Abstract
Stress-related psychological dysfunctions show a marked increase during adolescence, yet the mechanisms that mediate these vulnerabilities are unknown. Notably, however, adolescence is associated with changes in hormonal stress reactivity mediated by the hypothalamic-pituitary-adrenal (HPA) axis, which might contribute to these dysfunctions. Specifically, pre-adolescent animals display prolonged stress-induced HPA responses compared to adults. Previous experience with stressors further modify these changes in stress reactivity, such that repeated exposure to the same stressor results in an augmented HPA response prior to adolescence, but a habituated response in adulthood. It is unclear when during adolescence the habituated, adult-like response develops to a repeated stressor. Using male rats at various ages that span adolescence (30–70 days of age), we show that by mid-adolescence (i.e. 42 days of age), animals show neither a facilitated nor a habituated HPA hormonal response following four days of repeated restraint stress (4RS) compared to a single restraint session (1RS). We also show that the habituated HPA response to 4RS develops between late-adolescence and young adulthood (i.e. between 56 and 70 days of age, respectively). Further, we find age- and experience-dependent changes in progesterone and testosterone secretion, indicating that the interaction between development and experience affects stress-induced hormonal responses outside of canonical HPA-related hormones. Despite these hormonal differences mediated by age and experience, repeated restraint stress resulted in decreased fecal boli production at all four ages, suggesting dissociation between hormonal and autonomic reactivity during adolescence. These data indicate that HPA plasticity is significantly affected by adolescence and that a habituated hormonal response to homotypic stress does not occur until young adulthood. A greater appreciation of these changes in stress reactivity will contribute to our understanding of the psychological vulnerabilities often associated with stressful adolescence.
1. Introduction
Stress-related psychological and physiological disorders, ranging from anxiety and depression to drug abuse and obesity, show a marked increase in prevalence during adolescence (Dahl & Gunnar, Citation2009; Lee et al., Citation2014; McCormick & Green, Citation2013; Patton & Viner, Citation2007; Poyrazoglu et al., Citation2014; Spear, Citation2000). Though the mechanisms that mediate the increase in these dysfunctions are unclear, human and non-human animal studies indicate significant shifts in hormonal stress reactivity during the pubertal and adolescent stages of development (Dahl & Gunnar, Citation2009; McCormick et al., Citation2010, Citation2017; Romeo, Citation2018; Romeo et al., Citation2016). Numerous experiments have shown that peri-adolescent rats and mice show protracted HPA-mediated adrenocorticotropic hormone (ACTH) and corticosterone responses compared to adults following a variety of stressors, including hypoxia, foot shock, and restraint (Goldman et al., Citation1973; Romeo, Lee, Chhua, et al., Citation2004; Romeo et al., Citation2013, Citation2016; Vazquez & Akil, Citation1993). In addition to development, previous experience with stressors also shapes hormonal stress reactivity. In adulthood, for instance, exposure to the same stressor (i.e. a homotypic stressor), such as repeated exposure to restraint stress, leads to a habituated HPA stress response (Bhatnagar et al., Citation2002; Grissom & Bhatnagar, Citation2009; Hauger et al., Citation1990). Conversely, prior to adolescence, homotypic stress leads to a sensitized hormonal stress response (Doremus-Fitzwater et al., Citation2009; Lui et al., Citation2012; Romeo et al., Citation2006). Thus, greater exposure to stress-induced hormonal responses during the peri-adolescent stages of development might contribute to the mental and physical vulnerabilities mentioned above.
Based on the significant difference in hormonal stress reactivity before and after adolescent development, studies were conducted later to determine when during adolescence these shifts in hormonal responsiveness occur. In the context of the protracted HPA response exhibited by pre-adolescent animals following a single, acute exposure to a stressor, we have shown that the adult-like corticosterone response in male rats does not completely emerge until young adulthood, at approximately 60 days of age (Foilb et al., Citation2011). However, the developmental time course of the switch to the sensitized response following a homotypic stress experience prior to adolescence to the habituated response observed in adulthood is currently unknown. Moreover, it is unclear if other hormonal responses known to be different before and after adolescent development, such as stress-induced testosterone and progesterone secretion (Foilb et al., Citation2011; Green et al., Citation2016; Romeo, Lee, Chhua, et al., Citation2004; Romeo et al., Citation2005), are modified by repeated experiences with stress.
To further our understanding of experience-dependent changes in hormonal stress reactivity during adolescence, we measured plasma ACTH and corticosterone in male rats before, during, and after adolescent development following either a single session of restraint stress or four consecutive days of a single session of restraint. We also measured plasma progesterone to assess whether other hormonal responses known to be affected by adolescent development are also modified by previous stress experience (Green et al., Citation2016; Romeo et al., Citation2005), and plasma testosterone to evaluate the pubertal maturation of these animals. As an index of autonomic activity (McCarty & Kopin, Citation1979), fecal boli production was also measured to examine whether hormonal stress reactivity and autonomic reactivity change in parallel. We hypothesize that the stage of adolescent development will significantly affect the way in which male rats respond to repeated restraint stress. Specifically, we predict that as animals experience pubertal onset, as indexed by increased testosterone secretion around 40 days of age (Foilb et al., Citation2011; Ketelslegers et al., Citation1978; Lescoat et al., Citation1982; Pignatelli et al., Citation2006), animals will begin to exhibit a more adult-like habituation response to repeated restraint.
