Abstract
Research over the last 10 years suggests that the brain’s reward system plays a crucial role in stress resilience. Notably, reward processing includes both an anticipatory (cue-triggered “wanting”) phase and a consummatory (“liking”) phase. However, previous studies manipulated rewards via direct reward administration, which makes it difficult to isolate the buffering effect of anticipating the reward stimulus. In the current study, we designed a paradigm to manipulate participants into generating reward anticipation or not and investigated whether reward anticipation can buffer psychological, neuroendocrine, and cardiovascular responses to psychosocial stress. A sample of 78 healthy young adults underwent the Trier Social Stress Test or placebo-Trier Social Stress Test after a reward anticipation task. Results showed that reward anticipation relieved subjective stress feelings, as well as the overall cortisol secretion and the increased heart rate induced by psychosocial stress. Taken together, these findings expanded our understanding of the role the reward system plays in stress resilience, and the possible psychological mechanism of the buffering effect for future stress study was also discussed.
Reward processing includes both an anticipatory and consummatory phases
The buffering effect of anticipating the reward stimulus requires elucidation
We examined if said anticipation buffers varied responses to psychosocial stress
Reward anticipation relieved subjective stress, cortisol secretion, and heart rate
We clarified the role of the reward system in stress resilience
HIGHLIGHTS
1. Introduction
Acute stress can leave people in a state of anxiety and trigger multiple biological reactions (Taverniers et al., Citation2010). The hypothalamic pituitary adrenal (HPA) axis is the major stress response and control system, and its final product—cortisol secreted from the adrenal cortex—is a biological marker of stress (Foley & Kirschbaum, Citation2010). Moreover, the sympatho-medullary system coordinates the release of catecholamines during stress, triggering increases in heart rate (Carroll et al., Citation2011). Notably, stress is implicated in the development and progression of a broad array of pathological conditions; therefore, reducing the stress response can protect individuals from negative physical and mental health impacts (Creswell & Lindsay, Citation2014).
Research over the last 10 years suggests that the brain’s reward system plays a crucial role in stress resilience, and activating the reward system through reward stimulation reduces stress physiology and behavior (Dutcher & Creswell, Citation2018). Predecessors have obtained convincing experimental evidence in animals and humans. Animal studies have shown that rats who regularly receive food and drink rewards exhibit reduced stress behavior (such as reduced painful vocalisation) and increased adaptive behavior (such as increased exploration of new environments) compared to those who do not receive the rewards (MacKay et al., Citation2017; Ulrich-Lai et al., Citation2010). In addition to primary rewards such as food, secondary rewards including positive social experiences that promote human development also show similar effects (Berridge & Robinson, Citation2003). For example, human neuroimaging studies have found that thinking about self-worth increases reward-related neural activity in the ventral striatum (VS; Dutcher et al., Citation2016) and also relieves neuroendocrine and behavioral responses to stress (Dutcher & Creswell, Citation2018). Moreover, recall of pleasant autobiographical memories also activated reward system and decreased cortisol responsiveness to stressors (Speer & Delgado, Citation2017).
Notably, reward processing is not a homogenous phenomenon, as it includes an anticipatory (cue-triggered “wanting”) phase and a consummatory (“liking”) phase (Berridge et al., Citation2009). “Wanting” refers to the drive toward a reward and is associated with approach motivation and anticipatory pleasure; whereas “liking” refers to the initial responsiveness to a reward associated with hedonic experience (Berridge et al., Citation2009). Animal and human studies indicate anatomical and neurochemical substrates were distinctly engaged in these phases (Dillon et al., Citation2008). However, previous studies manipulated rewards by administrating them directly (e.g. consuming a sweet drink or recalling pleasant memories) (e.g. Ulrich-Lai et al., Citation2010)—making it difficult to isolate the separate effects of the different reward processes. Thus, it remains unclear whether reward anticipation can buffer the acute psychosocial stress response.
Moreover, with regard to psychosocial stress, stimulation with risk of injury happens in unpredictable or uncontrollable situations (Dickerson & Kemeny, Citation2004), meaning that people cannot typically get an immediate reward when faced with real-life stressors. For example, to keep mental health and upward mood in the context of being criticized by the superior, people need to rely on their internal coping resources, rather than reward consumption to alleviate the stress response instantly (Gross, Citation2002). However, these strategies are not as effective and satisfying as a direct reward (Raio et al., Citation2013). Further, the pressured environment of real life often lacks reward stimuli, making it difficult to obtain direct reward stimulation to promote post stress recovery. For example, a traumatic childhood environment characterized by emotional or physical neglect makes it difficult for individuals to easily get warmth and rewards from their parents (Dennison et al., Citation2016). In this case, reward anticipation may be an effective way for them to foster stress resilience. Therefore, because we couldn’t get reward consumption immediately under acute stress or special circumstances, it is important to investigate whether reward anticipation can buffer stress response.
In the current study, we explored the buffering effect of reward anticipation on acute stress. Previous empirical studies found that anticipation of a beneficial outcome (such as better clinical efficacy for a patient) can have several therapeutic benefits, including alleviating anxiety as well as physical pain, and reducing heart rate response (e.g. Colloca et al., Citation2004; Kam-Hansen et al., Citation2014). For instance, inducing the anticipation of recovery in patients with irritable bowel syndrome can promote relief of symptoms and initiate overall improvement (Kaptchuk et al., Citation2008). Other researchers believed these effects to be closely related to reward anticipation (Liu, Citation2017). Therefore, we hypothesized that reward anticipation would buffer individuals’ overall psychosocial stress response—both in psychological and physiological aspects.
