Abstract
The wear and tear of the body caused by stressful events is subject of extensive research and can be measured by the allostatic load index (ALI). The aim of this cross-sectional study was to replicate an ALI-5 score in a population sample in the USA and to compare these findings with the original ALI-10 score. Data from the Midlife in the United States Study (MIDUS) assessed between 2004 and 2016 were used to calculate different ALI risk scores with 5 and 10 variables, respectively. Examinations included anthropometric data, heart rate variability (HRV), and blood and urine samples. Questionnaires assessed information on perceived stress and medical history. Logistic regression models estimated odds ratios (OR) with corresponding 95% confidence intervals (CIs) for the association between ALI indices and perceived stress, controlling for various confounders. Subgroup analysis explored the difference in gender and in three age clusters. Data of 1421 participants (43% male, 50.4 ± 9.3 years) were included in the analysis. Adjusted logistic regression models showed an odds ratio of 1.37 ± 0.19 (CI 1.05, 1.80; p=.022) for the association of ALI-5 with perceived stress. This association was stronger in females (OR = 1.62 ± 0.28, CI 1.15, 2.28; p = .006) and did not significantly differ between age clusters. Results for the original ALI-10 score did not reach significance. The streamlined ALI-5 score seems to be a reliable risk score and is strongly associated with perceived stress in life. Longitudinal studies should further elaborate this association in different samples.
Lay summary: Stress from different sources can lead to serious diseases. A short composite index comprising of five medical variables is highly associated with perceived stress. This index is able to serve as an early indicator to detect people who are at risk to develop stress-related diseases.
Introduction
Psychosocial stress is common and can lead to serious physical and mental diseases such as cardiovascular disease (Theorell et al., Citation2016) and depression (Siegrist et al., Citation2012).
In 1993, McEwen and Stellar (Citation1993) described the concept of “allostatic load” to explain adverse biological impacts of repeated and chronic stress exposure. Allostasis explains efforts to maintain homoeostasis by adapting body functions to psychosocial stressors. In 1997, Teresa Seeman et al. (Citation1997) developed a 10-variable allostatic load index (ALI) to measure stress-related wear and tear of the human body. In the last 20 years, elevated ALI scores have been linked to various health disparities such as cardiovascular disease (Sabbah et al., Citation2008) and reduced mental health (McEwen, Citation2003).
Recently, we have developed and tested a streamlined 5-variable version (Mauss, Jarczok, et al., Citation2015) and replicated the findings in an extensive cohort study of 12,477 employees of different industries (Mauss et al., Citation2016). Both studies explored cross-sectional associations between work-related stress and ALI using the well-established Effort-Reward-Imbalance Model (Siegrist et al., Citation2004) in working populations in Germany.
Work-related loads are only one possible source of stress leading to disparities in health (Jarczok et al., Citation2020; Kivimäki et al., Citation2012; Theorell et al., Citation2015). Other sources may include negative life events (McEwen & Stellar, Citation1993), socioeconomic status (Gruenewald et al., Citation2012), and psychosocial challenges (Fava et al., Citation2019; McEwen, Citation2012). For example, worries in life such as social conditions, health, finance, and aging showed a dose–response relationship with incident coronary heart disease (CHD) (Kubzansky et al., Citation1997). A more general and widely used psychological instrument to measure perceived stress in life is the perceived stress scale (PSS) (Cohen et al., Citation1983). PSS is a self-report questionnaire designed to assess one’s level of stress perception during the last 4 weeks regarding unpredictability, lack of control, and overload. While there are three different validated PSS versions with 4-, 10-, and 14-items, Cohen and Williamson recommended the 10-item version in terms of psychometric properties (Cohen, Citation1988).
So far, only one small study has assessed the association of PSS and ALI in the past (D’Alonzo et al., Citation2019). D’Alonzo et al. explored 59 Mexican women aged 18–45 years, one group living in Mexico, one group as Mexicans in the USA. A composite measure for ALI included eight biological variables: systolic and diastolic blood pressure, body mass index, waist-to-hip ratio, total cholesterol, glycosylated hemoglobin, triglycerides, and C-reactive protein. Perceived stress was measured by the Spanish version of the 14-items PSS. ALI and perceived stress levels did not differ between both groups and no significant association could be found.
In this study, we sought to replicate and extend the knowledge on the association of perceived stress with a streamlined ALI-5 risk score. Specifically, we used a population sample from the USA measuring general stress perception (compared to work-associated stress in occupational samples as in previous publications). This should further support our approach of a reduced, handier set of allostatic load indicators (ALI-5) to avoid statistical misinterpretation and publication bias, as well as to build up valid and robust scientific evidence (Ferguson & Heene, Citation2012). Second, we aim to assess if the ALI-5 risk score is a consistent measurement for people at risk for biological impacts of stress compared to the original ALI-10 score.
