Abstract
Women are exposed to a variety of life stressors, particularly violence, during their lifetime which increases the risk of developing various psychiatric and somatic diseases, with the dysregulated secretion of cortisol as one potential biological mechanism. We examined the association between violence and other life stressors and hair cortisol concentration (HCC) in a population of urban women. We included 470 adult women (age = 21–86 years) attending the Cancer Detection Clinic in Iceland. The Life Stressor Checklist-Revised (LSC-R; 30-items) was used to assess exposure. HCC was measured with liquid chromatography coupled with tandem mass spectrometry. We used linear regression models to assess the association between life stressors and log-transformed HCC. The median HCC (pg/mg) in the study population was 4.9 (range 0.6–616.6). HCC was not associated with background covariates, including age (p = 0.868), education level (p = 0.824), marital status (p = 0.545), income (p = 0.363), occupation (p = 0.192), but associated with current smoking (p = 0.013). We noted a 3.3% (95% CI: 0.17–6.6%) associated increase in HCC per endorsed life stressor after adjusting for age and smoking, while non-violent life stressors were not associated with HCC. Per endorsed violence item, we observed a 10.2% (95% CI: 1.4–19.7%) associated increase in HCC after age and smoking adjustment. Women with lifetime exposure to both physical and sexual violence presented with higher HCC than unexposed women (p = 0.010), after age and smoking adjustment. Lifetime exposure to violence was associated with higher levels of HCC in a community sample of women. These findings need confirmation with prospective studies.
1. Introduction
Serious life stressors or trauma, such as exposure to violence, are associated with a risk of long-term psychological morbidities (Cerda et al., Citation2012; Chen et al., Citation2010; Lagdon et al., Citation2014), which may result in, and be maintained by, chronic dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis. Glucocorticoids, such as cortisol, are secreted by the HPA axis through a distinct diurnal pattern that regulates multiple functions, including glucose homeostasis (Kuo et al., Citation2015) and modulation of the immune system (Oppong & Cato, Citation2015). The HPA axis further reacts to environmental stressors by secreting cortisol (Spencer & Deak, Citation2017), spurring interest for cortisol as a potential biomarker of psychological stress. Evidence from multiple studies on animals and humans shows that the HPA axis becomes dysregulated when the organism cannot adequately respond to stressors (Lupien et al., Citation2009; Tsigos & Chrousos, Citation2002). While short-term stressors evoke an exaggerated cortisol response (Sapolsky et al., Citation2000), repeated or chronic stressors have been associated with low levels of cortisol or a flat diurnal rhythm (Adam et al., Citation2017). The dysregulation in cortisol secretion has traditionally been measured with multiple measures of saliva, blood, or urine daily, usually with great measurement error due to the pulsatile nature of the hormone. The advent of hair cortisol concentration (HCC) a decade back, lends promise for validly assessing average cortisol concentration, or HPA output, over weeks to months (Cirimele et al., Citation2000; Raul et al., Citation2004).
Findings from studies exploring the association between exposure to various life stressors and hair cortisol concentration have, however, been conflicting (Faresjo et al., Citation2013; Karlen et al., Citation2011). Hair cortisol measurements have been collected among adult victims of war and refugees in several studies, and most (Buchmüller et al., Citation2020; Schalinski et al., Citation2015; Steudte et al., Citation2011), but not all (Mewes et al., Citation2017), studies have found a significant positive association between trauma exposure and hair cortisol concentration. These studies examined highly traumatized individuals, but violence does not only occur in the context of war. Violence against women, globally, affects almost a third of women (World Health Organization et al., Citation2013), with similar estimates in high-income countries. Studies examining hair cortisol concentration in women exposed to violence have been few and shown inconsistent results, some reporting higher (Boeckel et al., Citation2017; Heller et al., Citation2018) and others lower (Alhalal and Falatah, Citation2020; Groer et al., Citation2016) hair cortisol levels while some have not found any association (Fischer et al., Citation2017). These studies have been relatively small and have targeted specific populations (e.g. sex workers, veterans), which may limit the reliability and generalizability of their findings.
Differing definitions of exposure, as well as possible differences in hair cortisol concentration by timing of violence (both in time from event as well as age at the event), may contribute to conflicting literature on life stressors, trauma, and hair cortisol concentration. A meta-analysis of adversity (including violence) found indications that the type and timing of adversity moderated the association between adversity and hair cortisol concentration, with the strongest association being a negative one between maltreatment in childhood and adversity (Khoury et al., Citation2019).
