Abstract
The cortisol awakening response (CAR) refers to a sharp rise in cortisol concentrations within the 45 min following morning awakening. Alterations in CAR have been associated with various internalizing symptoms and brain function. The current study aimed to investigate the association between CAR and neural activity in response to unpleasant emotional pictures. A total of 46 healthy adults (22.55 years ± 1.69) collected saliva samples at 0, 30, and 45 min post-awakening on two days to assess the CAR. In the afternoon after CAR measurement on the first day, electroencephalograms were recorded when the participants completed a passive viewing task. The results showed that a greater CAR was associated with a decreased late positive potential difference score between unpleasant and neutral stimuli. This finding indicates that a larger CAR may be associated with decreased attentional engagement to unpleasant emotional information in healthy adults.
1. Introduction
The CAR, which is defined as the spike in circulating cortisol within the 45 min following awakening, involves the natural response of the hypothalamic-pituitary-adrenal (HPA) axis (Pruessner et al., Citation1997). The CAR is a preparatory response to deal with impending day events before separate physiological HPA regulatory mechanisms take over (Fries et al., Citation2009). It is considered a reliable measure of cumulative or “allostatic” load on the body (Kudielka & Kirschbaum, Citation2005). Both higher and lower CARs have been linked to psychopathology and a moderate CAR is considered optimal (Chida & Steptoe, Citation2009; Vreeburg et al., Citation2009; Ulrike et al., Citation2013; Marin et al., Citation2019). A flat or blunted CAR might be a biological marker of chronic stress (Klein et al., Citation2016; Angelhoff et al., Citation2019).
The hippocampus, prefrontal cortex, and amygdala are involved in the regulation of the HPA axis, including CAR (Fries et al., Citation2009). Several studies have reported associations between the CAR and cognitive abilities such as memory and executive function that are known to be enabled by these regions (Aas et al., Citation2011; Almela et al., Citation2012; Evans et al., Citation2012; Law et al., Citation2020). Although the results of such studies have been inconsistent, most studies with accurate monitoring of sampling in the CAR period suggested that a greater CAR was associated with better cognitive functioning. For instance, a greater CAR has been observed with better working memory performance in older men (Almela et al., Citation2012). Similarly, CAR magnitude has been shown to be positively associated with episodic memory and executive functioning (Evans et al., Citation2012; Ennis et al., Citation2016).
Alterations in the CAR were also shown to be associated with various internalizing symptoms (Stetler & Miller, Citation2005; Rhebergen et al., Citation2015; Kofman et al., Citation2019; Stroud et al., Citation2019; Adams et al., Citation2020), but findings have been inconsistent. A meta-analysis showed that CAR was attenuated in patients with psychosis compared to healthy controls (Berger et al., Citation2016). Furthermore, recent research has addressed the question of whether the magnitude of the CAR is associated with emotional processing. For example, Lenaert et al. (Citation2016) showed that lower levels of self-reported emotional attentional control were associated with a higher CAR following exposure to a prolonged psychosocial stressor. For individuals who have difficulty controlling their attention in the presence of emotion may be more prone to prolonged HPA-axis hyperactivity after psychosocial stress. Recently, poorer inhibition of personally-relevant sad and angry facial expressions was associated with an increased CAR six months later (Wong et al., Citation2020). With respect to emotion regulation strategy, an elevated CAR was found to be associated with higher scores on the suppression scale which involves inhibiting or reducing outward expression of emotional arousal (Otto et al., Citation2018). As an adaptive emotion regulation strategy, higher use of problem-solving has also been associated with an elevated CAR (Gilbert et al., Citation2017). However, the majority of such studies studied emotional processing on a self-reported scale. Such manipulations ignore the brain activity underlying emotional processes. In addition, emotional experiences may change after interaction with information in the environment. Therefore, more research is needed to understand the association between CAR and negative affect at the neurophysiological level.
The late positive potential (LPP), an event-related potential (ERP) component that is sensitive to emotional processing, is a positive deflection that begins approximately 300 ms following stimulus onset (Cuthbert et al., Citation2000; Babkirk et al., Citation2015; Van Cauwenberge et al., Citation2017). It has been established as an indicator of emotional reactivity that complements other measures of self-reported emotion (Allan et al., Citation2019; Hajcak & Foti, Citation2020). LPP amplitude is larger following the presentation of unpleasant emotional stimuli compared to neutral stimuli, suggesting increased attention to emotional versus neutral stimuli (Hajcak et al., Citation2009; MacNamara, Citation2018). The time course of the LPP can be used to track the dynamic allocation of attention to emotional stimuli (Lang & Bradley, Citation2010). Prior source analysis studies suggested that neural substrates of the LPP involved cortical and subcortical brain areas, including the amygdala and prefrontal cortex (Liu et al., Citation2012; Hajcak & Foti, Citation2020), which are involved in the regulation of the CAR (Herman et al., Citation2005; Fries et al., Citation2009).
Regarding the association between the HPA axis and LPP, Alomari et al. (Citation2015) revealed an increase in the LPP to non-emotional pictures following acute stress, which suggested that stress exposure can impair the ability of the LPP to discriminate between emotional and non-emotional pictures. Weymar et al. (Citation2012) found that exposure to acute stress increased the LPP amplitude for unpleasant pictures, suggesting that acute stress primes the processing of unpleasant cues. Increased LPP amplitude toward unpleasant relative to neutral pictures was also associated with more severe post-traumatic stress symptoms after exposure to traumatic events (Lobo et al., Citation2014). Similarly, Klein et al. (Citation2019) found that youth with post‐traumatic stress disorder showed greater LPP amplitudes for socially threatening stimuli compared to controls. Recently, high levels of hair cortisol, indicating stronger chronic stress exposure, have been associated with larger LPP amplitudes and impaired recognition memory (Wirkner et al., Citation2019). However, little is known about the association between CAR, a reliable trait biomarker for HPA axis status, and LPP in healthy adults.