2. Material and methods
2.1. Participants
We used G*Power to estimate the sample size for F tests with α = 0.05 and power (1−β) = 0.8 (Faul et al., Citation2009), and so arrived at a sample size of n = 80. Eighty participants from a local University in China were recruited via advertisements. Two participants (both women) were excluded owing to their statistics on area in the curve for increase (AUCI) cortisol being outside the range of three standard deviations (SDs). The final sample consisted of 78 participants (54 women and 24 men) aged 18–23 years (Mean = 20.08, SD = 1.15). We ascertained eligibility, their current health status, and health behavior using potential participants’ self-reports. Exclusion criteria were acute or chronic psychiatric or somatic diseases, intake of psychotropic or glucocorticoid medication, alcohol/drug abuse, and enrollment in another Trier Social Stress Test (TSST) study. Participants were informed that the study was designed to investigate social cognitive function and that it would last about 1 h 30 min. Participants were randomly assigned to the Trier Social Stress Test in the reward anticipation group (TSST-RA), the TSST in the control group (TSST-NA), the placebo-TSST in the reward anticipation group (pTSST-RA), or the placebo-TSST in the control group (pTSST-NA). Demographic data for each group are presented in .
Table 1. Descriptive data of study variables and covariates.
Participants were asked not to smoke on the day of their appointment, and not to engage in strenuous exercise, drink alcohol or caffeine, eat or brush their teeth 1 hour before the session. This study was approved by the Research Ethics Committee of the Southwest University of China and was performed in line with the Declaration of Helsinki. Before the study, informed oral consent was obtained from all participants before testing. All participants received monetary compensation of 40 yuan for their participation, and participants in the reward anticipation group received a probabilistic extra monetary reward.
2.2. Reward anticipation manipulation
Participants were instructed to draw one of two kinds of tokens from a box: one was labeled “reward” for participants in the reward anticipation condition and the other “none” for participants in the control condition. The instruction for participants in the reward anticipation condition was, “you have a chance to enter a raffle after the experiment, and you can get a 10- to 50-yuan extra money reward”. The instruction for participants in the control condition was, “you did not get a chance for the raffle draw”. After the draw, participants returned to their seats, where they received the stress task instructions. In the reward anticipation condition, to make participants keep on anticipating the future reward during the recovery period and enhance the buffering effect of reward anticipation, a researcher showed participants the whole experiment process including the raffle to remind them of the chance for a raffle they got every 10 min after the stress task. And participants in the control condition were shown the experiment process without the lottery activity at the same time.
2.3. Stress and control treatment
The TSST is a standardized psychosocial stress test used to induce acute stress responses in laboratory research (Kirschbaum et al., Citation1993). It can effectively activate HPA axis responses (Dickerson & Kemeny, Citation2004). In the TSST condition, we set up two “interviewers” of different sexes, one camera, and one microphone. Participants were asked to prepare for a mock job interview, which required them to give a 5-min application speech in front of two interviewers, which would be video recorded. The interviewers remained impassive throughout the interview. After finishing the interview, participants were then asked to complete a 5-min continuous verbal subtraction task, such as subtracting 13 from 1022, as quickly and accurately as possible. If they made a mistake, the interviewer would interrupt them and ask them to start again.
We also employed a pTSST for the control group. The pTSST was set to be as similar to the TSST as possible in time course and activity, but not stressful (Het et al., Citation2009). Participants were asked to choose a topic they liked to talk about during the 10-min preparation period and speak for five minutes in an empty room. Next, participants were asked to complete a simple mental arithmetic task for five min.
2.4. Reward manipulation check
2.4.1. Positive and negative affects assessment
Before and after the reward anticipation tasks, participants’ moods were measured using the positive and negative affect schedule (PANAS) (Watson et al., Citation1988). PANAS is a 20-item scale, containing items measuring both positive and negative affects. Participants indicate how they currently feel using a 5-point Likert scale for each adjective: 1 (very slightly or not at all) to 7 (very much). And we calculated the total score of the positive and negative sub-scales respectively. Positive affect descriptors include “interested,” “excited,” “strong,” “enthusiastic,” “proud,” “alert,” “inspired,” “determined,” “attentive,” and “active”. Negative affect descriptors include “distressed,” “upset,” “guilty,” “scared,” “hostile,” “irritable,” “ashamed,” “nervous,” “jittery,” and “afraid”.
2.4.2. Reward anticipation assessment
At the end of the complete session, participants were asked to indicate on a 7-point scale, “how much are you looking forward to the raffle during the whole experiment after the reward anticipation task?” to examine the perceived level of reward anticipation.
2.5. Stress response measurement
2.5.1. Psychological assessment
Participants were asked to indicate their subjective stress level during the stress task on a 7-point Likert scale: 1 (not stressful) to 7 (very stressful).