Therefore, we aimed to assess the association between lifetime exposure to life stressors, including violence, and hair cortisol concentration in a larger urban population of women in a Nordic welfare state. To that end, we decided to assess the association between cumulative exposure to life stressors as well as focusing on the traumatic event of violence. We hypothesized that: (1) That there would be a positive association between cumulative exposure to life stressors and HCC and that the association would be stronger if violent events were examined vs. non-violent life stressors. (2) Those with the heaviest exposure to violence, e.g. both to physical and sexual violence would present with the highest HCC. (3) Levels of HCC would attenuate with time from the violence exposure so that exposure in childhood would yield lower levels of HCC.
2. Methods
2.1. Study population and design
In 2014, a random sample of 689 adult women living in the greater Reykjavík area and attending the national Cancer Detection Clinic was invited to participate in the study. All women had routine appointments for age-appropriate breast and cervical cancer screens from February to April of 2014. The Icelandic Cancer Society reports an ∼75% 5-year attendance rate with a small proportion of eligible women never attending screenings; 8% for cervical smears and 15% for mammographies (Society, Citation2016). After the appointment for screening was made (2–4 weeks in advance), the women were sent an introductory letter, then telephoned to discuss the study further and ask for their willingness to participate. To be included in the analysis, women had to answer at least two of seven questions on violence, have a valid measure of HCC, and could not be pregnant. The study was approved by the National Bioethics Committee (VSNb2013010025/03.07) and reported to the Data Protection Authority in Iceland.
2.2. Main outcomes and measures
Participants answered a web-based questionnaire, before their study visit, which was in conjunction with the cancer screening. The study visit consisted of a short interview and physical examination, measuring height, weight, and performing a blood draw and hair sampling.
In the web-based questionnaire, exposure to life stressors was assessed with the 30-item questionnaire: Life Stressor Checklist-Revised (LSC-R) (Wilson & Keane, Citation1997). It follows a yes/no format, with a range of life events tailored toward women, including violence and reproductive choices (Wilson & Keane, Citation2004). For each item, women were asked to specify their age when the event first and last occurred. The seven questions on violence were:
Before/After age 18, were you ever touched or made to touch someone else sexually because they forced you in some way or threatened to harm you if you didn’t?
Before/After age 18, did you ever have sex (oral, anal, genital) when you didn’t want to because someone forced you in some way or threatened to harm you if you didn’t?
Before/After age 18, were you ever abused (not sexually) or physically attacked (hit, slapped, choked, burned, or beat up) by someone you knew (for example, a parent, boyfriend, or husband)?
Have you ever been robbed, mugged, or physically attacked (not sexually) by someone you did not know?
From this questionnaire, different exposure definitions were employed. All thirty items were used as well as the seven violence items specifically and the 23 non-violence-related items. Violence exposure was further categorized as physical violence only, sexual violence only, and exposure to both physical and sexual violence. After each LSC-R question, the respondent was asked at what age this event first and last occurred. This item was used to determine the timing of exposure to violence in exposed women in two ways. Firstly, whether the first exposure to violence was before or after age eighteen, and secondly, the number of years since the woman was last exposed to violence (<10 years, 10–20 years, 20–40 years, or over forty years ago).
The main outcome assessed was hair cortisol concentration. Hair samples were collected during the study visit, taken from the posterior vertex if there was at least 3 cm of hair growth. The hair samples were stored at room temperature in a dark and dry environment. They were then sent to the Department of Psychology laboratory of TU Dresden, in Germany, to be assayed. The 3 cm closest to the scalp are assayed, representing the past three months of hair growth (Wennig, Citation2000). Liquid chromatography coupled with tandem mass spectrometry (LCMS/MS) was used to measure cortisol values in the hair samples; see Gao et al. (Citation2013) for a more detailed description. The inter-assay coefficient of variability was 15% (including pre-analytics) and the intra-assay coefficient of variability was 9.5%.
Information on potential covariates was obtained from questionnaire data and study visits. The sociodemographic variables explored were age, marital status, education, monthly income, and occupation. Age was used as a continuous variable (in years) as well as divided into tertiles. Marital status was divided into partnered (married, cohabitating, or in a relationship) and non-partnered (single or widowed). Education level was reported as highest level completed: primary (basic), secondary (high school, vocational training), or university. Income was divided into low, medium, and high based on national income from Statistics Iceland, with mean monthly income rated as medium (Statistics Iceland, Citation2016), with medium and high incomes merged into one category due to few participants indicating high incomes. Assessment of occupation was divided into employed and “other,” including student, homemaker, sick leave, disability, and retirement. Smoking status was characterized as current, former, and never smoker (<100 lifetime cigarettes). Mental health was assessed using two different measures, the Patient Health Questionnaire (PHQ-9) (Nease & Maloin, Citation2003) and the Primary Care PTSD screen (PC-PTSD) (Ouimette et al., Citation2008). The Patient Health Questionnaire, measuring depressive symptoms, has a maximum score of 27. A total score ranging from 0 to 9 was defined as no or mild symptomology present, referred to as no symptoms, and a score of 10 or more as moderate to severe depressive symptoms, referred to as depressive symptoms. The Primary Care PTSD screen is comprised of four items, with three or four positive items suggesting symptoms of post-traumatic stress disorder (PTSD) and categorized as a positive screen. At the study visit a registered nurse measured height (m) and weight (kg), enabling us to determine body mass index (BMI), calculated according to the formula weight/height2, and divided into normal weight (BMI < 25), overweight (BMI 25–30), and obese (BMI > 30). Women were asked to report their hair washing frequency (times per week) and whether they had dyed their hair in the last three months.
