrne.2003.07.001
- Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. https://doi.org/10.1002/cphy.c150015
- Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 29(8), 1201–1213. https://doi.org/10.1016/j.pnpbp.2005.08.006
- Herman, J. P., Patel, P. D., Akil, H., & Watson, S. J. (1989). Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Molecular Endocrinology (Baltimore, Md.), 3(11), 1886–1894. https://doi.org/10.1210/mend-3-11-1886
- Hill, M. N., McLaughlin, R. J., Pan, B., Fitzgerald, M. L., Roberts, C. J., Lee, T. T. Y., Karatsoreos, I. N., Mackie, K., Viau, V., Pickel, V. M., McEwen, B. S., Q. Song, Gorzalka, B.B, L., & Hillard, C. J. (2011). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. Journal of Neuroscience, 31(29):10506–10515. https://doi.org/10.1523/JNEUROSCI.0496-11.2011
- Hsu, D. T., Kirouac, G. J., Zubieta, J. K., & Bhatnagar, S. (2014). Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Frontiers in Behavioral Neuroscience, 8, 73. https://doi.org/10.3389/fnbeh.2014.00073
- Inoue, W., & Bains, J. S. (2014). Beyond inhibition: GABA synapses tune the neuroendocrine stress axis. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 36(6), 561–569. https://doi.org/10.1002/bies.201300178
- Jacobson, L., Akana, S. F., Cascio, C. S., Shinsako, J., & Dallman, M. F. (1988). Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology, 122(4), 1343–1348. https://doi.org/10.1210/endo-122-4-1343
- Jacobson, L., & Sapolsky, R. (1991). The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews, 12(2), 118–134. https://doi.org/10.1210/edrv-12-2-118
- Jafari, M., Seese, R. R., Babayan, A. H., Gall, C. M., & Lauterborn, J. C. (2012). Glucocorticoid receptors are localized to dendritic spines and influence local actin signaling. Molecular Neurobiology, 46(2), 304–315. https://doi.org/10.1007/s12035-012-8288-3
- Jaferi, A., Nowak, N., & Bhatnagar, S. (2003). Negative feedback functions in chronically stressed rats: Role of the posterior paraventricular thalamus. Physiology & Behavior, 78(3), 365–373. https://doi.org/10.1016/S0031-9384(03)00014-3
- James, W. (1994). The physical basis of emotion. Psychological Review, 101(2), 205–210. https://doi.org/10.1037//0033-295X.101.2.205
- Janak, P. H., & Tye, K. M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. https://doi.org/10.1038/nature14188
- Jasper, M. S., & Engeland, W. C. (1997). Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. American Journal of Physiology-Endocrinology and Metabolism, 273(2), E363–E368. https://doi.org/10.1152/ajpendo.1997.273.2.E363
- Joëls, M. (2006). Corticosteroid effects in the brain: U-shape it. Trends in Pharmacological Sciences, 27(5), 244–250. https://doi.org/10.1016/j.tips.2006.03.007
- Joëls, M., Angela Sarabdjitsingh, R., & Karst, H. (2012). Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow, and chronic modes. Pharmacological Reviews, 64(4), 901–938. https://doi.org/10.1124/pr.112.005892
- Johnson, L. R., Farb, C., Morrison, J. H., McEwen, B. S., & LeDoux, J. E. (2005). Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience, 136(1), 289–299. https://doi.org/10.1016/j.neuroscience.2005.06.050
- Karst, H., & Joëls, M. (2005). Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. Journal of Neurophysiology, 94(5), 3479–3486. https://doi.org/10.1152/jn.00143.2005
- Keller-Wood, M. E., & Dallman, M. F. (1984). Corticosteroid inhibition of ACTH secretion. Endocrine Reviews, 5(1), 1–24. https://doi.org/10.1210/edrv-5-1-1
- Koolhaas, J. M., Bartolomucci, A., Buwalda, B., de Boer, S. F., Flügge, G., Korte, S. M., Meerlo, P., Murison, R., Olivier, B., Palanza, P., Richter-Levin, G., Sgoifo, A., Steimer, T., Stiedl, O., van Dijk, G., Wöhr, M., & Fuchs, E. (2011). Stress revisited: A critical evaluation of the stress concept. Neuroscience & Biobehavioral Reviews, 35(5), 1291–1301. https://doi.org/10.1016/j.neubiorev.2011.02.003