2. Materials and methods
2.1. Animals and housing
Male Sprague-Dawley rats used in this experiment were obtained from our breeding colony at Barnard College. Animals were weaned at 21 days of age and housed 2 per cage (same age cage mates) in clear polycarbonate cages (45 × 25 × 20 cm) with heat dried corncob bedding (bed-o’cobs ¼ inch, Andersons Lab Bedding; Maumee, OH). All animals had ad libitum access to food (Lab Diet #5012; PMI Nutrition International, LLC; Brentwood, MO) and water and the animal room was maintained at 21 ± 2 °C and on a 12 h light-dark schedule (lights on at 0900 h). All procedures were carried out in accordance with the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals and the Animal Experimentation Guidelines from the Columbia University Institute of Comparative Medicine. All procedures were approved by the Institutional Animal Care and Use Committee of Columbia University.
2.2. Experimental design and tissue collections
Rats were exposed to a single 30 min session of restraint (1RS) or a single 30 min session of restraint for four consecutive days (4RS). This particular regimen of restraint was chosen for the homotypic stress condition because once daily 30 min sessions of restraint for four days leads to a sensitized restraint-induced corticosterone response in 30 day old male rats and a habituated restraint-induced corticosterone response in 60 day old male rats (Romeo, unpublished observation). Four age groups that span adolescent development were assessed: 28 days of age (d; pre-adolescent), 42d (mid-adolescent), 56d (late-adolescent), and 70d (post-adolescent). Thus, for the 1RS and 4RS groups, restraint commenced on 28d and 25d, 42d and 39d, 56d and 53d, and 70d and 67d, respectively. Blood samples were collected via decapitation immediately before (i.e. 0 min; n = 4 per age and stress condition) or immediately (i.e. 30 min; n = 6 per age and stress condition) or 40 min (i.e. 70 min; n = 6 per age and stress condition) after termination of the 30 min session of restraint stress. Fecal boli that had accumulated in the restrainer were counted in the 30 and 70 min groups (n = 12 per age and stress condition).
Restraint was administered in the same room (separate from the colony room) and at the same time of day (1100 h) for all ages. As described previously, restraint stress was administered by placing animals in the prone position in wire mesh restrainers, sized so that animals at these different ages were equally restrained physically (Lui et al., Citation2012). In an attempt to equate ambient environmental conditions across age groups, no cage changes were conducted on the four days during restraint and tissue collections.
Trunk blood was collected in Vacutainer K3 EDTA-coated tubes (Fisher Scientific, Pittsburgh, PA), spun down in a 4 °C refrigerated centrifuge and plasma was stored at −20 °C until radioimmunoassays were performed for ACTH, corticosterone, progesterone, and testosterone (see below). Body and paired-testis weights were collected to verify the maturational state of these animals, and measure any age- and stress experience-dependent changes in these tissues.
2.3. Radioimmunoassays
Radioimmunoassays for ACTH, corticosterone, progesterone, and testosterone were conducted using commercially available kits (MP Biomedicals; Solon, OH) and performed as indicated by the supplier (catalog # 07-106102, 07-120102, 07-170102, and 07-189102, respectively). These kits are reported to have 100% cross reactivity for the specific hormone measured and minimal cross reactivity for all other hormones. For all assays, samples were run in duplicate and values were averaged. All duplicate samples had a coefficient of variation (CV) under 10%. The intra-assay CV and lower limit of detectability for each assay was as follows: ACTH = 9.2% and 11.30 pg/ml; corticosterone = 12.6% and 24.75 ng/ml; progesterone = 14% and 0.17 ng/ml; and testosterone = 12.2% and 0.09 ng/ml. As reported previously, to capture an indirect measure of potential changes in adrenal sensitivity to ACTH, we conducted an analysis on the ratio of the plasma corticosterone to the log of the plasma ACTH (Cotella et al., Citation2020; Ulrich-Lai & Engeland, Citation2002).
2.4. Statistical analysis
The hormonal and somatic data were assessed with three-way ANOVAs (age x stress condition x stress time point). As the focus of this study was to assess potential age-related changes in hormonal stress reactivity in response to a single or four repeated sessions of restraint stress, follow up analyses of significant three-way ANOVAs were split by stress time point. Due to an issue with a single sample in the ACTH and corticosterone assays, one 28d 1RS 0 min time point ACTH sample and one 70d 4RS 30 min time point corticosterone sample are omitted from the ACTH and corticosterone analyses, respectively. The fecal boli data were analyzed with a two-way ANOVA (age x stress condition). F tests and Tukey's honestly significant difference post-hoc tests were used where appropriate. Data are reported as the mean ± SEM and differences were considered significant at p < 0.05. All statistical analyses were performed using R Studio (RStudio, Inc) or GraphPad PRISM, version 8.1 (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. Somatic measures
ANOVAs were conducted on body and paired-testis weights to assess the effects of age, stress experience, and stress time point on these peripheral measures. Three-way ANOVAs for both body and paired-testis weights revealed only a significant main effect of age (F(3,104) = 1244.11 and 1080.22, respectively, p < 0.001, ). Specifically, post hoc analyses revealed the expected significant age-related increase in these weights, with each age group showing progressively greater body and paired-testis weights (all p < 0.001, ). These data indicate significant pubertal and adolescent maturation occurred in these animals during the 42 days encompassing the tissue collections.