Methods
Study sample
Data are taken from the second and third wave of the Midlife in the United States Study (MIDUS). MIDUS is longitudinally designed to determine how psychological, behavioral, and social factors impact mental and physical health. Of the 3191 study participants, 1255 agreed to participate in the MIDUS II biomarker project (Project 4), which required a 2D medical examination at one of the three following clinical research centers between July 2004 and May 2009: University of California at Los Angeles, Georgetown University, and University of Wisconsin (ICPSR 29282; update level V9 [2019-03-27]). Between 2012 and 2016, data from 863 respondents were collected for the MIDUS Refresher Biomarker study (Project 4) including questionnaires and biomarkers in the same study setup (ICPSR 36901; update level V6 [2019-11-18]). The study was approved by the Institutional Review Board at each participating center. All study protocols were conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants (Dienberg Love et al., Citation2010).
Data collection
Data collection included a medical history (telephone interviews and self-administered questionnaires), a physical examination as well as blood- and urine-based measurements of biomarkers. The study protocol is described in detail elsewhere (Dienberg Love et al., Citation2010).
Sample collection
Fasting blood samples were collected from each participant before breakfast. To ensure consistency, all samples were collected using standardized procedures. All samples were frozen and stored in a −60 °C to −80 °C freezer until shipped on dry ice to the central MIDUS Biocore laboratory.
Biomarkers
Biomarkers included fasting blood samples exploring total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, DHEA-S, creatinine, glycosylated hemoglobin (HbA1c), and 12-h overnight urine samples assessing cortisol, epinephrine, norepinephrine, and creatinine.
Physical examination
Waist circumference was measured directly on skin over a single layer of clothing if the garment was a camisole or undershirt. It was measured at the narrowest point between ribs and the iliac crest. Hip circumference was measured over a single layer of clothing, typically subject’s underwear, at the iliac crest. Blood pressure was assessed in a sitting position allowing a maximum of 30 s between each measurement. The average value of three measurements was calculated and used for further analyses.
Heart rate variability
Cardiovascular reactivity was assessed in the morning after a light breakfast with no caffeinated beverages via continuous measurement of a 3-lead electrocardiogram (ECG). ECG electrodes were placed on the left and right collarbones, and in the left lower quadrant of the abdomen. Beat-to-beat ECG waveforms were then analyzed to calculate heart rate variability (HRV). HRV reflects activity of the parasympathetic nervous system with higher values usually indicating a better health status. HRV is operationalized as variability in the series of intervals between consecutive R waves. Analog beat-to-beat ECG signals were digitized at a sampling rate of 500 Hz by a National Instruments A/D board and passed to a microcomputer for collection. ECG waveforms were submitted to an R-wave detection routine implemented by a proprietary event detection software (Graphical Marking, Delano McFarlane), resulting in an RR interval series. Research staff visually reviewed all ECG waveforms to correct interactively any software errors in identifying normal R waves. The resulting series of normal RR intervals were used to calculate HRV. Time-domain index of RR interval variability was calculated as root mean squared successive differences (RMSSD).
Stress measurement
Stress level was assessed by the self-rated 10-item PSS (Cohen, Citation1988; Smith et al., Citation2014) using a 5-point Likert scale (1 = never; 2 = almost never; 3 = sometimes; 4 = fairly often; 5 = very often) (). Items 4, 5, 7, and 8 were reverse-coded so that higher scores indicate a higher stress level. All items were equally weighted and summed up to a scale score ranging from 10 to 50 (Cronbach’s α = 0.86). This score was binary recoded with the lower three quartiles as reference group. As shown in earlier studies, perceived stress depends on different variables such as age and gender, indicating greater stress‐related health risks among women and younger adults (Cohen & Janicki-Deverts, Citation2012).
Table 1. Items of the perceived stress scale.
Allostatic load
In order to adapt body functions to perceived stress, neuroendocrine, neurophysiological, and anti-inflammatory variables (“primary mediators”) are released and a shift in sympathovagal balance can be observed. In a second step, “secondary outcomes” are characterized by subclinical disturbances such as increased cardiovascular, immunological, and metabolic biomarkers. Later, these disturbances may lead to clinical adverse health problems, so-called “tertiary outcomes.” ALI serves as a composite risk score representing different regulatory systems of the human body including primary mediators (e.g. neuroendocrine and neurophysiological) and secondary outcomes (e.g. cardiovascular, inflammatory, and metabolic) to have the best predictive power for physiological strain (Mauss, Li, et al., Citation2015). According to our previous studies, a streamlined ALI was calculated using five variables such as RMSSD as an indicator for the human neurophysiological system, HbA1c as a metabolic indicator and risk factor for type 2 diabetes as well as waist circumference, LDL, and diastolic blood pressure as cardiovascular indicators (Mauss, Jarczok, et al., Citation2015; Mauss et al., Citation2016). Based on these studies, we used predefined subclinical cutoff values for each variable as reported in . ALI was calculated as the number of variables for which the participant was outside these cutoff values or is taking prescribed medication (i.e. blood glucose-lowering, blood pressure-lowering, or lipid-lowering drugs). Every participant scored “1” for being outside the range (all variables higher than the cutoff except RMSSD, for which lower values indicate higher risk) and “0” for being in a normal range. The sum score could therefore range from 0 to 5, with higher scores indicating higher allostatic load. This score was binary recoded with participants scoring 4 or 5 in one group and participants scoring lower than 4 as reference group.