2.5.2. Biological measures
Salivary cortisol was collected as the neuroendocrine indicator of the stress response. A saliva collector (salivate SARSTEDT, Germany) was used to store samples. Participants were asked to put cotton buds into their mouth, chew for 1 min, and spit them back into the sampler. We reminded participants not to touch the cotton buds with their hands or other objects during this process to avoid contaminating the sample. All samples were kept in a refrigerator at −20 °C until subsequent analysis. Finally, the concentrations of cortisol in saliva samples were analyzed by an enzyme-linked immunosorbent assay (IBL-Hamburg, Germany) following the manufacturer’s instructions. The sensitivity of the cortisol assay was 0.005 μg/dl. The inter and intra assay coefficients of variation for the cortisol assay were 3.1% and 6.4% respectively.
Heart rate was monitored continuously at a sampling rate of 1 kHz using a Biopac MP150 system and analyzed with the AcqKnowledge software package (Biopac Systems, Goleta, CA). Specifically, participants’ cardiovascular activity was recorded using the electrocardiogram amplifier module and three disposable electrodes positioned on the chest, left armpit, and abdomen, visually inspected for artifacts. Abnormal or biologically implausible data were excluded. Heart rate was reported in beats per minute.
2.6. Procedure
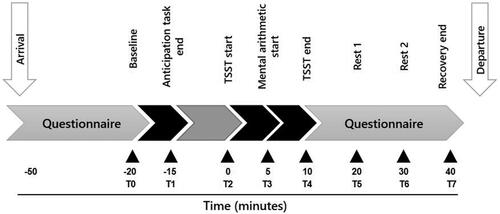
Experiments were conducted between 2:30 pm and 5:00 pm to control for the diurnal rhythm of cortisol. outlines the experimental procedure. The experiment was conducted in a quiet room and participants were asked to rest for at least 30 min upon arrival (while completing a questionnaire). Following the acclimation period, participants provided a baseline saliva sample for the assessment of cortisol levels. Participants then completed the reward anticipation task and evaluated their emotions before and after the task using the PANAS. Next, participants prepared for the stress task. Ten minutes later, the stress experiment officially began, and participants were sent to the testing room to complete the TSST or pTSST. After finishing the test, participants were instructed to go back to the waiting room and rest. In our experiment, participants were free to withdraw at any time.
During the entire experiment, the physiological and psychological data were collected eight times (): T0 = −20 min (baseline measurement), T1 = −15 min (reward anticipation task end), T2 = 0 min (TSST/pTSST start), T3 = 5 min (mental arithmetic start), T4 = 10 min (TSST/pTSST end), T5 = +20 min (Rest 1), T6 = +30 min (Rest 2), T7 = +40 min (recovery end). Specifically, saliva samples were obtained three times: T0, T5, and T7; subjective stress reportswere collected seven times: T0, T1, T2, T4, T5, T6, and T7; and heart rate was collected continuously and computed at all points.
2.7. Statistical analyses
Analysis of demographic, psychometric, and physiological variables was performed using SPSS Statistics 20 (IBM, Armonk, NY, USA). All variables were examined for distributional properties and cases were deleted for univariate outliers. The area under the curve with respect to increases from baseline (AUCI) was used to analyze overall stress responses which including salivary cortisol levels, heart rates, and subjective stress levels in the whole experiment (Pruessner et al., Citation2003). We selected AUCI as our measure because it takes into account both time-related changes and overall intensity of the stress response (Fekedulegn et al., Citation2007). To examine the buffering effect of reward anticipation on psychosocial stress response, analyses of variance were used with stress (TSST/pTSST) and reward (reward anticipation/control) as between-participant variables. We also included demographic variables as covariates in our analyses, including age and gender.
3. Results
3.1. Manipulation Check
Participants’ reward anticipation and mood are listed in . Independent-sample t-tests showed that people in the reward (vs. non-reward) anticipation group reported a higher level of reward anticipation (MRA = 4.61, SD = 0.86; MNA = 1.25, SD = 0.44; t(1,76) = 21.96, p < 0.001, d = 4.97, 95% CI: 4.08–5.87) and greater positive feeling (MRA = 25.63, SD = 3.26; MNA = 23.28, SD = 3.00; t(1,76) = 3.33, p = 0.001, d = 0.75, 95% CI: 0.29–1.21) after the reward anticipation task, regardless of whether they experienced the stress task. However, the two groups did not show any significant difference in positive feelings before the reward anticipation task (MRA = 23.74, SD = 2.32; MNA = 24.20, SD = 2.45; t(1,76) = 0.86, p = 0.395, d = −0.19, 95% CI: −0.64–0.25) or negative feelings both before (MRA = 15.58, SD = 2.90; MNA = 15.93, SD = 2.63; t(1,76) = 0.55, p = 0.582, d = −0.12, 95% CI: −0.57–0.32) or after the reward anticipation task (MRA = 15.00, SD = 2.63; MNA = 15.28, SD = 2.24, t(1,76) = 0.50, p = 0.620, d = −0.11, 95% CI: −0.56–0.33).
Table 2. Descriptive data of study variables and covariates.