Table 1. Mean (± SEM) body (g) and paired-testis (g) weights in 28d, 42d, 56d, and 70d male rats.
3.2. Hormonal measures
3.2.1. ACTH and corticosterone
In an effort to examine age-, experience-, and time point-dependent changes in hormonal stress reactivity, three-way ANOVAs were applied to plasma ACTH and corticosterone values, as well as to the ratio of plasma corticosterone to the log of plasma ACTH. For ACTH, a significant three-way interaction was observed between age, stress condition, and stress time point (F(6,103) = 3.42, p < 0.004). Follow up analyses were separated by stress time point, which showed no effect of age or stress condition at the 0 min stress time point. However, significant effects of age and stress condition on ACTH responses were noted at the 30 min stress time point (F(3,40) =14.44, p < 0.001 and F(1,40) = 4.18 p < 0.04, ) with 70d animals showing the lowest ACTH levels, independent of stress condition, and animals exposed to 4RS showed lower ACTH levels compared to the animals exposed to 1RS, independent of age. Analyses at the 70 min stress time point showed a significant interaction between age and stress condition (F(3,40) = 4.58, p < 0.008, ), such that 28d animals had higher ACTH levels than the other three age groups and that within 28d animals, those exposed to 4RS had significantly lower levels than those exposed to 1RS (p < 0.01, ).
Figure 1. Mean (± SEM) plasma ACTH (pg/ml; A top row), corticosterone (ng/ml; B middle row), and corticosterone by log ACTH levels ([CORT]/log[ACTH]; C bottom rows) in 28d, 42d, 56d, and 70d male rats exposed to a single 30 min session of restraint stress (1RS; open circles) or a single 30 min session of restraint stress on four consecutive days (4RS, closed squares) before (0 min), immediately after termination of a 30 min session of restraint stress (30 min), or 40 min after termination of a 30 min session of restraint stress (70 min). Asterisks indicate that 4RS is significantly less than 1RS at that age and “#” indicates that 4RS is significantly greater than 1RS at that age. Note that for ease of visualization, significant main effects of age or stress condition are not indicated.
![Figure 1. Mean (± SEM) plasma ACTH (pg/ml; A top row), corticosterone (ng/ml; B middle row), and corticosterone by log ACTH levels ([CORT]/log[ACTH]; C bottom rows) in 28d, 42d, 56d, and 70d male rats exposed to a single 30 min session of restraint stress (1RS; open circles) or a single 30 min session of restraint stress on four consecutive days (4RS, closed squares) before (0 min), immediately after termination of a 30 min session of restraint stress (30 min), or 40 min after termination of a 30 min session of restraint stress (70 min). Asterisks indicate that 4RS is significantly less than 1RS at that age and “#” indicates that 4RS is significantly greater than 1RS at that age. Note that for ease of visualization, significant main effects of age or stress condition are not indicated.](/cms/asset/b6e634df-0932-470d-9e10-6b95903d41e1/ists_a_1873945_f0001_b.jpg)
For corticosterone, a significant three-way interaction was observed between age, stress condition, and stress time point (F(6,103) = 6.71, p < 0.0001). Follow up analyses were divided by stress time point, which showed a significant effect of stress condition at the 0 min stress time point (F(1, 24) = 18.59, p < 0.0002), such that independent of age, animals exposed to the 4RS condition had significantly higher levels of plasma corticosterone at the 0 min time point than animals exposed to the 1RS condition (). At the 30 min stress time point, there was a significant interaction between age and stress condition (F(3,39) =6.78, p < 0.0009). Here it was noted that 28d animals exposed to 4RS had significantly higher levels of plasma corticosterone than the 28d animals exposed to the 1RS. Conversely, 70d animals exposed to 4RS had significantly lower corticosterone levels at the 30 min stress time point than the 70d animals exposed to 1RS (all p < 0.01). There were no significant differences between the 4RS and 1RS conditions in either the 42d or 56d animals at the 30 min stress time point (). There was also a significant interaction between age and stress condition at the 70 min stress time point (F(3,40) = 4.40, p < 0.009) with the 28d animals exposed to 4RS showing significantly lower corticosterone levels than the 28d animals exposed to 1RS (p < 0.02, ).
For the ratio of plasma corticosterone to the log of plasma ACTH, a significant three-way interaction was observed between age, stress condition, and stress time point (F(6,103) = 5.46, p < 0.0001) and the follow up analyses at the stress time points revealed the identical pattern of results as those obtained for plasma corticosterone. Specifically, there was a significant effect of stress condition at the 0 min stress time point (F(1, 23) = 17.02, p < 0.0004), such that independent of age, animals exposed to the 4RS condition had significantly higher plasma corticosterone to log plasma ACTH values than animals exposed to the 1RS condition (). At the 30 min stress time point, there was a significant interaction between age and stress condition (F(3,39) = 5.55, p < 0.003), in which the 28d animals exposed to 4RS had significantly higher values than the 28d animals exposed to 1RS, while 70d animals exposed to 4RS had significantly lower values at the 30 min stress time point than the 70d animals exposed to 1RS (all p < 0.05). There were no significant differences between the 4RS and 1RS conditions in either the 42d or 56d animals at the 30 min stress time point (). There was also a significant interaction between age and stress condition at the 70 min stress time point (F(3,40) = 3.09, p < 0.04) with the 28d animals exposed to 4RS showing significantly lower plasma corticosterone to log plasma ACTH values than the 28d animals exposed to 1RS (p < 0.05, ).
