Abstract
Anticipation of stress induces physiological, behavioral and cognitive adjustments that are required for an appropriate response to the upcoming situation. Additional research examining the response of cardiopulmonary parameters and stress hormones during anticipation of stress in different chronic stress adaptive models is needed. As an addition to our previous research, a total of 57 subjects (16 elite male wrestlers, 21 water polo player and 20 sedentary subjects matched for age) were analyzed. Cardiopulmonary exercise testing (CPET) on a treadmill was used as the laboratory stress model; peak oxygen consumption (VO2) was obtained during CPET. Plasma levels of adrenocorticotropic hormone (ACTH), cortisol, alpha-melanocyte stimulating hormone (alpha-MSH) and N-terminal-pro-B type natriuretic peptide (NT-pro-BNP) were measured by radioimmunometric, radioimmunoassay and immunoassay sandwich technique, respectively, together with cardiopulmonary measurements, 10 minutes pre-CPET and at the initiation of CPET. The response of diastolic blood pressure and heart rate was different between groups during stress anticipation (p = 0.019, 0.049, respectively), while systolic blood pressure, peak VO2 and carbon-dioxide production responses were similar. ACTH and cortisol increased during the experimental condition, NT-pro-BNP decreased and alpha-MSH remained unchanged. All groups had similar hormonal responses during stress anticipation with the exception of the ACTH/cortisol ratio. In all three groups, ΔNT-pro-BNP during stress anticipation was the best independent predictor of peak VO2 (B = 36.01, r = 0.37, p = 0.001). In conclusion, the type of chronic stress exposure influences the hemodynamic response during anticipation of physical stress and the path of hormonal stress axis activation. Stress hormones released during stress anticipation may hold predictive value for overall cardiopulmonary performance during the stress condition.
LAY SUMMARY
The study revealed differences in hormonal and hemodynamic responses during anticipation of stress between athletes and sedentary participants. Stress hormones released during stress anticipation may hold predictive value for overall cardiopulmonary performance during the stress condition.
Introduction
Stress is considered to be a state of threatened homeostasis following exposure to intrinsic or extrinsic adverse events. Acute stress may be sustained from minutes or hours, whereas chronic stress persists for a longer time period. (Hyun et al., Citation2019; Neupert & Bellingtier, Citation2019; Pulopulos et al., Citation2018; Quent et al., Citation2018). Anticipation of stress induces physiological, behavioral and cognitive adjustments that are necessary to deal with the upcoming acute stress situation (Hyun et al., Citation2019; Neupert & Bellingtier, Citation2019; Pulopulos et al., Citation2018; Quent et al., Citation2018). Physiologically preparing for an impending stressful event is of a highest priority; the integrity of the prestress response has the potential to either enhance or undermine psychobiological resilience to oxidative damage, the latter potentially contributing to increased morbidity and mortality (Aschbacher et al., Citation2013). Acute stress activates the sympatho-adrenal medullary system and hypothalamo–pituitary–adrenal (HPA) axis in parallel. Epinephrine and norepinephrine are well-known mediators of acute stress (Aschbacher et al., Citation2013). Chronic stress exposure promotes oxidative damage and increases the risk for adverse events through frequent and sustained activation of the HPA stress axis (Aschbacher et al., Citation2013). Acute and chronic stress reactions have also been shown to affect the immune system as indicated by an increase in various inflammatory markers (Hyun et al., Citation2019; Neupert & Bellingtier, Citation2019; Pulopulos et al., Citation2018; Quent et al., Citation2018). It has been shown that manageable levels of life stress may enhance psychobiological resilience to acute stress, which is proposed as a model of eustress (Aschbacher et al., Citation2013; Parker & Ragsdale, Citation2015). This implies the importance of the body's prior exposure to chronic stress in order to cope with stress situations and its’ negative effects.
The exposure to stress is highly variable in everyday life and includes a wide range of situations including stress at work, stress in family life, stress related to low socio-economic status as well as physical, metabolic and immunologic stress. The response to these stress situations is influenced by genetic factors, type of personality, exercise training and nutritional status (Grande et al., Citation2012; Herbison et al., Citation2016; Piepoli et al., Citation2016; Rutledge et al., Citation2016). A crucial role in the adaptation to stress is attributed to stress hormones, which allow the body to accommodate for the increased metabolic demands intrinsic to the stress condition (Lightman, Citation2008). Cortisol and adrenocorticotropic hormone (ACTH), released by activation of the HPA stress axis, are considered as primary stress hormones (Herman et al., Citation2016; Lightman, Citation2008). Moreover, hormones interact during stress, such as the interaction between alpha-melanocyte-stimulating hormone (alpha-MSH) and N-terminal-pro-B type natiuretic peptide (NT-pro-BNP) (Grimm et al., Citation2016; Popovic et al., Citation2013); these hormones plan a role in regulating carbohydrate, protein and lipid metabolism, energy homeostasis and body composition, inflammation and immune regulation, and autonomic system function, all of which are related to cardiopulmonary function (Cain & Cidlowski, Citation2017; Pivonello et al., Citation2016; Stimson et al., Citation2017; van Ockenburg et al., Citation2016).