- Korte, S. M., Koolhaas, J. M., Wingfield, J. C., & McEwen, B. S. (2005). The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews, 29(1), 3–38. https://doi.org/10.1016/j.neubiorev.2004.08.009
- Kovacs, K. J., & Mezey, E. (1987). Dexamethasone inhibits cortioctropin-releasing factor gene expression in the rat paraventricular nucleus. Neuroendocrinology, 46(4), 365–368. https://doi.org/10.1159/000124846
- Lachize, S., Apostolakis, E. M., Van Der Laan, S., Tijssen, A. M. I., Xu, J., De Kloet, E. R., & Meijer, O. C. (2009). Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America, 106(19), 8038–8042. https://doi.org/10.1073/pnas.0812062106
- Laryea, G., Schütz, G., & Muglia, L. J. (2013). Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Molecular Endocrinology (Baltimore, Md.), 27(10), 1655–1665. https://doi.org/10.1210/me.2013-1187
- Levin, N., Shinsako, J., & Dallman, M. F. (1988). Corticosterone acts on the brain to inhibit adrenalectomy-induced adrenocorticotropin secretion. Endocrinology, 122(2), 694–701. https://doi.org/10.1210/endo-122-2-694
- Li, H. Y., Ericsson, A., & Sawchenko, P. E. (1996). Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2359–2364. https://doi.org/10.1073/pnas.93.6.2359
- Lightman, S. L., Birnie, M. T., & Conway-Campbell, B. L. (2020). Dynamics of ACTH and cortisol secretion and implications for disease. Endocrine Reviews, 41(3), 470–490. https://doi.org/10.1210/endrev/bnaa002
- Li, S., & Kirouac, G. J. (2012). Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Structure & Function, 217(2), 257–273. https://doi.org/10.1007/s00429-011-0360-7
- Liu, J., Garza, J. C., Truong, H. V., Henschel, J., Zhang, W., & Lu, X. Y. (2007). The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology, 148(11), 5531–5540. https://doi.org/10.1210/en.2007-0745
- Luyck, K., Arckens, L., Nuttin, B., & Luyten, L. (2020). It takes two: Bilateral bed nuclei of the stria terminalis mediate the expression of contextual fear, but not of moderate cued fear. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 101, 109920. https://doi.org/10.1016/j.pnpbp.2020.109920
- Mains, R. E., & Eipper, B. A. (1999). Neuropeptide functions and regulation. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999. Available from: https://www.ncbi.nlm.nih.gov/books/NBK28128/
- Maniscalco, J. W., & Rinaman, L. (2017). Interoceptive modulation of neuroendocrine, emotional, and hypophagic responses to stress. Physiology & Behavior, 176, 195–206. https://doi.org/10.1016/j.physbeh.2017.01.027
- McEwen, B. S., Nasca, C., & Gray, J. D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(1), 3–23. https://doi.org/10.1038/npp.2015.171
- McEwen, B. S., & Stellar, E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. https://doi.org/10.1001/archinte.1993.00410180039004
- McKay, L. I., & Cidlowski, J. A. (1998). Cross-talk between nuclear factor-κB and the steroid hormone receptors: Mechanisms of mutual antagonism. Molecular Endocrinology (Baltimore, Md.), 12(1), 45–56. https://doi.org/10.1210/mend.12.1.0044
- McKlveen, J. M., Morano, R. L., Fitzgerald, M., Zoubovsky, S., Cassella, S. N., Scheimann, J. R., Ghosal, S., Mahbod, P., Packard, B. A., Myers, B., Baccei, M. L., & Herman, J. P. (2016). Chronic stress increases prefrontal inhibition: A mechanism for stress-induced prefrontal dysfunction. Biological Psychiatry, 80(10):754–764. https://doi.org/10.1016/j.biopsych.2016.03.2101
- McKlveen, J. M., Myers, B., Flak, J. N., Bundzikova, J., Solomon, M. B., Seroogy, K. B., & Herman, J. P. (2013). Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biological Psychiatry, 74(9), 672–679. https://doi.org/10.1016/j.biopsych.2013.03.024
- Meijer, O. C., Kalkhoven, E., Van Der Laan, S., Steenbergen, P. J., Houtman, S. H., Dijkmans, T. F., Pearce, D., & De Kloet, E. R. (2005). Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology, 146(3), 1438–1448. https://doi.org/10.1210/en.2004-0411