3.2.2. Progesterone
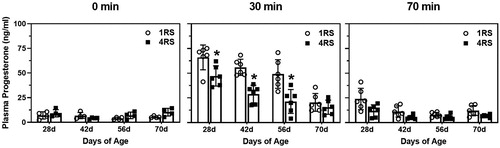
The three-way ANOVA on plasma progesterone revealed a significant interaction among the three variables (F(6,104) = 2.12, P = < 0.05). Follow up analyses showed that there were no effects of age or stress condition on plasma progesterone levels at the 0 min stress time point (). However, at the 30 min stress time point, there were significant effects between age and stress condition (F(3,40) = 3.04, p < 0.04), such that 28d, 42d, and 56d animals exposed to the 4RS condition had significantly lower plasma progesterone levels compared to the animals exposed to 1RS (all p < 0.01), while 70d animals were unaffected by stress condition. At the 70 min stress time point there were significant effects of age and stress condition (F(3,40) = 10.58, p < 0.0001 and F(1,40) = 17.23, p < 0.0002) with 28d animals having higher levels of progesterone than the other three ages, and animals exposed to the 4RS had significantly lower progesterone levels than animals exposed to 1RS (all p < 0.01).
Figure 2. Mean (± SEM) plasma progesterone (ng/ml) in 28d, 42d, 56d, and 70d male rats exposed to a single 30 min session of restraint stress (1RS; open circles) or a single 30 min session of restraint stress on four consecutive days (4RS, closed squares) before (0 min), immediately after termination of a 30 min session of restraint stress (30 min), or 40 min after termination of a 30 min session of restraint stress (70 min). Asterisks indicate that 4RS is significantly less than 1RS at that age. Note that for ease of visualization, significant main effects of age or stress condition are not indicated.
3.2.3. Testosterone
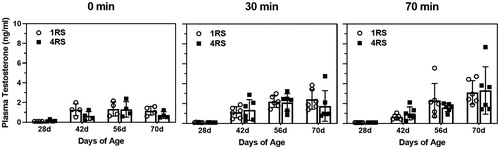
For testosterone, there was no significant three-way interaction (P = 0.72), but there was a significant two-way interaction between age and stress time point (F(6,104) = 3.87, p < 0.002). Specifically, plasma testosterone levels were significantly higher at all three stress time points in the 42d, 56d, and 70d animals compared to the 28d animals, with the greatest elevation at the 70 min stress time point ().
Figure 3. Mean (± SEM) plasma testosterone (ng/ml) in 28d, 42d, 56d, and 70d male rats exposed to a single 30 min session of restraint stress (1RS; open circles) or a single 30 min session of restraint stress on four consecutive days (4RS, closed squares) before (0 min), immediately after termination of a 30 min session of restraint stress (30 min) or 40 min after termination of a 30 min session of restraint stress (70 min). Note that for ease of visualization, significant main effects of age or stress condition are not indicated.
3.2.4. Fecal Boli
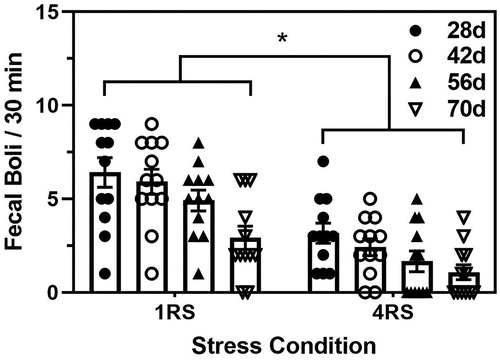
The two-way ANOVA conducted on the number of fecal boli revealed significant main effects of both age and stress condition (F(3,88) = 8.60 and (1,88) = 51.37, both p < 0.0001) Specifically, independent of stress condition, 70d animals had a lower number of fecal boli than all other ages (p < 0.05), while independent of age, animals exposed to the 4RS condition had significantly fewer fecal boli than the animals exposed to the 1RS condition (p < 0.001, )
Figure 4. Mean (± SEM) fecal boli per 30 min of the terminal stress session in 28d (closed circles), 42d (open circles), 56d (closed triangles), and 70d (open triangles) male rats exposed to a single 30 min session of restraint stress (1RS) or a single 30 min session of restraint stress on four consecutive days (4RS). Asterisks indicates a significant difference between the stress conditions.