Despite extensive theoretical knowledge regarding stress hormones, and the awareness that stress may be the cause of many diseases and adverse events, clinical management has lagged behind, in part because there is currently a lack of accurate quantification of stress hormones as well as the inability to define standard approaches to treatment (Piepoli et al., Citation2016; Schnohr et al., Citation2015). In order to expand our understanding of active stress management, we aimed to examine how different types of intentional chronic stress exposure affect acute stress responses. We hypothesized that cardiopulmonary responses and stress axis activation during stress anticipation may differ depending on adaptation to chronic stress exposure and cardiorespiratory fitness. To test this hypothesis, we assessed cardiopulmonary parameters and stress hormones (ACTH, cortisol, alpha-MSH, NT-pro-BNP), as well as the activation of stress axis (ACTH/cortisol ratio) during anticipation of stress and the ability of these hormones to reflect cardiorespiratory fitness.
Methods
Subjects
Our study is addition to our already published research (Popovic et al., Citation2013, Citation2014). In short, 57 healthy male subjects of similar age were included [16 elite wrestlers (W), 21 elite water polo players (WP), and 20 sedentary control subjects (C)]. Subjects in the W and WP groups included in the present study are athletes have been successfully competitive at the international level; they have all trained intensively for more than 10 years. Both W and WP subjects performed combined endurance and strength training protocols. At the time of the study, both W and WP subjects were in a period of preparation for international competition. Subjects in the W group performed four hours of power training in the gym, nine hours of wrestling a week and 4 hours of high intensity running a week. Subjects in the WP group trained 12 hours a week in the pool, where they swimmed at least 2 km per each training session, and they also spent three hours a week in the gym where they performed both endurance and power exercises. Subjects in the C group were not engaged in sporting activities; they reported less then two hours a week of physical activity for the last 10 years. All subjects provided detailed personal and family history of illness. They did not declare any risk factors or diseases which were considered as exclusion criteria, such as hypertension, hypertrophic cardiomyopathy, diabetes, arrhythmias, renal disease, infections, anabolic steroids usage and smoking. All subjects provided an informed consent and the local Ethical Committee approved the study.
Study protocol
After initial screening, all subjects underwent anthropometric measurements. The stress anticipation situation was simulated in laboratory conditions using cardiopulmonary exercise testing (CPET) as a laboratory stress model (Fletcher et al., Citation2013; Svensson et al., Citation2016). The stress anticipation period was considered 10 minutes before the beginning of CPET. CPET was conducted approximately at 1 PM for all subjects. Cardiopulmonary and hormonal measurements were taken: (1) 10 minutes before CPET - at rest; and (2) at the beginning of CPET.
Anthropometry
Body weight (BW), fat mass (FM) and fat-free mass (FFM) were obtained by bioelectrical impedance analysis using Tanita weight (phase-sensitive multi-frequency analyzer Data Input GmbH 2000, software Nutri 3). The Du Bois and Du Bois formula was used to calculate body surface area (BSA). (Du Bois & Du Bois, Citation1916).
CPET procedure
All the subjects underwent maximal CPET on a treadmill, according to current recommendations (American Thoracic Society, American College of Chest Physicians, Citation2003). The protocol consisted of: (1) 2 min at a speed 6 km/h and 2% inclination; (2) 2 min at a speed 9 km/h and 2% inclination; and (3) an increase of inclination for 2% every 2 min thereafter until criteria for maximal exertion was reached. Ventilatory expired gas analysis was performed using a Cardiovit CS 200 (Schiller, Baar, Switzerland) throughout the assessment with three phases of primary interest: (1) 10 minutes before CPET at rest; (2) at the beginning of CPET; and (3) at the peak effort. Standard 12-lead electrocardiograms were obtained in all phases of CPET, as well as systolic (SBP) and diastolic arterial blood pressure (DBP) which was measured using a standard cuff sphygmomanometer. The following variables were determined from CPET: (1) heart rate (HR); (2) oxygen uptake (VO2); (3) carbon dioxide production (VCO2); and (4) minute ventilation (VE). VE, VO2, and VCO2 were acquired breath-by-breath, averaged over 30 seconds, and printed using rolling averages every 10 seconds. The changes in cardiopulmonary and hormonal variables during anticipation of stress were considered the differences between measurement obtained at rest and the beginning of CPET. Peak respiratory exchange ratio (RER) was calculated as the highest 10-second averaged sample obtained during the last 20 seconds of testing, and was ≥1.1 in all subjects.
