Abstract
Several studies have focused on the cortisol levels in fingernail samples as a possible index of cumulative hormone production; however, the biological validity of fingernail cortisol has not been fully established. We investigated the association between cortisol levels in fingernail samples and other biological specimens, including hair and saliva samples, in healthy young adults to determine whether fingernail cortisol was associated with past cumulative hormone production. Participants were 23 adults (14 men and 9 women; mean age = 22.7 ± 2.8 years). Saliva samples were collected three times per day for 30 days, and hair samples (1 cm) from participants’ scalps were obtained. Fingernail samples were repeatedly collected for 8 months, considering growth rate of fingernail and time lag for fingernails to fully extend from the nail matrix. Cortisol levels in hair samples were significantly associated with the levels in fingernail samples that were obtained 3 months after hair collection (r = .48, p < .05). The 30-day integrated area under the curve, based on salivary cortisol levels at awakening and bedtime, were significantly associated with cortisol levels in fingernail samples that were collected 2–5 months after saliva collection. This finding was especially significant after adjusting for the awakening time when the saliva was collected. This study provided evidence that fingernail cortisol was associated with cumulative hormone levels measured several months before but not those in the present. The samples may be useful for endocrinological evaluation in the investigation of chronic stress, cortisol levels, and health; moreover, the use of fingernail samples would permit larger-scale studies.
1. Introduction
Acute psychosocial stress triggers the activation of the hypothalamic-pituitary-adrenal axis. In response, the cortisol levels in blood and saliva increase 20–40 min from the onset of acute psychological stress (Dickerson & Kemeny, Citation2004). Cortisol levels measured in hair samples are an index of cumulative hormone levels. Scalp hair grows at an average rate of 1 cm/month; therefore, 1 cm of scalp hair could be used to measure hormone levels in 1 month (Russell et al., Citation2012). Hair cortisol levels are associated with saliva cortisol levels (Short et al., Citation2016; Sugaya et al., Citation2020), and chronically stressed people (e.g. dementia caregivers, unemployed men) exhibited elevated hair cortisol levels (Dettenborn et al., Citation2012; Stalder et al., Citation2014). While saliva and blood samples revealed hormone levels over a short period and exhibited large diurnal variations with higher levels in the morning and lower levels in the afternoon, hair samples provide an index of cumulative hormone exposure over a longer period, which informs the investigation of cortisol levels and chronic stress.
Many studies focused on cortisol levels in fingernail samples as another index of cumulative hormone production. Neutrally charged endogenous hormones passively diffuse from capillaries into the nail matrix, and are incorporated into keratin during nail formation (Berker et al., Citation2007). Fingernails grow at an average rate of 3 mm/month (e.g. 3.47 mm, Yaemsiri et al., Citation2010). Generally, it takes approximately 3–6 months for a whole nail to replace itself; that is, 3–6 months is required for nails to fully extend from the nail matrix; however, there may be a 3–4 month lag time (Buzalaf et al., Citation2006). Therefore, cortisol levels measured from the free edge of a nail may reflect cortisol levels several months before clipping (Warnock et al., Citation2010). Compared to hair, fingernail samples are easy to collect and fewer samples are required. Higher fingernail cortisol levels were associated with major depressive episodes (Herane-Vives et al., Citation2018) and the onset of an acute coronary syndrome (Izawa et al., Citation2019). Consistently, stressful experiences in the past are associated with higher fingernail cortisol levels (Doan et al., Citation2018; Izawa et al., Citation2017; Wu et al., Citation2018).
However, the validity of fingernail cortisol requires elucidation. Only two studies reported correlations between fingernail cortisol and saliva or hair cortisol (). One reported an association between fingernail and saliva cortisol (collected in the morning and early afternoon; Frugé et al., Citation2018). Another reported that the area under the curve of salivary cortisol across two days was correlated with cortisol levels in fingernail samples collected 3–5 months after collecting saliva (Izawa et al., Citation2015, Study 2). That study also reported a moderate correlation between cortisol levels in a 6-cm hair segment and fingernails that were grown for 2 weeks (Izawa et al., Citation2015, Study 1). However, in these studies, the time windows of saliva and hair samples did not correspond to those of fingernails. For example, for the validation of cortisol level in fingernails grown for one month, saliva samples could be collected for one month. However, in the previous study, saliva samples were collected for only two days (Izawa et al., Citation2015, Study 2). Further studies are needed to thoroughly investigate the validity of fingernail cortisol.