Table 2. Sample characteristics (N = 1421).
Based on the original model of Seeman et al. (Citation1997) a second ALI was calculated using 10 variables. Each variable within the highest risk quartile (highest quartile for each variable except DHEA-S and HDL, for which the lowest quartile indicates the highest risk) scored “1” and these in the other quartiles scored “0.” The sum score could therefore range from 0 to 10 with a higher score indicating a higher wear and tear of the body. This score was binary recoded with participants scoring 5 or higher in one group and participants scoring lower than 5 as reference group. To better compare the results, a third ALI was calculated with the five variables of the streamlined ALI-5 using the statistical method of risk quartiles. This means that values for diastolic blood pressure, HbA1c, LDL, and waist circumference in the highest quartile, and values for RMSSD in the lowest quartile scored “1” and these in the other quartiles scored “0.”
Exclusion criteria
Participants with missing data on any of the variables under study were excluded from the analysis. To better compare the results with previous research on ALI-5, participants older than 65 years were excluded as well.
Cutoff values
According to our earlier studies on ALI-5 (Mauss, Jarczok, et al., Citation2015; Mauss et al., Citation2016), we have predefined subclinical ALI-5 cutoff values as reported in based on following references: components of the metabolic syndrome (diastolic blood pressure and waist circumference) were defined referring to the Joint Statement (Alberti et al., Citation2009) and European guidelines (Williams et al., Citation2018), other metabolic variables based on standard definitions including LDL (Grundy et al., Citation2004) and glycosylated hemoglobin (World Health Organization, Citation2011). RMSSD as a marker for HRV was based on previous empirical data (Nunan et al., Citation2010; Shaffer & Ginsberg, Citation2017) using a cutoff of 20 ms. Our earlier studies on ALI-5 used a much younger sample (MICS) and therefore a cutoff of 30 ms. As the MIDUS sample is on average a decade older, we lowered the cutoff value from 30 to 20 ms in contrast to our earlier studies based on reference data above.
Statistical analysis
We present descriptive, univariate analysis using means, standard deviations, and range (minimum and maximum values) where appropriate. If indicated by a combined test for normality based on skewness and on kurtosis (ladder command in Stata), the according linear transformation was applied to better approximate a normal distribution. We conducted bivariate correlations of all ALI variables with the sum score of the PSS.
The usefulness of a new risk marker can be assessed by several statistical methods (Pencina et al., Citation2010). Recommendations for the reporting of novel risk markers have been proposed (Vasan, Citation2006). In particular, the guidelines of the American Heart Association give recommendations for the reporting of studies that evaluate novel biomarkers (here ALI-5 score). This includes observational studies and the report of odds ratio with according confidence intervals (CIs) or related statistics (Hlatky et al., Citation2009). Therefore, three logistic regression models tested the relationship of binary ALI risk scores (ALI-5 and ALI-10) as dependent variable with perceived stress as independent variable, adjusting for age (years), sex (male and female), and current smoking (no, yes). ALI sum scores were divided into two categories with the lower two tertiles as reference category (ALI-5: 4–5 and ALI-10: 4–10), resulting in upper tertile values of >3 (ALI-5 and ALI-10). Model 1 (based on subclinical cutoffs) and model 2 (based on the highest risk quartile) included all five variables of the streamlined ALI-5, model 3 included the 10 variables of the original ALI-10 risk score. Subgroup analysis for sex and age cluster (26–46, 47–56, and 57–65 years) explored this association further. We used Stata 15.1 SE (StataCorp LP, College Station, TX) for data management and statistical analysis.
Results
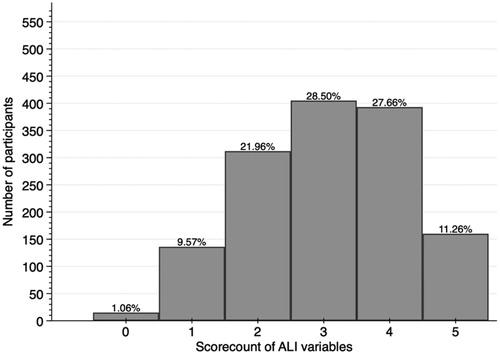
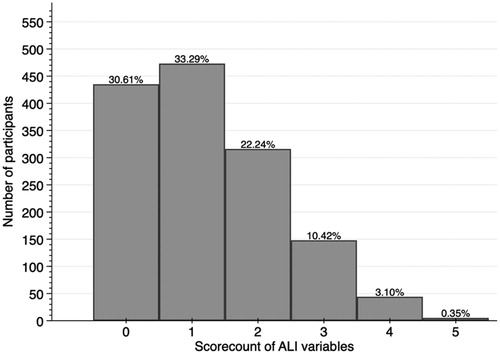
A total of N = 1421 (43% male) were included in the analysis with an average age of 50.4 ± 9.3 years. The sample characteristics are reported in . The average value of ALI-5 (subclinical) was 3.1 ± 1.2, of ALI-5 (statistical) was 1.2 ± 1.1, and of ALI-10 2.6 ± 1.7. Distribution of all variables for both ALI-5 scores is presented in and .