4. Discussion
These data indicate significant alterations in experience-dependent hormonal stress reactivity during adolescence in male rats. Specifically, the sensitized homotypic-induced corticosterone response exhibited by pre-adolescent males (Doremus-Fitzwater et al., Citation2009; Lui et al., Citation2012; Romeo et al., Citation2006) is no longer expressed by 42 days of age, while the adult-like habituated corticosterone response to a homotypic stressor (Bhatnagar et al., Citation2002; Grissom & Bhatnagar, Citation2009; Hauger et al., Citation1990) develops between 56 and 70 days of age. It should be noted, however, that others have found that male rats exposed to homotypic isolation/confinement stress from 30d to 45d do show habituated stress-induced corticosterone responses, thus indicating age differences in habituation are sensitive to the type and duration of the homotypic stress experience (Hodges & McCormick, Citation2015). Interestingly, our ACTH data show a slightly different pattern than our corticosterone data, particularly at the 30 min stress time point. That is, following experience with a homotypic stress, ACTH levels in 28d animals do not display a sensitized response and ACTH levels in 70d animals do not display a habituated response, though 70d animals do show a decreased response at this time point. These data suggest that changes in adrenal sensitivity to ACTH might, at least in part, be responsible for the sensitized and habituated corticosterone responses following homotypic stress exposure before and after adolescence, respectively. In fact, our results obtained from the ratio of plasma corticosterone to the log of plasma ACTH, a measure thought to reflect adrenal sensitivity to ACTH (Cotella et al., Citation2020; Ulrich-Lai & Engeland, Citation2002), support this notion. Our ACTH and corticosterone data at the 70 min stress time point also replicate our previous observations, such that only 28d animals show an extended hormonal stress response after a single stressor compared to animals ≥ 40 days of age, and that recovery of the response at this age is affected by a homotypic stress experience (Foilb et al., Citation2011; Lui et al., Citation2012; Romeo et al., Citation2006). The current data also indicate that the pattern of progesterone secretion is modified by the developmental state of the animals and their stress history, while testosterone secretion is only significantly affected by stress in males that have achieved pubertal onset. Despite these hormonal differences, repeated restraint resulted in reduced fecal boli production throughout adolescent development, suggesting dissociation between hormonal and autonomic reactivity during adolescence. Though the physiological and neurobehavioral implications of these age- and experience-dependent changes in hormone secretion are unclear, these data highlight the significant malleability of the hormonal stress response following stressful experiences during adolescence.
The neuroendocrine mechanisms that mediate this developmental switch between a homotypic stress-induced sensitized versus habituated HPA response are unclear. However, as these changes in hormonal stress reactivity are associated with increases in testosterone secretion (Foilb et al., Citation2011; Ketelslegers et al., Citation1978; Lescoat et al., Citation1982; Pignatelli et al., Citation2006), and peripheral markers of gonadal development, such as increased pair-testis weights (Foilb et al., Citation2011), it is possible that pubertal changes in testosterone modulate HPA responsiveness to repeated stressors. Though we have not found any influence of testosterone on hormonal stress reactivity prior to puberty in male rats (Romeo, Lee, Chhua, et al., Citation2004), androgens have been shown to affect HPA reactivity in response to both acute and repeated stressors in mid-adolescent (Gomez et al., Citation2004) and adult males (Gray et al., Citation2010; Handa et al., Citation2013; McCormick et al., Citation2002; Viau, Citation2002; Viau & Meaney, Citation1996). Furthermore, exposure to gonadal hormones, including the central conversion of testosterone to estradiol, early in development can organize later HPA reactivity to stressors in adulthood (Bingham et al., Citation2011; Bingham & Viau, Citation2008; McCormick et al., Citation1998). Despite these associations between gonadal hormones and stress reactivity, the neural substrate(s) mediating this developmental change are unknown, though these hormones have been noted to act directly in the medial preoptic nucleus (MPN) to influence HPA reactivity in adults (Williamson et al., Citation2010; Williamson & Viau, Citation2008). Along with the MPN, additional brain regions, such as posterior paraventricular nucleus of the thalamus (Bhatnagar et al., Citation2002; Bhatnagar & Dallman, Citation1998), rostral posterior hypothalamus (Nyhuis et al., Citation2016), and medial prefrontal (Jackson & Moghaddam, Citation2006; Weinberg et al., Citation2010) and orbitofrontal cortices (Campeau et al., Citation2002), could also be involved, as these areas have been reported to play a role in experience-induced changes in hormonal stress reactivity in adults. Though little is known about changes in these brain regions in context of HPA function and their sensitivity to androgens or estrogens during adolescent development, studies utilizing hormone implants, lesions, or transient inactivation of these structures in adolescent animals exposed to homotypic stress would be helpful in assessing their role in these age- and experience-dependent hormonal changes.
It is important to note that, in addition to central mechanisms, peripheral mechanisms may also play a role mediating these age- and experience-dependent changes in HPA reactivity. For instance, we have previously shown pubertal changes in adrenal sensitivity to ACTH (Romeo et al., Citation2014) and our current data, in the context of the ratio of plasma corticosterone to the log of plasma ACTH, would support the idea that changes in adrenal sensitivity to ACTH and/or steroidogenic capability of the adrenal might contribute to the sensitized response following homotypic stress at this stage of development. Moreover, splanchnic nerve innervation of the adrenal gland might also contribute to some of these changes in hormonal stress reactivity, as has been noted in adult rats (Ulrich-Lai & Engeland, Citation2002). Future studies utilizing peripheral hormone administration paradigms and splanchnic nerve transection in animals exposed to homotypic stress would help elucidate this possibility.