Table 1. Summary of previous findings about the associations of fingernail cortisol with saliva or hair cortisol.
The purpose of this study is to investigate the association between cortisol levels in fingernail samples and other biological specimens, including hair and saliva samples, in healthy young adults to determine the validity of fingernail cortisol as a measure of cumulative hormone production. One-cm long hair samples from participants’ scalps were obtained and saliva samples were collected for 30 days to estimate cumulative cortisol levels for one month. Considering the growth rate of fingernails, we posited that cortisol levels assessed by hair and saliva samples would correlate with cortisol levels in fingernail samples that were collected 3–6 months after the hair and saliva collections. Fingernail samples were repeatedly collected for 8 months. We hypothesized that one-month cortisol levels assessed by hair and saliva samples would be associated with cortisol levels in fingernail samples that were collected 3–6 months after but not with those collected during the other periods. We additionally investigated these associations after adjusting for possible confounding factors (e.g. sex, body mass index, and frequency of washing hands).
2. Methods
2.1. Participants
In this 8-month observational study, healthy young adults, without dyed hair, manicured nails, routine medication use, and without a history of an adrenal disease were recruited. This study was conducted from November 2017 to January 2019; that is, before the COVID-19 pandemic. Twenty-seven young adults were enrolled in this study (16 men and 11 women, mean age = 22.6 ± 2.7 years). We analyzed the data from 23 participants after excluding an individual with poor adherence to the online survey during the period of saliva collection, two dropouts, and one whose nail cortisol levels could not be measured as it was over the standard range. This study was approved by the ethical committee of Yokohama City University (no. B171000031) and the Nagaoka University of Technology (no. H29-9). Written informed consent was obtained from all participants. The portion of data was identical to that used in a previous study (Sugaya et al., Citation2020), in which an association between salivary and hair cortisol was investigated.
2.2. Procedure
Saliva and hair samples were collected in the first month of an 8-month period, and fingernail samples were collected over the entire 8 months. Participants were also asked to report the frequency of washing hair, as well as the frequency of washing hands using soap or hand cleanser, when hair and fingernail samples were collected.
2.2.1. Saliva collection
The saliva collection methods were described in our previous study (Sugaya et al., Citation2020). Saliva sample collections were conducted for 30 days. Participants were asked to collect saliva upon awakening (t1), 30 min after awakening (t2), and at bedtime (t3) on each day by using the passive drool method (Granger et al., Citation2007). Participants were asked to pool saliva in their mouths for 3 min and expectorate saliva through a short plastic straw into a microtube. We followed the practical procedures described in the consensus guideline for cortisol awakening response (Stalder et al., Citation2016). Participants were instructed not to eat, drink, or brush their teeth for 30 min after awakening or one hour before bedtime. Furthermore, participants were required to access an online survey (i.e. Google form) during the time of pooling saliva, in which the actual times of saliva collections were recorded in the online server.
Before study commencement, participants were instructed by two investigators (SI and NS) to ensure that the sampling protocol was well understood. They were sent reminders regarding saliva collection via e-mail every day. Saliva samples were kept in the participants’ home freezers and transported frozen to the laboratory at the end of the collection period.
2.2.2. Hair collection
A few days after the end of the saliva collection period, participants’ hair strands were collected by carefully cutting, with fine scissors as close as possible to the scalp, from a posterior vertex region. One-cm long hair samples, approximately 50 mg in total, were obtained to estimate cortisol levels for the previous month—based on the notion that hair growth rate is approximately 1 cm per month (Wennig, Citation2000). The samples were stored at room temperature and covered with aluminum foil until the day of the cortisol measurements. Hair samples were collected in December 2017 for 11 participants, in January 2018 for 5 participants, in March 2018 for 6 participants, and in June 2018 for 2 participants.
2.2.3. Fingernail collection
Participants were asked to grow their fingernails for 15 days and to provide samples from every digit by clipping the nail directly into a resealable poly bag to avoid losing any part of the sample. Fingernail collection was conducted 16 times for 8 months. On the day of the fingernail collection, participants were reminded of the collection via e-mail and asked to report the completion of each collection on the online survey form. The samples were stored at room temperature and transported to the laboratory once a month.