- Miles, O. W., & Maren, S. (2019). Role of the bed nucleus of the stria terminalis in PTSD: Insights from preclinical models. Frontiers in Behavioral Neuroscience, 13, 68. https://doi.org/10.3389/fnbeh.2019.00068
- Mitra, R., Jadhav, S., Mcewen, B. S., Vyas, A., & Chattarji, S. (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences, 102(26), 9371–9376. https://doi.org/10.1073/pnas.0504011102
- Mizoguchi, K., Ishige, A., Aburada, M., & Tabira, T. (2003). Chronic stress attenuates glucocorticoid negative feedback: Involvement of the prefrontal cortex and hippocampus. Neuroscience, 119(3), 887–897. https://doi.org/10.1016/S0306-4522(03)00105-2
- Mueller, N. K., Dolgas, C. M., & Herman, J. P. (2004). Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology, 145(8), 3763–3768. https://doi.org/10.1210/en.2004-0097
- Myers, Brent, Scheimann, J. R., Franco-Villanueva, A., & Herman, J. P. (2017). Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience & Biobehavioral Reviews, 74(Pt B), 366–375. https://doi.org/10.1016/j.neubiorev.2016.05.011
- Myers, B., Carvalho-Netto, E., Wick-Carlson, D., Wu, C., Naser, S., Solomon, M. B., Ulrich-Lai, Y. M., & Herman, J. P. (2016). GABAergic signaling within a limbic-hypothalamic circuit integrates social and anxiety-like behavior with stress reactivity. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(6), 1530–1539. https://doi.org/10.1038/npp.2015.311
- Myers, B., Mark Dolgas, C., Kasckow, J., Cullinan, W. E., & Herman, J. P. (2014a). Central stress-integrative circuits: Forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Structure & Function, 219(4), 1287–1303. https://doi.org/10.1007/s00429-013-0566-y
- Myers, B., McKlveen, J. M., & Herman, J. P. (2012). Neural regulation of the stress response: The many faces of feedback. Cellular and Molecular Neurobiology. https://doi.org/10.1007/s10571-012-9801-y
- Myers, B., McKlveen, J. M., & Herman, J. P. (2014b). Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Frontiers in Neuroendocrinology, 35(2), 180–196. https://doi.org/10.1016/j.yfrne.2013.12.003
- Myers, B., McKlveen, J. M., Morano, R., Ulrich-Lai, Y. M., Solomon, M. B., Wilson, S. P., & Herman, J. P. (2017). Vesicular glutamate transporter 1 knockdown in infralimbic prefrontal cortex augments neuroendocrine responses to chronic stress in male rats. Endocrinology, 158(10), 3579–3591. https://doi.org/10.1210/en.2017-00426
- Nahar, J., Haam, J., Chen, C., Jiang, Z., Glatzer, N. R., Muglia, L. J., Dohanich, G. P., Herman, J. P., & Tasker, J. G. (2015). Rapid nongenomic glucocorticoid actions in male mouse hypothalamic neuroendocrine cells are dependent on the nuclear glucocorticoid receptor. Endocrinology, 156(8), 2831–2842. https://doi.org/10.1210/en.2015-1273
- Novaes, L. S., Dos Santos, N. B., Batalhote, R. F., Malta, M. B., Camarini, R., Scavone, C., & Munhoz, C. D. (2017). Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology, 113, 457–466. https://doi.org/10.1016/j.neuropharm.2016.10.026
- Nyhuis, T. J., Masini, C. V., Day, H. E. W., & Campeau, S. (2016). Evidence for the integration of stress-related signals by the rostral posterior hypothalamic nucleus in the regulation of acute and repeated stress-evoked hypothalamo-pituitary-adrenal response in rat. The Journal of Neuroscience, 36(3), 795–805. https://doi.org/10.1523/JNEUROSCI.3413-15.2016
- Oitzl, M. S., Van Haarst, A. D., & De Kloet, E. R. (1997). Behavioral and neuroendocrine responses controlled by the concerted action of central mineralocorticoid (MRS) and glucocorticoid receptors (GRS). Psychoneuroendocrinology, 22, S87–S93. https://doi.org/10.1016/S0306-4530(97)00020-6
- Ostrander, M. M., Richtand, N. M., & Herman, J. P. (2003). Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Research, 990(1–2), 209–214. https://doi.org/10.1016/j.brainres.2003.07.001