3.2. Stress reactivity
3.2.1. Salivary cortisol
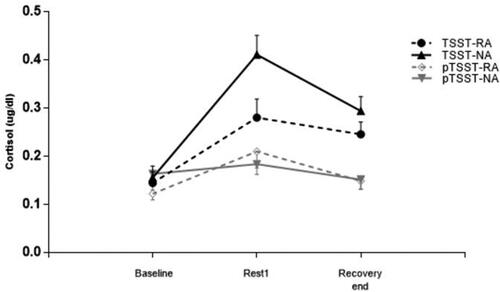
The cortisol concentration levels during the complete experiment are illustrated in . Results suggested a significant main effect of stress (F(1,72) = 21.05, p < 0.001, ηp2 = 0.23), indicating the AUCI of cortisol in TSST groups was higher than that in the pTSST groups. We also found a significant interaction effect (F(1,72) = 7.81, p = 0.007, ηp2 = 0.10). The simple effect analysis revealed that the AUCI of cortisol in the TSST-RA group was lower than that in the TSST-NA group (MRA = 0.18, SD = 0.16; MNA = 0.32, SD = 0.26; F(1,72) = 6.19, p = 0.015, ηp2 = 0.08); however, the AUCI of cortisol did not differ between the pTSST-RA and pTSST-NA groups (MRA = 0.10, SD = 0.17; MNA = 0.01, SD = 0.14; F(1,72) = 1.91, p = 0.171, ηp2 = 0.03). Moreover, we did not find a significant main effect of reward anticipation (F(1,72) = 0.74, p = 0.394, ηp2 = 0.01).
Figure 2. Neuroendocrine responses to psychosocial stress. Cortisol concentrations at all time points in the reward anticipation and control conditions of individuals in TSST and pTSST groups. RA: reward anticipation condition; NA: no reward anticipation (control) condition; TSST: Trier Social Stress Test; pTSST: placebo-Trier Social Stress Test.
3.2.2. Heart rates
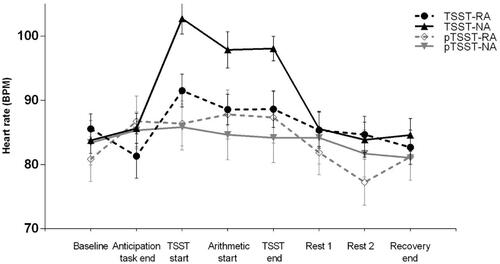
Participants’ heart rates during the whole experiment are illustrated in . Results suggested a significant main effect of stress (F(1,72) = 4.15, p = 0.045, ηp2 = 0.05), indicating the AUCI of heart rates in the TSST groups was higher than that in the pTSST groups. Results also suggested a significant main effect of reward anticipation (F(1,72) = 21.13, p < 0.001, ηp2 = 0.23), indicating that the AUCI of heart rates in the reward anticipation condition was lower than that in control condition. Moreover, we found a significant interaction effect (F(1,72) = 5.47, p = 0.022, ηp2 = 0.07); post-hoc analysis revealed that the AUCI of heart rates in the TSST-RA group was lower than that in the TSST-NA group (MRA = 3.45, SD = 31.37; MNA = 56.69, SD = 34.58; F(1,72) = 22.78, p < 0.001, ηp2 = 0.24); however, the AUCI of heart rates did not differ between the pTSST-RA and pTSST-NA groups (MRA = 4.26, SD = 48.94; MNA = 22.49, SD = 43.52; F(1,72) = 2.92, p = 0.092, ηp2 = 0.04).
Figure 3. Cardiovascular responses to psychosocial stress. Heart rates at all time points in the reward anticipation and control conditions of individuals in TSST and pTSST groups. BPM: Beat Per Minute; RA: reward anticipation condition; NA: no reward anticipation (control) condition; TSST: Trier Social Stress Test; pTSST: placebo-Trier Social Stress Test.
3.2.3. Subjective stress report
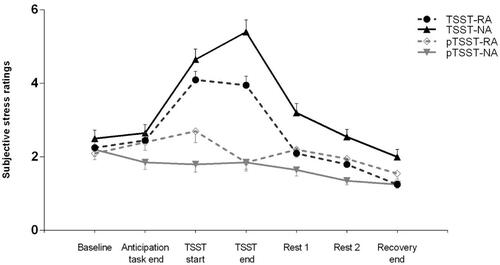
The subjective stress levels during the whole experiment are illustrated in . Results suggested a significant main effect of stress (F(1,72) =18.40, p < 0.001, ηp2 = 0.20), indicating the AUCI of subjective stress levels in the TSST groups was higher than that in pTSST groups. We also found a significant interaction effect (F(1,72) = 17.01, p < 0.001, ηp2 = 0.19); the simple effect analysis revealed that the AUCI of subjective stress levels in the TSST-RA group was lower than that in the TSST-NA group (MRA = 1.47, SD = 5.15; MNA = 6.95, SD = 5.99; F(1,72) = 8.94, p = 0.004, ηp2 = 0.11), and the AUCI of subjective stress levels in the pTSST-RA group was higher than that in the pTSST-NA group (MRA = 1.13, SD = 4.06; MNA = −2.98, SD = 4.17; F(1,72) = 7.68, p = 0.007, ηp2 = 0.10). Moreover, we did not find a significant main effect of reward anticipation (F(1,72) = 0.08, p = 0.780, ηp2 = 0.001).
Figure 4. Psychological responses to psychosocial stress. Subjective stress ratings at all time points in the reward anticipation and control conditions of individuals in TSST and pTSST groups. RA: reward anticipation condition; NA: no reward anticipation (control) condition; TSST: Trier Social Stress Test; pTSST: placebo-Trier Social Stress Test.
