 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Since medical communication can be perceived as stressful, the assessment of patients’ physiological arousal and behavior during anamnesis interviews may lead to a better understanding of doctor-patient interactions. Therefore, the aim of this study was to test physiological arousal and word use in a laboratory anamnesis interview. In total, sixty-five participants with a mean age of 25.0 years were randomly assigned either to an experimental group (n = 35, 65.7% women) in which they underwent an anamnesis interview or to a control group (n = 30, 73.3% women). Physiological arousal was assessed by salivary cortisol, salivary alpha-amylase (sAA), heart rate (HR) and heart rate variability (HRV). Psychological arousal was assessed using the Positive and Negative Affect Schedule (PANAS). Anamnesis interviews were analyzed using the Linguistic Inquiry and Word Count text analysis tool (LIWC). Participants of the experimental group showed an increase of sAA, HR and negative affect (p’s ≤.0.05). Moreover, higher cortisol area under the curve with respect to ground (AUCg) was associated with lesser use of positive emotion words during the interview and subsequent higher negative affect (p’s <.05). These results indicate that talking about one’s own and family’s medical history in anamnesis interview induces physiological arousal. Our findings suggest that anamnesis interviews could not only induce higher negative affect, but also induce physiological arousal, underscoring the importance of good doctor-patient communication.
1. Introduction
As medical settings come along with many uncertainties for the patient (e.g. new interventions, hospitalization, upcoming procedures), and often include loss of control, it is the healthcare providers’ responsibility to provide a setting that fosters understandable communication and ensures good decision making. Before starting a care plan or undergoing any procedure, patients are confronted with consultations, but our knowledge on the experience of stress (and its consequences) during medical consultations is limited. Previous research has shown that patient’s reactions to medical situations as well as poor medical communication has negative consequences for the adherence to a treatment plan leading to less health literacy and poor compliance (Arbuthnott & Sharpe, Citation2009; Esfandiari et al., Citation2020; García-Llana et al., Citation2014; Karvinen et al., Citation2013; Miller, Citation2016). Emotional arousal induced by breaking bad news for instance impaired the recall of relevant medical information (e.g. van Osch et al., Citation2014). Moreover, research on cancer patients has shown that poor communication could severely impact psychological well-being of patients, leading to social, emotional and high economic costs (Lehmann et al., Citation2009; Thorne et al., Citation2005).
Taken together, patients in medical settings are confronted with situations, in which the characteristics of these situations can provoke feelings of stress (Blascovich & Tomaka, Citation1996; Dickerson et al., Citation2004; Dickerson & Kemeny, Citation2004; Dienstbier, Citation1989; Henry & Grim, Citation1990; Mason, Citation1968; Rose, Citation1980; Sapolsky, Citation1993; Sapolsky et al., Citation2000). Moreover, the social self-preservation theory suggests that situations which pose a threat to one’s social-self elicit physiological reactions if the situation (1) contains a social evaluative threat and (2) is interpreted as uncontrollable (Dickerson & Kemeny, Citation2004). However, research in this field, with few exceptions (e.g. van Stegeren et al., Citation2006), is lacking.
1.1. Present study
As medical communication plays a key role in many health outcomes and research on patients’ physiological stress levels during medical consultations is limited, a standardized experimental approach could improve our knowledge of individual responses during doctor-patient interactions. The goal of the present study was to investigate psychophysiological arousal in doctor-patient interactions under standardized circumstances. Therefore, participants were randomly assigned to an experimental group (EG), in which they underwent an audiotaped semi-structured anamnesis interview where they were asked about their own and their family’s medical history, or to a control group (CG), in which they watched a documentary. Salivary cortisol, salivary alpha-amylase (sAA), heart rate (HR) and heart rate variability (HRV) were analyzed to assess physiological arousal. Changes in participants’ affect were assessed, and verbal behavior during the interview was assessed via word analysis (Pennebaker et al., Citation2015; Tausczik & Pennebaker, Citation2010; Weintraub, Citation1989).
We expected that a medical consultation would result in higher physiological arousal as compared to a control condition. Specifically, we expected an increase of sAA, cortisol, HR and negative affect due to the anamnesis interview, as well as a decrease of HRV during the anamnesis interview and a decrease of positive affect after the interview. Moreover, we hypothesized that patients' affective state would be associated with elevated physiologial arousal. Regarding word use, we expected that emotional words as well as health-related words would be associated with stress responses.