Blood analysis
An intravenous cannula was placed in brachial vein three hours before the test, in order to take blood samples avoiding acute hormonal stimulation by needle punctuation. Participants were free of drink and food at least three hours before collecting blood samples. Blood was taken in two phases of the CPET: (1) 20 ml at rest − 10 min before CPET; and (2) 20 ml at the beginning of CPET. Samples were centrifuged on 4000 rpm and kept at −80 °C. Immunoradiometric method (ELSA-ACTH, CIS BioInternational, Gif-Sur-Yvette Cedex, France) was used to measure ACTH (lower sensitivity limit 2 ng/l). Radioimmunoassay (CORT-CT2, CIS BioInternational, Gif-Sur-Yvette Cedex, France) was used to measure plasma cortisol (lower sensitivity limit 4.6 nmol/l). Immunoassay sandwich technique (pro-BNP II, Cobas, Roche, Burgess Hill, England) was used to measure NT-pro-BNP (lower sensitivity limit 5 pg/ml). Radioimnunoassay technique (EURIA-α-MSH, Euro-Diagnostica, Malme, Sweden) was used to measure alpha-MSH (lower sensitivity limit of 3 pmol/l). Intra- and interassay coefficient of variation was less than 10% for all assays.
Statistical analysis
The Kolmogorov–Smirnov test was used to perform the analysis of distribution of the observed variables. Classic descriptive parameters were used to express the results, such as median for non-normally distributed variables and mean and standard deviation for normally distributed variables. The analysis of variance (ANOVA) was used to assess the differences between the groups; post hoc multiple group comparisons were assessed with LSD and Bonferronìs method. Kruskal–Wallis nonparametric ANOVA followed by the Mann–Whitney test was used for non-normally distributed variables. The general linear model for repeated measures was used to analyze hormonal responses during anticipation of stress. Correlations between variables were examined by Spearman`s rank and Pearson`s correlation tests. The difference was considered significant when a p value was <0.05, and highly significant when a p value was <0.01. SPSS software (SPSS version 10.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
The participants had similar age, SBP and DBP; there were significant differences between subjects in BW, BSA, FM and FFM as shown in .
Table 1. Baseline characteristics of the study groups.
Cardiopulmonary parameters at rest are shown in . HR at rest was higher in controls, and similar in the W and WP groups. VO2 rest and VE/VCO2 rest were similar in all groups, while VCO2 rest and VE/VO2 rest were higher in the W group compared to the C group. There was no difference in HR peak between groups; VO2 peak was higher in the W group compared to the C group and the highest in the WP group.
Table 2. Cardiopulmonary parameters.
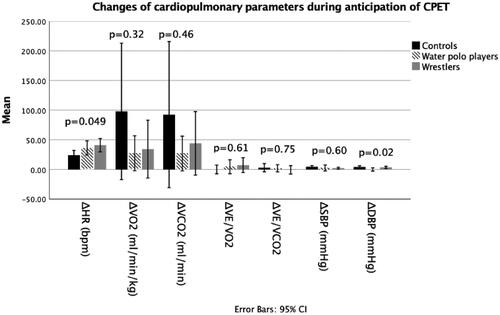
There was no difference between subjects in the change of VO2, VCO2, VE/VO2, VE/VCO2 and SBP during anticipation of CPET, as shown at . The increase in HR was significantly higher in the W group compared to controls (p = 0.033), while it was not significantly different between the WP and C groups (p = 0.088), as well as between the W and WP groups (p = 1.000). DBP increased more in the C group than in the WP group during anticipation of CPET (p = 0.006), while the difference in DBP change was not significant between W and C groups (p = 0.079) and the W and WP groups (p = 0.238).
Figure 1. Cardiopulmonary parameters during anticipation of stress. C: control group; CPET: cardiopulmonary exercise test; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure; VCO2: carbon-dioxide production; VE: minute ventilation; VO2: oxygen uptake; W: wrestlers; WP: water polo players.
Plasma levels of stress hormones at rest and the changes during anticipation of CPET are shown in and .
Figure 2. Changes of stress hormones during anticipation of stress. ACTH: adrenocorticotropic hormone; C: control group; CPET: cardiopulmonary exercise test; MSH: melanocyte stimulating hormone; NT-pro-BNP: N-terminal-pro B type natriuretic pepide; W: wrestlers; WP: water polo players.
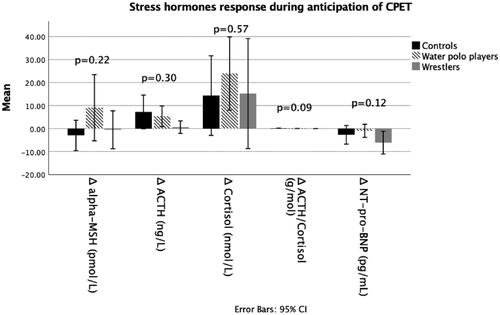
Table 3. Stress hormones at rest.