4. Discussion
Many studies have shown that being given primary reward stimuli and performing secondary reward tasks can both effectively buffer the acute stress response induced in a laboratory scenario (Dutcher & Creswell, Citation2018). Based on previous studies, the present study provides the evidence that reward anticipation can also effectively buffer subjective stress feelings, as well as the neuroendocrine and cardiovascular responses to psychosocial stress. Specifically, individuals who anticipated future rewards showed a dampened rise in cortisol levels and heart rates as well as reduced subjective stress levels brought on by psychosocial stressors in the whole experimental process. By contrast, the control group under stress showed evidence of heightened cortisol, heart rates, and subjective stress levels that mirrored an acute stress response.
Consistent with our hypotheses, our results indicated that reward anticipation can significantly reduce individuals’ stress response. Firstly, anticipation of a future reward arouses pleasant feelings (Berridge & Robinson, Citation2003; Heller et al., Citation2009; Kringelbach & Berridge, Citation2009), and the enhancement of positive emotions after the reward anticipation task was also found in the present study. Positive emotions have significant adaptational functions in stress resilience (Folkman & Moskowitz, Citation2000). For example, positive emotions can promote the regulation of emotional responses to stressors and involve meaning-based stress coping (Folkman & Moskowitz, Citation2000; Waugh, Citation2020). Furthermore, the state of positive emotions will build psychological resources, such as optimism and ego resilience (Fredrickson, Citation2001; Speer & Delgado, Citation2017; Utsey et al., Citation2008), which has been shown to offset the potentially damaging psychological concomitants of negative effects, such as social-evaluative threat which is a crucial factor behind the social-psychological stress response (Dickerson & Kemeny, Citation2004; Trope & Pomerantz, Citation1998). In addition to positive emotions, a recent review suggested the motivational component in the reward-related processes may play an important role in the development of perceived control (Fu & Depue, Citation2019; Ly et al., Citation2019). And the enhancement of controllability could facilitate adaptive stress coping which can relieve excessive psychological and physical stress responses (Folkman et al., Citation1986; Sinha et al., Citation2016).
Another possible explanation may come from the perspective of cognitive reappraisal. When someone is in a state of stress, expecting good things to happen in the future can broaden their cognitive perspective and cause them to make positive reappraisals (Aldwin et al., Citation1994; Nicolson et al., Citation2020; Pierce et al., Citation1992), Additionally, the behavioral activation system activated by reward anticipation also has a positive direct effect on stress appraisal and alleviating detrimental effects (Espedido & Searle, Citation2020). According to the transactional stress model, the subject’s reaction to stressors is determined, in part, by their appraisal of the stressor (Delawalla, Citation2011), and cognitive appraisal processes are not only crucial for comprehending the stressors (Gaab et al., Citation2005; Lazarus & Folkman, Citation1984), they also modulate the extent that HPA axis reactivity to stress is habituated. Previous studies suggest that changing healthy individuals’ cognitive appraisal of stress can reduce the neuroendocrine stress response to an acute stressor (Gaab et al., Citation2003). For example, research among community samples has revealed that subjects feel less stressed if they perceive a stressor as a challenge, rather than as a threat (Delawalla, Citation2011). In this case, they are more likely to adopt a kind of active coping strategy and have more coping resources, which are related to faster recovery after stress (Folkman & Moskowitz, Citation2000; Sinha et al., Citation2016).
Interestingly, our results showed that reward anticipation increased participants’ subjective stress levels in the pTSST groups. Although seeming counterintuitive at first glance, the increased subjective stress ratings reflected awareness of stress signals and interoceptive experience, which are conscious steps toward regaining perceived and emotional control over stress (Gross & John, Citation2003). And the mechanism could be similar to using mindfulness for increasing perceived control over stress, pain, and other stress-related conditions (Segal & Walsh, Citation2016; Teasdale et al., Citation2002). In the present study, pTSST was regarded as a “low stress” control condition (e.g. Morris et al., Citation2012). On the one hand, the positive emotion aroused by reward anticipation may help individuals to detect the threat cues in the “low stress” environment by broadening the scope of attention (Fredrickson & Branigan, Citation2005). On the other hand, a previous study indicated that increased self-reported stress feeling originated from internal awareness which has been found positively related to active stress coping (Sinha et al., Citation2016). And we speculated reward anticipation could drive detection of internal state by promoting resilient coping.
It should be noted that the buffering effect may be influenced by the severity of acute stressors. Some studies suggested that acute stress is linked to increases in “incentive-triggered motivation” (Kumar et al., Citation2014; Lemos et al., Citation2012). Neurobiologically, the corticotropin-releasing factor (CRF), a neuropeptide released in response to acute stressors, causes dopamine release through co-activation of the receptors CRFR1 and CRFR2, which, in turn, facilitates “cue-triggered motivation” (Lemos et al., Citation2012). However, one study indicated that severe stress selectively abolishes the ability of CRF to modulate dopamine levels, specifically in the Nucleus accumbens (NAc), and it is thought to switch “cue-triggered appetitive motivation” into “aversive motivation” (Lemos et al., Citation2012). As approach motivation is a crucial factor in the processing of reward anticipation which is affected oppositely by stressors of different severity (Berridge & Robinson, Citation2003), the buffering effect of reward anticipation could be weakened by severe stress and enhanced by mild stress. The TSST paradigm used in this study was a relatively moderate stressor and we found reward anticipation had a good buffering effect, future research should examine the buffering effect of reward anticipation on severe acute stress for promoting individuals’ resilience under distinct kinds of stress conditions.