2. Method
2.1. Participants
Participants were recruited from the local community from March to December 2017. Inclusion criteria were: (1) above 18 years of age, (2) not taking medications, (3) no major psychiatric disorders, (4) not pregnant or breast feeding and (5) no coronary heart disease, cardiovascular disease or acute infectious disease. Nine participants did not meet the inclusion criteria and were excluded from analysis. In total 16 women reported intake of hormonal contraceptives. The study sample consisted of 65 participants (69% female) which were randomly assigned to two groups: Standardized anamnesis interview (experimental group, EG; n = 35) or control group (CG; n = 30). A sensitivity power analysis performed with G*Power indicated 95% power to detect an effect size of f = 0.18 (4 time points for saliva samples) and an effect size of f = 0.17 (5 time points for HR/HRV time segments) in an rmANOVA with ɑ = 0.05, two tailed (Faul et al., Citation2009). See description of the study sample in . The study protocol was approved by the local ethics committee (Friedrich-Alexander University Erlangen-Nürnberg; 332_16 B) and carried out in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants at study entry. Participants received monetary compensation for participation.
Table 1. Sociodemographic characteristics of participants.
2.2. Procedure
Participants were scheduled for their lab visit between 2 pm and 7 pm to control for diurnal rhythms of cortisol and sAA (Rohleder & Nater, Citation2009). Participants were instructed to refrain from exercising 24 h before their scheduled appointment, and from eating or drinking anything except water one hour before their visit. Upon arrival, participants were escorted to equally furnished testing rooms and written informed consent was obtained.
For HR recording, participants were fitted with a Polar watch (Polar Electro) with a heart rate sensor around their chest. Basic demographics and current affect were assessed using an online survey tool (“Unipark”, Questback, Germany). They were then randomly assigned either to the EG or to the CG. Participants in the EG were informed that they will undergo an anamnesis interview followed by a venipuncture, while the remaining participants were informed that they had been assigned to the CG. Afterwards, 45 min after arrival, all participants started watching a documentary (BBC one Planet Earth program, Episode 2 “Mountains”), which was presented on a tablet (Samsung) for five minutes without audio. After four minutes of watching the documentary, the first saliva sample was taken.
Participants in the CG continued watching for another 15 minutes. This protocol for the CG is based on our experience with other acute stress studies, and optimized to not elicit arousal and to not activate physiological stress systems. Participants in the EG stopped watching the documentary and were escorted into a separate room with one male and one female study team member wearing white lab coats. As a cover story, participants were told that the anamnesis interview would take place to learn about their own and their family’s medical history to then perform a venipuncture to assess inflammation. For the next 15 minutes, they underwent an audiotaped semi-structured anamnesis interview. After the interview, participants returned to their original testing room. Affect was assessed shortly afterwards in both groups. Saliva samples were taken 5, 15, and 20 minutes after the intervention. After that, participants’ height, weight and body composition were measured. Participants were explicitly debriefed and informed about the cover-story.
2.2.1. Semi-structured anamnesis interview and word use analysis
As a trained paramedic, one of the authors (S.Schm.) conducted the semi-structured interviews, while another team member recorded the interview. The two interviewers were instructed to behave neutrally toward participants. The testing room was furnished with a desk and a treatment table to simulate a doctor’s office. To accentuate the atmosphere of a medical setting, a second table next to the treatment table presented typical medical instruments for performing venipunctures (tourniquet, needles, tubes, swabs, band-aids). The interview took between 8 and 18 minutes (M = 14:06, SD = 02:03). All interviews were audiotaped using dictation devices (Philips and GLTECK) and covered the following topics (tailored follow-up questions were asked individually, if necessary). First, we asked for previous illnesses, surgeries as well as physical or mental complaints in the last 6 months. Then, participants’ current drug intake as well as gender specific medication were assessed (men: intake of potency drugs; women: intake of hormonal contraceptives), followed by several questions to cover for common risk factors like smoking, alcohol consumption and diseases prevalent in the family. Further, participants’ willingness to attend preventive medical checkups was recorded (e.g. cancer screening, colonoscopy) or if they had attended one recently. Assessment of allergies, intolerances and common vaccinations followed as well as any negative experiences with doctors or medical treatments in the past. To steer the conversation to the venipuncture, participants were asked about previous reactions to venipunctures. Participants were then informed about risks (e.g. swelling, bleeding, infection, artery puncture) to increase threat perceptions. In order to perform the upcoming venipuncture, the interviewer told them, he would measure blood pressure first. Blood pressure was measured using a common oscillometric blood pressure monitor (boso medicus X, BOSCH + SOHN, Jungingen, Germany). The interviewer then announced blood pressure levels and explained that he had now gained enough information about the participants health and that the venipuncture would not be performed. Participants then returned to the original testing room and were debriefed.
Audiotaped interviews were transcribed and analyzed using the Linguistic Inquiry and Word Count (LIWC; Pennebaker et al., Citation2001; Wolf et al., Citation2008) text analysis tool (dictionary version 2015; Pennebaker et al., Citation2015). The transcripts contained exclusively the speaking parts of the participants, in which colloquial language was translated into standard German. Our targets were the following word categories and its subcategories: “Biological Processes” (e.g. eat, blood, pain), with subcategories “Health” (e.g. clinic, flu, pill) and “Body” (e.g. cheek, hands, spit). Furthermore, the word category “Affective Processes” (e.g. happy, cried) with subcategories “Positive Emotion” (e.g. love, nice, sweet) and “Negative Emotion” (e.g. hurt, ugly, nasty) was considered.