Perceived stress showed an average score of 22.9 ± 6.4. Age, HRV, HDL, and waist circumference were significantly correlated with perceived stress (). Adjusted logistic regression models showed an odds ratio (OR 1.37 ± 0.19, CI 1.05, 1.80; p=.0122) for ALI-5 (subclinical cutoff) with perceived stress, whereas odds ratio of ALI-10 with PSS did not reach significance (OR 1.27 ± 0.18, CI 0.95, 1.69, p=.10) (). A second ALI-5 score based on the statistical distribution of risk quartiles showed an odds ratio of 0.85 ± 0.32, CI 0.40, 1.80; p=.676 with perceived stress.
Table 3. Pearson’s correlation of perceived stress and various ALI variables.
Table 4. Logistic regression models, allostatic load index (ALI) as dependent variable, perceived stress as independent variable, adjusted for age, gender, and smoking, ALI-5 stratified by gender and age.
Subgroup analysis of the ALI-5 (subclinical cutoff) presented a stronger association in females (OR = 1.62 ± 0.28, CI 1.15, 2.28; p=.006) and, although not significant, younger age groups (26–46 years) (OR = 1.52 ± 0.36, CI 0.96, 2.41; p=.076), as reported in .
Discussion
The ALI is used as a composite risk score of cumulative health burden. Aim of this cross-sectional study was to replicate earlier research findings on the association of perceived stress with a streamlined ALI-5 score in a general population sample from the USA and to compare these results with the original ALI-10 score.
First, in addition to our findings regarding ALI and work-related stress (Mauss, Jarczok, et al., Citation2015; Mauss et al., Citation2016) we can confirm the ALI-5 as a reliable measurement associated with perceived stress in life. This index composed of five variables such as diastolic blood pressure, HbA1c, LDL, RMSSD, and waist circumference seems to be well defined and valid. Although most of these 5 variables were not significantly correlated to perceived stress, the composite score of allostatic load still indicates physiological adaptions to higher stress levels. This supports the hypothesis that ALI is a better predictor of later health than single biomarkers (Seeman et al., Citation2001). All these five variables have been used in earlier studies, e.g. diastolic blood pressure and HbA1c are part of the original ALI of 1997 (Seeman et al., Citation1997). Common understanding of ALI research is that different pathways such as metabolic (e.g. waist circumference), neurophysiological (e.g. HRV), and cardiovascular (e.g. HbA1c) should be covered in a comprehensive risk score (Juster et al., Citation2010; Mauss et al., Citation2016) to have a strong predictive power for future diseases.
Surprisingly, the HRV parameter RMSSD was not negatively correlated with the PSS stress measure in the MIDUS sample using parametric and nonparametric correlation (Supplementary Figure 1). Usually, more perceived stress means lower vagal activity. However, we previously found a measure of depressive symptoms to be negatively correlated to measures of HRV in men, but positively in women (Jarczok et al., Citation2018). Similar, higher depressive symptoms are usually reported to be associated with lower vagal activity. The positive association between HRV and PSS remains when using other HRV parameters from the 5-min resting baseline (SDNN, HF-power, and LF-power ) in nonparametric correlations. Other experimental phases are available (e.g. stress and recovery) to derive reactivity. The here reported positive association warrant further analysis that are beyond the scope of this manuscript. We still would strongly recommend to use vagally-mediated HRV parameters as a neurophysiological variable for the calculation of allostatic load based on growing evidence that HRV is a very valuable indicator for work-related (Jarczok et al., Citation2013, Citation2020; Järvelin-Pasanen et al., Citation2018; Tonello et al., Citation2014) and non-work-related stress (Glei et al., Citation2013).
Second, our standardized set of five variables contributes to a set of practical tools for occupational physicians. Our findings support the hypothesis that the streamlined ALI based on subclinical thresholds is stronger associated with various sources of stress than the ALI-5 or ALI-10 based on statistical thresholds. In addition, most variables of earlier studies calculating an ALI risk score are difficult to measure due to missing standards, a lack of (sub-)clinical thresholds, or high costs. For example, earlier attempts for a streamlined ALI with seven (Johansson et al., Citation2007) or eight (Langelaan et al., Citation2007; Näswall et al., Citation2012) variables included no primary mediators. Others with four or five variables included those that are expensive and hard to assess such as cortisol, epinephrine, norepinephrine, and DHEA-S (Gersten, Citation2008), or thrombin/antithrombin III complex, d-dimer, tissue-type plasminogen activator antigen, von Willebrand factor, and plasminogen activator inhibitor 1 antigen (Känel et al., Citation2003). In addition, it is beneficial to use a limited number of biomarkers and still covering neurophysiologic (e.g. HRV), metabolic (e.g. waist circumference), and cardiovascular (e.g. glycosylated hemoglobin) pathways. The benefit of our streamlined ALI-5 is the easy accessibility and low expenses as well as thresholds based on subclinical values according to clinical guidelines and evidence. This approach allows usability in day-to-day business as it is not related to statistical distribution in bigger population samples based on the highest risk quartile (below the 25th percentile for RMSSD, above the 75th percentile for diastolic blood pressure, waist circumference, HbA1c, and LDL). On the other hand side, empirically defined thresholds may enable examinations of whether other gradients are associated with health variables (Thomson et al., Citation2019). A statistical approach to calculate an ALI risk score is important and useful but should be limited to empirical studies and not to the daily routine of occupational health practitioners.