The plasma progesterone and testosterone data indicate that in addition to the canonical HPA hormonal responses often measured, these hormonal responses change with stress experience and age as well. The hormonal responses to the single bout of restraint stress agree with previously published data that shows greater stress-induced testosterone, but less stress-induced progesterone secretion, in adult compared to prepubertal males (Foilb et al., Citation2011; Green et al., Citation2016; Romeo, Lee, Chhua, et al., Citation2004; Romeo et al., Citation2005). It is noteworthy that stress-induced progesterone responses are modified by stress experience, while testosterone responses are not, but these differences might be related to the changes in adrenal steroidogenesis and/or adrenal responsiveness more generally, as stress-induced progesterone responses have been noted in gonadectomized (Romeo, Lee, & McEwen, Citation2004; Romeo et al., Citation2005), but not adrenalectomized (Hueston & Deak, Citation2014), rats. Though the central and/or peripheral mechanisms responsible for these age- and stress-related differences in progesterone and testosterone secretion are unknown, future studies will be needed to examine the influence of these hormonal changes on the physiology and behavior of both adolescent and adult animals.
The fecal boli data suggest that despite different experience-dependent changes in hormonal stress reactivity noted throughout adolescence, autonomic responsiveness shows the same experience-dependent changes regardless of the animal’s stage of adolescent development. These data are similar to what we have found in the context of behavioral reactivity in response to acute versus homotypic stress before and after adolescent development. That is, both prepubertal and adult male rats show reduced struggling behavior in the restraint device following exposure to homotypic stress in spite of the hormonal habituation occurring only in adults (Lui et al., Citation2012). Thus, in conjunction with these previous behavioral results (Lui et al., Citation2012) and the current fecal boli results, there appear to be dissociations between stress experience-dependent hormonal changes and behavioral and autonomic changes, at least as indexed by restraint-induced struggling and fecal boli production. It would also be interesting to investigate whether homotypic exposure to an appetitive or neutral stimulus would result in similar developmental differences in habituation on other physiological or behavioral parameters and whether these differences would be as diametrically opposed as they are for these HPA responses to an aversive stimulus.
A limitation of these current findings is that only male subjects were examined. Adult male and female rats both exhibit reduced hormonal stress reactivity following repeated homotypic stressors, such as restraint and loud noise (Babb et al., Citation2014). Moreover, similar to prepubertal males, prepubertal females also show a relative lack of hormonal habituation after repeated restraint (Doremus-Fitzwater et al., Citation2009). However, it is possible that adolescent females will show different developmental trajectories in experience-dependent hormonal changes following a homotypic stressor compared to adolescent males. Additional studies including females in the experimental design could help address any potential sex differences in responsiveness that might exist following homotypic stress during adolescence and the role that gonadal hormones may play in experience-dependent changes in hormonal stress responsiveness prior to adulthood.
5. Conclusion
In summary, these results indicate that adolescence and stress experience interact in male rats to shape hormonal stress reactivity. A major conclusion drawn from these data is that by mid-adolescence, animals show neither a facilitated nor a habituated HPA hormonal response following exposure to homotypic stress and that the habituated response develops between late-adolescence and young adulthood. These data suggest that further development of the neural and/or peripheral substrates that mediate these experience-dependent changes may be sensitive to the gradual and sustained increase in gonadal hormones that occur at this stage of maturation. Moreover, progesterone and testosterone responses show unique patterns of secretion depending on the age and stress experience of the animal, indicating adolescence is associated with hormonal changes outside of the canonical HPA axis and that some of these changes are sensitive to previous stress exposure. Though the consequences of these hormonal responses remain unclear, further study of these endocrine changes may provide much-needed information on the neurobehavioral perturbations often associated with stressful adolescence.
Acknowledgments
We would like to thank Gregory Pearson for his help with the statistical analyses and Page Buchanan for his expert animal care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Russell D. Romeo
RDR designed and conducted the experiments, while RKS helped conduct the experiments. Both authors contributed to the writing and editing of the manuscript.
Rose K. Sciortino
RDR designed and conducted the experiments, while RKS helped conduct the experiments. Both authors contributed to the writing and editing of the manuscript.