2.3. Measures
2.3.1. Salivary cortisol and sAA
Saliva was collected via Salivettes (Sarstedt, Nümbrecht, Germany) and stored at −30 °C until cortisol concentrations were assessed in duplicates using a chemiluminescence immunoassay (CLIA, IBL International, Hamburg, Germany). sAA was measured by a quantitative enzyme kinetic method (Roche Diagnostics, Mannheim, Germany; Becker & Rohleder, Citation2020). Intra- and inter-assay coefficients of variation were below 10%.
2.3.2. HR and HRV
To analyze HR and HRV, five segments of 5 minutes each were selected and processed using Kubios HRV (Kubios Ltd.) premium version 3.0.2 (Tarvainen et al., Citation2014). HR and HRV Data was visually checked for artifacts and corrected if necessary. As detrending method the “Smooth N priors algorithm” was used. FFT was calculated via Welch’s periodogram using 256 s window with 50% overlap. Due to one outlier regarding artifacts as well as some missing data, HR and HRV results below are reported on a sample size of N = 60. First segment: 5-minute documentary prior to the anamnesis interview. Interviews were split into three 5-minute segments, followed by one recovery segment (similarly timed segments in CG). We refer to the aforementioned segments as phases (1–5). Time-domain measures included HR and Root Mean Squared of Successive Differences (RMSSD), as well as proportion of successive normal-to-normal interbeat-intervals that differed by more than 50 ms divided by the total number of NN intervals (pNN50). Frequency domain parameters were high frequency power (HF; 0.15–0.4 Hz) and the ratio of low frequency to HF power (LF/HF). RMSSD, pNN50 as well as HF-HRV represent the vagal tone, whereas LF-HRV represents a mix of both sympathetic and vagal activity and baroreflex activity (Laborde et al., Citation2017).
2.3.3. Positive and negative affect
To assess mood changes, the 20-item German version of the Positive and Negative Affect Schedule (Krohne et al., Citation1996; Watson et al., Citation1988) was completed before and after the anamnesis interview. Positive affect (PA) was further subdivided into joy, interest and activation (Egloff et al., Citation2003). Internal consistency of both time points was very good for PA, α = 0.81 and α = 0.88 and acceptable for negative affect (NA), α = 0.62 and α = 0.77.
2.4. Data analysis
All analysis were conducted in R (R Core Team, Citation2020) 3.6.3, RStudio 1.4.1103 and IBM SPSS Statistics 28. Normal distributions of variables were calculated with the Shapiro-Wilk test and we did not find outliers. For cortisol and sAA increase we computed the second sample minus first sample, as well as cortisol AUCg (Pruessner et al., Citation2003). HRV variables were log-transformed due to skewed distributions. For HR increase we computed the average HR of phase 2 (first phase of intervention) minus phase 1 (baseline). For HR and HRV comparisons between the intervention phase and the baseline we computed the average of phases 2–4 minus phase 1. Analyses of variances included the between-subjects factor group, the within subjects factor time (2 levels for affect, 4 levels for saliva samples and 5 levels for HR/HRV) as well as the following covariates: BMI, disease of the hepatobiliary system and metabolic disorders. All analyses regarding physiological parameters were calculated both including and excluding covariates to test for robustness and are reported using Greenhouse-Geisser correction. The level of significance for all analyses was α ≤ 0.05. Results reported below are controlled for covariates. Of note, the pattern of main findings was similar when covariates were not included, exceptions are noted. Moreover, we refer to effect sizes of partial Eta-squared = 0.02 as small;
= 0.08 as medium; and
= 0.16 as large (Cohen, Citation1977).
3. Results
3.1. Salivary cortisol and sAA
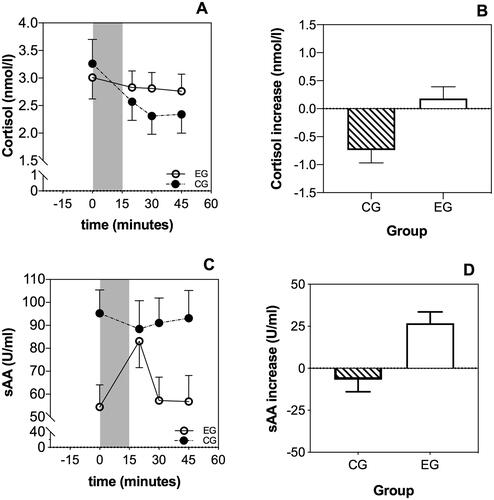
Salivary cortisol levels showed significant differences over time (4 levels) between the EG and CG (FTime*group(1.61,80.45) = 4.22, p = .025, = 0.08; ). This significant difference was based on a significantly stronger decrease in the CG as compared to the EG (i.e. significantly lower increase; Fgroup(1,51) = 7.06, p = .011,
= 0.12). However, groups did not significantly differ regarding total cortisol output, assessed by AUCg (Fgroup(1,55) = 0.23, p = .632,
= 0.005), suggesting that overall cortisol levels did not differ between the two groups. sAA levels showed a significant difference over time (4 levels) between the EG and CG (FTime*group(2.65,153.65) = 6.65, p < .001,
= 0.10). sAA increases after the intervention were significantly higher in the EG vs. CG (Fgroup(1,58) = 15.476, p < .001,
= 0.21). However, CG showed significantly higher baseline sAA levels than EG (see ; Fgroup(1,58) = 8.62, p = .005,
= 0.13).