Third, our findings indicate gender-related differences regarding the association of perceived stress in life and indicators of individual health status. While our earlier studies showed that work-related stress is stronger associated with allostatic load in men (Mauss, Jarczok, et al., Citation2015; Mauss et al., Citation2016), women in the MIDUS sample exhibited a stronger association of perceived stress and ALI. This finding is in line with earlier findings from the SEBAS sample in elderly Taiwanese (Goldman et al., Citation2005). Cohen and Janicki-Deverts polled more than 2000 U.S. residents over the age of 18 between 1983 and 2009. The results indicate that women report more stress in all three surveys. They also show that as Americans age, they experience less stress and that retirees consistently report low levels of stress, indicating that retirement is not experienced as an adverse event (Cohen & Janicki-Deverts, Citation2012). While job empowerment and equal gender rights might add some burden on employed women it may as well be endemically stressful for modern women balancing both work and private life (Juster & Lupien, Citation2012). Stress reaction of both genders may differ following behavioral patterns due to divergent roles and genetic disparities. While Taylor et al. (Citation2000) mentioned that women are more likely to act supportive and assisting (tend-and-befriend), men’s behavior are usually related to confrontation or flight (fight-or-flight). Although the basic neuroendocrine stress responses do not vary substantially between males and females, these behavioral patterns may certainly have an impact on physiological stress reactions and therefore on allostatic load. Recent studies have shown sex differences in perceived stress of patients with acute myocardial infarction (Xu et al., Citation2015) and depression (Nayak et al., Citation2019). In the last 50 years, gender roles, family roles, and family structure have undergone dramatic shifts from traditional role expectations of “men as breadwinner” and “women as caregiver” (Xu et al., Citation2015). Other changes in social context such as increased labor force participation among women and higher divorce rates and single parenthood might be additional stressors, which certainly have implications on women’s stress perception and health (Williams & Kurina, Citation2002). Finally, we found a stronger association of perceived stress and ALI in participants aged 26–46 years (OR 1.52), although not significant (p=.076). While the ALI in general is age-dependent due to age-dependency of all included variables rising until the age of 60 and remaining on the same level up to 90 years (Crimmins et al., Citation2003), the association of stress and ALI may show a different pattern. The fact that the association of stress in life and ALI is strongest in younger age groups indicates that the vulnerability may be higher in younger people. The “window of opportunity” between the age of 20 and 60 years mentioned by Juster might be even tighter and more important to address stress-relieving activities in younger populations (Juster et al., Citation2010).
Our study presents different strengths. First, this study compares the relatively new and streamlined ALI-5 with the original ALI-10 risk score to find the best way to calculate the index. Second, the age range and size of the MIDUS sample allowed us to test for age and gender differences in the association of perceived stress and allostatic load, although we excluded participants older than 65 years. A supplementary analysis (data not shown) included these participants in the age range of 66–86 years (n = 352) resulting in a full sample size of 1773 participants. This analysis showed similar results and led us to the same conclusions. Third, due to the broad measurement of variables in the MIDUS biomarker project including multiple indicators of neurophysiological, endocrine, cardiovascular, and metabolic activity (Gruenewald et al., Citation2012), we were able to use one of the most comprehensive data sets to calculate different indices for allostatic load. That is especially exceptional as neuroendocrine biomarkers of the ALI-10 are expensive and hard to measure and are usually not part of routine datasets.
Limitations
Besides several strengths, this study has some limitations worth noting. First, although the MIDUS sample is diverse in respect to age, gender, and socioeconomic status, generalizability is limited to a U.S. population according to possible racial/ethnic differences. Second, this study has a cross-sectional study design. Therefore, it is difficult to draw any causal conclusions about the direction of observed associations. While we concluded, based on existing literature, that work-related or general perceived stress in life leads to wear and tear of the body, it may be that physical and psychological complains increase perceived stress levels of participants. Third, the association of perceived stress and ALI could depend on estimations and methods to define a high risk versus low-risk ALI as well as binary coding of the PSS. Nevertheless, this association was independent of the group split for ALI-10 variables (0–6 and 7–10 variables versus 0–4 and 5–10 variables as reference group). Fourth, there is limited evidence on cutoff values. For example, the parameters for HRV vary across age. Using several literature sources and own data from more than 12,000 office clerks (under revision), the average (i.e. 50th percentile) RMSSD value for male and female participants aged 50–54 is between 20 and 22 ms (Nunan et al., Citation2010; Shaffer & Ginsberg, Citation2017), while that for participants aged 40–44 years is 28–29 ms. Our previous two published MICS papers apply a cutoff value for RMSSD of 30 ms, but the average age of the underlying German sample is around 41 years, matching the chosen cutoff value of 30 ms. As the MIDUS sample is on average a decade older, we lowered the cutoff value to 20 ms based on the observation described above. Future studies could differentiate between age clusters using age-dependent cutoff values for HRV. Finally, today’s level of perceived stress or as assessed by PSS, stress level of the last 4 weeks, is not likely to represent earlier stress exposures, whereas an observed level of physiological dysregulation is likely to reflect a vast number of influences experienced over the life course.
