Abstract
Oral ingestion of a glucose solution following severe stress is a simple and effective way of preventing several of the negative sequelae of stress in rats. Similar resilience is obtained through hormetic training – pre-exposure to mild-to-moderate stress prior to severe stress. Here, we examined whether hormetic training is facilitated when a glucose solution is available following each hormetic training session. In Experiment 1, all rats were pre-exposed to a 30 min hormetic session of 25 inescapable tailshocks on each of 3 days. The schedule or hormesis differed between groups. The hormetic sessions occurred on either 3 consecutive days or with an interpolated day of rest between each hormetic session. Furthermore, in each of these conditions, one group had access to water and one group had access to a 40% glucose solution immediately after each hormetic session to complete a 2x2 factorial design. All groups were exposed to 100 inescapable tailshocks on the day following the end of hormetic training. Shuttle-escape testing occurred 24 h later. In Experiment 2, rats received two consecutive days of 100 inescapable tailshocks. Water or glucose was available following each session. Testing occurred 24 h after the second shock exposure. Experiment 1 replicated previous findings that rats exposed to hormetic training with interpolated rest did not show exaggerated fear responding or shuttle-escape deficits that normally result from 100 inescapable tailshocks, but training was ineffective if no rest was given between stress sessions. However, all post-stress glucose groups showed an elimination of helpless behavior. In Experiment 2, it was revealed that even 100 tailshocks can be made hormetic by post-stress glucose consumption.
Introduction
Exposure to traumatic stress results in a number of physiological and psychological changes in both human and non-human species (Minor et al., Citation2006; Selye, Citation1946). These changes are often deleterious in nature and can endure throughout a lifetime. As such, interest in practical interventions aimed at treating resulting pathology or increasing resilience to the damaging effects of traumatic stress has increased substantially in recent years.
Hormesis refers to a process by which small stresses build resilience to large stresses. Originally, the term was used in the context of the immunity to poisoning that develops when an individual ingests a small amount of the toxin over an extended period of time (Calabrese et al., Citation2007; Southam, Citation1943). The term has been used more recently to describe the benefits of exercise, dietary restriction, and oxidative stress in preventing bodily disease and improving emotional health (Li & He, Citation2009; Mattson, Citation2008; Radak et al., Citation2008).
We previously reported a benefit of hormetic training given prior to severe stress on subsequent shuttle-escape performance (Plumb et al., Citation2015). A set of parametric studies in rats determined that exposure to mild restraint stress or a moderate shock stress before a more severe shock stress prevented the enhanced fear responding and shuttle-escape deficits normally observed following severe stress. Furthermore, we found that while rest between hormetic training sessions was not necessary if the hormetic stressor was mild restraint stress, interpolated rest was critical when the stressor was the more intense moderate shock stress exposure.
A second intervention that mitigates the deleterious effects of severe stress is post-stress glucose consumption. A number of findings suggest that metabolic homeostasis is challenged by exposure to severe uncontrollable stress (Bliss & Sapolsky, Citation2001; Horner et al., Citation1990; Minor, Chang, et al., Citation1994; Minor, Winslow, et al., Citation1994; Plumb et al., Citation2013). Minor and Saade (Citation1997), hypothesized that simply giving rats ad libitum access to glucose following severe stress would restore energy homeostasis and eliminate the negative sequelae of stress. They demonstrated that rats given 18 h access to a concentrated glucose solution immediately following exposure to inescapable, severe shock stress exhibited escape latencies during shuttle-box testing equal to that of controls. Thus, simply giving rats ad libitum access to glucose after a severe stressor eliminated the behavioral deficits normally observed following stress. Therefore, we hypothesized that glucose may have the potential to facilitate the hormetic exposure of moderate shock stress without interpolated days of rest that we previously found to be necessary (Plumb et al., Citation2015). We suggest that glucose would exert this facilitatory effect by restoring metabolic homeostasis in a stress schedule that otherwise does not allow time for a return to homeostasis. In fact, we suggest that post-stress glucose consumption may work in this way to make exposure to severe stress hormetic.
The following experiments aimed to determine whether post-stress glucose facilitates hormetic stress training, as well as resilience to multiple severe stress exposures. Experiment 1 determined whether the efficacy of hormetic preconditioning is enhanced when a 40% glucose solution is ingested after each training session. Experiment 2 expanded on the work of Minor and Saade (Citation1997) and examined whether glucose consumption following one severe stressor protects against future stressors.