In the current study, we aimed to investigate the relationship between individual differences in CAR and neural activity during unpleasant emotional picture processing using ERPs. Previous studies have suggested that elevated cortisol might be mood protective, helping individuals cope with the emotional load of situations by reducing negative emotional responses (Hoyt et al., Citation2016). The CAR has been proposed to help individuals cope with the challenges of the upcoming day (Fries et al., Citation2009). Because LPP is considered to reflect motivated selective attention to unpleasant stimuli (Hajcak et al., Citation2009), it was hypothesized that a higher CAR would be associated with attenuated affective stimulus processing, as indicated by the LPP.
2. Methods
2.1. Participants
Fifty healthy participants (18 females) between the ages of 18 and 25 were recruited as part of a wide-ranging study that addressed the relationship between the HPA axis and brain function (Shi et al., Citation2018). Of these, one participant withdrew from the experiment in the passive viewing paradigm due to the nature of the images, and three participants were excluded due to insufficient saliva quantity. Thus, data for 46 adults (16 females, M = 22.55 years, SD = 1.69) were available for the assessment. All participants were recruited on the basis that they were healthy and were free of medication and had no history of chronic illness (e.g. diabetes, asthma, cardiovascular disease). According to a study by Wolfram et al. (Citation2011), the CAR was elevated during ovulation while there were no differences in the CAR between the follicular and luteal phases. Thus, female participants were asked to report the last two dates of their menses, and then the appointment for the experiment was arranged to avoid the ovulation phase of the menstrual cycle. None of the female participants took the contraceptive pill during the time of the study. In addition, female participants were excluded in cases of pregnancy. All volunteers signed written informed consent and received monetary compensation for participation. Ethical approval was approved by the Ethics Committee of Human Experimentation of the Institute of Psychology, Chinese Academy of Sciences.
2.2. General procedures
After participants were recruited, the dates were set for saliva collection and ERP testing. During their first visit, detailed instructions, including techniques for collecting saliva samples, were explained to the participants face-to-face. Then, they were provided with a pack containing a written version of instructions for saliva collection, MotionWatch 8 (Camntech, UK), the Salivette collection device (Sarstedt, Germany), and MEMS TrackCap containers (MEMS 6 TrackCap Monitor, Aardex Ltd. Switzerland). The CAR was assessed repeatedly over two days within one week. On each of the 2 study days of saliva collection, participants were instructed to collect three saliva samples upon awakening, and at 30, 45 min following awakening. In the afternoon after CAR measurement on the first day, the participants arrived at the lab between 1:00 p.m. and 2:00 p.m. to minimize circadian variations in hormone levels (Schreiber et al., Citation2006). After a short introduction, the participants prepared for electroencephalogram (EEG) recording. The EEG was collected while the participants performed tasks including the passive viewing task. Another saliva sample was collected before the ERP task began to measure the baseline cortisol level before the task.
2.3. Passive viewing task
The passive viewing task was run on a computer with a 17-inch monitor. The entire task consisted of 3 blocks including 60 pictures (30 unpleasant and 30 neutral each). The pictures used in our study were selected from the International Affective Picture System (Lang et al., Citation1997). The unpleasant picture set included images of bodily mutilation, threat and attack scenes, whereas the neutral picture set incorporated images of landscapes and neutral facesFootnote1. Unpleasant and neutral pictures differed in terms of valence (means of 2.48 ± 0.56 for unpleasant and 5.03 ± 0.33 for neutral; t(58) = −21.112, p < 0.001) and arousal (means of 5.66 ± 0.53 for unpleasant and 2.92 ± 0.49 for neutral; t(58) = 20.479, p < 0.001). Each trial began with a 1000 ms presentation with a random interstimulus interval of 1200–1800 ms. Pictures were presented with a viewing distance of approximately 70 cm. Participants were instructed to passively view each presented picture and did not need to make any response.
2.4. Salivary cortisol and analysis
The CAR was sampled on two days, and participants were asked to collect at awakening, +30, and +45 min following awakening on each day. Participants were warned to avoid eating, drinking stimulants (such as tea, coffee, or alcohol) and brushing teeth during the sampling procedure (Stalder et al., Citation2016). The Salivette sampling tubes used for passive drool were stored in a medication event monitoring bottle (MEMS 6 TrackCap Monitor, Aardex Ltd. Switzerland), and self-report information was recorded at the time each sample was taken. In addition, in a subsample of 29 participants, we assessed compliance with the timing of saliva collection with wrist-worn activity-recording devices (Cambridge Neurotechnology, Cambridge, UK) to monitor awakening times. All delay times between electronically monitored waking times and self-reported collection times were less than 5 min. The participants were also asked to complete a morning diary including a 10 cm visual analogue scale where 0 cm was “very bad” and 10 cm was “very good” to report their perceived sleep quality during the previous night.
Returned saliva samples were stored at −20 °C until analysis. Saliva samples were thawed and centrifuged for 5 min at 3000 g. Cortisol concentrations were analyzed using an electrochemiluminescence immunoassay method (ECLIA, Cobas e601, Roche Diagnostics, Mannheim, Germany) in line with the manufacturer’s instructions (Salimetrics, Newmarket, UK). Inter- and intra-assay coefficients of variation were both below 8%.