2.3. Measurements of hair, fingernail, and salivary cortisol levels
We mainly followed the protocol of previous studies (Izawa et al., Citation2015, Citation2016; Sugaya et al., Citation2020) to determine hair and nail cortisol levels. Hair samples were washed 3 times in 2.5 mL of isopropanol, and the nail samples were washed twice in 5 ml of isopropanol; then, these samples were dried overnight. Hair and nail samples were grounded using a cell disruptor (Multi Beads Shocker®; MB901RK, Yasui Kikai, Japan) at 2500 rpm for 3 min and 2 min, respectively. Fifteen milligrams of hair and nail powder were weighed, and 1.5 ml of pure methanol was added for cortisol extraction throughout 48 h under slow rotation. Following this, the samples were spun in a microcentrifuge at 10 000 rpm for 2 min, and 0.5 ml of the clear supernatant was evaporated at 60 °C until completely dry.
The cortisol levels of hair and nail samples was determined in singlicate by an enzyme immunoassay method utilizing the EIA Kit (Salimetrics LLC, USA). The evaporated samples were re-suspended in 50 μl of the assay diluent included in the EIA Kit, and the levels of cortisol in the diluent were analyzed according to the manufacturer's instructions. For hair cortisol, the intra-assay and inter-assay variations were 8.6% and 4.4%, respectively. For nail cortisol, the intra-assay and inter-assay variations were 5.5% and 4.0%, respectively. The findings are presented as pg cortisol/mg hair or fingernail (pg/mg).
Saliva samples were centrifuged at 3000 rpm for 15 min before the assay. The concentration of cortisol within saliva (nmol/l) was determined using the EIA Kit (Salimetrics LLC, USA). The inter- and intra-assay variations were 8.4% and 5.3%, respectively.
2.4. Statistical analyses
For integrated measures of salivary cortisol for 30 days (90 samples in total), the area under the curve (AUC) with respect to ground (AUCt123) and the AUC excluding time 2 data (AUCt1&3) were calculated according to the previous study (Sugaya et al., Citation2020). AUCt123 was a conventional AUC with respect to ground, and AUCt1&3 was the AUC excluding awakening response, representing the AUC of the basal level. AUCt123 and AUCt1&3 were adjusted for the total number of hours the AUC spanned for each day to compensate for the difference in the time from awakening to bedtime for 30 days and across the participants. Details for the calculation of AUC and the handling of missing or invalid cortisol data were described previously (Sugaya et al., Citation2020).
The data for 16 fingernail cortisol levels across 8 months were obtained for each participant. We calculated the average monthly fingernail cortisol levels to compare with hair and salivary cortisol. For the calculation, the averages were weighted according to the fingernail volume obtained in each collection.
Correlation analyses (Pearson’s correlations) were conducted to investigate whether one-month cortisol levels estimated by the hair and saliva samples were associated with the cortisol level in the fingernails collected for the 8 months. Partial correlation analyses were also conducted to investigate those associations adjusting for possible confounders, such as sex, body mass index (BMI), frequency of washing hands with soap (e.g. Madry et al., Citation2014), frequency of hair washing (e.g. Hamel et al., Citation2011), or awakening time when the saliva was collected (e.g. Kudielka & Kirschbaum, Citation2003). Each confounder was independently adjusted because of the small sample size in this study. Repeated measures of analyses of variance with Greenhouse-Geisser correction (in the case of lack of sphericity) were also conducted to investigate the variations in salivary cortisol levels upon awakening, 30 min after awakening, and at bedtime.
3. Results
3.1. Participants’ demographic data
The demographic variables and descriptive cortisol data are shown in . Salivary cortisol levels significantly increased from awakening to 30 min after awakening, and the levels decreased at bedtime (F(1.3/22.0) = 86.1, p < .01).
Table 2. Participants’ demographic data.
3.2. Correlations between fingernail and hair cortisol
Correlations between hair and fingernail cortisol levels over 8 months are shown in . Cortisol levels in hair samples significantly correlated with the levels in fingernail samples that were collected 3 months after the hair collection (r = .48, p < .05; ). Cortisol levels in hair samples also marginally correlated with fingernail cortisol levels at 1, 2, and 4 months. These associations remained after controlling for possible confounders, such as sex, BMI, or frequency of washing hands with soap and washing hair.