Conclusion
The findings underline the approach of using predefined subclinical cutoff values instead of statistical methods based on risk quartiles to calculate an ALI risk score. In addition, the five variables including diastolic blood pressure, HbA1c, LDL, RMSSD, and waist circumference seem to be reliable biomarkers associated with perceived stress in life. The index could be used as an indicator for different sources of stress, work-related and non-work-related, and should be validated in longitudinal study settings.
Acknowledgments
The authors thank the staff of the Clinical Research Centers at the University of Wisconsin-Madison, UCLA, and Georgetown University for their support in conducting the MIDUS study.
Disclosure statement
No potential conflict of interest was by reported the author(s).
Additional information
Funding
Notes on contributors
Daniel Mauss
Daniel Mauss is specialized in occupational health and does research on health outcomes of stress at work. In the last 15 years he has been working as chief medical officer of different international industrial companies.
Marc N. Jarczok
Marc N Jarczok has a PhD in social sciences and does research in Psychobiology and Public Health. His current projects are on circadian variation parameters of cardiac autonomic activity.
References
- Xu, X., Bao, H., Strait, K., Spertus, J. A., Lichtman, J. H., D’Onofrio, G., Spatz, E., Bucholz, E. M., Geda, M., Lorenze, N. P., Bueno, H., Beltrame, J. F., & Krumholz, H. M. (2015). Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation, 131(7), 614–623. https://pubmed.ncbi.nlm.nih.gov/25679303
- Alberti, K. G. M. M., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., Fruchart, J. C., James, W. P. T., Loria, C. M., & Smith, S. C, International Association for the Study of Obesity. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 120(16), 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644
- Cohen, S. (1988). Perceived stress in a probability sample of the United States. The social psychology of health (pp. 31–67). Sage Publications, Inc. (The Claremont Symposium on Applied Social Psychology).
- Cohen, S., & Janicki-Deverts, D. (2012). Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. Journal of Applied Social Psychology, 42(6), 1320–1334. https://doi.org/10.1111/j.1559-1816.2012.00900.x
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Crimmins, E. M., Johnston, M., Hayward, M., & Seeman, T. (2003). Age differences in allostatic load: An index of physiological dysregulation. Experimental Gerontology, 38(7), 731–734. https://doi.org/10.1016/S0531-5565(03)00099-8
- D’Alonzo, K. T., Munet-Vilaro, F., Carmody, D. P., Guarnaccia, P. J., Linn, A. M., & Garsman, L. (2019). Acculturation stress and allostatic load among Mexican immigrant women. Revista Latino-Americana De Enfermagem, 27, e3135. https://doi.org/10.1590/1518-8345.2578.3135
- Dienberg Love, G., Seeman, T. E., Weinstein, M., & Ryff, C. D. (2010). Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22(8), 1059–1080. https://doi.org/10.1177/0898264310374355
- Fava, G. A., McEwen, B. S., Guidi, J., Gostoli, S., Offidani, E., & Sonino, N. (2019). Clinical characterization of allostatic overload. Psychoneuroendocrinology, 108, 94–101. https://doi.org/10.1016/j.psyneuen.2019.05.028
- Ferguson, C. J., & Heene, M. (2012). A vast graveyard of undead theories: Publication bias and psychological science’s aversion to the null. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 7(6), 555–561. https://doi.org/10.1177/1745691612459059
- Gersten, O. (2008). Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Social Science & Medicine, 66(3), 507–519. https://doi.org/10.1016/j.socscimed.2007.09.004
- Glei, D. A., Goldman, N., Shkolnikov, V. M., Jdanov, D., Shkolnikova, M., Vaupel, J. W., & Weinstein, M. (2013). Perceived stress and biological risk: Is the link stronger in Russians than in Taiwanese and Americans? Stress, 16(4), 411–420. https://doi.org/10.3109/10253890.2013.789015
- Goldman, N., Glei, D. A., Seplaki, C., Liu, I. W., & Weinstein, M. (2005). Perceived stress and physiological dysregulation in older adults. Stres, 8(2), 95–105. https://doi.org/10.1080/10253890500141905
- Gruenewald, T. L., Karlamangla, A. S., Hu, P., Stein-Merkin, S., Crandall, C., Koretz, B., & Seeman, T. E. (2012). History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine, 74(1), 75–83. https://doi.org/10.1016/j.socscimed.2011.09.037
- Grundy, S. M., Cleeman, J. I., Merz, C. N. B., Brewer, H. B., Clark, L. T., Hunninghake, D. B., Pasternak, R. C., Smith, S. C., & Stone, N. J, Coordinating Committee of the National Cholesterol Education Program. (2004). A summary of implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(8), 1329–1330. https://doi.org/10.1161/01.ATV.0000139012.45265.e0
- Hlatky, M. A., Greenland, P., Arnett, D. K., Ballantyne, C. M., Criqui, M. H., Elkind, M. S. V., Go, A. S., Harrell, F. E., Hong, Y., Howard, B. V., Howard, V. J., Hsue, P. Y., Kramer, C. M., McConnell, J. P., Normand, S. L. T., O’Donnell, C. J., Smith, S. C., & Wilson, P. W. F, American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. (2009). Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation, 119(17), 2408–2416. https://doi.org/10.1161/CIRCULATIONAHA.109.192278
- Jarczok, M. N., Aguilar-Raab, C., Koenig, J., Kaess, M., Borniger, J. C., Nelson, R. J., Hall, M., Ditzen, B., Thayer, J. F., & Fischer, J. E. (2018). The Heart’s rhythm ‘n’ blues: Sex differences in circadian variation patterns of vagal activity vary by depressive symptoms in predominantly healthy employees. Chronobiology International, 35(7), 896–909. https://doi.org/10.1080/07420528.2018.1439499
- Jarczok, M. N., Jarczok, M., & Thayer, J. F. (Eds.). (2020). Work stress and autonomic nervous system activity: Handbook of socioeconomic determinants of occupational health - from macro-level to micro-level evidence. Springer International Publishing.