Method
Experiment 1
Our previous work on hormetic stress provided evidence that three days of moderate shock stress (25 tailshocks) with intermittent days of rest afforded the greatest protection against severe stress (100 tailshocks; (Plumb et al., Citation2015). Three days of moderate stress without rest immediately preceding severe stress afforded no protection. Experiment 1 aimed to determine if post-stress glucose consumption facilitates hormetic stress training in two ways. First, will post-stress glucose facilitate an already effective hormetic stress procedure (3 days of shock with intermittent days of rest)? Second, will glucose exposure make a previously ineffective hormetic stress procedure (3 consecutive days of shock immediately before severe stress exposure) now effective in eliminating exaggerated fear conditioning and shuttle-escape deficits following severe stress?
Subjects
Thirty-two male Sprague-Dawley albino rats (290–320 g) from Harlan Laboratories (Indianapolis, IN) were housed in individual cages with free access to food and water in a room maintained on a 12:12 h light/dark cycle for one week prior to experimental treatment. Experimentation occurred during the light portion of the cycle. All protocols in this paper were pre-approved by the UCLA IACUC.
Apparatus
Tail shock occurred in clear Plexiglas restraining tubes, measuring 23 cm in length and 6 cm in diameter. Adjustable front walls prevented the rats from moving forward in the tubes. A rat's tail extended through the rear door of each tube and was taped to a plastic rod. Unscrambled electric shocks were delivered from one of four constant-current shock generators (Lafayette Instrument Co., Model 82400, Lafeyette, IN) through electrodes attached to the rat's tail with electrode paste and tape. Each tube was housed in a sound-attenuating enclosure containing an exhaust fan that masked extraneous noises. A 7-W house light located in the center of the attenuating enclosure’s rear wall provided constant illumination.
Escape testing occurred in a (45 cm x 20 cm x 20 cm) shuttle box (BRS-LVE model 146-40). The shuttle box was divided into two equal compartments by a metal barrier that had an 8 × 7 cm center opening flush with the grid floor. The floor consisted of 2-mm diameter stainless-steel rods spaced 1.1 cm apart center to center. Scrambled shock was delivered to the grid floor from a Grason-Stadler (Series 700, West Concord, MA) shock generator. The floor pivoted in the center and a response was recorded when a rat's front paws applied 300-g of force to the center grid in a compartment. Two 6-W lamps located in the center of each end wall provided constant illumination. The shuttle box was housed in a sound-attenuating chest, containing an exhaust fan that masked extraneous noise.
Procedure
Rats were assigned randomly to one of four groups of 8 rats each. All groups underwent 3 days of pre-exposure to the glucose solution, with 7 days intervening between the end of pre-exposure and the start of hormetic training. This was done to establish a flavor preference, as rats often refuse to drink novel substances following stress (Minor and Saade, Citation1997). All groups received hormetic training in the form of 3, 30 min sessions (1 session per day) of 25, 1.0 mA variable-duration (mean = 8.0 s; range: 3 to 15 s) inescapable tailshocks on a variable-time 60-s schedule (range: 20 to 150 s) in the restraining tubes prior to exposure to severe stress exposure. These groups differed with respect to pattern of rest (0 or 3 rest days) and what fluid was available to drink (water or a glucose solution) following each 30-min shock session in a 2 × 2 factorial design (See ). Each subject was given ad libitum access to either 100 mL of tap water or 100 mL of a concentrated 40% (wt/vol) glucose solution (with 1 drop of artificial vanilla extract added for a distinct odor and flavor) upon immediate return to the home cage at the end of each shock session. The glucose solution was available for a period of 18 h immediately following the session. Fluid consumption for all groups was measured after the 18 h time period. All bottles were replaced with tap water for the remaining 6 h, to ensure that glucose was not onboard during testing.
Figure 1. Experimental Timeline. In the first experiment, groups were given three moderate shock stress sessions with interpolated days of rest (I) or on consecutive days with no rest (N). Following each day of moderate shock stress, groups were given access to water (W) or glucose (G). In the second experiment, all groups received two severe shock stress sessions. Groups either received water following both stressors (WW), glucose following both stressors (GG), water following the first stressor and glucose the second (WG), or glucose following the first stressor and water the second (GW). S: 100 shock stressor, s: 25 shock stressor, T: shuttle-box testing, w: water was given after stress, g: Glucose was given after stress.
All four groups received severe shock stress the day following the termination of hormetic training. Severe shock stress consisted of exposure to 100, 1.0 mA variable-duration (mean = 8.0 s; range: 3 to 15 s) inescapable tailshocks on a variable-time 60-s schedule (range: 20 to 150 s) in restraining tubes over 1.83 h as this has been previously shown to be effective in producing the shuttle-escape deficit quintessential to learned helplessness (Minor et al., Citation1994).