Figure 1. Scatter plots illustrating the relationship between the cortisol levels in the 1-cm hair segment and the cortisol levels in fingernail samples collected 3 months later.
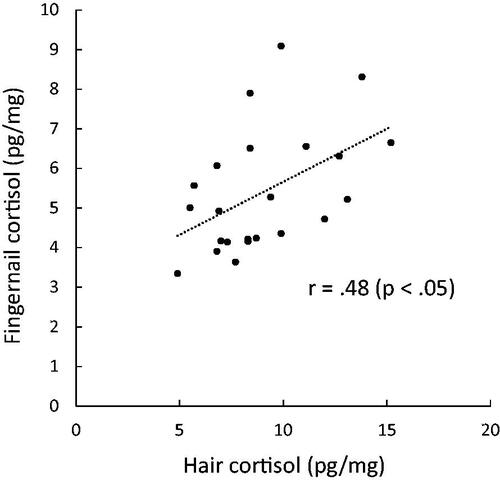
Table 3. Correlations between hair cortisol levels and fingernail cortisol levels over 8 monthsTable Footnotea.
3.3. Correlations between fingernail and salivary cortisol
The correlations between two AUCs of salivary cortisol and fingernail cortisol levels over the 8 months are shown in and . AUCt123 of salivary cortisol did not correlate with fingernail cortisol significantly. AUCt1&3 was associated with fingernail cortisol levels at 2 months (r = .44, p < .05), 4 months (r = .39, p < .10), and 5 months (r = .44, p < .05) after saliva collection (). Furthermore, the correlations became rather stronger after controlling for the frequency of washing hands with soap or upon awakening time of saliva collection days, and the basal AUC was moderately and significantly associated with fingernail cortisol levels at 2, 3, 4, and 5 months as well as those of all months ().
Figure 2. Scatter plots illustrating the relationship between the 30-day integrated area under the curve based on salivary cortisol levels at awakening and bedtime (AUCt1&3) and the cortisol levels in fingernail samples collected 2 months (A) and 5 months (B) later.
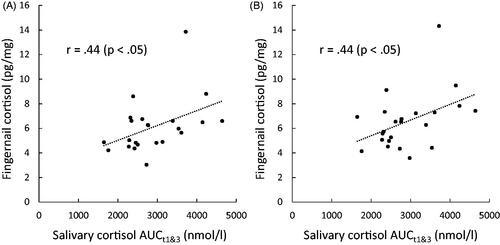
Table 4. Correlations between salivary cortisol levels (AUCt123) and fingernail cortisol levels over 8 monthsTable Footnotea,Table Footnoteb.
Table 5. Correlations between baseline salivary cortisol levels (AUCt1&3) and fingernail cortisol levels over 8 monthsTable Footnotea,Table Footnoteb.
For post-hoc analyses of salivary cortisol, we further investigated the association of salivary cortisol levels at awakening, 30 min after awakening, and at bedtime, which was averaged for 30 days, with fingernail cortisol levels. Cortisol levels at awakening were associated with fingernail cortisol levels at 2 months (r = .47, p < .05), 4 months (r = .42, p < .05), and 5 months (r = .51, p < .05). After controlling the awakening time of the saliva collection days, cortisol levels at awakening were also associated with fingernail cortisol levels at 2 (r = .53, p < .05), 3 (r = .44, p < .05), 4 (r = .47, p < .05), and 5 months (r = .58, p < .01). We did not find significant correlations between salivary cortisol levels at 30 min after awakening and at bedtime and fingernail cortisol.
We also investigated the associations between salivary cortisol awakening response (CAR) and diurnal slope, which was averaged for 30 days, with fingernail cortisol levels. The calculation of CAR and diurnal slope were descried previously (Sugaya et al., Citation2020). CAR did not exhibit any significant correlations with fingernail cortisol (Pearson’s correlations ranged from .13 to .15). Cortisol diurnal slope from awakening to bedtime was associated with fingernail cortisol levels at 2 (r = .51, p < .05), 4 (r = .44, p < .05), and 5 months (r = .57, p < .01).