- Jarczok, M. N., Jarczok, M., Mauss, D., Koenig, J., Li, J., Herr, R. M., & Thayer, J. F. (2013). Autonomic nervous system activity and workplace stressors – A systematic review. Neuroscience and Biobehavioral Reviews, 37(8), 1810–1823. https://doi.org/10.1016/j.neubiorev.2013.07.004
- Järvelin-Pasanen, S., Sinikallio, S., & Tarvainen, M. P. (2018). Heart rate variability and occupational stress-systematic review. Industrial Health, 56, 500–511. https://doi.org/10.2486/indhealth.2017-0190
- Johansson, G., Huang, Q., & Lindfors, P. (2007). A life-span perspective on women’s careers, health, and well-being. Social Science & Medicine, 65(4), 685–697. https://doi.org/10.1016/j.socscimed.2007.04.001
- Juster, R. P., & Lupien, S. (2012). A sex- and gender-based analysis of allostatic load and physical complaints. Gender Medicine, 9(6), 511–523. https://doi.org/10.1016/j.genm.2012.10.008
- Juster, R. P., McEwen, B. S., & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. https://doi.org/10.1016/j.neubiorev.2009.10.002
- Känel, R. v., Dimsdale, J. E., Patterson, T. L., & Grant, I. (2003). Acute procoagulant stress response as a dynamic measure of allostatic load in Alzheimer caregivers. Annals of Behavioral Medicine, 26(1), 42–48. https://doi.org/10.1207/S15324796ABM2601_06
- Kivimäki, M., Nyberg, S. T., Batty, G. D., Fransson, E. I., Heikkilä, K., Alfredsson, L., Bjorner, J. B., Borritz, M., Burr, H., Casini, A., Clays, E., de Bacquer, D., Dragano, N., Ferrie, J. E., Geuskens, G. A., Goldberg, M., Hamer, M., Hooftman, W. E., Houtman, I. L., Joensuu, M., … Theorell, T. (2012). Job strain as a risk factor for coronary heart disease: A collaborative meta-analysis of individual participant data. The Lancet , 380(9852), 1491–1497. https://doi.org/10.1016/S0140-6736(12)60994-5
- Kubzansky, L. D., Kawachi, I., Spiro, A., Weiss, S. T., Vokonas, P. S., & Sparrow, D. (1997). Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the normative aging study. Circulation, 95(4), 818–824. https://doi.org/10.1161/01.cir.95.4.818
- Langelaan, S., Bakker, A. B., Schaufeli, W. B., van Rhenen, W., & van Doornen, L. J. P. (2007). Is burnout related to allostatic load? International Journal of Behavioral Medicine, 14(4), 213–221. https://doi.org/10.1007/BF03002995
- Mauss, D., Jarczok, M. N., & Fischer, J. E. (2015). A streamlined approach for assessing the Allostatic Load Index in industrial employees. Stress, 18(4), 475–483. https://doi.org/10.3109/10253890.2015.1040987
- Mauss, D., Jarczok, M. N., & Fischer, J. E. (2016). The streamlined Allostatic Load Index: A replication of study results. Stress, 19(6), 553–558. https://doi.org/10.1080/10253890.2016.1219718
- Mauss, D., Li, J., Schmidt, B., Angerer, P., & Jarczok, M. N. (2015). Measuring allostatic load in the workforce: A systematic review. Industrial Health, 53(1), 5–20. https://doi.org/10.2486/indhealth.2014-0122
- McEwen, B. S. (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. https://doi.org/10.1016/S0006-3223(03)00177-X
- McEwen, B. S. (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America, 109(2), 17180–17185. https://doi.org/10.1073/pnas.1121254109
- McEwen, B. S., & Stellar, E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. https://doi.org/10.1001/archinte.1993.00410180039004
- Näswall, K., Lindfors, P., & Sverke, M. (2012). Job insecurity as a predictor of physiological indicators of health in healthy working women: An extension of previous research. Stress and Health: Journal of the International Society for the Investigation of Stress, 28(3), 255–263. https://doi.org/10.1002/smi.1430
- Nayak, A. S., Parkar, S. R., Nachane, H. B., Sangoi, B. A., & Shinde, R. G. (2019). Gender variability of perceived stress and negative inferential feedback in depression. Indian Journal of Psychological Medicine, 41(4), 331–337. https://pubmed.ncbi.nlm.nih.gov/31391665
- Nunan, D., Sandercock, G. R. H., & Brodie, D. A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33(11), 1407–1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