Shuttle-escape testing occurred 24 h after severe stress. The test consisted of five trials during which a rat had to cross from one side of the central barrier to the other in order to terminate shock (Fixed Ratio (FR)-1 trials). 60 s intervened between the end of the previous shock and onset of the subsequent shock . A trained observer scored defensive freezing, defined as the absence of all bodily and vibrissae movement except for that related to respiration, every five seconds during each inter-trial interval using a time-sampling procedure. FR-1 trials were followed three minutes later by 25 FR-2 trials during which a rat had to cross from one side of the central barrier and then return to terminate shock. Shock terminated automatically if the appropriate response contingency was not met within 40 s of shock onset on a given trial. Escape latencies were recorded on each trial. Shock intensity was set at 0.6 mA with a variable time 60 s intertrial interval schedule (range: 20 to 230 s timed from shock termination to shock onset, cf., Minor & LoLordo Citation1984).
Experiment 2
Minor and Saade’s (Citation1997) original experiment showed that glucose consumption following severe stress prevented the characteristic shuttle-escape deficits. Experiment 2 investigated post-stress glucose following exposure to two inescapable, severe stress sessions 24 h apart in an effort to push the envelope and assess glucose’s effectiveness following a more extreme stressor.
Subjects and apparatus
Thirty-two male Sprague-Dawley albino rats (290–320 g) were housed as in Experiment 1. The apparatus was the same as described above.
Procedure
All groups received two severe shock stress sessions, with the second session occurring 24 h after the first. Each session consisted of the same severe shock stress procedure as described in the first experiment. Following each session, rats were returned to their home cage where they had ad libitum access to either 100 mL of tap water or a glucose solution as described in the first experiment.
Rats were randomly assigned to one of four groups of 8 rats each. Each group differed on what was available to drink following the two stress sessions (water or the glucose solution) in a 2 × 2 factorial design (See ). For example, one group received water following the first stress session, and the glucose solution following the second stress session (Group WG). Shuttle-box testing occurred 24 h after the second stress session for all groups.
Statistical Analysis. Software package SPSS (SAS Institute, Inc., Version 16.0, Cary, NC, USA) was used for statistical analyses. One-way, two-way, and mixed-design ANOVAs were used when appropriate. Following significant interactions, Bonferroni post-hoc analyses are reported. Statistical significance was noted when p values were less than 0.05, after correction for multiple comparisons. Data is presented as group means with error bars denoting group mean +/− SEM. No statistical outliers were removed from the data. Animals were excluded solely based on equipment malfunction. Data is uploaded at <seurld>https://github.com/mconoscenti/glucose_hormesis</seurld>.
Results
Experiment 1
Baseline glucose solution consumption, as well as post-stress fluid consumption, for individual rats ranged between 25 and 45 ml. Mean intake was similar among groups across pre-exposure days and hormetic training days (Fs <1.5).
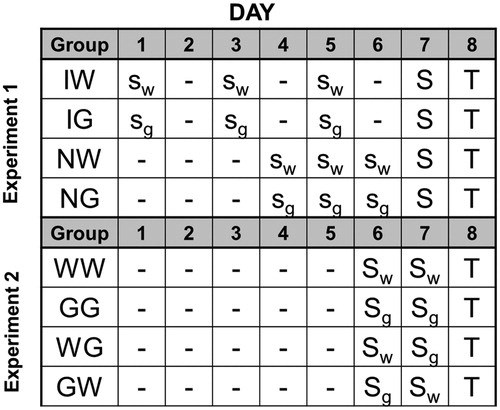
The left panel of shows mean percent post-trial freezing in each group. No significant difference in freezing was observed between rats given water during hormetic training with interpolated days of rest (IW) and those given hormetic training with no rest (NW). Glucose consumption during hormetic training resulted in reduced fear conditioning relative to those with access to water only. Furthermore, freezing was reduced in rats given glucose during hormetic training with interpolated days of rest (Group IG) when compared to rats given glucose during training with no rest (Group NG).
A mixed-design analysis of variance (ANOVA: Hormesis Schedule x Fluid Type x Trial) on freezing yielded significant main effects of Trial, F(4, 112) = 26.648, p < 0.001, Hormesis Schedule, F(1,28) = 12.145, p = 0.002, and Fluid Type, F(1,28) = 69.736, p < 0.001. There were no statistically significant interactions. Bonferroni post-hoc contrasts on grand mean freezing suggested the following ordered relationship among group means: NW = IW > NG > IG.
The right panel of shows mean escape latencies across blocks of five trials in each group. As typically observed (Jackson & Minor, Citation1988), FR-1 escape latencies did not differ among groups, F < 1. Group NW exhibited the standard impairment of escape performance with inescapable shock. Water consumption following hormetic training with interpolated rest afforded a moderate amount of protection (IW). Glucose consumption during hormetic training eliminated helplessness, regardless of pattern of rest.