Figure 1. Time course of salivary cortisol and sAA. Participants in the control group (CG) showed a significantly lower increase of cortisol after the intervention than the experimental group (EG) (A and B). Moreover, participants in the EG showed a significant increase of sAA levels after the interview (C and D).
3.2. HR and HRV
3.2.1. Time domain analyses
HR showed significant differences over time (5 levels) between the EG and CG (FTime*group(3.00,161.51) = 17.18, p < .001, = 0.24). In detail, differences emerged between phase 1 (baseline) and phase 2 (first phase of intervention), as well as between phase 4 (last phase of intervention) and phase 5 (recovery). On average, participants of the EG showed a significant increase in HR (; ) of M = 6.35 bpm (SD = 6.39) at the beginning of the intervention (Fgroup(1,54) = 13.97, p < .001,
= 0.21) and a significant decrease after the intervention as compared to the CG (Fgroup(1,54) = 41.58, p < .001,
= 0.44). pNN50, showed significant differences over time (5 levels) between EG and CG (FTime*group(2.79,150.59) = 5.21, p = .002,
= 0.10). In detail, differences emerged between phase 2 and phase 3 and between phase 4 and 5. On average, participants of the EG showed a significant decrease at the beginning of the intervention (Fgroup(1,54) = 5.62, p = .021,
= 0.10) and a significant increase after the intervention as compared to the CG (Fgroup(1,54) = 8.36, p = .006,
= 0.14). Regarding RMSSD, we did not find any group differences over time (p’s >.05) ().
Figure 2. Average HR assessed over time. The experimental group (EG) exhibited a significantly higher HR during the intervention as compared to the control group (A). HR of the EG increased by M = 6.35 bpm at the beginning of the intervention relative to baseline (B).
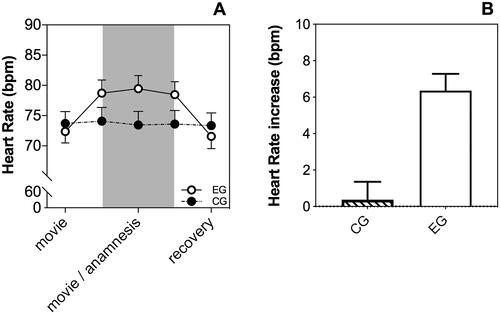
Table 2. Average salivary cortisol and salivary alpha-amylase (sAA) through study protocol.
Table 3. Average HR and HRV parameters through study protocol.
3.2.2. Frequency domain analyses
HF-power did not differ significantly over time between both groups (p > .05). However, the ratio of LF/HF showed significant differences over time (5 levels) between both groups as became evident by an interaction of time by group (FTime*group(3.5,191.59) = 7.11, p < .001, = 0.12). More precisely, the ratio during the intervention (phases 2–4) was statistically higher in the EG (M = 2.20, SD = 1.24) than in the CG (M = 0.53, SD = 1.61) (Fgroup(1,54) = 15.71, p < .001,
= 0.23). Moreover, the EG showed a higher decrease after the intervention than the CG (Fgroup(1,59) = 10.34, p = .001,
= 0.16).
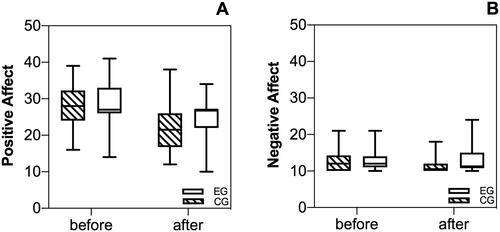
3.3. PA and NA
At baseline both groups reported similar NA (Fgroup(1,63) = 0.0, p = .971, = 0.0) and PA (overall PA: Fgroup(1,63) = 0.50, p = .481,
= 0.008; joy: Fgroup(1,63) = 1.41, p = .240,
= 0.02; activation: Fgroup(1,63) = 0.25, p = .619,
= 0.004; interest: Fgroup(1,63) = 1.36, p = .248,
= 0.02). After the anamnesis interview, participants of the EG reported significantly higher NA than controls (FTime*group(1,63) = 8.23, p = .006,
= 0.12). Further, PA as well as the subscales of PA were significantly higher in the EG after the anamnesis interview as compared to the CG (overall PA: FTime*group(1,63) = 21.54, p < .001,
= 0.26; joy: Fgroup(1,63) = 4.51, p = .038,
= 0.07; activation: Fgroup(1,63) = 19.29, p < .001,
= 0.23; interest: Fgroup(1,63) = 10.0, p = .002,
= 0.14). Change scores of PA and NA are visualized in .