3.4. Stability of fingernail cortisol across 8 months
Correlations between fingernail cortisol levels across 8 months were calculated (). Overall, fingernail cortisol levels were moderately or strongly associated with each other. Fingernail cortisol levels at 0 months exhibited weaker correlations with the other months; however, one participant exhibited relatively higher fingernail cortisol levels (19.6 pg/mg), which affected the results of correlation analyses. When excluding that participant’s data, Pearson’s correlations between 0 months and the other months became stronger (ranging from .62 to .85).
Table 6. Intercorrelations between fingernail cortisol across 8 months.
4. Discussion
In this study, we comprehensively investigated the associations of one-month cortisol levels estimated by hair and saliva samples with eight-month fingernail cortisol in a sample of 23 healthy young adults. We found that the one-month cortisol levels, estimated by the hair samples, were significantly and moderately associated with cortisol levels in fingernails that were collected 3 months after the hair collection. Additionally, the 30-day integrated AUC, based on salivary cortisol levels at awakening and bedtime, was significantly associated with cortisol levels in fingernail samples that were collected 2–5 months after the saliva collection. This study demonstrated evidence that fingernail cortisol was retrospectively associated with cumulative hormone levels.
We observed moderate correlations between hair cortisol and fingernail cortisol, and the association was not altered by any confounding factors. This finding was consistent with our previous study (Izawa et al., Citation2015), demonstrating a moderate relationship between cortisol levels in hair and fingernails. This study strictly estimated one-month cortisol production with hair samples and indicated that the strength of correlation was highest in the third month. Considering the overall validity and reliability of hair cortisol as an index of cumulative hormone secretion (Stalder et al., Citation2017), our results indicated that fingernail cortisol from clippings represents the hormone levels from 3 months earlier. A recent study (Binz et al., Citation2018) reported no associations between toenail cortisol levels and hair cortisol levels in a sample of 120 individuals. It takes longer for toenails to fully extend from the nail matrix (e.g. 10 months for the great toenail; Yaemsiri et al., Citation2010). In Binz et al. (Citation2018), hair and toenail samples were collected at the same time, which might explain the finding. Future studies should investigate the differences between fingernail and toenail cortisol in other specimens.
We also found associations between the basal AUC of salivary cortisol and fingernail cortisol with a time lag of 2–5 months, and the associations became clearer after adjusting for the effects of hand-washing frequency or awakening time when the saliva was collected. This finding was consistent with our preliminary study (Izawa et al., Citation2015), demonstrating that fingernail cortisol is retrospectively associated with salivary cortisol with a time lag of 4–5 months. The previous study collected eight saliva samples across 2 days, which did not accurately correspond to the week- or month-long duration that fingernails are thought to reflect. In the current study, we collected saliva samples for 30 days—a strength of this study.
However, we found relatively large variations in the time lag in the association between salivary and fingernail cortisol (2–5 months) and did not observe a clear peak of correlation, which was contrary to that observed for hair cortisol. Furthermore, we did not find significant correlations between salivary cortisol AUC including awakening response and fingernail cortisol. Salivary cortisol responds more readily to acute stress and the levels are affected by the time of the day as well as the sleep-wake cycle. It is well known that cortisol secretion shows a marked circadian rhythm with higher levels in the morning; it rapidly increases 30–45 min after awakening and modestly declines over the daytime to the nadir around midnight. Such large variations in salivary cortisol levels could make it difficult to strictly assess cumulative cortisol secretion and possibly affect the results. Although we determined the association between the basal AUC of salivary cortisol and fingernail cortisol and the association was largely not altered by confounding factors, the time lag for fingernail and salivary cortisol needs to be carefully interpreted.
The biological mechanism for incorporating cortisol into nails needs to be considered. Previously, it was hypothesized that endogenous cortisol passively diffuses from capillaries into the nail matrix, and they are incorporated into keratin during nail formation (Warnock et al., Citation2010). Generically, it requires approximately 3–6 months for a whole nail to replace itself, suggesting that 3–6 months were required for nails to fully extend from the nail matrix. In this study, we found a 3-month delay for the associations between cortisol levels of fingernails and hair, which could support this hypothesis to an extent. Previous studies investigating some drugs, such as zolpidem, also indicated the role of the nail bed as another incorporating mechanism. For example, an elevation of the zolpidem level in the fingernail was observed a few weeks after the ingestion of the drug (Madry et al., Citation2014). Furthermore, sweat containing cortisol could be a possible factor for the contamination of fingernails, even if the samples were washed by solvents before the measurement (Madry et al., Citation2014). Therefore, daily hygiene, such as the frequency of washing hands, must be carefully considered. In this study, the correlations between fingernail and salivary cortisol became stronger after controlling for the frequency of washing hands with soap; however, the frequency did not significantly correlate with fingernail cortisol levels (ranging from −.31 to .05). Data regarding the incorporating mechanism is needed.