A mixed-design ANOVA (Hormesis Schedule x Fluid Type x Trial Block) on FR-2 escape latencies yielded significant main effects of Hormesis Schedule, F(1,28) = 7.544, p = 0.010 and Fluid Type, F(1,28) = 32.864, p < 0.001. There were no statistically significant interactions. Bonferroni post-hoc contrasts on grand mean FR-2 escape latencies suggested the following ordered relation among group means: NW > IW > IG = NG.
Experiment 2
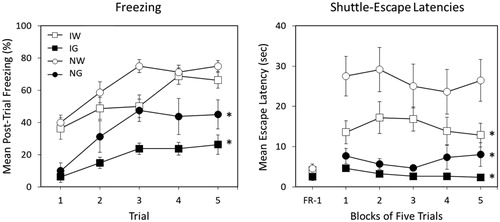
Baseline glucose solution consumption, as well as post-stress fluid consumption, for individual rats ranged between 25 and 50 milliliters. Mean intake was similar among groups across pre-exposure days (F < 1.5). However, rats that received glucose following the first day of inescapable shock exhibited higher fluid consumption rates when compared to their water-drinking counterparts (). This same effect was not observed following the second day of shock. A mixed-design ANOVA yielded a significant Group x Day interaction, F (3, 23) = 3.396, p = .035. Bonferroni post-hoc contrasts on Day 1 fluid consumption suggested the following ordered relationship among group means: GG = GW > WG = WW.
Figure 2. Percent freezing (left panel) and shuttle escape latencies (right panel) for 4 groups in Experiment 1. All groups received hormetic training in the form of 3, 30 min sessions of 25 inescapable tailshocks (1 session per day) prior to severe stress exposure. Group IW and Group IG received 100 mL of water or glucose, respectively, following each hormetic session with interpolated days of rest. Group NW and Group NG received 100 mL of water or glucose, respectively, after each hormetic session with no days of rest. Shuttle-escape testing occurred 24 h later. Freezing was measured over 5 trials (FR-1) at the start of the shuttle-escape test. Impaired escape performance was assessed over the next 25 trials (FR-2). Error bars denote mean ± SEM. * denotes p < 0.05 using Bonferroni post hoc comparisons of simple main effects of groups compared to NW.
The left panel of shows mean percent post-trial freezing in each of the four groups in Experiment 2. The group that received water following both days of shock (WW) showed exaggerated fear conditioning throughout the five FR-1 trials compared to all other groups.
Figure 3. Post-stress fluid consumption for 4 groups in Experiment 2. All groups were exposed to two severe stress sessions 24 h apart. Two groups received either 100 mL of water or 100 mL of a concentrated 40% (wt/vol) glucose solution following both stress sessions (Group WW and GG, respectively). One group received water following the first stress session and glucose following the second stress session (Group WG). The remaining group received glucose following the first stress session and water following the second session (Group GW). Total volume of fluid consumed was measured in milliliters (mL). Error bars denote mean ± SEM. * denotes p < 0.05 using Bonferroni post hoc comparisons of simple main effects of groups compared to WW.
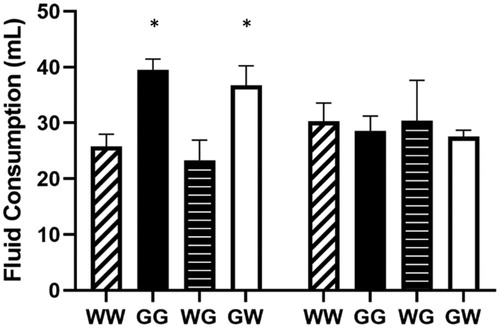
A mixed-design ANOVA (Fluid 1 × Fluid 2 × Trial) yielded a significant Fluid 1 × Fluid 2 interaction, F(1,28) = 6.406, p = 0.017, and a significant main effect of Trial, F(4, 112) = 20.047, p < 0.001, but no significant three-way interaction. Bonferroni post-hoc contrasts on grand mean freezing suggested the following ordered relation among group means: WW > GW = WG = GG.
Figure 4. Percent freezing (left panel) and shuttle escape latencies (right panel) for 4 groups in Experiment 2. All groups were exposed to two severe stress sessions 24 h apart. Two groups received either 100 mL of water or 100 mL of a concentrated 40% (wt/vol) glucose solution following both stress sessions (Group WW and GG, respectively). One group received water following the first stress session and glucose following the second stress session (Group WG). The remaining group received glucose following the first stress session and water following the second session (Group GW). Shuttle-escape testing occurred 24 h later. Freezing was measured over 5 trials (FR-1) at the start of the shuttle-escape test. Impaired escape performance was assessed over the next 25 trials (FR-2). Error bars denote mean ± SEM. * denotes p < 0.05 using Bonferroni post hoc comparisons of simple main effects of groups compared to WW.