Circulating ACTH increased between the two phases of CPET (p = 0.008) in all groups (W, WP and C), whereas alpha-MSH was not significantly changed (p = 0.238). Plasma ACTH and alpha-MSH were not different among groups at any phase of the test (p > 0.05). The changes in plasma ACTH and alpha-MSH level during anticipation of CPET were similar in all groups (p = 0.301, 0.219, respectively), which indicates similar response.
Circulating cortisol increased from rest to the beginning of CPET (p < 0.001). Plasma cortisol was lower in the C group compared to the W and WP groups during both phases of the test (p = 0.001 and 0.028, respectively at rest; p = 0.001 and 0.045, respectively at the beginning of the test), while the W and WP groups had similar values (p = 0.610 at rest, p = 0.281 at the beginning of the test). The change of cortisol between the two phases of CPET was not different among the groups (p = 0.569).
The ACTH/cortisol ratio at rest was highest in the WP group, and higher in the C group compared to the W group, while the W and WP groups similar values, as shown in . The change of this ratio during anticipation of CPET was significantly different between the C and W groups (p = 0.033), while the WP and C groups had similar values, as well as the W and WP groups (p = 0.141, 0.123, respectively).
Plasma level of NT-pro-BNP significantly decreased from rest to the beginning of CPET (p = 0.002). Plasma levels of NT-pro-BNP were significantly higher in the C group compared to the W group in both phases of the test (C vs. W at rest p = 0.017 and at the beginning of CPET p = 0.013); The WP group did not have significantly different values compared to the W and C groups during both phases of the test (p > 0.05). The change in NT-pro-BNP plasma level between the two phases of CPET in all groups was similar (p = 0.119), which indicates a similar response.
In all three groups combined, the change of NT-pro-BNP during anticipation of the CPET correlated with VO2 peak (r = 0.40, p = 0.003). Other significant correlations of hormonal variables with HR peak and VO2 peak were not found (p > 0.05).
On multiple regression analysis, which included hormonal and anthropometric variables, in all three groups combined, the best independent predictor of VO2 peak was ΔNT-pro-BNP (B = 36.01, r = 0.37, p = 0.001).
Discussion
The results of our study demonstrate that during anticipation of stress different types of chronic stress exposure models led to variability in HR and DBP responses, while the response of VO2, VCO2, VE/VO2, VE/VCO2 and SBP were similar. The increase in HR was higher in the W group compared to controls, while the WP group did not differ from C and W. DBP increased more in the C group compared to the WP group during anticipation of CPET, while the difference in DBP change was not significant between W and the other two groups. Circulating ACTH and cortisol significantly increased during anticipation of stress, while plasma levels of NT-pro-BNP significantly decreased. The response of ACTH, alpha-MSH, cortisol and NT-pro-BNP was similar during anticipation of stress in all groups, while the ACTH/cortisol ratio was significantly different between the C and W groups. In all three groups combined, the change of NT-pro-BNP during anticipation of CPET was the best independent predictor of VO2 peak.
It is worth noting that the anticipation of disruption of homeostasis or a threat to well-being, even before confronting with the stressor, may trigger various physiological responses (Engert et al., Citation2013; Pulopulos et al., Citation2020; Zandara et al., Citation2018). These are adaptive responses that allow individuals to make cognitive, behavioral, and physiological prearrangements that are necessary to encounter the stressful situation (Turan, Citation2015; Turan et al., Citation2015). Understanding the physiologic responses during the anticipation of stress are important, as they significantly impact the physiological response to the actual stress event (Engert et al., Citation2013). For instance, it was shown that positive expectancy or anticipatory stress appraisal attenuate the response of cortisol during the stressful situation (Gaab et al., Citation2005; Pulopulos et al., Citation2018). The anticipation of a stressful event has been shown to be associated with prolonged prefrontal activation, which decreases amygdala activation and improves the stress response regulating the activation of the HPA axis (De Raedt and Hooley, Citation2016). Limited evidence has also shown stress anticipation increases cortisol during this phase (Gossett et al., Citation2018), similar to the findings in the current study. The physiological effects of cortisol are well known and involve regulation of cardiopulmonary parameters (Popovic et al., Citation2013; Whitworth et al., Citation2005). Knowing the effects of cortisol, the increase in HR, blood pressure, VO2 and VCO2 during the anticipation of stress observed in the present study is not surprising. Moreover, our primary hypothesis was to determine whether individuals with different exercise training histories (i.e. two different types of athlete groups and a control group) respond differently to an anticipatory stressful stimulus. This was indeed the case for HR and DBP responses. Interestingly, no differences were observed in the hormonal responses, except for sensitivity of the HPA axis, measured by the ACTH/cortisol ratio. The explanation for diverse hormonal responses in different chronic stress adaptive models may be partially explained by autonomic regulation (Thayer et al., Citation2009). For instance, a higher vagal activity which is related to better stress adaptability and emotion regulation; previously, athletes demonstrated inhibitory control of the prefrontal cortex on the amygdala which impacts the stress response (Thayer et al., Citation2009). Bidirectional connections between the vagal nuclei in the medulla oblongata and the hypothalamus have been reported, suggesting an association of autonomic regulation and the HPA stress response (La Marca et al., Citation2011). These are important findings given that understanding the factors that contribute to stress reaction is fundamental to the prevention and treatment of stress-related disorders (Pulopulos et al., Citation2018).