2.3. Statistical analysis
Of 689 women that were invited to participate, 474 (68.8%) responded to the online questionnaire and provided a usable hair sample. Three women were excluded due to current pregnancy. When examining the data, we found that one woman had an abnormally high hair cortisol concentration of 1782 pg/mg and was excluded as a clear outlier before continuing our analysis. Not all women answered all seven questions on violence, our main exposure, though all answered at least 75% of the LSC-R. Therefore, we imputed missing data for 23 women with predictive mean matching using the mice package in R v3.4.2. The hair cortisol data were positively skewed and were therefore log-transformed. Hair cortisol concentration (pg/mg) before log-transformation was used, with medians, for descriptive purposes in the tables but all regressions and further calculations use log-transformed hair cortisol concentration (logHCC) as means with standard deviation. We also present percentage differences in HCC after exponentiating the estimate of logHCC models. Traditionally, HCC values higher than three standard deviations from the mean (N = 11) and values three times higher than cortisone (N = 25) have been excluded from further analyses. However, in this sample they were associated with the main exposure of interest; thus, rather than excluding these individuals altogether, we performed a sensitivity analysis with these more restrictive exclusion criteria (N = 434) while using the entire sample in our main analyses (N = 470).
We contrasted socio-demographic characteristics along with health behaviors of women across groups defined by the number of life stressors: 0–1, 2–4, 5–6, and 7+. We repeated the exercise but grouped it by violence exposure. In both scenarios, we used the chi-square test and Wilcoxon test (for categorical and continuous variables, respectively) to identify differences in socio-economic factors, health behaviors, mental health, hair characteristics, number of 30-item life stressors experienced, and logHCC. We used linear regression to evaluate the difference in logHCC by both exposures to various types of life stressors and the timing of the exposure. We also created box plots to show hair cortisol concentration by different violence categories. To assess which covariates to include in our models, we calculated the differences in hair cortisol concentration across covariate levels in the study population which indicated smoking as a potential confounder while other suggested covariates, including hair washing and dying (Stalder et al., Citation2017), were not. We, therefore, used age and smoking-adjusted model.
3. Results
Of the 470 women who were included in the analyses, 41.9% (n = 197) reported that they had experienced sexual and/or physical violence and 7.7% (n = 36) reported to have been raped. Exposure to sexual violence only was reported by 58 (12.3%) women, physical violence only by 80 (17.0%) women, and 59 (12.6%) women were exposed to both sexual and physical violence (). The mean number of life stressors endorsed by all 470 women was 4.8 ± 3.4. Women with outlier cortisol values (N = 36) had experienced a higher number of life stressors (average 5.3 ± 3.6), with 44% experiencing violence and 22% endorsing both physical and sexual violence.
Table 1. Background characteristics of the study population by exposure to life stressors and trauma, including exposure to violence specifically.
0–1n = 512–4n = 252
Background characteristics of all women by the number of life stressors as well as exposure to violence specifically are presented in . The mean age was 52.9 ± 11.0 years, with the majority of women being partnered (75.7%) and employed (76.4%). The prevalence of current smokers was 13.0% and the mean BMI was 27.6 ± 5.5. The prevalence of PTSD symptoms was 9.1%. Women who had been exposed to at least seven life stressors were less likely to be partnered, with lower income, not employed, more likely to smoke, and have a higher BMI, experience symptoms of depression and PTSD when compared with women exposed to none or one life stressor (all p’s <0.05). Women exposed to violence specifically were less likely to be employed, more likely to smoke, and less likely to have dyed hair (all p’s <0.05). Women exposed to violence had also experienced more non-violent life stressors (5.4 vs. 3.0, p = <0.001) when compared with women with no violence exposure.