Figure 3. Positive and negative affect before and after the intervention. Participants of the experimental group reported both increases of positive affect as well as negative affect after the intervention compared to the control group.
Higher NA after the interview was associated with higher cortisol AUCg (r = 0.51, p = .004), whereas higher PA after the interview was associated with a higher ratio of LF/HF (r = 0.26, p = .042) during the interview. Other associations were not significant (p’s >.05).
3.4. Word use analyses
In total, thirty-three of thirty-five interviews could be transcribed with an average duration of 14:06 minutes (SD = 2:03). The words used by participants ranged between 219 and 1,144 words (M = 734, SD = 232). Our target word categories showed the following associations: Higher cortisol output was associated with less use of Positive Emotion words (r = –0.51, p = .005). NA assessed with the PANAS before the interview, was associated with Biological Processes (r = –0.37, p = .035), more precisely with the subcategory Health (r = –0.37; p = .036). Apart from these associations, we found that total word count was associated with the word category Biological Processes (r = 0.37, p = .033) and the subcategory Body (r = 0.42, p = .014). The use of informal language (e.g. swear words, filler words) was associated with Affective Processes (r = 0.68; p < .001), more precisely with Negative Emotion (r = 0.76; p < .001).
4. Discussion
This study aimed to investigate psychophysiological indicators of stress during laboratory anamnesis interviews in a sample of young adults. With regard to physiological arousal, we found a significant increase of HR and sAA in the EG as compared to the CG. We further found a higher ratio of LF/HF power during the intervention within the EG compared to the CG. However, we did not find any group differences in the remaining HRV parameters. Moreover, cortisol AUCg did not differ between groups. Looking at psychological changes, participants in the EG showed both increases in NA as well as PA after the interview. Finally, word analyses showed that lesser use of positive emotion words was associated with higher overall cortisol.
Our results indicate that the anamnesis interview might have been appraised as a threat and therefore activated a generalized stress response of the SNS and the HPA axis. As mentioned earlier, patients in real medical settings are confronted with situations, in which the characteristics of these situations can provoke feelings of stress: The stressors may occur in a novel context (Rose, Citation1980), the situation itself may seem uncontrollable (Dickerson & Kemeny, Citation2004; Henry & Grim, Citation1990; Sapolsky, Citation1993) and may hold a threat to the physical self and thereby have the potential for physical harm (Blascovich & Tomaka, Citation1996; Dickerson et al., Citation2004; Dienstbier, Citation1989; Sapolsky et al., Citation2000), and the outcome of the situation is likely to be unpredictable (Mason, Citation1968). Therefore, experience of stress activates central and peripheral systems, when a stressor has specific aforementioned characteristics and exceeds the individual threshold (Chrousos & Gold, Citation1992, Citation1998; Dickerson & Kemeny, Citation2004; Lazarus & Folkman, Citation1984). Moreover, Dickerson and Kemeny (Citation2004) suggest that a threat either to the physical or the social self does induce activation of stress systems. In this study, the cover story of the announced venipuncture following the anamnesis interview could have contributed to the perception of physical threat. Moreover, in the anamnesis interview, participants talked about their own and their family’s medical history in front of two individuals and were audio-taped while telling private and delicate details about their personal life. The situation itself and the content of the interview could have been experienced as uncomfortable or shameful and therefore, be perceived as a threat to the social self.
Although most of our results are in accordance with the literature, we did not find the expected decrease of HRV during the intervention. Specifically, HRV, assessed by RMSSD and HF power did not show any significant differences between the two groups. However, the ratio of LF/HF power was higher during the interview within the EG as compared to the CG. Of note, the interpretation of the LF/HF-ratio as an indicator of autonomic balance has been criticized recently as the contribution of sympathetic efference to LF-HRV has been questioned (e.g. Reyes del Paso et al., Citation2013; Shaffer et al., Citation2014). Of note, some authors suggest that sympathetic and parasympathetic influences of LF-HRV could be attributed to the lower (0.06–0.1 Hz) and upper frequency band (0.1 − 0.14 Hz), respectively (Schwerdtfeger et al., Citation2020). Unless more research accumulates on the autonomic origin of the LF-band, the current results should be interpreted with caution. It should further be noted that HRV was not controlled for breathing and participants of the EG were speaking during the entire intervention, while participants of the CG remained quietly seated to ensure the resting state. Breathing and speaking patterns could alter HRV, thus compromising interpretation of the data (e.g. Laborde et al., Citation2017; Lehrer & Gevirtz, Citation2014; Schwerdtfeger et al., Citation2020). Taken together, our results indicate that the intervention did probably not lead to parasympathetic withdrawal. In accordance with the aforementioned self-preservation theory, especially the beginning of the anamnesis interview seemed to be perceived as a stressor due to the novel setting and thus, could have primarily affected the SNS.