While the increase in NT-pro-BNP during stress is well documented (Ohba et al., Citation2001; Popovic et al., Citation2013), the behavior and the role of NT-pro-BNP during anticipation of stress is largely unexplored. This hormone is secreted in cardiac chambers due to increased wall stress, however there are studies demonstrating an interface with HPA axis (Popovic et al., Citation2013). Complex hormonal interrelations during anticipation of stress apparently lead to a decrease in plasma NT-pro-BNP observed in the present study, which should have a role in the regulation of cardiopulmonary function. Previous studies found a relation between an NT-pro-BNP increase with exercise and higher cardiorespiratory fitness (Mottram et al., Citation2004). The increase in plasma levels of this hormone in patients after cardiac surgery was found to be associated with reduced cardiorespiratory fitness (Salustri et al., Citation2011). Our study demonstrated that the change in NT-pro-BNP during anticipation of CPET was the best independent predictor of VO2 peak, suggesting that hormonal changes during stress anticipation prepare the body for coping with the stress stimulus and that cardiac hormonal response may be a key element in overall performance during stress.
Further, our results demonstrated differences mainly between the control group and one of the athlete groups, leaving unclear whether exercise in general has a favorable impact to stress anticipation through cross-stressor adaptation. It seems that in this regard different athletic population adapt differently to exercise stimulus. There are reports demonstrating the impact of training level to HR response to anxiety, however, the differences between different athletic population are highly unexplored (Ensari et al., Citation2020). There is reasonable point of view that physical activity should be the natural means to prevent the consequences of stress, assuming that the stress response is a neuroendocrine mechanism triggered in anticipation of physical action. Indeed, positive effects of exercise to stress reduction are already proven (Arvidson et al., Citation2020). Inverse relationship between stress and exercise may vary from person to person, warranted precision medicine research for further concluding (Burg et al., Citation2017).
Limitations
There is high physiological variability of stress system activation, depending on age, genetic factors, gender, race, nutritional status, psychological factors, and physical activity habits (Bergh et al., Citation2015; Bernard et al., Citation2017; Bossé et al., Citation2015; Garrido, Citation2011; Lightman, Citation2008; Popovic et al., Citation2013, Citation2014; Wittert et al., Citation1996). Physical activity is a common condition for athletes allowing better understanding of what the exercise will involve and how they will respond to it. Confounding effects of possible psychological differences between athletes and controls cannot be ruled out. However, different types of chronic stress adaptation models, in this case different athletic groups, acted differently to the anticipation of an acute physical stress and we do not generalize our findings. Further studies involving other laboratory stress as an intervention are warranted. Moreover, in the present study, resting parameters were defined as measurements taken 10 min before testing. Of note, stress cannot be fully accounted for in that time point, since any information of being in the study may produce stress. However, even these time points distinguished the groups well enough in terms of their stress responsiveness. The results of present study have to be confirmed and explored in greater detail in larger clinical trials. Specifically, future research is warranted to better define the potential of stress hormone analysis during anticipation of stress to guide medical care and treatment options.
Conclusion
The type of chronic stress exposure influences hemodynamic responses during anticipation of physical stress and the path of hormonal stress axis activation. While stress hormones, such as ACTH and cortisol, increase during anticipation of stress, NT-pro-BNP decreases, and the amount of this change holds predictive value for overall cardiopulmonary performance during a stress condition, demonstrating the importance of the anticipatory phase in coping with stress.
| Abbreviations | ||
| ACTH | = | adrenocorticitropic hormone |
| BSA | = | body surface area |
| BW | = | body weight |
| C | = | controls |
| CPET | = | cardiopulmonary exercise test |
| DBP | = | diastolic arterial blood pressure |
| FFM | = | fat-free mass |
| FM | = | fat mass |
| HR | = | heart rate |
| MSH | = | melanocyte-stimulating hormone |
| NT-pro-BNP | = | N terminal-pro-B type natriuretic peptide |
| SBP | = | systolic arterial blood pressure |
| VCO2 | = | carbon dioxide production |
| VE | = | minute ventilation |
| VO2 | = | oxygen consumption |
| W | = | wrestlers |
| WP | = | water polo players |
Disclosure statetement
The authors have nothing to disclose.