Overall, the median HCC was 4.9 pg/mg (25–75th percentiles: 2.9–9.0) with a range of 0.6–616.6 pg/mg. Women exposed to one or no life stressors had a median HCC of 4.9 pg/mg (25–75th percentiles: 2.5–9.8 pg/mg), while women exposed to seven or more life stressors had a median of 5.8 pg/mg (25–75th percentiles: 3.1–12.5 pg/mg) (p = 0.127) (). Median HCC was 4.9 pg/mg (25–75th percentiles: 3.0–8.6 pg/mg) in women unexposed to violence and 5.0 pg/mg (25–75th percentiles: 2.9–10.0 pg/mg) in women exposed to any violence. Hair cortisol concentrations of the entire sample by different background characteristics are presented in . Current smokers had higher HCC (5.2 vs. 4.7 pg/mg, p = 0.013) than never smokers, but the distribution of HCC was similar for other variables including age, BMI, symptoms of depression and PTSD, weekly hair washing, hair dying, and socioeconomic status (all p’s >0.20).
Table 2. Differences in hair cortisol concentration by background characteristics (n = 470).
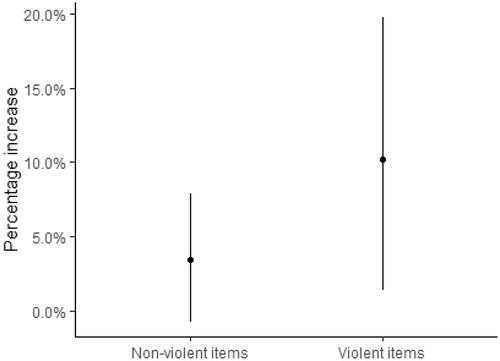
When the association between the number of life stressors (all 30-items) and logHCC is examined, a positive association is observed after age- and smoking adjustment, with an associated increase of 3.3% (95% CI: 0.17–6.6%) per additional item of LSC-R endorsed (). In , the association is further examined by observing the percentage increase when either a non-violent or violent item was endorsed. For each violent item endorsed there was a percentage increase of 10.2% (95% CI: 1.4–19.7%) with a non-significant associated increase with the 23 non-violent items of 3.5% (95% CI: −0.7, 7.8), though the two categories of life stressors (violent and non-violent) are highly correlated [r(168) = 0.78, p = <0.001]. In a sensitivity analysis excluding women with HCC three standard deviations above the mean and excluding women that had cortisol values that were three times higher than their cortisone value (N = 434), we observed a non-significant percentage increase in HCC per additional life stressor endorsed (All 30 items: 2.0%; 95% CI: −0.2–4.2%) as well as by an increased number of violence items endorsed (4.7%; 95% CI: −1.2–11.1%).
Figure 1. Linear regression of log-transformed cortisol and the 30-item Life Stressor Checklist with 95% confidence intervals, after adjusting for age and smoking. presents a positive association between logHCC and the 30-item LSC-R after adjusting for age and smoking (p = 0.039), with an associated increase of 3.3% (95% CI: 0.17–6.6%).
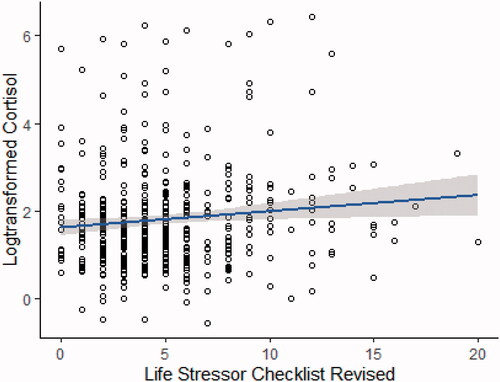
Figure 2. Differences in hair cortisol concentration by exposure to nonviolent and violent life stressors. presents the percentage increase of hair cortisol for each nonviolent and violent item endorsed with 95% confidence intervals (95% CI) after adjusting for age and smoking. There is a significant associated increase in hair cortisol concentration (10.2%; 95% CI: 1.4–19.7%) per additional violence item endorsed. A non-significant associated increase in HCC by 23 nonviolent life stressors (3.5%, 95% CI: −0.7, 7.8).
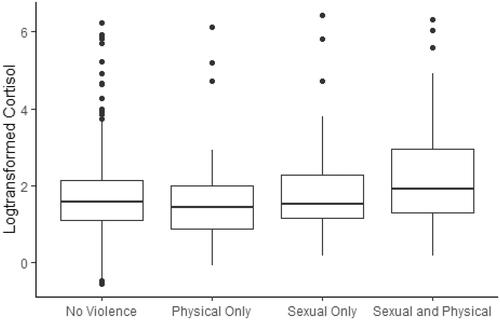
When the group was divided by type of violence exposure in we see a significantly higher median logHCC among women exposed to sexual and physical violence (2.0 vs. 1.6, p = 0.010) compared with women unexposed to violence (mean logHCC 2.3 vs. 1.8, ). No difference was found when comparing physical violence only and sexual violence only with unexposed women (p = 0.166 and p = 0.707, respectively). When using more stringent inclusion criteria (N = 434), there was still a higher median logHCC among women exposed to both sexual and physical violence (p = 0.034), with no differences seen in exposure to physical violence only (p = 0.303) or sexual violence only (p = 0.884).