Additionally, participants in the EG showed both increases in NA as well as PA after undergoing the interview. Our findings regarding increases of NA are in line with current literature regarding laboratory stress settings (e.g. Het et al., Citation2012; Minkley et al., Citation2014), however, to the best of our knowledge not fully investigated in this setting yet (e.g. Krohne, Citation2017; Krohne et al., Citation1996), whereas the increase of PA might be unexpected at the first glance. However, PA does not only imply feelings of joy – it rather considers states such as feeling alert or attentive (Egloff et al., Citation2003). Therefore, it seems reasonable to assume that undergoing an anamnesis interview also increases activated PA. Certain questions during the interview may have provoked certain feelings like shame or nervousness, whereas other questions may have increased one’s alertness and kept participants actively involved in the interaction. Therefore, the anamnesis interview enhanced both affective domains simultaneously.
Furthermore, we found that affect after the interview was associated with some of the physiological variables. In particular, NA assessed after the interview was associated with higher cortisol AUCg, while PA was associated with a higher ratio of LF/HF-power assessed during the interview. As mentioned earlier, LF/HF ratio represents a mix of sympathetic and vagal activity (Laborde et al., Citation2017). Therefore, higher PA might have indicated a more activated emotional state (e.g. alertness, attentiveness), which could have been associated with a higher sympathetic than parasympathetic activation.
Finally, our multimodal approach of investigating physiological and psychological arousal during anamnesis interviews was further supported through word content analyses of the performed interviews. We found that lesser use of positive emotion words was associated with higher cortisol output (AUCg), supporting the aforementioned finding of the association of NA, assessed by the PANAS, with cortisol AUCg.
4.1. Limitations
Of course, our study has some limitations. First, our study sample predominantly represents healthy young adults and due to limited access to our lab the sample size is smaller than a priori determined. Therefore, our results should not be generalized to an older sample seeking primary care. However, the chosen study population was necessary in this first study to decrease confounding variables due to age and health issues. Second, the control condition used in this study does not closely mirror the procedures in the experimental condition. However, we decided for this approach in this first study addressing this topic in order to compare responses to the interview with a complete resting state. In future studies, we intend to better understand these differences by including further control conditions. Third, although our results indicate that talking about one’s own medical history may elicit physiological and psychological arousal, given our study design, we cannot for certain disentangle which specific aspect of our laboratory anamnesis interviews caused the arousal. We strongly assume that the topic of the anamnesis interview in combination with the medical setup and the recording of the interview lead to the increase of arousal. However, we cannot rule out the mere fact that talking to a “doctor” (e.g. white coat effect) caused arousal.
4.2. Practice implications and future directions
Taken together, transactions with medical settings can be perceived as stressful, induce uncertainties and may evoke physiological stress reactions (Del Piccolo et al., Citation2019; Dickerson & Kemeny, Citation2004, Han et al., Citation2019). Previous research has shown that patient’s reactions to medical situations as well as poor medical communication has negative consequences for the adherence to a treatment plan leading to less health literacy and poor compliance as well as impaired recall of relevant medical information (Arbuthnott & Sharpe, Citation2009; Esfandiari et al., Citation2020; García-Llana et al., Citation2014; Karvinen et al., Citation2013; Miller, Citation2016; van Osch et al., Citation2014). Future research should therefore investigate how stress impacts patient’s attention to and comprehension of medical information during doctor-patient-interactions. In this study, mainly healthy young adults participated and provided information about their medical history. The physiological and psychological reactions observed here could therefore be quite strong when actual patients with specific health complaints undergo anamnesis interviews. Upcoming studies should consider the assessment of real patients during anamnesis interviews and provide more details about the narration of patients during these interviews, for instance, provide video-recordings to assess behavior changes not only via word analyses, but rather speaking patterns in general. Furthermore, future studies for instance could either compare an anamnesis interview to a neutral interview topic or compare two communication styles used in anamnesis interviews to focus more on the doctor-patient interaction (e.g. patient-centered communication; Hashim, Citation2017; King & Hoppe, Citation2013). Moreover, future study designs could elaborate inter- and intrapersonal differences when all participants prior to the intervention run through a waiting phase in which they occupy themselves as they would in a real doctor-patient setting when waiting for their appointment (e.g. reading, sending text messages, listening to music) and afterwards they undergo an interview condition. This addition would allow a deeper intrapersonal comparison between resting state, coping mechanisms and possible arousal during the intervention phase.
4.3. Conclusion
Previous research indicates that many medical procedures carry uncertainty and anxiety, which in turn could evoke feelings of fear (e.g. loss of control), despair and also worry (Del Piccolo et al., Citation2019; Han et al., Citation2019). Transactions with medical settings could therefore be perceived both as physical threat and threat to the social-self and may thus trigger physiological stress reactions (Dickerson & Kemeny, Citation2004). Our novel multimodal approach in this laboratory study significantly adds to our understanding of psychological experiences and physiological responses during a medical anamnesis interview, showing that such an interview can induce psychological and physiological stress responses. These results underscore the importance of managing and reducing patients’ stress experiences during medical procedures. For this research field, it is important to gain further information from experimental and longitudinal studies about patients psychological and physiological adaptation to the medical setting and assess generalized stress responses of the HPA and SNS to optimize current standard of procedures and medical communication training.