Compared with saliva and hair samples, the use of fingernail samples may have some advantages. Salivary cortisol reveals current hormone levels, and the levels are affected by several state-like confounders such as time of day, sleep-wake cycle, eating, and drinking. Salivary cortisol could be suitable for the assessment of individuals’ physiological response to acute stress in laboratory settings but not to chronic stress in daily life. Hair cortisol reveals cumulative hormone levels. However, hair collection is sometimes unacceptable owing to religious or socio-cultural reasons, and it is impossible for people who have no hair. Further, hair self-sampling may be difficult for participants. Fingernail samples are easily collected by participants and only a small amount is required. Fingernail samples may be useful for endocrinological evaluation in the investigation of chronic stress, cortisol levels, and health; the use of fingernail samples would permit larger-scale studies.
This study had certain limitations that may affect the interpretation of the findings. First, because of the intensive nature of the study (e.g. frequent saliva collections, fingernail collections for longer duration), we included a small number of participants. Second, we only collected two post-awakening saliva samples; although, guidelines for CAR recommended three post-awakening saliva samples (Stalder et al., Citation2016). Furthermore, saliva cortisol levels were assessed only two or three times a day to calculate AUC. The cortisol diurnal rhythm is not linear when going from the waking value to the bedtime value and determining the intermediate time points could be useful. This process was altered because we had considered that more frequent saliva collections with various restrictions for 30 days could yield poorer adherence of the participants to the protocols and result in them dropping out of the study. Third, this study only demonstrated the correlations between fingernail cortisol and hair and saliva cortisol. For hair cortisol, marginally significant correlations were found at 1-, 2-, and 4-month periods, which might be because fingernail cortisol levels were intra-individually stable and correlated, as demonstrated in . Another possibility for the marginally significant correlations could be owing to variations in the nail growth rate between individuals and between digits (Gupta et al., Citation2005; Yaemsiri et al., Citation2010), although the nail growth rates were not evaluated in this study. Future studies could employ more convincing strategies, such as pharmacological administration, to methodically investigate the incorporating mechanisms of fingernail cortisol. Fourth, we cut hair strands as close as possible to the scalp to estimate cortisol levels for the previous month; however, the strands beneath the scalp could not be collected (3–5 mm; Russell et al., Citation2012). Furthermore, a few millimeters of strands from the scalp might be technically difficult to cut completely. Therefore, rather older hair segments could have been possibly collected and the association between hair and fingernail cortisol might be more time-lagged with a range of a few weeks. Finally, we found significant correlations between a larger cortisol diurnal slope and fingernail cortisol. However, diurnal slope was strongly correlated with AUC t1&3 (r = .82) and cortisol levels at awakening (r = .95). This was not surprising because diurnal slope and AUC t1&3 were calculated by using the value of awakening cortisol level. Further studies are needed to explore what aspects of cortisol diurnal rhythm are associated with fingernail cortisol by collecting saliva samples more frequently.
5. Conclusions
We demonstrated that one-month cortisol levels assessed by the hair and salivary samples were associated with cortisol levels in fingernails collected 2–5 months after the hair and saliva collections but not with those collected during other periods, and the associations were not largely altered by possible confounding factors. Although this study had some limitations, the findings supported that fingernail cortisol is retrospectively associated with cumulative hormone levels to a certain extent. The samples may be useful for endocrinological evaluation in the investigation of chronic stress, cortisol levels, and health; moreover, the use of fingernail samples would permit larger-scale studies.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
Notes on contributors
Shuhei Izawa
Shuhei Izawa, PhD is a researcher of National Institute of Occupational Safety and Health, Japan. His research interests include job stress, cortisol, and stress-related diseases.
Nagisa Sugaya
Nagisa Sugaya, PhD is an assistant professor of public health specializing in research on mental health and stress-related physiological response.