References
- Babb, J. A., Masini, C. V., Day, H. E. W., & Campeau, S. (2014). Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress, 17(3), 224–234. https://doi.org/10.3109/10253890.2014.905534
- Bhatnagar, S., & Dallman, M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience, 84(4), 1025–1039. https://doi.org/10.1016/s0306-4522(97)00577-0
- Bhatnagar, S., Huber, R., Nowak, N., & Trotter, P. (2002). Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology, 14(5), 403–410. https://doi.org/10.1046/j.0007-1331.2002.00792.x
- Bingham, B., Gray, M., Sun, T., & Viau, V. (2011). Postnatal blockade of androgen receptors or aromatase impair the expression of stress hypothalamic-pituitary-adrenal axis habituation in adult male rats. Psychoneuroendocrinology, 36(2), 249–257. https://doi.org/10.1016/j.psyneuen.2010.07.015
- Bingham, B., & Viau, V. (2008). Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology, 149(7), 3581–3591. https://doi.org/10.1210/en.2007-1796
- Campeau, S., Dolan, D., Akil, H., & Watson, S. J. (2002). c-fos mRNA induction in acute and chronic audiogenic stress: Possible role of the orbitofrontal cortex in habituation. Stress, 5(2), 121–130. https://doi.org/10.1080/10253890290027895
- Cotella, E. M., Morano, R. L., Wulsin, A. C., Martelle, S. M., Lemen, P., Fitzgerald, M., Packard, B. A., Moloney, R. D., & Herman, J. P. (2020). Lasting impact of chronic adolescent stress and glucocorticoid receptor selective modulation in male and female rats. Psychoneuroendocrinology, 112, 104490 https://doi.org/10.1016/j.psyneuen.2019.104490
- Dahl, R. E., & Gunnar, M. R. (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21(1), 1–6. https://doi.org/10.1017/S0954579409000017
- Doremus-Fitzwater, T. L., Varlinskaya, E. I., & Spear, L. P. (2009). Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & Behavior, 97(3–4), 484–494. https://doi.org/10.1016/j.physbeh.2009.03.025
- Foilb, A. R., Lui, P., & Romeo, R. D. (2011). The transformation of hormonal stress responses throughout puberty and adolescence. The Journal of Endocrinology, 210(3), 391–398. https://doi.org/10.1530/JOE-11-0206
- Goldman, L., Winget, C., Hollingshead, G. W., & Levine, S. (1973). Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology, 12(3), 199–211. https://doi.org/10.1159/000122169
- Gomez, F., Manalo, S., & Dallman, M. F. (2004). Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology, 145(1), 59–70. https://doi.org/10.1210/en.2003-0565
- Gray, M., Bingham, B., & Viau, V. (2010). A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. Journal of Neuroendocrinology, 22(2), 92–101. https://doi.org/10.1111/j.1365-2826.2009.01941.x
- Green, M. R., Nottrodt, R. E., Simone, J. J., & McCormick, C. M. (2016). Glucocorticoid receptor translocation and expression of relevant genes in the hippocampus of adolescent and adult male rats. Psychoneuroendocrinology, 73, 32–41. https://doi.org/10.1016/j.psyneuen.2016.07.210
- Grissom, N., & Bhatnagar, S. (2009). Habituation to repeated stress: Get used to it. Neurobiology of Learning and Memory, 92(2), 215–224. https://doi.org/10.1016/j.nlm.2008.07.001
- Handa, R. J., Kudwa, A. E., Donner, N. C., McGivern, R. F., & Brown, R. (2013). Central 5-alpha reduction of testosterone is required for testosterone's inhibition of the hypothalamo-pituitary-adrenal axis response to restraint stress in adult male rats. Brain Research, 1529, 74–82. https://doi.org/10.1016/j.brainres.2013.07.021
- Hauger, R. L., Lorang, M., Irwin, M., & Aguilera, G. (1990). CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Research, 532(1–2), 34–40. https://doi.org/10.1016/0006-8993(90)91738-3
- Hodges, T. E., & McCormick, C. M. (2015). Adolescent and adult male rats habituate to repeated isolation, but only adoelscents sensitize to partner familiarity. Hormones and Behavior, 69, 16–30. https://doi.org/10.1016/j.yhbeh.2014.12.003
- Hueston, C. M., & Deak, T. (2014). On the time course, generality, and regulation of plasma progesterone release in male rats by stress exposure. Endocrinology, 155(9), 3527–3537. https://doi.org/10.1210/en.2014-1060
- Jackson, M. E., & Moghaddam, B. (2006). Distinct patterns of plasticity in prefrontal cortex neurons that encode slow and fast responses to stress. The European Journal of Neuroscience, 24(6), 1702–1710. https://doi.org/10.1111/j.1460-9568.2006.05054.x
- Ketelslegers, J. M., Hetzel, W. D., Sherins, R. J., & Catt, K. J. (1978). Developmental changes in testicular gonadotropin receptors: Plasma gonadotropins and plasma testosterone in the rat. Endocrinology, 103(1), 212–222. https://doi.org/10.1210/endo-103-1-212
- Lee, F. S., Heimer, H., Giedd, J. N., Lein, E. S., Sestan, N., Weinberger, D. R., & Casey, B. J. (2014). Mental health. Adolescent mental health-opportunity and obligation. Science, 346(6209), 547–549. https://doi.org/10.1126/science.1260497
- Lescoat, G., Lescoat, D., & Garnier, D. H. (1982). Influence of adrenalectomy on maturation of gonadotrophin function in the male rat. The Journal of Endocrinology, 95(1), 1–6. https://doi.org/10.1677/joe.0.0950001
- Lui, P., Padow, V. A., Franco, D., Hall, B. S., Park, B., Klein, Z. A., & Romeo, R. D. (2012). Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiology & Behavior, 107(1), 104–111. https://doi.org/10.1016/j.physbeh.2012.06.011