References
- American Thoracic Society, American College of Chest Physicians (2003). ATS/ACCP statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine, 167(2), 211. https://doi.org/10.1164/rccm.167.2.211
- Arvidson, E., Dahlman, A. S., Börjesson, M., Gullstrand, L., & Jonsdottir, I. H. (2020). The effects of exercise training on hypothalamic-pituitary-adrenal axis reactivity and autonomic response to acute stress-a randomized controlled study. Trials, 21(1), 888. https://doi.org/10.1186/s13063-020-04803-3
- Aschbacher, K., O’Donovan, A., Wolkowitz, O. M., Dhabhar, F. S., Su, Y., & Epel, E. (2013). Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38(9), 1698–1708. https://doi.org/10.1016/j.psyneuen.2013.02.004
- Bergh, C., Udumyan, R., Fall, K., Almroth, H., & Montgomery, S. (2015). Stress resilience and physical fitness in adolescence and risk of coronary heart disease in middle age. Heart, 101(8), 623–629. https://doi.org/10.1136/heartjnl-2014-306703
- Bernard, K., Frost, A., Bennett, C. B., & Lindhiem, O. (2017). Maltreatment and diurnal cortisol regulation: A meta-analysis. Psychoneuroendocrinology, 78, 57–67. https://doi.org/10.1016/j.psyneuen.2017.01.005
- Bossé, S., Masciotra, V., Solomon, C., Stalder, T., & D’Antono, B. (2015). Childhood trauma, perceived stress, and hair cortisol in adults with and without coronary artery disease. Psychoneuroendocrinology, 61, 36. https://doi.org/10.1016/j.psyneuen.2015.07.488
- Burg, M. M., Schwartz, J. E., Kronish, I. M., Diaz, K. M., Alcantara, C., Duer-Hefele, J., & Davidson, K. W. (2017). Does stress result in you exercising less? Or does exercising result in you being less stressed? Or is it both? Testing the bi-directional stress-exercise association at the group and person (N of 1) level. Annals of Behavioral Medicine, 51(6), 799–809. https://doi.org/10.1007/s12160-017-9902-4
- Cain, D. W., & Cidlowski, J. A. (2017). Immune regulation by glucocorticoids. Nature Reviews. Immunology, 17(4), 233–247. https://doi.org/10.1038/nri.2017.1
- De Raedt, R., & Hooley, J. M. (2016). The role of expectancy and proactive control in stress regulation: A neurocognitive framework for regulation expectation. Clinical Psychology Review, 45, 45–55. https://doi.org/10.1016/j.cpr.2016.03.005
- Du Bois, D., & Du Bois, E. F. (1916). Clinical calorimetry. A formula 17: to estimate the approximate surface area if height and weight be known. Arch Intern Med, 17, 863–871.
- Engert, V., Efanov, S. I., Duchesne, A., Vogel, S., Corbo, V., & Pruessner, J. C. (2013). Differentiating anticipatory from reactive cortisol responses to psychosocial stress. Psychoneuroendocrinology, 38(8), 1328–1337. https://doi.org/10.1016/j.psyneuen.2012.11.018
- Ensari, I., Schwartz, J. E., Edmondson, D., Duran, A. T., Shimbo, D., & Diaz, K. M. (2020). Testing the cross-stressor hypothesis under real-world conditions: exercise as a moderator of the association between momentary anxiety and cardiovascular responses. Journal of Behavioral Medicine, 43(6), 989–1001. https://doi.org/10.1007/s10865-020-00155-0
- Fletcher, G. F., Ades, P. A., Kligfield, P., Arena, R., Balady, G. J., Bittner, V. A., Coke, L. A., Fleg, J. L., Forman, D. E., Gerber, T. C., Gulati, M., Madan, K., Rhodes, J., Thompson, P. D., & Williams, M. A, American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention (2013). Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation, 128(8), 873–934. https://doi.org/10.1161/CIR.0b013e31829b5b44
- Gaab, J., Rohleder, N., Nater, U. M., & Ehlert, U. (2005). Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology, 30(6), 599–610. https://doi.org/10.1016/j.psyneuen.2005.02.001
- Garrido, P. (2011). Aging and stress: past hypotheses, present approaches and perspectives. Aging and Disease, 2(1), 80–99. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3295041/
- Gossett, E. W., Wheelock, M. D., Goodman, A. M., Orem, T. R., Harnett, N. G., Wood, K. H., Mrug, S., Granger, D. A., & Knight, D. C. (2018). Anticipatory stress associated with functional magnetic resonance imaging: Implications for psychosocial stress research. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 125, 35–41. https://doi.org/10.1016/j.ijpsycho.2018.02.005