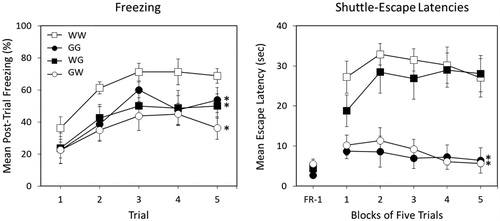
The right panel of shows mean escape latencies across blocks of five trials in each group. FR-1 escape latencies did not differ, F < 1. Glucose consumption following the first severe stress session dramatically improved escape performance, regardless of whether the type of fluid consumed after the second stress session was water (GW) or glucose (GG). Consuming glucose following only the second stress session (WG) did not improve performance.
A mixed-design ANOVA (Fluid 1 × Fluid 2 × Trial Block) yielded a significant Fluid 1 × Trial Block interaction, F(4, 112) = 4.670, p = 0.002. Bonferroni post-hoc contrasts on FR-2 escape latencies suggested the following ordered relation among group means on Trial Blocks 2–5: WW = WG > GG = GW.
Discussion
The experiments described above provide evidence that post-stress glucose consumption is a simple and effective method of building stress resilience. Post-stress glucose facilitates hormetic stress training and renders previously ineffective training procedures now effective in preventing the exaggerated fear conditioning and behavioral depression that result from severe stress. Post-stress glucose ingestion also builds resilience to back-to-back severe stress sessions. Interestingly, glucose consumption following the first stressor was the critical factor in building resilience to the second stressor. While the fluid consumed following the second stressor reduced freezing, it yielded no benefit for shuttle-escape. This suggests that glucose’s mechanism of action on freezing and shuttle escape behaviors are likely dissociable. Also, it should be noted that we observed significantly greater fluid consumption following the first stressor in groups given glucose. Though this effect is of interest, we have not seen a reliable effect of fluid type on post-stress consumption in past experiments where glucose has been effective (Conoscenti et al., Citation2017; Conoscenti et al., Citation2019; Minor & Saade, Citation1997). Therefore, we do not suspect the glucose effects are due to this increased fluid ingestion.
There are a number of potential mechanisms through which post-stress glucose consumption might increase resilience to severe stress. The work of Plumb et al. (Citation2015) on hormetic stress highlighted the importance of rest between stressor exposures if the stressor was severe. This observation is consistent with McEwen and colleagues’ argument that rest is necessary to repair the physiological damage inflicted by severe stress and a sustained rise in catabolic hormones (McEwen & Gianaros, Citation2011; McEwen & Stellar, Citation1993). The recovery process presumably requires not only a decrease in catabolic hormones, but an increase in anabolic hormones (e.g. growth hormone), which repair the damage due to stress. Growth hormone levels normally increase at night in direct proportion to the quality of sleep (Jarrett et al., Citation1990; Perras et al., Citation1999; Sassin et al., Citation1969; Takahashi et al., Citation1968). Thus, the benefit of interpolated rest when the hormetic stressor is moderate to severe may be that it affords the opportunity for rest and an increase in anabolism. Glucose, however, may circumvent this process. Chandler et al. (Citation1994), reported that carbohydrate ingestion following weight-lifting training significantly increased blood growth hormone concentrations above control levels within six hours of the exercise. Thus, post-stress glucose ingestion following mild to moderate hormetic stress may eliminate the need for rest by facilitating anabolism and the recovery of a non-stress baseline.
Perhaps the benefit of post-stress glucose comes instead from its ability to prevent metabolic exhaustion. Fear is an intensely catabolic state and rapidly challenges brain metabolic homeostasis (Hanff et al., Citation2010; Minor, Chang, et al., Citation1994; Minor et al., Citation2006; Minor et al., Citation2008; Minor, Winslow, et al., Citation1994; Plumb et al., Citation2013). Under these circumstances, adenosine is released to inhibit further activity in an effort to prevent cell death. Minor and colleagues have shown that adenosine A2A receptors are involved in the conservation-withdrawal symptoms normally observed following severe stress exposure. Post-stress glucose has been shown to uniquely restore blood glucose levels and liver glycogen stores following inescapable shock when compared to fructose, thereby increasing energy availability for the brain (Conoscenti et al., Citation2019). Importantly, 2-deoxy-D-glucose- induced glucoprivation has been shown to mimic the effects of inescapable shock- an effect that is reversed by systemic administration of an adenosine antagonist (Hanff et al., Citation2010). These data taken together suggest that glucose consumption following severe stress might restore metabolic homeostasis, thereby eliminating the necessity for the compensatory adenosine response. It should be noted that stress-induced upregulation of A2A receptor activity is both necessary and sufficient for the shuttle escape deficits, but not increased freezing behavior following stress (Briones et al., Citation2014; Minor, Chang, et al., Citation1994; Minor, Winslow, et al., Citation1994; Plumb et al., Citation2013). Therefore, this is not a likely route for glucose’s effect on freezing.