Namiko Ogawa
Namiko Ogawa, PhD is a research fellow of Advanced Research Center for Human Sciences, Waseda University. Her research interests include psychosocial stress, happiness, and cortisol.
Kentaro Shirotsuki
Kentaro Shirotsuki, PhD is Associate Professor of Musashino University. His research subjects are social anxiety disorder, stress, cognitive behavior therapy, and psychosomatic medicine. He studies the basic mechanism of social anxiety, the treatment effect of cognitive behavior therapy on social anxiety symptoms and the effect of cortisol on anxiety.
Shusaku Nomura
Shusaku Nomura, PhD is a Professor of Nagaoka University of Technology. His research interests include man-machine system, biological information processing, and stress related hormones.
References
- Berker, D. A., André, J., & Baran, R. (2007). Nail biology and nail science. International Journal of Cosmetic Science, 29(4), 241–275. https://doi.org/https://doi.org/10.1111/j.1467-2494.2007.00372.x
- Binz, T. M., Gaehler, F., Voegel, C. D., Hofmann, M., Baumgartner, M. R., & Kraemer, T. (2018). Systematic investigations of endogenous cortisol and cortisone in nails by LC-MS/MS and correlation to hair. Analytical and Bioanalytical Chemistry, 410(20), 4895–4903. https://doi.org/https://doi.org/10.1007/s00216-018-1131-6
- Buzalaf, M. A., Pessan, J. P., & Alves, K. M. (2006). Influence of growth rate and length on fluoride detection in human nails. Caries Research, 40(3), 231–238. https://doi.org/https://doi.org/10.1159/000092231
- Dettenborn, L., Muhtz, C., Skoluda, N., Stalder, T., Steudte, S., Hinkelmann, K., Kirschbaum, C., & Otte, C. (2012). Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress, 15(3), 348–353. https://doi.org/https://doi.org/10.3109/10253890.2011.619239
- Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. https://doi.org/https://doi.org/10.1037/0033-2909.130.3.355
- Doan, S. N., DeYoung, G., Fuller-Rowell, T. E., Liu, C., & Meyer, J. (2018). Investigating relations among stress, sleep and nail cortisol and DHEA. Stress (Amsterdam, Netherlands), 21(2), 188–193. https://doi.org/https://doi.org/10.1080/10253890.2018.1429398
- Frugé, A. D., Cases, M. G., Howell, C. R., Tsuruta, Y., Smith-Johnston, K., Moellering, D. R., & Demark-Wahnefried, W. (2018). Fingernail and toenail clippings as a non-invasive measure of chronic cortisol levels in adult cancer survivors. Cancer Causes & Control: CCC, 29(1), 185–191. https://doi.org/https://doi.org/10.1007/s10552-017-0989-5
- Granger, D. A., Kivlighan, K. T., Fortunato, C., Harmon, A. G., Hibel, L. C., Schwartz, E. B., & Whembolua, G. L. (2007). Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiology & Behavior, 92, 583–590.
- Gupta, G. R., Dhruw, V. K., Athawal, B. K., Siddiqui, P., Yousuf Agrawal, H. K., & Chandra, H. (2005). Human nail growth pattern and medicolegal aspect. Indian Academy of Forensic Medicine, 27, 87–91.