Acknowledgements
We thank the study team members for contributing: Jana Dörr, Anette Malapally, Carsten Jakob, Rebecca Koller, Alexander Maßen, Sabrina Mellerowic, Irene Schießler, Maike Stumpf, Jana Welling.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Sarah Sturmbauer, upon reasonable request.
Additional information
Notes on contributors
Sarah C. Sturmbauer
Sarah Sturmbauer, MSc graduated from University of Graz in Austria, in 2016. She is a psychologist currently working on her PhD supervised by Nicolas Rohleder at the Friedrich-Alexander University of Erlangen-Nürnberg on the assessment of stress in medical settings.
Andreas R. Schwerdtfeger
Andreas R. Schwerdtfeger is professor and head of the health psychology unit at the University of Graz, Austria. He received his Diploma in 1996 at the University of Freiburg, Germany, and his Ph.D. at the University of Wuppertal, Germany. His research activities focus on cardiovascular psychophysiology, stress and emotion, psychosocial resources and resilience, and psychophysiological ambulatory assessment.
Simon Schmelzle
Simon Schmelzle, BSc, a trained paramedic, studies psychology at Friedrich-Alexander University Erlangen-Nürnberg. Currently he is working on his master thesis in clinical psychology.
Nicolas Rohleder
Nicolas Rohleder, Ph.D. graduated from University of Trier in Germany, in 2003. He is a psychologist with a focus on biological and health psychology. His main research interest is on the pathways between Central Nervous System (CNS) states such as stress, depression, and trauma and pathophysiological changes in the organism. He currently directs the chair of Health Psychology at Friedrich-Alexander University Erlangen-Nürnberg.
References
- Arbuthnott, A., & Sharpe, D. (2009). The effect of physician-patient collaboration on patient adherence in non-psychiatric medicine. Patient Education and Counseling, 77(1), 60–67. https://doi.org/10.1016/j.pec.2009.03.022
- Becker, L., & Rohleder, N. (2020). Associations between attention and implicit associative learning in healthy adults: The role of cortisol and salivary alpha-amylase responses to an acute stressor. Brain Sciences, 10(8), 544. https://doi.org/10.3390/brainsci10080544
- Blascovich, J., & Tomaka, J. (1996). The biopsychosocial model of arousal regulation. In M. P. Zanna (Ed.), Advances in experimental social psychology (Vol. 28, pp. 1–51). Academic Press.
- Chrousos, G. P., & Gold, P. W. (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA, 267(9), 1244–1252. https://doi.org/10.1001/jama.1992.03480090092034
- Chrousos, G. P., & Gold, P. W. (1998). A healthy body in a healthy mind-and vice versa-the damaging power of “uncontrollable” stress. The Journal of Clinical Endocrinology and Metabolism, 83(6), 1842–1845. https://doi.org/10.1210/jcem.83.6.4908
- Cohen, J. (1977). Statistical power analysis for the behavioral sciences (rev. ed.) Lawrence Erlbaum Associates. Inc., Hillsdale, NJ, England.
- Del Piccolo, L., Mazzi, M. A., Mascanzoni, A., Lonardi, M., De Felice, M., Danzi, O. P., Buizza, C., Ghilardi, A., Bottacini, A., & Goss, C. (2019). Factors related to the expression of emotions by early-stage breast cancer patients. Patient Education and Counseling, 102(10), 1767–1773. https://doi.org/10.1016/j.pec.2019.04.002
- Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. https://doi.org/10.1037/0033-2909.130.3.355
- Dickerson, S. S., Gruenewald, T. L., & Kemeny, M. E. (2004). When the social self is threatened: Shame, physiology, and health. Journal of Personality, 72(6), 1191–1216. https://doi.org/10.1111/j.1467-6494.2004.00295.x
- Dienstbier, R. A. (1989). Arousal and physiological toughness: Implications for mental and physical health. Psychological Review, 96(1), 84–100. https://doi.org/10.1037/0033-295X.96.1.84
- Egloff, B., Schmukle, S. C., Burns, L. R., Kohlmann, C.-W., & Hock, M. (2003). Facets of dynamic positive affect: Differentiating joy, interest, and activation in the positive and negative affect schedule (PANAS). Journal of Personality and Social Psychology, 85(3), 528–540. https://doi.org/10.1037/0022-3514.85.3.528
- Esfandiari, M., Faramarzi, M., Nasiri-Amiri, F., Parsian, H., Chehrazi, M., Pasha, H., Omidvar, S., & Gholinia, H. (2020). Effect of supportive counseling on pregnancy-specific stress, general stress, and prenatal health behaviors: A multicenter randomized controlled trial. Patient Education and Counseling, 103(11), 2297–2304. https://doi.org/10.1016/j.pec.2020.04.024
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
- García-Llana, H., Remor, E., Del Peso, G., & Selgas, R. (2014). The role of depression, anxiety, stress and adherence to treatment in dialysis patients health-related quality of life: A systematic review of the literature. Nefrologia, 34(5), 637–657. https://doi.org/10.3265/Nefrologia.pre2014.Jun.11959
- Han, P. K. J., Babrow, A., Hillen, M. A., Gulbrandsen, P., Smets, E. M., & Ofstad, E. H. (2019). Uncertainty in health care: Towards a more systematic program of research. Patient Education and Counseling, 102(10), 1756–1766. https://doi.org/10.1016/j.pec.2019.06.012
- Hashim, M. J. (2017). Patient-centered communication: Basic skills. American Family Physician, 95(1), 29–34..