Another possible explanation for the effects of post-stress glucose on helplessness is increased context discrimination. Minor & LoLordo (Citation1984), demonstrated that the helplessness effect is eliminated when rats can discriminate the training context, in which inescapable shock is delivered, from the shuttle-escape testing context. Contextual learning critically depends on hippocampal processing (Fanselow, Citation2000). An increase in circulating glucocorticoids during stress impairs glucose uptake into the hippocampus and severely impairs contextual processing (Bliss & Sapolsky, Citation2001; Dash et al., Citation1996; Horner et al., Citation1990; Patel et al., Citation2001; Sapolsky, Citation1996). Such deficits are reversed by increasing hippocampal glucose concentrations by any of a number of means (Gold, Citation2015). Thus, post-stress glucose consumption may be allowing veridical encoding of the context in the hippocampus, resulting in less generalization between the two contexts. Indeed, glucose-enhanced discrimination has been demonstrated using a fear discrimination task in humans (Glenn et al., Citation2014). However, it should be noted that a study by Luyten et al. (Citation2016) observed no effect of involuntarily-administered glucose (gavage) on context discrimination in rats.
A simple, reasonable hypothesis is that the post-stress effects of glucose are due to its appetitive properties. Indeed, glucose ingestion has been shown to activate reward pathways and increase release of neurotransmitters involved in reinforcement, such as dopamine (Blum et al., Citation2014; Koekkoek et al., Citation2017). However, other sweet solutions have proved ineffective in ameliorating the negative sequelae of stress exposure. The non-nutritive, artificial sweetener saccharine (matched for taste), as well as fructose (matched for caloric density), have exhibited no behavioral benefit when given after exposure to inescapable shock (Conoscenti, Citation2020; Conoscenti & Fanselow, Citation2019). This suggests that glucose is likely exerting its beneficial effects due to a property other than its sweet taste.
Future research should expand upon the burgeoning stress-glucose literature. While we have shown the behavioral benefits of glucose-enhanced hormetic stress, we did not elucidate the physiological mechanisms of this effect. While we may draw hypotheses from physiological data describing the effects of glucose following severe shock stress, we cannot be sure that there is a similar mechanism at play when glucose is given following repeated, moderate shock stress. This should be directly tested. Further experimentation elucidating glucose administration parameters should be performed to resolve why the timing of glucose administration was critical for shuttle-escape, but not freezing, behavior. Additional studies investigating other parameters, such as route of administration, and the effects of pre-stress glucose, are also important to further our understanding of glucose’s effects and constraints.
Conclusion
The present experiments provided evidence that post-stress glucose consumption facilitates stress resilience. Plumb et al. (Citation2015) demonstrated that hormetic training with mild or moderate stress before severe stress prevented the negative sequelae of stress. Experiment 1 showed that giving an glucose solution immediately following hormetic stress sessions facilitated their ability to build resilience to severe stress exposure. Post-stress glucose was also able to make a previously-ineffective hormetic stress procedure now effective. Glucose also had the ability to make severe stress hormetic. Experiment 2 demonstrated that giving glucose following the first stress session builds resilience to a second session 24 h later. It was discovered that glucose consumption following the first stressor was critical. While there was an effect on freezing if glucose was given only after the second stress session, there was no effect on shuttle escape. Clearly, the present experiments demonstrate that post-stress glucose consumption is a simple and effective method of building stress resilience.
Acknowledgements
This is a posthumous authorship for Dr. Thomas Minor who made major contributions to the study design and data collection.
Disclosure statement
Dr Fanselow is a Board member of Neurovation, Inc). All of the other authors reported no biomedical financial interests or potential conflicts of interest.
Additional information
Funding
References
- Bliss, T. M., & Sapolsky, R. M. (2001). Interactions among glucose, lactate and adenosine regulate energy substrate utilization in hippocampal cultures. Brain Research, 899(1–2), 134–141. https://doi.org/10.1016/S0006-8993(01)02218-1
- Blum, K., Thanos, P. K., & Gold, M. S. (2014). Dopamine and glucose, obesity, and reward deficiency syndrome. Frontiers in Psychology, 5, 919. https://doi.org/10.3389/fpsyg.2014.00919
- Briones, B., Plumb, T., & Minor, T. (2014). Adenosine’s autacoid function in the central nervous system and the behavioral state of conservation-withdrawal. Autacoids, 3(106), 479–2161.