2.5. ERP recording and signal preprocessing
On the day of ERP testing, participants returned to take part in the electroencephalographic (EEG) portion of the study. Wearing an elastic EEG cap (Neuroscan Inc., Charlotte, NC), each participant performed the passive viewing task. With a reference electrode attached to the left mastoid, the EEG activity was recorded from 64 Ag/AgCl electrodes. The EEG amplifier was set with a digitization rate of 1000 Hz and the EEG signals were filtered with a 0.05–100 Hz band-pass filter. Eye movements and blinks were measured by electrodes placed 1 cm from the outer canthus of each eye (horizontal eye movements) and 1 cm above and below the left eye (vertical eye movements). All electrode impedances were maintained at less than 5 kΩ.
During offline processing, all EEG data were re-referenced to the average of the left and right mastoids. Collected EEGs were digitally filtered with a 30 Hz low-pass filter and no high-pass filter was applied. The effect of blinks was reduced using Neuroscan software’s regression-based algorithm. Stimulus-locked data were then segmented into epochs ranging from 200 ms pre-stimulus to 1000 ms post-stimulus, with a 200 ms baseline correction. Prior to averaging, trials with voltages above 100 μV or below −100 μV were rejected.
2.6. Data analysis
ERPs were constructed by separately averaging unpleasant and neutral picture trials. The LPP was scored as the average amplitude of the ERP within the time window of 300–1000 ms at Pz. The electrode sites and the temporal window were selected based on visual inspection of topographical maximum sites of LPP and previous research (Cuthbert et al., Citation2000; Keil et al., Citation2002). Difference waves were also computed to examine discrepancies between unpleasant and neutral conditions (ΔLPP: unpleasant minus neutral).
Salivary cortisol values were log-transformed to normalize skewed distributions prior to analysis. Cortisol concentrations for each assessment time were averaged over the 2 days, as correlations across days were significant (r = 0.437, 0.615, 0.584, respectively, all p < 0.01). CAR was calculated by subtracting the cortisol sample value at awakening from the peak cortisol concentration after awaking (Steptoe & Ussher, Citation2006).
Statistical analyses were performed using SPSS version 20.0. Correlation analyses were used to investigate the relation between CAR and LPP. Because of prior research on the importance of pre-task cortisol, sex (Pruessner et al., Citation1997; Wust et al., Citation2000; Hoyt et al., Citation2015) and sleep quality for CAR (Lasikiewicz et al., Citation2008; Fekedulegn et al., Citation2018; Tsai et al., Citation2019), hierarchical regression analyses were then used to investigate the relation between CAR and LPP, while sex, pre-task cortisol and sleep quality were entered as covariates.
3. Results
3.1. General descriptives
presents the descriptive statistics on day 1 and day 2 for both the cortisol sample and sleep quality. As expected, on average, the peak in cortisol was found at 30 min post-awakening, followed by a decline until 45 min post-awakening. The average CAR across two days (baseline to peak increase) was 5.16 nmol/l (log-transformed: 0.11) 45 min after awakening. Of the 46 total samples, 63.04% of the participants showed a positive CAR (n = 29) on both days.
Table 1. Descriptive statistics for all relevant variables.
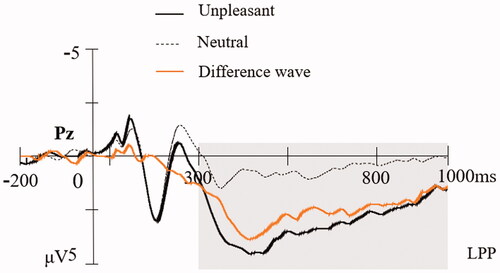
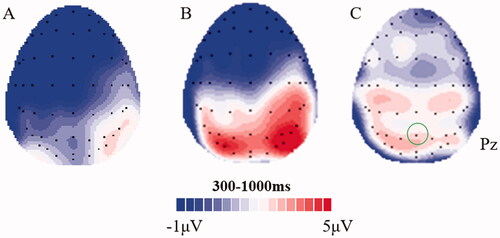
As expected, the LPP was modulated by emotion (t(45) = 10.96, p < 0.001), with significantly larger LPP amplitudes for unpleasant pictures (M = 3.09, SE = 3.51) than for neutral pictures (M = 0.57, SE = 2.77). The mean amplitude (±SD) of the LPP difference wave (unpleasant minus neutral) was 2.52 (±1.56) μV. The grand average ERP waveforms locked to unpleasant, neutral pictures, as well as the difference wave at electrode Pz are shown in . The scalp distributions of neutral, unpleasant, and different LPP waveforms are shown in .
3.2. Sex differences in CAR and LPP
Sex differences were tested using Student's t-tests for independent samples. No significant difference was observed in the cortisol baseline levels at the time of awakening on each day. Regarding the CAR, there was a marginally significant difference between sexes, as females showed higher CAR (t(44) = 1.986, p = 0.053). As for the LPP, males had significantly larger amplitudes to unpleasant pictures (t(44) = 2.202, p = 0.033) as well as neutral pictures (t(44) = 2.911, p = 0.006) than females. However, LPP difference amplitudes (unpleasant minus neutral) appear not to differ between males and females (t(44) = 0.022, p = 0.982).