- Grande, G., Romppel, M., & Barth, J. (2012). Association between type D personality and prognosis in patients with cardiovascular diseases: a systematic review and meta-analysis. Annals of Behavioral Medicine : a Publication of the Society of Behavioral Medicine, 43(3), 299–310. https://doi.org/10.1007/s12160-011-9339-0
- Grimm, G., Resl, M., Heinisch, B. B., Hülsmann, M., Luger, A., Clodi, M., & Vila, G. (2016). B-type natriuretic peptide increases cortisol and catecholamine concentrations in healthy subjects. Journal of Applied Physiology, 122(5), 1249–1254. jap-00360. https://doi.org/10.1152/japplphysiol.00360.2016
- Herbison, C. E., Henley, D., Marsh, J., Atkinson, H., Newnham, J. P., Matthews, S. G., & Pennell, C. E. (2016). Characterization and novel analysis of acute stress response patterns in a population-based cohort of young adults: influence of gender, smoking and BMI. Stress, 19(2), 139–150. https://doi.org/10.3109/10253890.2016.1146672
- Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. https://doi.org/10.1002/cphy.c150015
- Hyun, J., Sliwinski, M. J., & Smyth, J. M. (2019). Waking up on the wrong side of the bed: The effects of stress anticipation on working memory in daily life. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 74(1), 38–46. https://doi.org/10.1093/geronb/gby042
- La Marca, R., Waldvogel, P., Thörn, H., Tripod, M., Wirtz, P. H., Pruessner, J. C., & Ehlert, U. (2011). Association between cold face test-induced vagal inhibition and cortisol response to acute stress. Psychophysiology, 48(3), 420–429. https://doi.org/10.1111/j.1469-8986.2010.01078.x
- Lightman, S. L. (2008). The neuroendocrinology of stress: a never ending story. Journal of Neuroendocrinology, 20(6), 880–884. https://doi.org/10.1111/j.1365-2826.2008.01711.x
- Mottram, P. M., Haluska, B. A., & Marwick, T. H. (2004). Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: correlation with cardiac function, hemodynamics, and workload. American Heart Journal, 148(2), 365–370. https://doi.org/10.1016/j.ahj.2004.02.012
- Neupert, S. D., & Bellingtier, J. A. (2019). Daily stressor forecasts and anticipatory coping: Age differences in dynamic, domain-specific processes. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 74(1), 17–28. https://doi.org/10.1093/geronb/gby043
- Ohba, H., Takada, H., Musha, H., Nagashima, J., Mori, N., Awaya, T., Omiya, K., & Murayama, M. (2001). Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. American Heart Journal, 141(5), 751–758. https://doi.org/10.1067/mhj.2001.114371
- Parker, K. N., & Ragsdale, J. M. (2015). Effects of distress and eustress on changes in fatigue from waking to working. Applied Psychology. Health and Well-Being, 7(3), 293–315. https://doi.org/10.1111/aphw.12049
- Piepoli, M. F., Hoes, A. W., Agewall, S., Albus, C., Brotons, C., Catapano, A. L., Cooney, M.-T., Corrà, U., Cosyns, B., Deaton, C., Graham, I., Hall, M. S., Hobbs, F. D. R., Løchen, M.-L., Löllgen, H., Marques-Vidal, P., Perk, J., Prescott, E., Redon, J., Binno, S., ESC Scientific Document Group. (2016). 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR)). European Heart Journal, 37(29), 2315–2381. https://doi.org/10.1093/eurheartj/ehw106
- Pivonello, R., De Martino, M. C., Iacuaniello, D., Simeoli, C., Muscogiuri, G., Carlomagno, F., … Colao, A. (2016). Metabolic alterations and cardiovascular outcomes of cortisol excess. In Cortisol excess and insufficiency (Vol. 46, pp. 54–65). Karger Publishers. https://doi.org/10.1159/000443864
- Popovic, D., Plecas-Solarovic, B., Pesic, V., Petrovic, M., Vujisic-Tesic, B., Popovic, B., Ignjatovic, S., Ristic, A., & Damjanovic, S. S. (2014). How does stress possibly affect cardiac remodeling? Peptides, 57, 20–30. https://doi.org/10.1016/j.peptides.2014.04.006
- Popovic, D., Popovic, B., Plecas-Solarovic, B., Pešić, V., Markovic, V., Stojiljkovic, S., Vukcevic, V., Petrovic, I., Banovic, M., Petrovic, M., Vujisic-Tesic, B., Ostojic, M. C., Ristic, A., & Damjanovic, S. S. (2013). The interface of hypothalamic–pituitary–adrenocortical axis and circulating brain natriuretic peptide in prediction of cardiopulmonary performance during physical stress. Peptides, 47, 85–93. https://doi.org/10.1016/j.peptides.2013.07.009