Figure 3. Boxplot of hair cortisol concentration by type of violence exposure. The figure presents boxplots of hair cortisol concentration by no violence exposure vs. by violence type (physical only, sexual only, sexual and physical). Compared to none, there is no difference in HCC by exposure to physical violence only (p = 0.166) or sexual violence only (p = 0.707), but a significant difference for exposure to sexual and physical violence (p = 0.010) after adjusting for age and smoking.
Finally, we examined HCC with respect to age at first violence exposure as well as the duration of time from the last exposure (). The majority (70%) were first exposed in childhood, and the mean time from last violence exposure was 28 ± 14 (range = 1–61) years. Neither younger age at first exposure nor shorter duration of time from last violence exposure were statistically significantly associated with hair cortisol concentration.
Table 3. Differences in hair cortisol concentration by age at first exposure and time from last exposure.
4. Discussion
In this study of Icelandic women from a general urban population, we found that the number of experienced life stressors was associated with higher hair cortisol concentration, with a greater increase in hair cortisol levels by the history of violence than for other kinds of life stressors. This increase was mainly due to higher hair cortisol concentration in women exposed to both sexual and physical violence during their lifetime. Neither age at first violence exposure, nor time from last exposure were associated with hair cortisol concentration.
A handful of studies have examined violence against women and hair cortisol concentration. A study of 81 US female veterans found that women who had been sexually abused as children had lower hair cortisol concentration compared with veterans with no such history (Groer et al., Citation2016). Conversely, a study of 43 female refugees in Germany, all with stress-related disorders, found an increase in hair cortisol concentration among women who had been sexually abused in childhood and an increase in the number of traumatic event types (Schalinski et al., Citation2015). A study of 141 female sex workers in Kenya found that women with recent violence exposure (<12 months) had higher hair cortisol concentration when compared with women with more remote or no violence exposure (Heller et al., Citation2018). Two studies focusing on intimate partner violence had divergent results, the first with 59 Brazilian women, half recruited at a domestic violence clinic, finding an association with higher hair cortisol concentration (Boeckel et al., Citation2017), while a Saudi study of 156 women found lower levels of hair cortisol with increased exposure severity (Alhalal & Falatah, Citation2020). Finally, a study of 139 Londoners, 72% women, did not find an association between critical life events and hair cortisol concentration (Fischer et al., Citation2017). However, only one question was included to assess lifetime exposure to violence. These studies are all quite small, and their contradicting findings show a need to perform larger studies with more detailed information on trauma history, particularly on violence.
To our knowledge, this is the largest study to address the association between exposure to life stressors, including violence, and hair cortisol concentration in an unselected cohort of women from the general population. Based on a qualitative review of existing smaller studies on trauma and hair cortisol concentration (42–242 participants) (Steudte-Schmiedgen et al., Citation2016), the authors suggested that traumatization and post-traumatic stress disorder might lead to an initial increase in hair cortisol concentration after the traumatic event which over time (years) is attenuated, leading to hypocortisolism. This hypothesis is not confirmed by our results indicating higher hair cortisol levels among women exposed to violence, often as far as decades back. We also did not find an association between experiencing symptoms of depression or PTSD and hair cortisol, though there was a clear association with multiple exposures to violence. Yet, in line with our results, the findings of this review suggest that exposure to a potentially traumatic stressor per se (including exposure to violence) may drive the association with hair cortisol concentration.
We did not only study violence, which has a high likelihood of traumatization, but also 23 other life stressors of the LSC-R, representing a broad variety of adverse experiences. In this way, our paper echoes an earlier study on 180 pregnant women that used a weighted LSC-R score to determine life stressor exposure. They found higher hair cortisol concentration among black women, the ethnic group that was most traumatized (Schreier et al., Citation2016). More broadly, a meta-analysis from 2019 examining adversity (including violence, maltreatment, and natural disasters) found both a negative and positive association with hair cortisol concentration (Khoury et al., Citation2019), with a negative association with childhood maltreatment. This finding was not replicated in our cohort, however, maltreatment encompasses a broader range of experiences which may explain why we do not see lower hair cortisol concentration among women first exposed to violence in childhood. Khoury et al. also did not find one maltreatment subtype, including physical, emotional, and sexual abuse, to be significantly associated with hair cortisol concentration. Indeed, four studies were underpinning the negative effects on HCC in this meta-analysis (Khoury et al., Citation2019), while 16 other studies on childhood maltreatment showed null or trend toward a positive association. The non-significant increase in hair cortisol concentration observed with increasing exposure to non-violent life stressors may indicate that these events have a weaker or no association with hair cortisol concentration, or that bidirectional differences exist that cancel each other out when examined broadly.