Our study had a few limitations. First, we failed to investigate the possible psychological/neurological path of these effects, which is very important for understanding the mechanism of reward anticipation on the stress response. Second, cortisol was collected at only three time-points. Although previous studies have found that this is enough to support the buffering effect of the reward system (Speer & Delgado, Citation2017), the limitations of sample collection prevented us from analyzing the effect of reward anticipation on neuroendocrine responses in different stress periods. Some studies suggested that further identifying the temporal dynamics within cortisol levels (i.e. anticipatory and reactive hormone surges) is important (e.g. Dickerson & Kemeny, Citation2004). For example, anticipatory stress cortisol interacted with a history of depressive episodes to predict depression trajectories (Morris et al., Citation2012). In future studies, more cortisol data at multiple time points can be collected to analyze the effect of reward anticipation on HPA axis response to anticipatory stress—especially for adolescents, whose cortisol response shifted from speech delivery toward anticipation (van den Bos et al., Citation2014). Third, the effectiveness of reward anticipation was only checked through a single item measurement. Although previous empirical studies have found that anticipation of therapeutic reward generated by simple manipulation can relieve physiological and psychological symptoms of patients (e.g. Colloca et al., Citation2004; Kam-Hansen et al., Citation2014), further study of direct manipulation of reward anticipation is required to provide more compelling evidence.
5. Conclusion
Previous studies have not examined the effect of the anticipatory phase of reward processing on stress response. By designing an experimental paradigm, the present study revealed the buffering effects of reward anticipation on subjective, cardiovascular, and neuroendocrine aspects of acute psychosocial stress responses in young adults. In sum, the findings elucidate the role that the reward system plays in stress resilience by demonstrating the important buffering effect of reward anticipation on acute psychosocial stress response.
Author contributions
Conceptualization: Weiyu Hu and Juan Yang
Experimental design: Weiyu Hu and Juan Yang
Data collection and analyzing: Weiyu Hu
Writing–original draft: Weiyu Hu
Writing–review and editing: Weiyu Hu and Juan Yang
Acknowledgements
We are grateful to Yang He, Xiaolin Zhao, Xuehan Zhang, Mei Zeng, Mengxue Lan, Mengning Zhang, Xiaohan Zhang, Yadong Liu, Haopeng Chen, Jiwen Li, and Xi Ren for helping to complete the experiment.
Disclosure statement
This manuscript has not been published or presented elsewhere in part or entirety and is not under consideration by another journal. All the authors approved the manuscript, agree with its submission to your esteemed journal, and contributed significantly to its creation. All study participants provided informed consent, and the study design was approved by the appropriate ethics review board. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
Additional information
Funding
Notes on contributors
Weiyu Hu
Weiyu Hu is Graduate student in Psychology.
Juan Yang
Juan Yang is Professor of Psychology of Southwest University (Chongqing, China).
References
- Aldwin, C. M., Levenson, M. R., & Spiro, A. (1994). Vulnerability and resilience to combat exposure: Can stress have lifelong effects? Psychology and Aging, 9(1), 34–44. https://doi.org/https://doi.org/10.1037/0882-7974.9.1.34
- Berridge, K. C., & Robinson, T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513. https://doi.org/https://doi.org/10.1016/S0166-2236(03)00233-9
- Berridge, K. C., Robinson, T. E., & Aldridge, J. W. (2009). Dissecting components of reward:‘liking’,‘wanting’, and ‘learning’. Current Opinion in Pharmacology, 9(1), 65–73. https://doi.org/https://doi.org/10.1016/j.coph.2008.12.014
- Carroll, D., Phillips, A. C., Der, G., Hunt, K., & Benzeval, M. (2011). Blood pressure reactions to acute mental stress and future blood pressure status: Data from the 12-year follow-up of the West of Scotland Study. Psychosomatic Medicine, 73(9), 737–742. https://doi.org/https://doi.org/10.1097/PSY.0b013e3182359808
- Colloca, L., Lopiano, L., Lanotte, M., & Benedetti, F. (2004). Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. The Lancet Neurology, 3(11), 679–684. https://doi.org/https://doi.org/10.1016/S1474-4422(04)00908-1
- Creswell, J. D., & Lindsay, E. K. (2014). How does mindfulness training affect health? A mindfulness stress buffering account. Current Directions in Psychological Science, 23(6), 401–407. https://doi.org/https://doi.org/10.1177/0963721414547415
- Delawalla, Z. (2011). Stress reactivity, stress appraisal and coping responses in schizophrenia. Dissertation Abstracts International: Section B: The Sciences and Engineering, 71(8), 5117.