- Pulopulos, M. M., Baeken, C., & De Raedt, R. (2020). Cortisol response to stress: The role of expectancy and anticipatory stress regulation. Hormones and Behavior, 117, 104587. https://doi.org/10.1016/j.yhbeh.2019.104587
- Pulopulos, M. M., Vanderhasselt, M. A., & De Raedt, R. (2018). Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology, 94, 63–71. https://doi.org/10.1016/j.psyneuen.2018.05.004
- Quent, J. A., McCullough, A. M., Sazma, M., Wolf, O. T., & Yonelinas, A. P. (2018). Reward anticipation modulates the effect of stress-related increases in cortisol on episodic memory. Neurobiology of Learning and Memory, 147, 65–73. https://doi.org/10.1016/j.nlm.2017.11.007
- Rutledge, T., Kenkre, T. S., Thompson, D. V., Bittner, V. A., Whittaker, K., Eastwood, J.-A., Eteiba, W., Cornell, C. E., Krantz, D. S., Pepine, C. J., Johnson, B. D., Handberg, E. M., & Bairey Merz, C. N. (2016). Psychosocial predictors of long-term mortality among women with suspected myocardial ischemia: the NHLBI-sponsored women's ischemia syndrome evaluation. Journal of Behavioral Medicine, 39(4), 687–693. https://doi.org/10.1007/s10865-016-9737-7
- Salustri, A., Cerquetani, E., Piccoli, M., Pastena, G., Amici, E., La Carrubba, S., Bakir, S., & Al Mahmeed, W. A. (2011). B-type natriuretic peptide levels predict functional capacity in postcardiac surgery patients. Journal of Cardiovascular Medicine, 12(3), 167–172. https://doi.org/10.2459/JCM.0b013e328341d061
- Schnohr, P., Marott, J. L., Kristensen, T. S., Gyntelberg, F., Gronbaek, M., Lange, P., Jensen, M. T., Jensen, G. B., & Prescott, E. (2015). Ranking of psychosocial and traditional risk factors by importance for coronary heart disease: the Copenhagen City Heart Study. European Heart Journal, 36(22), 1385–1393. https://doi.org/10.1093/eurheartj/ehv027
- Stimson, R. H., Anderson, A. J., Ramage, L. E., Macfarlane, D. P., de Beaux, A. C., Mole, D. J., Andrew, R., & Walker, B. R. (2017). Acute physiological effects of glucocorticoids on fuel metabolism in humans are permissive but not direct. Diabetes, Obesity and Metabolism, 19(6), 883–891. https://doi.org/10.1111/dom.12899
- Svensson, M., Rosvall, P., Boza-Serrano, A., Andersson, E., Lexell, J., & Deierborg, T. (2016). Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiology of Stress, 5, 8–18. https://doi.org/10.1016/j.ynstr.2016.09.002
- Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of behavioral medicine: a publication of the. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 37(2), 141–153. https://doi.org/10.1007/s12160-009-9101-z
- Turan, B. (2015). Predictors of anticipatory cortisol reactivity to subsequent stressors. Physiology and Behavior, 149, 239–246. https://doi.org/10.1016/j.physbeh.2015.06.011
- Turan, B., Foltz, C., Cavanagh, J. F., Wallace, B. A., Cullen, M., Rosenberg, E. L., Jennings, P. A., Ekman, P., & Kemeny, M. E. (2015). Anticipatory sensitization to repeated stressors: The role of initial cortisol reactivity and meditation/emotion skills training. Psychoneuroendocrinology, 52, 229–238. https://doi.org/10.1016/j.psyneuen.2014.11.014
- van Ockenburg, S. L., Rosmalen, J. G., Bakker, S. J., de Jonge, P., & Gans, R. O. (2016). Effects of urinary cortisol levels and resting heart rate on the risk for fatal and nonfatal cardiovascular events. Atherosclerosis, 248, 44–50. https://doi.org/10.1016/j.atherosclerosis.2016.02.030
- Whitworth, J. A., Williamson, P. M., Mangos, G., & Kelly, J. J. (2005). Cardiovascular consequences of cortisol excess. Vascular Health and Risk Management, 1(4), 291–299. https://doi.org/10.2147/vhrm.2005.1.4.291
- Wittert, G. A., Livesey, J. H., Espiner, E. A., & Donald, R. A. (1996). Adaptation of the hypothalamopituitary adrenal axis to chronic exercise stress in humans. Medicine and Science in Sports and Exercise, 28(8), 1015–1019. https://doi.org/10.1097/00005768-199608000-00011
- Zandara, M., Garcia-Lluch, M., Villada, C., Hidalgo, V., & Salvador, A. (2018). Searching for a job: Cardiac responses to acute stress and the mediating role of threat appraisal in young people. Stress and Health : journal of the International Society for the Investigation of Stress, 34(1), 15–23. https://doi.org/10.1002/smi.2757