- Calabrese, E. J., Bachmann, K. A., Bailer, A. J., Bolger, P. M., Borak, J., Cai, L., Cedergreen, N., Cherian, M. G., Chiueh, C. C., Clarkson, T. W., Cook, R. R., Diamond, D. M., Doolittle, D. J., Dorato, M. A., Duke, S. O., Feinendegen, L., Gardner, D. E., Hart, R. W., Hastings, K. L., … Mattson, M. P. (2007). Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicology and Applied Pharmacology, 222(1), 122–128. https://doi.org/10.1016/j.taap.2007.02.015
- Chandler, R., Byrne, H., Patterson, J., & Ivy, J. (1994). Dietary supplements affect the anabolic hormones after weight-training exercise. Journal of Applied Physiology, 76(2), 839–845. https://doi.org/10.1152/jappl.1994.76.2.839
- Conoscenti, M. A. (2020). The parametric determinants of heterogeneity in the behavioral and neurobiological impact of stress. https://escholarship.org/uc/item/0bb0t290
- Conoscenti, M. A., & Fanselow, M. S. (2019). Dissociation in effective treatment and behavioral phenotype between stress-enhanced fear learning and learned helplessness. Frontiers in Behavioral Neuroscience, 13, 104. https://doi.org/10.3389/fnbeh.2019.00104
- Conoscenti, M. A., Williams, N. M., Turcotte, L. P., Minor, T. R., & Fanselow, M. S. (2019). Post-stress fructose and glucose ingestion exhibit dissociable behavioral and physiological effects. Nutrients, 11(2), 361. https://doi.org/10.3390/nu11020361
- Conoscenti, M., Hart, E., Smith, N., & Minor, T. (2017). Temporal parameters of post-stress prophylactic glucose treatment in rats. Stress, 20(3), 265–276. https://doi.org/10.1080/10253890.2017.1334052
- Dash, R., Lawrence, M., Ho, D., & Sapolsky, R. (1996). A herpes simplex virus vector overexpressing the glucose transporter gene protects the rat dentate gyrus from an antimetabolite toxin. Experimental Neurology, 137(1), 43–48. https://doi.org/10.1006/exnr.1996.0005
- Fanselow, M. S. (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research, 110(1–2), 73–81. https://doi.org/10.1016/S0166-4328(99)00186-2
- Glenn, D. E., Minor, T. R., Vervliet, B., & Craske, M. G. (2014). The effect of glucose on hippocampal-dependent contextual fear conditioning. Biological Psychiatry, 75(11), 847–854. https://doi.org/10.1016/j.biopsych.2013.09.022
- Gold, P. W. (2015). The organization of the stress system and its dysregulation in depressive illness. Molecular Psychiatry, 20(1), 32–47. https://doi.org/10.1038/mp.2014.163
- Hanff, T. C., Furst, S. J., & Minor, T. R. (2010). Biochemical and anatomical substrates of depression and sickness behavior. Israel Journal of Psychiatry and Related Sciences, 47(1), 64–71.
- Horner, H. C., Packan, D. R., & Sapolsky, R. M. (1990). Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology, 52(1), 57–64.
- Jackson, R. L., & Minor, T. R. (1988). Effects of signaling inescapable shock on subsequent escape learning: Implications for theories of coping and" learned helplessness. Journal of Experimental Psychology: Animal Behavior Processes, 14(4), 390. https://doi.org/10.1037/0097-7403.14.4.390
- Jarrett, D. B., Greenhouse, J. B., Miewald, J. M., Fedorka, I. B., & Kupfer, D. J. (1990). A reexamination of the relationship between growth hormone secretion and slow wave sleep using delta wave analysis. Biological Psychiatry, 27(5), 497–509. https://doi.org/10.1016/0006-3223(90)90441-4
- Koekkoek, L. L., Mul, J. D., & la Fleur, S. E. (2017). Glucose-sensing in the reward system. Frontiers in Neuroscience, 11, 716. https://doi.org/10.3389/fnins.2017.00716
- Li, G., & He, H. (2009). Hormesis, allostatic buffering capacity and physiological mechanism of physical activity: A new theoretic framework. Medical Hypotheses, 72(5), 527–532. https://doi.org/10.1016/j.mehy.2008.12.037
- Luyten, L., Schroyens, N., Luyck, K., Fanselow, M., & Beckers, T. (2016). No effect of glucose administration in a novel contextual fear generalization protocol in rats. Translational Psychiatry, 6(9), e903–e903. https://doi.org/10.1038/tp.2016.183
- Mattson, M. P. (2008). Hormesis defined. Ageing Research Reviews, 7(1), 1–7. https://doi.org/10.1016/j.arr.2007.08.007