3.3. Association between CAR and LPP
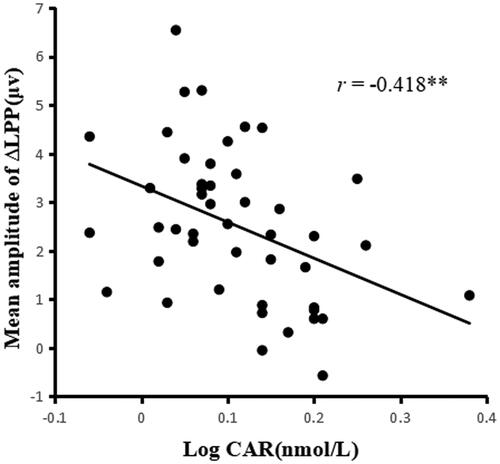
Bivariate Pearson correlation analysis indicated that the CAR was inversely associated with LPP response to unpleasant pictures (r = −0.390, p = 0.007). No significant association was observed between CAR and LPP amplitude for neutral pictures (r = −0.260, p = 0.081). More importantly, the CAR was inversely associated with the mean amplitude of ΔLPP (r = −0.418, p = 0.004), which indicated that a greater CAR was associated with a reduced LPP difference score. In , a scatterplot is given to show that the CAR is negatively related to the ΔLPP mean amplitude.
Figure 3. Scatter plot of the bivariate correlation between CAR and the ΔLPP mean amplitude (n = 46).
In , the results of the hierarchical regression analysis are presented. In the first step of the model, we included control variables (sex, sleep quality, and pretest cortisol level). In the second step, we included the CAR to investigate the association between the CAR and the ΔLPP amplitude. The results showed that there was a significant negative linear association between the CAR and the ΔLPP mean amplitude (β = −0.464, p < 0.01).
Table 2. Regression analysis (with covariates) with CAR as the predictor and ΔLPP mean amplitude as the dependent variable.
4. Discussion
Previous research has focused on HPA dysregulation as an endocrine marker of psychological distress. However, no known studies have attempted to link variations in CAR, the most straightforward measure of dynamics of the HPA axis, with the neural activity during negative emotional information processing in healthy adults. Considering this gap, the current study sought to examine the association between CAR and the neural activity in response to emotional information reflected by the amplitude of LPP in healthy adults. As we expected, the results showed that unpleasant pictures elicited significantly greater amplitudes of LPP than neutral pictures. More importantly, the individuals who had larger increases in cortisol concentrations in the morning displayed diminished differences between LPP amplitudes of unpleasant and neutral stimuli. In addition, our results were significant after controlling for a number of potentially confounding variables, including sex, pre-task cortisol, and sleep quality.
In the present study, CAR explained 19.6% of the variance in LPP difference scores. Research on the LPP shows that LPP differences between emotional and neutral pictures are thought to reflect facilitated attention to emotional stimuli and encoding of emotional information (Dolcos & Cabeza, Citation2002; Brown et al., Citation2012). The results in the current study seem to suggest that participants who have larger CARs showed decreased attentional engagement to negative emotional information. It has been proposed that the circadian rhythm of glucocorticoid secretion may promote internal homeostasis and optimal brain function, and moderate CAR is considered optimal. The CAR was a marker of neuroendocrine activation as the individual contemplated the challenges of the day ahead (Adam et al., Citation2006; Fries et al., Citation2009; Nader et al., Citation2010). The lower CAR may indicate a lack of a “boost,” or energetic resources. The findings of the current study indicate that CAR as a trait might be a protective factor in that it helps individuals cope with the emotional load of situations by shifting their attention away from unpleasant emotional pictures.
The results in the current study were similar to prior work demonstrating that a higher CAR was associated with higher average use of problem-solving as an adaptive emotion regulation strategy (Gilbert et al., Citation2017) and less aggressive behavior (Böhnke et al., Citation2010). Support for the association between CAR and brain activity was also found in previous studies. Consistent with our study, Duan et al. (Citation2019) showed that a higher CAR was associated with more left-sided cortical activity at frontocentral sites under the resting state which is involved in more positive emotion processing as well as more active coping. By contrast, Quevedo et al. (Citation2017) found that higher CAR was associated with elevated levels of dorsal anterior cingulate cortex (dACC) activity during processing of negative self-descriptors among maltreated depressed youth, which suggested that a heightened arousal system (i.e. higher dACC activity) might increase preparedness for negative events as indicated by elevated levels of CAR. These inconsistent findings could be due to differences in the sample composition and the participants in these studies, focusing on clinical samples or participants facing chronic stressors. Our current study extends this work and suggests that the CAR is negatively associated with neural activity related to negative emotion in healthy adults.
The mechanisms underlying the association between CAR and LPP amplitude are not yet clear. Previous work has demonstrated that corticosteroids can reduce the anxiety-driven selective attention to threat (Putman et al., Citation2007) and protect mood by reducing negative emotional responses (Hoyt et al., Citation2016). Prior fMRI studies also indicated that corticosteroids facilitated prefrontal cortex (PFC) function and acted on the neural correlates of attentional processing (Henckens et al., Citation2010). They decreased activity in the cuneus (i.e. a smaller lobe in the occipital lobe involved in attentional processing), probably suggesting reduced bottom-up attentional processing (Henckens et al., Citation2012). In our study, the most prominent and dynamic element of the circadian pattern of cortisol secretion (i.e. CAR) was associated with neural activity during negative emotional information processing. It has been suggested that the CAR mobilizes energy for the day (Pruessner et al., Citation1997). Thus, we speculate that CAR, an indicator of physiological arousal, may modulate the neural correlates of sustained attention to emotional input and position individuals in one way or another to pay attention to contextual stimuli at the moment.