A strength of our study included the large population-based sample of urban women with detailed, validated measures on violence and other life stressors and hair cortisol concentration. Conversely, there are limitations involved with sampling women who attend appointments for age-appropriate cancer screenings. The anticipation of a cancer screen could possibly lead to a transient increase in stress levels, but as the hair cortisol concentration measurement represent the average concentration over the previous three months this should have a minimal effect. Our exposure measure is retrospective, with the potential for recall bias. Yet, studies have reported a moderate concordance between retrospective and prospective reporting (Colman et al., Citation2016; Reuben et al., Citation2016). Underreporting of trauma in early life may be an issue (Hardt & Rutter, Citation2004), which in our study would result in attenuation of any real association between early life trauma and hair cortisol concentration. Despite this study is one of the largest studies to date on life stressors and hair cortisol concentration, lack of power—in addition to possible survivor bias—may underlie our null association with regards to the timing of violence. However, we believe that this bias should be minimal, or at least non-differential, as the women are blinded with respect to their actual measure of hair cortisol.
Our study is also cross-sectional in design, so a temporal relationship cannot be assumed and causality cannot be inferred. Nevertheless, this field of research is in dire need of prospective studies, and studies that can take advantage of the retrospective nature of hair cortisol concentration. There is also a need for more work regarding hair cortisol itself, laying down the groundwork on normal and extreme values. In our case, values that have traditionally been excluded were associated with our exposure, highlighting the need for careful consideration before altering the data. We, therefore, chose not to exclude them from the primary analysis as it is possible that these “extreme” values may be a sign of great allostatic load (McEwen, Citation1998). Our sensitivity analysis excluding women with extreme HCC values (n = 434) indeed showed non-significant associations with all 30 life stressor-items, while exposure to both sexual and physical violence was still associated with significantly higher cortisol levels, in line with our hypothesis.
Another concern is the selection of covariates. The literature is inconsistent but a recent meta-analysis shows a weak association with age, BMI, hair washing frequency, and hair treatment (Stalder et al., Citation2017). These associations were tested but were not significant in our cohort. Other variables tested here, such as socio-economic factors, mental health status, and BMI, we a priori expected to be on the causal pathway between violence and hair cortisol concentration, but were not associated with hair cortisol concentration in this study. However, the great number of potential covariates tested suggest that hair cortisol concentration in this cohort is quite stable.
In summary, the findings from this community-based study of 470 urban-dwelling women suggest a positive association between the number of life stressors experienced and hair cortisol concentration assessed several years later, with women exposed to both sexual and physical violence presenting with the highest levels. These findings, therefore, suggest that exposure to violence—which at least one in every three women world-wide are exposed to—may permanently disrupt the function of the HPA-axis, potentially representing an important mechanism for deteriorated health in this population. Our findings highlight the importance of acknowledging cumulative trauma in hair cortisol concentration research and lend support to previous research that the violence exposure itself may alter hair cortisol concentration.
Supplemental Material
Download MS Word (18.4 KB)Acknowledgments
We further wish to acknowledge the invaluable contribution of our research nurses (Elsa Björnsdóttir, Jóna Ellen Valdimarsdóttir, Þuríður Anna Guðnadóttir), other research staff (Hildur Guðný Ásgeirsdóttir, Hrafnhildur Eymundsdóttir, and Þórunn Guðmundsdóttir), and the participating women. .
Disclosure statement
The authors R. Lynch, T. Aspelund, M. Kormáksson, M. Flores-Torres, A. Hauksdóttir, F. Arnberg, C. Kirscbaum, and U. Valdimarsdóttir reported no biomedical financial interests or potential conflicts of interest. M. Lajous received and investigator-initiated non-restricted grant from AstraZeneca.