- Hamel, A. F., Meyer, J. S., Henchey, E., Dettmer, A. M., Suomi, S. J., & Novak, M. A. (2011). Effects of shampoo and water washing on hair cortisol concentrations. Clinica Chimica Acta; International Journal of Clinical Chemistry, 412(3–4), 382–385. https://doi.org/https://doi.org/10.1016/j.cca.2010.10.019
- Herane-Vives, A., Fischer, S., de Angel, V., Wise, T., Cheung, E., Chua, K. C., Arnone, D., Young, A. H., & Cleare, A. J. (2018). Elevated fingernail cortisol levels in major depressive episodes. Psychoneuroendocrinology, 88, 17–23. https://doi.org/https://doi.org/10.1016/j.psyneuen.2017.10.026
- Kudielka, B. M., & Kirschbaum, C. (2003). Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology, 28(1), 35–47. https://doi.org/https://doi.org/10.1016/S0306-4530(02)00008-2
- Izawa, S., Matsudaira, K., Miki, K., Arisaka, M., & Tsuchiya, M. (2017). Psychosocial correlates of cortisol levels in fingernails among middle-aged workers. Stress (Amsterdam, Netherlands), 20(4), 386–389. https://doi.org/https://doi.org/10.1080/10253890.2017.1342808
- Izawa, S., Miki, K., Tsuchiya, M., Mitani, T., Midorikawa, T., Fuchu, T., Komatsu, T., & Togo, F. (2015). Cortisol level measurements in fingernails as a retrospective index of hormone production. Psychoneuroendocrinology, 54, 24–30. https://doi.org/https://doi.org/10.1016/j.psyneuen.2015.01.015
- Izawa, S., Miki, K., Tsuchiya, M., Yamada, H., & Nagayama, M. (2019). Hair and fingernail cortisol and the onset of acute coronary syndrome in the middle-aged and elderly men. Psychoneuroendocrinology, 101, 240–245. https://doi.org/https://doi.org/10.1016/j.psyneuen.2018.11.021
- Izawa, S., Yoshida, R., Ohira, M., Yamaguchi, A., & Nomura, S. (2016). Quantitative measurements of fingernail cortisol: Effects of ground-fingernail grain size and extraction time. Japanese Journal of Physiological Psychology and Psychophysiology, 34(3), 245–249. in Japanese) https://doi.org/https://doi.org/10.5674/jjppp.1615tn
- Madry, M. M., Steuer, A. E., Binz, T. M., Baumgartner, M. R., & Kraemer, T. (2014). Systematic investigation of the incorporation mechanisms of zolpidem in fingernails. Drug Testing and Analysis, 6(6), 533–541. https://doi.org/https://doi.org/10.1002/dta.1558
- Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. https://doi.org/https://doi.org/10.1016/j.psyneuen.2011.09.009
- Short, S. J., Stalder, T., Marceau, K., Entringer, S., Moog, N. K., Shirtcliff, E. A., Wadhwa, P. D., & Buss, C. (2016). Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology, 71, 12–18. https://doi.org/https://doi.org/10.1016/j.psyneuen.2016.05.007
- Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wüst, S., Dockray, S., Smyth, N., Evans, P., Hellhammer, D. H., Miller, R., Wetherell, M. A., Lupien, S. J., & Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. https://doi.org/https://doi.org/10.1016/j.psyneuen.2015.10.010
- Stalder, T., Tietze, A., Steudte, S., Alexander, N., Dettenborn, L., & Kirschbaum, C. (2014). Elevated hair cortisol levels in chronically stressed dementia caregivers. Psychoneuroendocrinology, 47, 26–30. https://doi.org/https://doi.org/10.1016/j.psyneuen.2014.04.021
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., Kirschbaum, C., & Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. https://doi.org/https://doi.org/10.1016/j.psyneuen.2016.12.017
- Sugaya, N., Izawa, S., Ogawa, N., Shirotsuki, K., & Nomura, S. (2020). Association between hair cortisol and diurnal basal cortisol levels: A 30-day validation study. Psychoneuroendocrinology, 116, 104650. https://doi.org/https://doi.org/10.1016/j.psyneuen.2020.104650
- Warnock, F., McElwee, K., Seo, R. J., McIsaac, S., Seim, D., Ramirez-Aponte, T., Macritchie, K. A., & Young, A. H. (2010). Measuringcortisol and DHEA in fingernails: A pilot study. Neuropsychiatric Disease and Treatment, 6, 1–7.
- Wennig, R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Science International, 107(1–3), 5–12. https://doi.org/https://doi.org/10.1016/S0379-0738(99)00146-2
- Wu, H., Zhou, K., Xu, P., Xue, J., Xu, X., & Liu, L. (2018). Associations of perceived stress with the present and subsequent cortisol levels in fingernails among medical students: A prospective pilot study. Psychology Research and Behavior Management, 11, 439–445. https://doi.org/https://doi.org/10.2147/PRBM.S181541
- Yaemsiri, S., Hou, N., Slining, M. M., & He, K. (2010). Growth rate of human fingernails and toenails in healthy American young adults. Journal of the European Academy of Dermatology and Venereology, 24(4), 420–423. https://doi.org/https://doi.org/10.1111/j.1468-3083.2009.03426.x