- McCarty, R., & Kopin, I. J. (1979). Stress-induced alterations in plasma catecholamines and behavior of rats: Effects of chlorisondamine and bretylium. Behavioral and Neural Biology, 27(3), 249–265. https://doi.org/10.1016/S0163-1047(79)92314-8
- McCormick, C. M., Furey, B. F., Child, M., Sawyer, M. J., & Donohue, S. M. (1998). Neonatal sex hormones have 'organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Research. Developmental Brain Research, 105(2), 295–307. https://doi.org/10.1016/s0165-3806(97)00155-7
- McCormick, C. M., & Green, M. R. (2013). From the stressed adolescent to the anxious and depressed adult: Investigations in rodent models. Neuroscience, 249, 242–257. https://doi.org/10.1016/j.neuroscience.2012.08.063
- McCormick, C. M., Green, M. R., & Simone, J. J. (2017). Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiology of Stress, 6, 31–43. https://doi.org/10.1016/j.ynstr.2016.08.003
- McCormick, C. M., Linkroum, W., Sallinen, B. J., & Miller, N. W. (2002). Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress, 5(4), 235–247. https://doi.org/10.1080/1025389021000061165
- McCormick, C. M., Mathews, I. Z., Thomas, C., & Waters, P. (2010). Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition, 72(1), 73–85. https://doi.org/10.1016/j.bandc.2009.06.003
- Nyhuis, T. J., Masini, C. V., Day, H. E. W., & Campeau, S. (2016). Evidence for the integration of stress-related signals by the rostral posterior hypothalamic nucleus in the regulation of acute and repeated stress-evoked Hypothalamo-Pituitary-Adrenal Response in Rat. The Journal of Neuroscience, 36(3), 795–805. https://doi.org/10.1523/JNEUROSCI.3413-15.2016
- Patton, G. C., & Viner, R. (2007). Pubertal transitions in health. Lancet, 369(9567), 1130–1139. https://doi.org/10.1016/S0140-6736(07)60366-3
- Pignatelli, D., Xiao, F., Gouveia, A. M., Ferreira, J. G., & Vinson, G. P. (2006). Adrenarche in the rat. The Journal of Endocrinology, 191(1), 301–308. https://doi.org/10.1677/joe.1.06972
- Poyrazoglu, S., Bas, F., & Darendeliler, F. (2014). Metabolic syndrome in young people. Current Opinion in Endocrinology, Diabetes, and Obesity, 21(1), 56–63. https://doi.org/10.1097/01.med.0000436414.90240.2c
- Romeo, R. D. (2018). The metamorphosis of adolescent hormonal stress reactivity: A focus on animal models. Frontiers in Neuroendocrinology, 49, 43–51. https://doi.org/10.1016/j.yfrne.2017.12.003
- Romeo, R. D., Bellani, R., Karatsoreos, I. N., Chhua, N., Vernov, M., Conrad, C. D., & McEwen, B. S. (2006). Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology, 147(4), 1664–1674. https://doi.org/10.1210/en.2005-1432
- Romeo, R. D., Bellani, R., & McEwen, B. S. (2005). Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress, 8(4), 265–271. https://doi.org/10.1080/10253890500489320
- Romeo, R. D., Kaplowitz, E. T., Ho, A., & Franco, D. (2013). The influence of puberty on stress reactivity and forebrain glucocorticoid receptor levels in inbred and outbred strains of male and female mice. Psychoneuroendocrinology, 38(4), 592–596. https://doi.org/10.1016/j.psyneuen.2012.07.019
- Romeo, R. D., Lee, S. J., Chhua, N., McPherson, C. R., & McEwen, B. S. (2004). Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology, 79(3), 125–132. https://doi.org/10.1159/000077270
- Romeo, R. D., Lee, S. J., & McEwen, B. S. (2004). Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology, 80(6), 387–393. https://doi.org/10.1159/000084203
- Romeo, R. D., Minhas, S., Svirsky, S. E., Hall, B. S., Savenkova, M., & Karatsoreos, I. N. (2014). Pubertal shifts in adrenal responsiveness to stress and adrenocorticotropic hormone in male rats. Psychoneuroendocrinology, 42, 146–152. https://doi.org/10.1016/j.psyneuen.2014.01.016
- Romeo, R. D., Patel, R., Pham, L., & So, V. M. (2016). Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neuroscience and Biobehavioral Reviews, 70, 206–216. https://doi.org/10.1016/j.neubiorev.2016.05.020
- Spear, L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24(4), 417–463. https://doi.org/10.1016/s0149-7634(00)00014-2
- Ulrich-Lai, Y. M., & Engeland, W. C. (2002). Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology, 76(2), 79–92. https://doi.org/10.1159/000064426
- Vazquez, D. M., & Akil, H. (1993). Pituitary-adrenal response to ether vapor in the weanling animal: Characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatric Research, 34(5), 646–653. https://doi.org/10.1203/00006450-199311000-00017
- Viau, V. (2002). Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of Neuroendocrinology, 14(6), 506–513. https://doi.org/10.1046/j.1365-2826.2002.00798.x
- Viau, V., & Meaney, M. J. (1996). The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. The Journal of Neuroscience, 16(5), 1866–1876. https://doi.org/10.1523/JNEUROSCI.16-05-01866.1996
- Weinberg, M. S., Johnson, D. C., Bhatt, A. P., & Spencer, R. L. (2010). Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience, 168(3), 744–756. https://doi.org/10.1016/j.neuroscience.2010.04.006
- Williamson, M., Bingham, B., Gray, M., Innala, L., & Viau, V. (2010). The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. The Journal of Neuroscience, 30(35), 11762–11770. https://doi.org/10.1523/JNEUROSCI.2852-10.2010
- Williamson, M., & Viau, V. (2008). Selective contributions of the medial preoptic nucleus to testosterone-dependant regulation of the paraventricular nucleus of the hypothalamus and the HPA axis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 295(4), R1020–R1030. https://doi.org/10.1152/ajpregu.90389.2008