Additional information
Funding
References
- Adam, E. K., Quinn, M. E., Tavernier, R., McQuillan, M. T., Dahlke, K. A., & Gilbert, K. E. (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. https://doi.org/10.1016/j.psyneuen.2017.05.018
- Alhalal, E., & Falatah, R. (2020). Intimate partner violence and hair cortisol concentration: A biomarker for HPA axis function. Psychoneuroendocrinology, 122, 104897. https://doi.org/10.1016/j.psyneuen.2020.104897
- Boeckel, M. G., Viola, T. W., Daruy-Filho, L., Martinez, M., & Grassi-Oliveira, R. (2017). Intimate partner violence is associated with increased maternal hair cortisol in mother-child dyads. Comprehensive Psychiatry, 72, 18–24. https://doi.org/10.1016/j.comppsych.2016.09.006
- Buchmüller, T., Lembcke, H., Busch, J., Kumsta, R., Wolf, O. T., & Leyendecker, B. (2020). Exploring hair steroid concentrations in asylum seekers, internally displaced refugees, and immigrants. Stress, 23(5), 538–538. https://doi.org/10.1080/10253890.2020.1737008
- Cerda, M., Digangi, J., Galea, S., & Koenen, K. (2012). Epidemiologic research on interpersonal violence and common psychiatric disorders: Where do we go from here? Depression and Anxiety, 29(5), 359–385. https://doi.org/10.1002/da.21947
- Chen, L. P., Murad, M. H., Paras, M. L., Colbenson, K. M., Sattler, A. L., Goranson, E. N., Elamin, M. B., Seime, R. J., Shinozaki, G., Prokop, L. J., & Zirakzadeh, A. (2010). Sexual abuse and lifetime diagnosis of psychiatric disorders: Systematic review and meta-analysis. Mayo Clinic Proceedings, 85(7), 618–629. https://doi.org/10.4065/mcp.2009.0583
- Cirimele, V., Kintz, P., Dumestre, V., Goulle, J. P., & Ludes, B. (2000). Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectrometry. Forensic Science International, 107(1–3), 381–388. https://doi.org/10.1016/S0379-0738(99)00180-2
- Colman, I., Kingsbury, M., Garad, Y., Zeng, Y., Naicker, K., Patten, S., Jones, P. B., Wild, T. C., & Thompson, A. H. (2016). Consistency in adult reporting of adverse childhood experiences. Psychological Medicine, 46(3), 543–549. https://doi.org/10.1017/S0033291715002032
- Faresjo, A., Theodorsson, E., Chatziarzenis, M., Sapouna, V., Claesson, H. P., Koppner, J., & Faresjo, T. (2013). Higher perceived stress but lower cortisol levels found among young Greek adults living in a stressful social environment in comparison with Swedish young adults. PLoS One, 8(9), e73828. https://doi.org/10.1371/journal.pone.0073828
- Fischer, S., Duncko, R., Hatch, S. L., Papadopoulos, A., Goodwin, L., Frissa, S., Hotopf, M., & Cleare, A. J. (2017). Sociodemographic, lifestyle, and psychosocial determinants of hair cortisol in a South London community sample. Psychoneuroendocrinology, 76, 144–153. https://doi.org/10.1016/j.psyneuen.2016.11.011
- Gao, W., Stalder, T., Foley, P., Rauh, M., Deng, H., & Kirschbaum, C. (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 928, 1–8. https://doi.org/10.1016/j.jchromb.2013.03.008
- Groer, M. W., Kostas-Polston, E. A., Dillahunt-Aspillaga, C., Beckie, T. M., Johnson-Mallard, V., Duffy, A., & Evans, M. E. (2016). Allostatic perspectives in women veterans with a history of childhood sexual assault. Biological Research for Nursing, 18(4), 454–464. https://doi.org/10.1177/1099800416638442
- Hardt, J., & Rutter, M. (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45(2), 260–273. https://doi.org/10.1111/j.1469-7610.2004.00218.x
- Heller, M., Roberts, S. T., Masese, L., Ngina, J., Chohan, N., Chohan, V., Shafi, J., McClelland, R. S., Brindle, E., & Graham, S. M. (2018). Gender-based violence, physiological stress, and inflammation: A cross-sectional study. Journal of Women's Health, 27(9), 1152–1161. https://doi.org/10.1089/jwh.2017.6743
- Karlen, J., Ludvigsson, J., Frostell, A., Theodorsson, E., & Faresjo, T. (2011). Cortisol in hair measured in young adults – A biomarker of major life stressors? BMC Clinical Pathology, 11, 12. https://doi.org/10.1186/1472-6890-11-12
- Khoury, J. E., Bosquet Enlow, M., Plamondon, A., & Lyons-Ruth, K. (2019). The association between adversity and hair cortisol levels in humans: A meta-analysis. Psychoneuroendocrinology, 103, 104–117. https://doi.org/10.1016/j.psyneuen.2019.01.009
- Kuo, T., McQueen, A., Chen, T. C., & Wang, J. C. (2015). Regulation of glucose homeostasis by glucocorticoids. Advances in Experimental Medicine and Biology, 872, 99–126. https://doi.org/10.1007/978-1-4939-2895-8
- Lagdon, S., Armour, C., & Stringer, M. (2014). Adult experience of mental health outcomes as a result of intimate partner violence victimisation: A systematic review. European Journal of Psychotraumatology, 5(1), 24794. https://doi.org/10.3402/ejpt.v5.24794
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. https://doi.org/10.1038/nrn2639
- McEwen, B. S. (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338(3), 171–179. https://doi.org/10.1056/NEJM199801153380307
- Mewes, R., Reich, H., Skoluda, N., Seele, F., & Nater, U. M. (2017). Elevated hair cortisol concentrations in recently fled asylum seekers in comparison to permanently settled immigrants and non-immigrants. Translational Psychiatry, 7(3), e1051. https://doi.org/10.1038/tp.2017.14
- Nease, D. E. Jr., & Maloin, J. M. (2003). Depression screening: a practical strategy. The Journal of Family Practice, 52(2), 118–124.