In addition, it is possible that the same neural functions that mediate the CAR may also mediate LPP. Wirkner et al.’s results (2019) suggested that prefrontal and amygdala function might be crucial for engaging in both emotion processing and long-term systemic cortisol levels. On the one hand, it is proposed that the amygdala and PFC may play a critical role in the regulation of the CAR (Herman et al., Citation2005; Fries et al., Citation2009). On the other hand, prior functional neuroimaging studies found that the LPP amplitude was coupled with BOLD in the medial prefrontal cortex and amygdala (Keil et al., Citation2002; Liu et al., Citation2012; Sabatinelli et al., Citation2013), which suggests that LPP is generated and modulated by the structures associated with CAR. Perhaps, increased functions in the PFC and amygdala might have contributed to both higher CAR and reduced differences in LPP amplitude.
There were several limitations that should be mentioned. First, we did not measure ERPs evoked by pleasant pictures in our study. Positive and negative affect have been found to relate differently to the levels of salivary cortisol concentrations (Smyth et al., Citation1998; Hoyt et al., Citation2015; Miller et al., Citation2016). Future work should aim to extend the findings to a wider range of emotions, such as positive affect. Second, the association between CAR and emotion processing might vary among different individuals. For example, the personality trait of extraversion has been associated with CAR, with higher extraversion scores associated with greater CAR (Hauner et al., Citation2008; Hill et al., Citation2013). Additionally, components of dispositional mindfulness have been shown to moderate the relation between CAR and negative emotions. There is a significant positive correlation between CAR and negative emotions at lower levels of mindfulness but not at higher levels (Daubenmier et al., Citation2014). Therefore, it is necessary to further explore the moderating effect of various dispositional factors on the relation between CAR and negative emotions in future studies. Thirdly, our finding was based on a small sample and included only 16 females, the results need to be interpreted with caution. Finally, CAR values were only on two days and one of these days occurred after the experimental session in the current study. Since state differences account for variance in CAR magnitude (Hellhammer et al., Citation2007; Law et al., Citation2013) and responses to unpleasant stimuli on day 1 might affect CAR values on day 2, future studies could assess CAR at more than two days before the experimental session to achieve a reliable trait measure of the CAR.
In conclusion, we examined the association between the CAR and emotion processing using the high temporal resolution ERP technique in the current study. The results showed that higher CAR was associated with reduced LPP amplitudes evoked by unpleasant pictures compared to neutral pictures, suggesting a decrease in sustained attention toward unpleasant pictures. Such an association may provide a better understanding of the function of the CAR as a promising biomarker in psychophysiological research and pave the way for targeted prevention.
Author Contributors
Author S.X. collected and analyzed the data and wrote the manuscript. Author N.X. participated in the interpretation of the data. Author W.J.H. designed the study and revised the manuscript. All authors approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 List of stimuli: Unpleasant (1111, 1275, 2751, 3015, 3051, 3062, 3064, 3102, 3130, 3160, 3550, 6244, 6530, 6834, 9007, 9120, 9180, 9253, 9400, 9405, 9415, 9430, 9432, 9433, 9500, 9520, 9530, 9592, 9611, 9920) and neutral (2214, 2215, 2372, 2381, 2383, 2440, 2480, 2495, 2514, 2516, 2580, 2749, 2850, 2870, 2880, 5520, 5530, 5740, 6150, 7004, 7006, 7031, 7034, 7060, 7090, 7185, 7187, 7205, 7234, 7950).
References
- Aas, M., Dazzan, P., Mondelli, V., Toulopoulou, T., Reichenberg, A., Forti, M. D., Fisher, H. L., Handley, R., Hepgul, N., Marques, T., Miorelli, A., Taylor, H., Russo, M., Wiffen, B., Papadopoulos, A., Aitchison, K. J., Morgan, C., Murray, R. M., & Pariante, C. M. (2011). Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychological Medicine, 41(3), 463–476. https://doi.org/10.1017/S0033291710001170
- Adam, E. K., Hawkley, L. C., Kudielka, B. M., & Cacioppo, J. T. (2006). Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 17058–17063. https://doi.org/10.1073/pnas.0605053103
- Adams, G. C., Wrath, A. J., von Dewitz, B., Marciniuk, K., Roesler, A., & Napper, S. (2020). Attachment impacts cortisol awakening response in chronically depressed individuals. Psychoneuroendocrinology, 120, 104778. https://doi.org/10.1016/j.psyneuen.2020.104778
- Allan, N. P., Judah, M. R., Albanese, B. J., Macatee, R. J., Sutton, C. A., Bachman, M. D., Bernat, E. M., & Schmidt, N. B. (2019). Gender differences in the relation between the late positive potential in response to anxiety sensitivity images and self-reported anxiety sensitivity. Emotion, 19(1), 70–83. https://doi.org/10.1037/emo0000420