- Dennison, M. J., Sheridan, M. A., Busso, D. S., Jenness, J. L., Peverill, M., Rosen, M. L., & McLaughlin, K. A. (2016). Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. Journal of Abnormal Psychology, 125(8), 1201–1212. https://doi.org/https://doi.org/10.1037/abn0000215
- Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355. https://doi.org/https://doi.org/10.1037/0033-2909.130.3.355
- Dillon, D. G., Holmes, A. J., Jahn, A. L., Bogdan, R., Wald, L. L., & Pizzagalli, D. A. (2008). Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology, 45(1), 36–49. https://doi.org/https://doi.org/10.1111/j.1469-8986.2007.00594.x
- Dutcher, J. M., & Creswell, J. D. (2018). The role of brain reward pathways in stress resilience and health. Neuroscience & Biobehavioral Reviews, 95, 559–567. https://doi.org/https://doi.org/10.1016/j.neubiorev.2018.10.014
- Dutcher, J. M., Creswell, J. D., Pacilio, L. E., Harris, P. R., Klein, W. M., Levine, J. M., & Eisenberger, N. I. (2016). Self-affirmation activates the ventral striatum: A possible reward-related mechanism for self-affirmation. Psychological Science, 27(4), 455–466. https://doi.org/https://doi.org/10.1177/0956797615625989
- Espedido, A., & Searle, B. J. (2020). Daily proactive problem-solving and next day stress appraisals: The moderating role of behavioral activation. Anxiety, Stress, & Coping, 33(4), 416–413. https://doi.org/https://doi.org/10.1080/10615806.2020.1751828
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/https://doi.org/10.3758/BRM.41.4.1149
- Fekedulegn, D. B., Andrew, M. E., Burchfiel, C. M., Violanti, J. M., Hartley, T. A., Charles, L. E., & Miller, D. B. (2007). Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine, 69(7), 651–659. https://doi.org/https://doi.org/10.1097/PSY.0b013e31814c405c
- Foley, P., & Kirschbaum, C. (2010). Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neuroscience & Biobehavioral Reviews, 35(1), 91–96. https://doi.org/https://doi.org/10.1016/j.neubiorev.2010.01.010
- Folkman, S., Lazarus, R. S., Dunkel-Schetter, C., DeLongis, A., & Gruen, R. J. (1986). Dynamics of a stressful encounter: Cognitive appraisal, coping, and encounter outcomes. Journal of Personality and Social Psychology, 50(5), 992–1003. https://doi.org/https://doi.org/10.1037/0022-3514.50.5.992
- Folkman, S., & Moskowitz, J. T. (2000). Stress, positive emotion, and coping. Current Directions in Psychological Science, 9(4), 115–118. https://doi.org/https://doi.org/10.1111/1467-8721.00073
- Fredrickson, B. L. (2001). The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218–226. https://doi.org/https://doi.org/10.1037/0003-066X.56.3.218
- Fredrickson, B. L., & Branigan, C. (2005). Positive emotions broaden the scope of attention and thought‐action repertoires. Cognition & Emotion, 19(3), 313–332. https://doi.org/https://doi.org/10.1080/02699930441000238
- Fu, Y., & Depue, R. A. (2019). A novel neurobehavioral framework of the effects of positive early postnatal experience on incentive and consummatory reward sensitivity. Neuroscience & Biobehavioral Reviews, 107, 615–640. https://doi.org/https://doi.org/10.1016/j.neubiorev.2019.09.026
- Gaab, J., Blättler, N., Menzi, T., Pabst, B., Stoyer, S., & Ehlert, U. (2003). Randomized controlled evaluation of the effects of cognitive–behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology, 28(6), 767–779. https://doi.org/https://doi.org/10.1016/S0306-4530(02)00069-0
- Gaab, J., Rohleder, N., Nater, U. M., & Ehlert, U. (2005). Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology, 30(6), 599–610. https://doi.org/https://doi.org/10.1016/j.psyneuen.2005.02.001
- Gross, J. J. (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(3), 281–291. https://doi.org/https://doi.org/10.1017/S0048577201393198
- Gross, J. J., & John, O. P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348. https://doi.org/https://doi.org/10.1037/0022-3514.85.2.348
- Heller, A. S., Johnstone, T., Shackman, A. J., Light, S. N., Peterson, M. J., Kolden, G. G., & Davidson, R. J. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, 106(52), 22445–22450. https://doi.org/https://doi.org/10.1073/pnas.0910651106
- Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology, 34(7), 1075–1086. https://doi.org/https://doi.org/10.1016/j.psyneuen.2009.02.008
- Kam-Hansen, S., Jakubowski, M., Kelley, J. M., Kirsch, I., Hoaglin, D. C., Kaptchuk, T. J., & Burstein, R. (2014). Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science Translational Medicine, 6(218), 218ra5–218ra215. https://doi.org/https://doi.org/10.1126/scitranslmed.3006175
- Kaptchuk, T. J., Kelley, J. M., Conboy, L. A., Davis, R. B., Kerr, C. E., Jacobson, E. E., Kirsch, I., Schyner, R. N., Nam, B. H., Nguyen, L. T., Park, M., Rivers, A. L., McManus, C., Kokkotou, E., Drossman, D. A., Goldman, P., & Lembo, A. J. (2008). Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ, 336(7651), 999–1003. https://doi.org/https://doi.org/10.1136/bmj.39524.439618.25
- Kirschbaum, C., Pirke, K.-M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/https://doi.org/10.1159/000119004
- Kringelbach, M. L., & Berridge, K. C. (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences, 13(11), 479–487. https://doi.org/https://doi.org/10.1016/j.tics.2009.08.006
- Kumar, P., Berghorst, L. H., Nickerson, L. D., Dutra, S. J., Goer, F., Greve, D., & Pizzagalli, D. A. (2014). Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience, 266, 1–12. https://doi.org/https://doi.org/10.1016/j.neuroscience.2014.01.058
- Lazarus, R. S., & Folkman, S. (1984). Stress, appraisal, and coping. Springer publishing company.