- McEwen, B. S., & Gianaros, P. J. (2011). Stress-and allostasis-induced brain plasticity. Annual Review of Medicine, 62(1), 431–445. https://doi.org/10.1146/annurev-med-052209-100430
- McEwen, B. S., & Stellar, E. (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. https://doi.org/10.1001/archinte.1993.00410180039004
- Minor, T. R., & LoLordo, V. M. (1984). Escape deficits following inescapable shock: The role of contextual odor. Journal of Experimental Psychology: Animal Behavior Processes, 10(2), 168. https://doi.org/10.1037/0097-7403.10.2.168
- Minor, T. R., & Saade, S. (1997). Poststress glucose mitigates behavioral impairment in rats in the "learned helplessness" model of psychopathology. Biological Psychiatry, 42(5), 324–334. https://doi.org/10.1016/S0006-3223(96)00467-2
- Minor, T. R., Chang, W. C., & Winslow, J. L. (1994). Stress and adenosine: I. Effect of methylxanthine and amphetamine stimulants on learned helplessness in rats. Behavioral Neuroscience, 108(2), 254–264. https://doi.org/10.1037/0735-7044.108.2.254
- Minor, T. R., Dess, N. K., Ben-David, E., & Chang, W.-C. (1994). Individual differences in vulnerability to inescapable shock in rats. Journal of Experimental Psychology: Animal Behavior Processes, 20(4), 402. https://doi.org/10.1037/0097-7403.20.4.402
- Minor, T. R., Huang, Q., & Witt, A. E. (2006). Cytokine-purine interactions in traumatic stress, behavioral depression, and sickness. CNS & Neurological Disorders - Drug Targets, 5(5), 547–560. https://doi.org/10.2174/187152706778559282
- Minor, T. R., Rowe, M., Cullen, P. K., & Furst, S. (2008). Enhancing brain adenosine signaling with the nucleoside transport blocker NBTI (S-(4-nitrobenzyl)-6-theoinosine) mimics the effects of inescapable shock on later shuttle-escape performance in rats. Behavioral Neuroscience, 122(6), 1236–1247. https://doi.org/10.1037/a0013143
- Minor, T. R., Winslow, J. L., & Chang, W. C. (1994). Stress and adenosine: II. Adenosine analogs mimic the effect of inescapable shock on shuttle-escape performance in rats. Behavioral Neuroscience, 108(2), 265–276. https://doi.org/10.1037/0735-7044.108.2.265
- Patel, M., McIntosh, L., Bliss, T., Ho, D., & Sapolsky, R. (2001). Interactions among ascorbate, dehydroascorbate and glucose transport in cultured hippocampal neurons and glia. Brain Research, 916(1–2), 127–135. https://doi.org/10.1016/S0006-8993(01)02877-3
- Perras, B., Marshall, L., Köhler, G., Born, J., & Fehm, H. L. (1999). Sleep and endocrine changes after intranasal administration of growth hormone-releasing hormone in young and aged humans. Psychoneuroendocrinology, 24(7), 743–757. https://doi.org/10.1016/S0306-4530(99)00027-X
- Plumb, T. N., Cullen, P. K., & Minor, T. R. (2015). Parameters of hormetic stress and resilience to trauma in rats. Stress, 18(1), 88–95. https://doi.org/10.3109/10253890.2014.974154
- Plumb, T. N., Sterlace, S. R., Cavanaugh, K. A., & Minor, T. R. (2013). Stress, brain adenosine signaling, and fatigue-related behavioral processes (pp. 535–558). Springer.
- Radak, Z., Chung, H. Y., Koltai, E., Taylor, A. W., & Goto, S. (2008). Exercise, oxidative stress and hormesis. Ageing Research Reviews, 7(1), 34–42. https://doi.org/10.1016/j.arr.2007.04.004
- Sapolsky, R. M. (1996). Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress, 1(1), 1–19. https://doi.org/10.3109/10253899609001092
- Sassin, J., Parker, D., Mace, J., Gotlin, R., Johnson, L., & Rossman, L. (1969). Human growth hormone release: Relation to slow-wave sleep and sleep-waking cycles. Science, 165(3892), 513–515. https://doi.org/10.1126/science.165.3892.513
- Selye, H. (1946). The general adaptation syndrome and the diseases of adaptation. The Journal of Clinical Endocrinology, 6(2), 117–230. https://doi.org/10.1210/jcem-6-2-117
- Southam, C. M. (1943). Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology, 33, 517–524.
- Takahashi, Y., Kipnis, D., & Daughaday, W. (1968). Growth hormone secretion during sleep. Journal of Clinical Investigation, 47(9), 2079–2090. https://doi.org/10.1172/JCI105893