- Almela, M., van der Meij, L., Hidalgo, V., Villada, C., & Salvador, A. (2012). The cortisol awakening response and memory performance in older men and women. Psychoneuroendocrinology, 37(12), 1929–1940. https://doi.org/10.1016/j.psyneuen.2012.04.009
- Alomari, R. A., Fernandez, M., Banks, J. B., Acosta, J., & Tartar, J. L. (2015). Acute stress dysregulates the LPP ERP response to emotional pictures and impairs sustained attention: time-sensitive effects. Brain Sci, 5(2), 201–219. https://doi.org/10.3390/brainsci5020201
- Angelhoff, C., Edéll-Gustafsson, U., & Mörelius, E. (2019). The cortisol response in parents staying with a sick child at hospital. Nursing Open, 6(2), 620–625. https://doi.org/10.1002/nop2.245
- Babkirk, S., Rios, V., & Dennis, T. A. (2015). The late positive potential predicts emotion regulation strategy use in school-aged children concurrently and two years later. Developmental Science, 18(5), 832–841. https://doi.org/10.1111/desc.12258
- Berger, M., Kraeuter, A. K., Romanik, D., Malouf, P., Amminger, G. P., & Sarnyai, Z. (2016). Cortisol awakening response in patients with psychosis: Systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 68, 157–166. https://doi.org/10.1016/j.neubiorev.2016.05.027
- Böhnke, R., Bertsch, K., Kruk, M. R., & Naumann, E. (2010). The relationship between basal and acute HPA axis activity and aggressive behavior in adults. Journal of Neural Transmission, 117(5), 629–637. https://doi.org/10.1007/s00702-010-0391-x
- Brown, S. B., van Steenbergen, H., Band, G. P., de Rover, M., & Nieuwenhuis, S. (2012). Functional significance of the emotion-related late positive potential. Frontiers in Human Neuroscience, 6, 33. https://doi.org/10.3389/fnhum.2012.00033
- Chida, Y., & Steptoe, A. (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology, 80(3), 265–278. https://doi.org/10.1016/j.biopsycho.2008.10.004
- Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. https://doi.org/10.1016/S0301-0511(99)00044-7
- Daubenmier, J., Hayden, D., Chang, V., & Epel, E. (2014). It's not what you think, it's how you relate to it: Dispositional mindfulness moderates the relationship between psychological distress and the cortisol awakening response. Psychoneuroendocrinology, 48, 11–18. https://doi.org/10.1016/j.psyneuen.2014.05.012
- Dolcos, F., & Cabeza, R. (2002). Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, & Behavioral Neuroscience, 2(3), 252–263. https://doi.org/10.3758/CABN.2.3.252
- Duan, H., Fang, H., Zhang, Y., Shi, X., & Zhang, L. (2019). Associations between cortisol awakening response and resting electroencephalograph asymmetry. PeerJ, 7, e7059 https://doi.org/10.7717/peerj.7059
- Ennis, G. E., Moffat, S. D., & Hertzog, C. (2016). The cortisol awakening response and cognition across the adult lifespan. Brain and Cognition, 105, 66–77. https://doi.org/10.1016/j.bandc.2016.04.001
- Evans, P., Hucklebridge, F., Loveday, C., & Clow, A. (2012). The cortisol awakening response is related to executive function in older age. International Journal of Psychophysiology, 84(2), 201–204. https://doi.org/10.1016/j.ijpsycho.2012.02.008
- Fekedulegn, D., Innes, K., Andrew, M. E., Tinney-Zara, C., Charles, L. E., Allison, P., Violanti, J. M., & Knox, S. S. (2018). Sleep quality and the cortisol awakening response (CAR) among law enforcement officers: The moderating role of leisure time physical activity. Psychoneuroendocrinology, 95, 158–169. https://doi.org/10.1016/j.psyneuen.2018.05.034
- Fries, E., Dettenborn, L., & Kirschbaum, C. (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72(1), 67–73. https://doi.org/10.1016/j.ijpsycho.2008.03.014
- Gilbert, K., Mineka, S., Zinbarg, R. E., Craske, M. G., & Adam, E. K. (2017). Emotion regulation regulates more than emotion: Associations of momentary emotion regulation with diurnal cortisol in current and past depression and anxiety. Clinical Psychological Science, 5(1), 37–51. https://doi.org/10.1177/2167702616654437
- Hajcak, G., Dunning, J. P., & Foti, D. (2009). Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology, 120(3), 505–510. https://doi.org/10.1016/j.clinph.2008.11.028
- Hajcak, G., & Foti, D. (2020). Significance?…significance! empirical, methodological, and theoretical connections between the late positive potential and p300 as neural responses to stimulus significance: An integrative review. Psychophysiology, 57(7), e13570. https://doi.org/10.1111/psyp.13570
- Hauner, K. K. Y., Adam, E. K., Mineka, S., Doane, L. D., DeSantis, A. S., Zinbarg, R., Craske, M., & Griffith, J. W. (2008). Neuroticism and introversion are associated with salivary cortisol patterns in adolescents. Psychoneuroendocrinology, 33(10), 1344–1356. https://doi.org/10.1016/j.psyneuen.2008.07.011
- Hellhammer, J., Fries, E., Schweisthal, O. W., Schlotz, W., Stone, A. A., & Hagemann, D. (2007). Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology, 32(1), 80–86. https://doi.org/10.1016/j.psyneuen.2006.10.005
- Henckens, M., Van Wingen, G. A., Joels, M., & Fernandez, G. (2010). Time-dependent effects of corticosteroids on human amygdala processing. The Journal of Neuroscience, 30(38), 12725–12732. https://doi.org/10.1523/JNEUROSCI.3112-10.2010