- Oppong, E., & Cato, A. C. (2015). Effects of glucocorticoids in the immune system. Advances in Experimental Medicine and Biology, 872, 217–233. https://doi.org/10.1007/978-1-4939-2895-8
- Ouimette, P., Wade, M., Prins, A., & Schohn, M. (2008). Identifying PTSD in primary care: Comparison of the primary care-PTSD screen (PC-PTSD) and the General Health Questionnaire-12 (GHQ). Journal of Anxiety Disorders, 22(2), 337–343. https://doi.org/10.1016/j.janxdis.2007.02.010
- Raul, J. S., Cirimele, V., Ludes, B., & Kintz, P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry, 37(12), 1105–1111. https://doi.org/10.1016/j.clinbiochem.2004.02.010
- Reuben, A., Moffitt, T. E., Caspi, A., Belsky, D. W., Harrington, H., Schroeder, F., Hogan, S., Ramrakha, S., Poulton, R., & Danese, A. (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103–1112. https://doi.org/10.1111/jcpp.12621
- Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. https://doi.org/10.1210/edrv.21.1.0389
- Schalinski, I., Elbert, T., Steudte-Schmiedgen, S., & Kirschbaum, C. (2015). The cortisol paradox of trauma-related disorders: Lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS One, 10(8), e0136921. https://doi.org/10.1371/journal.pone.0136921
- Schreier, H. M., Bosquet Enlow, M., Ritz, T., Coull, B. A., Gennings, C., Wright, R. O., & Wright, R. J. (2016). Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress, 19(1), 45–52. https://doi.org/10.3109/10253890.2015.1117447
- Society, I.C. (2016). Yearly report 2015–2016. Reykjavik: Icelandic Cancer Society.
- Spencer, R. L., & Deak, T. (2017). A users guide to HPA axis research. Physiology & Behavior, 178, 43–65. https://doi.org/10.1016/j.physbeh.2016.11.014
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., Kirschbaum, C., & Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. https://doi.org/10.1016/j.psyneuen.2016.12.017
- Statistics Iceland (Ed.). (2016). Dreifing launa fullvinnandi launamanna eftir launþegahópi 2008–2014. Reykjavik: Statistics Iceland.
- Steudte, S., Kolassa, I. T., Stalder, T., Pfeiffer, A., Kirschbaum, C., & Elbert, T. (2011). Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology, 36(8), 1193–1200. https://doi.org/10.1016/j.psyneuen.2011.02.012
- Steudte-Schmiedgen, S., Kirschbaum, C., Alexander, N., & Stalder, T. (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience and Biobehavioral Reviews, 69, 124–135. https://doi.org/10.1016/j.neubiorev.2016.07.015
- Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. https://doi.org/10.1016/S0022-3999(02)00429-4
- Wennig, R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Science International, 107(1–3), 5–12. https://doi.org/10.1016/S0379-0738(99)00146-2
- Wilson, J. P., & Keane, T. M. (1997). Assessing psychological trauma and PTSD (pp. 192–238). Guilford Press.
- Wilson, J. P., & Keane, T. M. (2004). Assessing psychological trauma and PTSD (John P. Wilson &Terence M. Keane, Ed., 2nd ed.). Guilford Press.
- World Health Organization, García-Moreno, C., Pallitto, C., Devries, K., StöCkl, H., Watts, C., Abrahams, N., London School of Hygiene and Tropical Medicine, & South African Medical Research Council (2013). Global and regional estimates of violence against women: Prevalence and health effects of intimate partner violence and non-partner sexual violence (pp. vi, 50). World Health Organization.