- Pooley, J. R., Rivers, C. A., Kilcooley, M. T., Paul, S. N., Cavga, A. D., Kershaw, Y. M., Muratcioglu, S., Gursoy, A., Keskin, O., & Lightman, S. L. (2020). Beyond the heterodimer model for mineralocorticoid and glucocorticoid receptor interactions in nuclei and at DNA. PLoS One, 15(1), e0227520. https://doi.org/10.1371/journal.pone.0227520
- Radley, J. J., Gosselink, K. L., & Sawchenko, P. E. (2009). A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. Journal of Neuroscience, 29(22), 7330–7340. https://doi.org/10.1523/JNEUROSCI.5924-08.2009
- Radley, J. J., & Johnson, S. B. (2018). Anteroventral bed nuclei of the stria terminalis neurocircuitry: Towards an integration of HPA axis modulation with coping behaviors – Curt Richter Award Paper 2017. Psychoneuroendocrinology, 89, 239–249. https://doi.org/10.1016/j.psyneuen.2017.12.005
- Radley, J. J., & Sawchenko, P. E. (2011). A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. Journal of Neuroscience, 31(26), 9683–9695. https://doi.org/10.1523/JNEUROSCI.6040-10.2011
- Rainville, J. R., Weiss, G. L., Evanson, N., Herman, J. P., Vasudevan, N., & Tasker, J. G. (2019). Membrane-initiated nuclear trafficking of the glucocorticoid receptor in hypothalamic neurons. Steroids, 142, 55–64. https://doi.org/10.1016/j.steroids.2017.12.005
- Ressler, K. J., Mercer, K. B., Bradley, B., Jovanovic, T., Mahan, A., Kerley, K., Norrholm, S. D., Kilaru, V., Smith, A. K., Myers, A. J., Ramirez, M., Engel, A., Hammack, S. E., Toufexis, D., Braas, K. M., Binder, E. B., & May, V. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470(7335), 492–497. https://doi.org/10.1038/nature09856
- Reul, J. M., & de Kloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology, 117(6), 2505–2511. https://doi.org/10.1210/endo-117-6-2505
- Rinaman, L. (2011). Hindbrain noradrenergic A2 neurons: Diverse roles in autonomic, endocrine, cognitive, and behavioral functions. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 300(2), R222–R235. https://doi.org/10.1152/ajpregu.00556.2010
- Ritter, S., Watts, A. G., Dinh, T. T., Sanchez-Watts, G., & Pedrow, C. (2003). Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology, 144(4), 1357–1367. https://doi.org/10.1210/en.2002-221076
- Roland, B. L., & Sawchenko, P. E. (1993). Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. The Journal of Comparative Neurology, 332(1), 123–143. https://doi.org/10.1002/cne.903320109
- Russell, G. M., Kalafatakis, K., & Lightman, S. L. (2015). The importance of biological oscillators for hypothalamic-pituitary-adrenal activity and tissue glucocorticoid response: Coordinating stress and neurobehavioural adaptation. Journal of Neuroendocrinology, 27(6), 378–388. https://doi.org/10.1111/jne.12247
- Sah, P., Faber, E. S. L., Lopez De Armentia, M., & Power, J. (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83(3), 803–834. https://doi.org/10.1152/physrev.00002.2003
- Sapolsky, R. M., Krey, L. C., & McEwen, B. S. (1984). Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proceedings of the National Academy of Sciences of the United States of America, 81(19), 6174–6177. https://doi.org/10.1073/pnas.81.19.6174
- Sarabdjitsingh, R. A., Isenia, S., Polman, A., Mijalkovic, J., Lachize, S., Datson, N., De Kloet, E. R., & Meijer, O. C. (2010). Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology, 151(3), 1177–1186. https://doi.org/10.1210/en.2009-1119
- Sarabdjitsingh, R. A., Joëls, M., & de Kloet, E. R. (2012). Glucocorticoid pulsatility and rapid corticosteroid actions in the central stress response. Physiology & Behavior, 106(1), 73–80. https://doi.org/10.1016/j.physbeh.2011.09.017
- Sawchenko, P. E. (1987). Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Research, 403(2), 213–223. https://doi.org/10.1016/0006-8993(87)90058-8