- Henckens, M. J. A. G., van Wingen, G. A., Joels, M., & Fernandez, G. (2012). Time-dependent effects of cortisol on selective attention and emotional interference: A functional MRI study. Frontiers in Integrative Neuroscience, 6, 66. https://doi.org/10.3389/fnint.2012.00066
- Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 29(8), 1201–1213. https://doi.org/10.1016/j.pnpbp.2005.08.006
- Hill, E. M., Billington, R., & Krageloh, C. (2013). The cortisol awakening response and the big five personality dimensions. Personality and Individual Differences, 55(5), 600–605. https://doi.org/10.1016/j.paid.2013.05.010
- Hoyt, L. T., Craske, M. G., Mineka, S., & Adam, E. K. (2015). Positive and negative affect and arousal: Cross-sectional and longitudinal associations with adolescent cortisol diurnal rhythms. Psychosomatic Medicine, 77(4), 392–401. https://doi.org/10.1097/PSY.0000000000000178
- Hoyt, L. T., Zeiders, K. H., Ehrlich, K. B., & Adam, E. K. (2016). Positive upshots of cortisol in everyday life. Emotion, 16(4), 431–435. https://doi.org/10.1037/emo0000174
- Keil, A., Bradley, M. M., Hauk, O., Rockstroh, B., Elbert, T., & Lang, P. J. (2002). Large-scale neural correlates of affective picture processing. Psychophysiology, 39(5), 641–649. https://doi.org/10.1111/1469-8986.3950641
- Klein, F., Schindler, S., Neuner, F., Rosner, R., Renneberg, B., Steil, R., & Iffland, B. (2019). Processing of affective words in adolescent PTSD—attentional bias toward social threat. Psychophysiology, 56(11), e13444. https://doi.org/10.1111/psyp.13444
- Klein, L. C., Kim, K., Almeida, D. M., Femia, E. E., Rovine, M. J., & Zarit, S. H. (2016). Anticipating an easier day: Effects of adult day services on daily cortisol and stress. The Gerontologist, 56(2), 303–312. https://doi.org/10.1093/geront/gnu060
- Kofman, Y. B., Eng, Z. E., Busse, D., Godkin, S., Campos, B., Sandman, C. A., Wing, D., & Yim, I. S. (2019). Cortisol reactivity and depressive symptoms in pregnancy: The moderating role of perceived social support and neuroticism. Biological Psychology, 147, 107656. https://doi.org/10.1016/j.biopsycho.2019.01.016
- Kudielka, B. M., & Kirschbaum, C. (2005). Sex differences in HPA axis responses to stress: a review. Biological Psychology, 69(1), 113–132. https://doi.org/10.1016/j.biopsycho.2004.11.009
- Lang, P. J., & Bradley, M. M. (2010). Emotion and the motivational brain. Biological Psychology, 84(3), 437–450. https://doi.org/10.1016/j.biopsycho.2009.10.007
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International Affective Picture System (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 1, 39–58.
- Lasikiewicz, N., Hendrickx, H., Talbot, D., & Dye, L. (2008). Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology, 33(2), 143–151. https://doi.org/10.1016/j.psyneuen.2007.10.013
- Law, R., Evans, P., Thorn, L., Hucklebridge, F., Loveday, C., & Clow, A. (2020). The cortisol awakening response predicts a same-day index of executive function in healthy young adults. International Journal of Psychophysiology, 158, 27–33. https://doi.org/10.1016/j.ijpsycho.2020.08.004
- Law, R., Hucklebridge, F., Thorn, L., Evans, P., & Clow, A. (2013). State variation in the cortisol awakening response. Stress, 16(5), 483–492. https://doi.org/10.3109/10253890.2013.817552
- Lenaert, B., Barry, T. J., Schruers, K., Vervliet, B., & Hermans, D. (2016). Emotional attentional control predicts changes in diurnal cortisol secretion following exposure to a prolonged psychosocial stressor. Psychoneuroendocrinology, 63, 291–295. https://doi.org/10.1016/j.psyneuen.2015.10.013
- Liu, Y., Huang, H., McGinnis-Deweese, M., Keil, A., & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. https://doi.org/10.1523/JNEUROSCI.3109-12.2012
- Lobo, I., David, I. A., Figueira, I., Campagnoli, R. R., Volchan, E., Pereira, M. G., & de Oliveira, L. (2014). Brain reactivity to unpleasant stimuli is associated with severity of posttraumatic stress symptoms. Biological Psychology, 103, 233–241. https://doi.org/10.1016/j.biopsycho.2014.09.002
- MacNamara, A. (2018). In the mind's eye: The late positive potential to negative and neutral mental imagery and intolerance of uncertainty. Psychophysiology, 55(5), e13024 https://doi.org/10.1111/psyp.13024
- Marin, M. F., Geoffrion, S., Juster, R. P., Giguere, C. E., Marchand, A., Lupien, S. J., & Guay, S. (2019). High cortisol awakening response in the aftermath of workplace violence exposure moderates the association between acute stress disorder symptoms and PTSD symptoms. Psychoneuroendocrinology, 104, 238–242. https://doi.org/10.1016/j.psyneuen.2019.03.006
- Miller, K. G., Wright, A. G. C., Peterson, L. M., Kamarck, T. W., Anderson, B. A., & Kirschbaum, C., Marsland, A. L., Muldoon, M. F., & Manuck, S. B. (2016). Trait positive and negative emotionality differentially associate with diurnal cortisol activity. Psychoneuroendocrinology, 68, 177–185. https://doi.org/10.1016/j.psyneuen.2016.03.004
- Nader, N., Chrousos, G. P., & Kino, T. (2010). Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab, 21(5), 277–286. https://doi.org/10.1016/j.tem.2009.12.011
- Otto, L. R., Sin, N. L., Almeida, D. M., & Sloan, R. P. (2018). Trait emotion regulation strategies and diurnal cortisol profiles in healthy adults. Health Psychology, 37(3), 301–305. https://doi.org/10.1037/hea0000564