- Pencina, M. J., D’Agostino, R. B., & Vasan, R. S. (2010). Statistical methods for assessment of added usefulness of new biomarkers. Clinical Chemistry and Laboratory Medicine, 48(12), 1703–1711. https://doi.org/10.1515/CCLM.2010.340
- Sabbah, W., Watt, R. G., Sheiham, A., & Tsakos, G. (2008). Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: Evidence from the Third National Health and Nutrition Examination Survey. Journal of Epidemiology and Community Health, 62(5), 415–420. https://doi.org/10.1136/jech.2007.064188
- Seeman, T. E., McEwen, B. S., Rowe, J. W., & Singer, B. H. (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America, 98(8), 4770–4775. https://doi.org/10.1073/pnas.081072698
- Seeman, T. E., Singer, B. H., Rowe, J. W., Horwitz, R. I., & McEwen, B. S. (1997). Price of adaptation–allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine, 157(19), 2259–2268. https://doi.org/10.1001/archinte.1997.00440400111013
- Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. https://doi.org/10.3389/fpubh.2017.00258
- Siegrist, J., Lunau, T., Wahrendorf, M., & Dragano, N. (2012). Depressive symptoms and psychosocial stress at work among older employees in three continents. Globalization and Health, 8, 27. https://doi.org/10.1186/1744-8603-8-27
- Siegrist, J., Starke, D., Chandola, T., Godin, I., Marmot, M., Niedhammer, I., & Peter, R. (2004). The measurement of effort-reward imbalance at work: European comparisons. Social Science & Medicine, 58(8), 1483–1499. https://doi.org/10.1016/S0277-9536(03)00351-4
- Smith, K. J., Rosenberg, D. L., & Timothy Haight, G. (2014). An assessment of the psychometric properties of the perceived stress scale-10 (PSS10) with business and accounting students. Accounting Perspectives, 13(1), 29–59. https://doi.org/10.1111/1911-3838.12023
- Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A., & Updegraff, J. A. (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. https://doi.org/10.1037/0033-295x.107.3.411
- Theorell, T., Hammarström, A., Aronsson, G., Träskman Bendz, L., Grape, T., Hogstedt, C., Marteinsdottir, I., Skoog, I., & Hall, C. (2015). A systematic review including meta-analysis of work environment and depressive symptoms. BMC Public Health, 15(1), 1–14. https://doi.org/10.1186/s12889-015-1954-4
- Theorell, T., Jood, K., Jarvholm, L. S., Vingard, E., Perk, J., Ostergren, P. O., & Hall, C. (2016). A systematic review of studies in the contributions of the work environment to ischaemic heart disease development. European Journal of Public Health, 26(3), 470–477. https://doi.org/10.1093/eurpub/ckw025
- Thomson, E. M., Kalayci, H., & Walker, M. (2019). Cumulative toll of exposure to stressors in Canadians: An allostatic load profile. Health Reports, 30(6), 14–21. https://doi.org/10.25318/82-003-x201900600002-eng
- Tonello, L., Rodrigues, F. B., Souza, J. W. S., Campbell, C. S. G., Leicht, A. S., & Boullosa, D. A. (2014). The role of physical activity and heart rate variability for the control of work related stress. Frontiers in Physiology, 5, 67. https://doi.org/10.3389/fphys.2014.00067
- Vasan, R. S. (2006). Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation, 113(19), 2335–2362. https://doi.org/10.1161/CIRCULATIONAHA.104.482570
- Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M., Clement, D. L., Coca, A., de Simone, G., Dominiczak, A., Kahan, T., Mahfoud, F., Redon, J., Ruilope, L., Zanchetti, A., Kerins, M., Kjeldsen, S. E., Kreutz, R., Laurent, S., … Desormais, I. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal, 39(33), 3021–3104. https://doi.org/10.1093/eurheartj/ehy339
- Williams, K., & Kurina, L. M. (2002). The social structure, stress, and women’s health. Clinical Obstetrics and Gynecology, 45(4), 1099–1118. https://doi.org/10.1097/00003081-200212000-00018
- World Health Organization. (2011). Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Research and Clinical Practice, 93, 299–309. https://doi.org/10.1016/j.diabres.2011.03.012