- Scheimann, J. R., Moloney, R. D., Mahbod, P., Morano, R. L., Fitzgerald, M., Hoskins, O., Packard, B. A., Cotella, E. M., Hu, Y.-C., & Herman, J. P. (2019). Conditional deletion of glucocorticoid receptors in rat brain results in sex- specific deficits in fear and coping behaviors. eLife, 8, e44672. https://doi.org/10.7554/eLife.44672
- Schwaber, J. S., Kapp, B. S., Higgins, G. A., & Rapp, P. R. (1982). Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 2(10), 1424–1438. https://doi.org/10.1523/JNEUROSCI.02-10-01424.1982
- Selye, H. (1936). A syndrome produced by diverse nocuous agents [13]. Nature, 138(3479), 32–32. https://doi.org/10.1038/138032a0
- Selye, H. (1950). Stress and the general adaptation syndrome. British Medical Journal, 1(4667), 1383–1392. https://doi.org/10.1136/bmj.1.4667.1383
- Selye, H. (1956). The Stress of the Life. McGraw-Hill. https://doi.org/10.1177/0098628316662768
- Selye, H. (1975). Confusion and controversy in the stress field. Journal of Human Stress, 1(2):37–44. https://doi.org/10.1080/0097840X.1975.9940406
- Solomon, M. B., Loftspring, M., De Kloet, A. D., Ghosal, S., Jankord, R., Flak, J. N., Wulsin, A. C., Krause, E. G., Zhang, R., Rice, T., McKlveen, J., Myers, B., Tasker, J. G., & Herman, J. P. (2015). Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology, 156(8), 2843–2853. https://doi.org/10.1210/en.2015-1276
- Spencer, S. J., Buller, K. M., & Day, T. A. (2005). Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: Possible role of the bed nucleus of the stria terminalis. The Journal of Comparative Neurology, 481(4), 363–376. https://doi.org/10.1002/cne.20376
- Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wüst, S., Dockray, S., Smyth, N., Evans, P., Hellhammer, D. H., Miller, R., Wetherell, M. A., Lupien, S. J., & Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. https://doi.org/10.1016/j.psyneuen.2015.10.010
- Sterling, P., & Eyer, J. (1988). Allostasis: a new paradigm to explain arousal pathology. In S. Fisher & J. Reason (Eds.), Handbook of life stress, cognition and health (pp. 629–649). John Wiley & Sons.
- Sullivan, G. M., Apergis, J., Bush, D. E. A., Johnson, L. R., Hou, M., & Ledoux, J. E. (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. https://doi.org/10.1016/j.neuroscience.2004.06.015
- Sullivan, R. M., & Gratton, A. (1999). Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. The Journal of Neuroscience, 19(7), 2834–2840. https://doi.org/10.1523/JNEUROSCI.19-07-02834.1999
- Swanson, L. W., & Petrovich, G. D. (1998). What is the amygdala? Trends in Neurosciences, 21(8), 323–331. https://doi.org/10.1016/S0166-2236(98)01265-X
- Tauchi, M., Zhang, R., D’Alessio, D. A., Seeley, R. J., & Herman, J. P. (2008). Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Experimental Neurology, 210(2), 458–466. https://doi.org/10.1016/j.expneurol.2007.11.016
- Trapp, T., Rupprecht, R., Castren, M., Reul, J. M. H. M., & Holsboer, F. (1994). Heterodimerization between mineralocorticoid and glucocorticoid receptor: A new principle of glucocorticoid action in the CNS. Neuron, 13(6), 1457–1462. https://doi.org/10.1016/0896-6273(94)90431-6
- Tuvnes, F. A., Steffenach, H. A., Murison, R., Moser, M. B., & Moser, E. I. (2003). Selective hippocampal lesions do not increase adrenocortical activity. The Journal of Neuroscience, 23(10), 4345–4354. https://doi.org/10.1523/JNEUROSCI.23-10-04345.2003
- Ulrich-Lai, Y. M., Arnhold, M. M., & Engeland, W. C. (2006). Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 290(4), R1128–R1135. https://doi.org/10.1152/ajpregu.00042.2003
- Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. https://doi.org/10.1038/nrn2647
- van der Kooy, D., Koda, L. Y., McGinty, J. F., Gerfen, C. R., & Bloom, F. E. (1984). The organization of projections from the cortes, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. The Journal of Comparative Neurology, 224(1), 1–24. https://doi.org/10.1002/cne.902240102