- Pruessner, J. C., Wolf, O. T., Hellhammer, D. H., Buske-Kirschbaum, A., von Auer, K., Jobst, S., Kaspers, F., & Kirschbaum, C. (1997). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences, 61(26), 2539–2549. https://doi.org/10.1016/S0024-3205(97)01008-4
- Putman, P., Hermans, E. J., Koppeschaar, H., van Schijndel, A., & van Honk, J. (2007). A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology, 32(7), 793–802. https://doi.org/10.1016/j.psyneuen.2007.05.009
- Quevedo, K., Doty, J., Roos, L., & Anker, J. J. (2017). The cortisol awakening response and anterior cingulate cortex function in maltreated depressed versus non-maltreated depressed youth. Psychoneuroendocrinology, 86, 87–95. https://doi.org/10.1016/j.psyneuen.2017.09.001
- Rhebergen, D., Korten, N. C. M., Penninx, B. W. J. H., Stek, M. L., van der Mast, R. C., Oude Voshaar, R., & Comijs, H. C. (2015). Hypothalamic-pituitary-adrenal axis activity in older persons with and without a depressive disorder. Psychoneuroendocrinology, 51, 341–350. https://doi.org/10.1016/j.psyneuen.2014.10.005
- Sabatinelli, D., Keil, A., Frank, D. W., & Lang, P. J. (2013). Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology, 92(3), 513–519. https://doi.org/10.1016/j.biopsycho.2012.04.005
- Schreiber, J. E., Shirtcliff, E., Van Hulle, C., Lemery-Chalfant, K., Klein, M. H., Kalin, N. H., Essex, M. J., & Goldsmith, H., H. (2006). Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology, 31(9), 1131–1137. https://doi.org/10.1016/j.psyneuen.2006.07.005
- Shi, X., Sun, X., Yao, Z., Yuan, Y., Wu, J., & Clow, A. (2018). The cortisol awakening response predicts response inhibition in the afternoon of the same day. Psychoneuroendocrinology, 89, 23–29. https://doi.org/10.1016/j.psyneuen.2017.12.016
- Smyth, J., Ockenfels, M. C., Porter, L., Kirschbaum, C., Hellhammer, D. H., & Stone, A. A. (1998). Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology, 23(4), 353–370. https://doi.org/10.1016/S03064530(98)00008-0
- Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wust, S., Dockray, S., Smyth, N., Evans, P., Hellhammer, D. H., Miller, R., Wetherell, M. A., Lupien, S. J., & Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. https://doi.org/10.1016/j.psyneuen.2015.10.010
- Steptoe, A., & Ussher, M. (2006). Smoking, cortisol and nicotine. International Journal of Psychophysiology, 59(3), 228–235. https://doi.org/10.1016/j.ijpsycho.2005.10.011
- Stetler, C., & Miller, G. E. (2005). Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology, 114(4), 697–705. https://doi.org/10.1037/0021-843X.114.4.697
- Stroud, C. B., Vrshek-Shallhorn, S., Norkett, E. M., & Doane, L. D. (2019). The cortisol awakening response (CAR) interacts with acute interpersonal stress to prospectively predict depressive symptoms among early adolescent girls. Psychoneuroendocrinology, 107, 9–18. https://doi.org/10.1016/j.psyneuen.2019.04.017
- Tsai, J. C., Chou, K. R., Tsai, H. T., Yen, Y. C., & Niu, S. F. (2019). Effects of nocturnal sleep quality on diurnal cortisol profiles and attention in nurses: a cross-sectional study. Biological Research for Nursing, 21(5), 510–518. https://doi.org/10.1177/1099800419861695
- Ulrike, S., Reinhold, L., & Dirk, H. (2013). Major depression in young girls is related to altered cortisol awakening response. European Child & Adolescent Psychiatry, 22(6), 379–384. https://doi.org/10.1007/s00787-012-0371-9
- Van Cauwenberge, V., Van Leeuwen, K., Hoppenbrouwers, K., & Wiersema, J. R. (2017). Developmental changes in neural correlates of cognitive reappraisal: An ERP study using the late positive potential. Neuropsychologia, 95, 94–100. https://doi.org/10.1016/j.neuropsychologia.2016.12.015
- Vreeburg, S. A., Hoogendijk, W. J. G., van Pelt, J., Derijk, R. H., Verhagen, J. C. M., van Dyck, R., Smit, J. H., Zitman, F. G., & Penninx, B. W. J. H. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Archives of General Psychiatry, 66(6), 617–626. https://doi.org/10.1001/archgenpsychiatry.2009.50
- Weymar, M., Schwabe, L., Löw, A., & Hamm, A. O. (2012). Stress sensitizes the brain: Increased processing of unpleasant pictures after exposure to acute stress. Journal of Cognitive Neuroscience, 24(7), 1511–1518. https://doi.org/10.1162/jocn_a_00174
- Wirkner, J., Bort, C. V., Schwabe, L., Hamm, A., & Weymar, M. (2019). Chronic stress and emotion: Differential effects on attentional processing and recognition memory. Psychoneuroendocrinology, 107, 93–97. https://doi.org/10.1016/j.psyneuen.2019.05.008
- Wolfram, M., Bellingrath, S., & Kudielka, B. M. (2011). The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology, 36(6), 905–912. https://doi.org/10.1016/j.psyneuen.2010.12.006
- Wong, S. F., Trespalacios, F., & Ellenbogen, M. A. (2020). Poor inhibition of personally-relevant facial expressions of sadness and anger predicts an elevated cortisol response following awakening six months later. International Journal of Psychophysiology, 150, 73–82. https://doi.org/10.1016/j.ijpsycho.2020.02.005
- Wust, S., Wolf, J., Hellhammer, D. H., Federenko, I., Schommer, N., & Kirschbaum, C. (2000). The cortisol awakening response – normal values and confounds. Noise & Health, 2(7), 79–88.