- Lemos, J. C., Wanat, M. J., Smith, J. S., Reyes, B. A., Hollon, N. G., Van Bockstaele, E. J., & Phillips, P. E. (2012). Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature, 490(7420), 402–406. doi:https://doi.org/10.1038/nature1143 https://doi.org/https://doi.org/10.1038/nature11436
- Liu, T. (2017). Route of placebo administration: Robust placebo effects in laboratory and clinical settings. Neuroscience & Biobehavioral Reviews, 83, 451–457. https://doi.org/https://doi.org/10.1016/j.neubiorev.2017.09.018
- Ly, V., Wang, K. S., Bhanji, J., & Delgado, M. R. (2019). A reward-based framework of perceived control. Frontiers in Neuroscience, 13, 65. https://doi.org/https://doi.org/10.3389/fnins.2019.00065
- MacKay, J., Kent, P., James, J., Cayer, C., & Merali, Z. (2017). Ability of palatable food consumption to buffer against the short-and long-term behavioral consequences of social defeat exposure during juvenility in rats. Physiology & Behavior, 177, 113–121. https://doi.org/https://doi.org/10.1016/j.physbeh.2017.04.002
- Morris, M. C., Rao, U., & Garber, J. (2012). Cortisol responses to psychosocial stress predict depression trajectories: Social-evaluative threat and prior depressive episodes as moderators. Journal of Affective Disorders, 143(1–3), 223–230. https://doi.org/https://doi.org/10.1016/j.jad.2012.05.059
- Nicolson, N. A., Peters, M. L., & Yvo, M. (2020). Imagining a positive future reduces cortisol response to awakening and reactivity to acute stress. Psychoneuroendocrinology, 116, 104677. https://doi.org/https://doi.org/10.1016/j.psyneuen.2020.104677
- Pierce, G. R., Sarason, B. R., & Sarason, I. G. (1992). General and specific support expectations and stress as predictors of perceived supportiveness: An experimental study. Journal of Personality and Social Psychology, 63(2), 297. https://doi.org/https://doi.org/10.1037/0022-3514.63.2.297
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/https://doi.org/10.1016/S0306-4530(02)00108-7
- Raio, C. M., Orederu, T. A., Palazzolo, L., Shurick, A. A., & Phelps, E. A. (2013). Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences, 110(37), 15139–15144. https://doi.org/https://doi.org/10.1073/pnas.1305706110
- Segal, Z. V., & Walsh, K. M. (2016). Mindfulness based cognitive therapy for residual depressive symptoms and relapse prophylaxis. Current Opinion in Psychiatry, 29(1), 7. https://doi.org/https://doi.org/10.1097/YCO.0000000000000216
- Sinha, R., Lacadie, C. M., Constable, R. T., & Seo, D. (2016). Dynamic neural activity during stress signals resilient coping. Proceedings of the National Academy of Sciences, 113(31), 8837–8842. https://doi.org/https://doi.org/10.1073/pnas.1600965113
- Speer, M. E., & Delgado, M. R. (2017). Reminiscing about positive memories buffers acute stress responses. Nature Human Behaviour, 1(5), 0093. https://doi.org/https://doi.org/10.1038/s41562-017-0093
- Taverniers, J., Van Ruysseveldt, J., Smeets, T., & von Grumbkow, J. (2010). High-intensity stress elicits robust cortisol increases, and impairs working memory and visuo-spatial declarative memory in Special Forces candidates: A field experiment. Stress, 13(4), 324–334. https://doi.org/https://doi.org/10.3109/10253891003642394
- Teasdale, J. D., Moore, R. G., Hayhurst, H., Pope, M., Williams, S., & Segal, Z. V. (2002). Metacognitive awareness and prevention of relapse in depression: Empirical evidence. Journal of Consulting and Clinical Psychology, 70(2), 275. https://doi.org/https://doi.org/10.1037/0022-006X.70.2.275
- Trope, Y., & Pomerantz, E. M. (1998). Resolving conflicts among self-evaluative motives: Positive experiences as a resource for overcoming defensiveness. Motivation and Emotion, 22(1), 53–72. https://doi.org/https://doi.org/10.1023/A:1023044625309
- Ulrich-Lai, Y. M., Christiansen, A. M., Ostrander, M. M., Jones, A. A., Jones, K. R., Choi, D. C., & Davis, J. F. (2010). Pleasurable behaviors reduce stress via brain reward pathways. Proceedings of the National Academy of Sciences, 107(47), 20529–20534. https://doi.org/https://doi.org/10.1073/pnas.1007740107
- Utsey, S. O., Giesbrecht, N., Hook, J., & Stanard, P. M. (2008). Cultural, sociofamilial, and psychological resources that inhibit psychological distress in African Americans exposed to stressful life events and race-related stress. Journal of Counseling Psychology, 55(1), 49–62. https://doi.org/https://doi.org/10.1037/0022-0167.55.1.49
- van den Bos, E., De Rooij, M., Miers, A. C., Bokhorst, C. L., & Westenberg, P. M. (2014). Adolescents' increasing stress response to social evaluation: Pubertal effects on cortisol and alpha‐amylase during public speaking. Child Development, 85(1), 220–236. https://doi.org/https://doi.org/10.1111/cdev.12118
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063. https://doi.org/https://doi.org/10.1037/0022-3514.54.6.1063
- Waugh, C. E. (2020). The roles of positive emotion in the regulation of emotional responses to negative events. Emotion, 20(1), 54. https://doi.org/https://doi.org/10.1037/emo0000625