- Van Eekelen, J. A. M., Jiang, W., De Kloet, E. R., & Bohn, M. C. (1988). Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. Journal of Neuroscience Research, 21(1), 88–94. https://doi.org/10.1002/jnr.490210113
- Van Weert, L. T. C. M., Buurstede, J. C., Mahfouz, A., Braakhuis, P. S. M., Polman, J. A. E., Sips, H. C. M., Roozendaal, B., Balog, J., De Kloet, E. R., Datson, N. A., & Meijer, O. C. (2017). NeuroD factors discriminate mineralocorticoid from glucocorticoid receptor DNA binding in the male rat brain. Endocrinology, 158(5), 1511–1522. https://doi.org/10.1210/en.2016-1422
- Vertes, R. P., Linley, S. B., & Hoover, W. B. (2015). Limbic circuitry of the midline thalamus. Neuroscience & Biobehavioral Reviews, 54, 89–107. https://doi.org/10.1016/j.neubiorev.2015.01.014
- Viau, V., & Meaney, M. J. (1996). The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. The Journal of Neuroscience: The Oscience, 16(5), 1866–1876. https://doi.org/10.1523/JNEUROSCI.16-05-01866.1996
- Viltart, O., Sartor, D. M., & Verberne, A. J. M. (2006). Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Research Bulletin, 71(1-3), 51–59.
- Vyas, A., Mitra, R., Shankaranarayana Rao, B. S., & Chattarji, S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 22(15), 6810–6818. https://doi.org/10.1016/j.brainresbull.2006.07.016
- Waddell, J., Morris, R. W., & Bouton, M. E. (2006). Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: Aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience, 120(2), 324–336. https://doi.org/10.1037/0735-7044.120.2.324
- Walker, D. L., & Davis, M. (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience: The Oscience, 17(23), 9375–9383. https://doi.org/10.1523/JNEUROSCI.17-23-09375.1997
- Walker, D. L., Toufexis, D. J., & Davis, M. (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology, 463(1–3), 199–216. https://doi.org/10.1016/S0014-2999(03)01282-2
- Windle, R. J., Wood, S. A., Kershaw, Y. M., Lightman, S. L., & Ingram, C. D. (2013). Adaptive changes in basal and stress-induced HPA activity in lactating and post-lactating female rats. Endocrinology, 154(2), 749–761. https://doi.org/10.1210/en.2012-1779
- Yang-Yen, H. F., Chambard, J. C., Sun, Y. L., Smeal, T., Schmidt, T. J., Drouin, J., & Karin, M. (1990). Transcriptional interference between c-Jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell, 62(6), 1205–1215. https://doi.org/10.1016/0092-8674(90)90396-V
- Yuen, E. Y., Liu, W., Karatsoreos, I. N., Feng, J., McEwen, B. S., & Yan, Z. (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14075–14079. https://doi.org/10.1073/pnas.0906791106
- Yuen, E. Y., Liu, W., Karatsoreos, I. N., Ren, Y., Feng, J., McEwen, B. S., & Yan, Z. (2011). Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry, 16(2), 156–170. https://doi.org/10.1038/mp.2010.50
- Zalachoras, I., Grootaers, G., van Weert, L. T. C. M., Aubert, Y., de Kreij, S. R., Datson, N. A., van Roon-Mom, W. M. C., Aartsma-Rus, A., & Meijer, O. C. (2013). Antisense-mediated isoform switching of steroid receptor coactivator-1 in the central nucleus of the amygdala of the mouse brain. BMC Neuroscience, 14(1), 5. https://doi.org/10.1186/1471-2202-14-5
- Zhang, R., Jankord, R., Flak, J. N., Solomon, M. B., D’Alessio, D. A., & Herman, J. P. (2010). Role of glucocorticoids in tuning hindbrain stress integration. Journal of Neuroscience, 30(44), 14907–14914. https://doi.org/10.1523/JNEUROSCI.0522-10.2010
- Ziegler, D. R., & Herman, J. P. (2000). Local integration of glutamate signaling in the hypothalamic paraventricular region: Regulation of glucocorticoid stress responses. Endocrinology, 141(12), 4801–4804. https://doi.org/10.1210/endo.141.12.7949
- Zimmerman, J. M., & Maren, S. (2011). The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiology of Learning and Memory, 95(2), 199–205. https://doi.org/10.1016/j.nlm.2010.11.002