- Henry, J. P., & Grim, C. E. (1990). Psychosocial mechanisms of primary hypertension. Journal of Hypertension, 8(9), 783–793. https://doi.org/10.1097/00004872-199009000-00001
- Het, S., Schoofs, D., Rohleder, N., & Wolf, O. T. (2012). Stress-induced cortisol level elevations are associated with reduced negative affect after stress: Indications for a mood-buffering cortisol effect. Psychosomatic Medicine, 74(1), 23–32. https://doi.org/10.1097/PSY.0b013e31823a4a25
- Karvinen, K. H., Murray, N. P., Arastu, H., & Allison, R. R. (2013). Stress reactivity, health behaviors, and compliance to medical care in breast cancer survivors. Oncology Nursing Forum, 40(2), 149–156. https://doi.org/10.1188/13.Onf.149-156
- King, A., & Hoppe, R. B. (2013). “Best practice” for patient-centered communication: A narrative review. Journal of Graduate Medical Education, 5(3), 385–393. https://doi.org/10.4300/jgme-d-13-00072.1
- Krohne, H. W. (2017). Stress und Stressbewältigung bei Operationen. Springer-Verlag.
- Krohne, H. W., Egloff, B., Kohlmann, C.-W., & Tausch, A. (1996). Untersuchungen mit einer deutschen Version der “Positive and Negative Affect Schedule” (PANAS). Diagnostica-Gottingen, 42, 139–156.
- Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research – Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213. https://doi.org/10.3389/fpsyg.2017.00213
- Lazarus, R. S., & Folkman, S. (1984). Stress, appraisal, and coping. Springer publishing company.
- Lehmann, C., Koch, U., & Mehnert, A. (2009). [Impact of the doctor-patient-communication on distress and utilization of psychosocial services among cancer patients. A review of the current literature]. Psychotherapie Psychosomatik Medizinische Psychologie, 59(7), e3–e27. https://doi.org/10.1055/s-2008-1067443
- Lehrer, P. M., & Gevirtz, R. (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5, 756. https://doi.org/10.3389/fpsyg.2014.00756
- Mason, J. W. (1968). A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosomatic Medicine, 30(5, Pt. 2), 576–607. https://doi.org/10.1097/00006842-196809000-00020
- Miller, T. A. (2016). Health literacy and adherence to medical treatment in chronic and acute illness: A meta-analysis. Patient Educ Couns, 99(7), 1079–1086. https://doi.org/10.1016/j.pec.2016.01.020
- Minkley, N., Schröder, T. P., Wolf, O. T., & Kirchner, W. H. (2014). The socially evaluated cold-pressor test (SECPT) for groups: Effects of repeated administration of a combined physiological and psychological stressor. Psychoneuroendocrinology, 45, 119–127. https://doi.org/10.1016/j.psyneuen.2014.03.022
- Pennebaker, J. W., Boyd, R. L., Jordan, K., & Blackburn, K. (2015). The development and psychometric properties of LIWC2015. Austin, TX: University of Texas at Austin.
- Pennebaker, J. W., Francis, M. E., & Booth, R. J. (2001). Linguistic inquiry and word count: LIWC 2001. Lawrence Erlbaum Associates.
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/10.1016/s0306-4530(02)00108-7
- R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
- Reyes del Paso, G. A., Langewitz, W., Mulder, L. J., van Roon, A., & Duschek, S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology, 50(5), 477–487. https://doi.org/10.1111/psyp.12027
- Rohleder, N., & Nater, U. M. (2009). Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology, 34(4), 469–485. https://doi.org/10.1016/j.psyneuen.2008.12.004
- Rose, R. M. (1980). Endocrine responses to stressful psychological events. Psychiatric Clinics of North America, 3(2), 251–276. https://doi.org/10.1016/S0193-953X(18)30965-1
- Sapolsky, R. M. (1993). Endocrinology alfresco: Psychoendocrine studies of wild baboons. Recent Progress in Hormone Research, 48, 437–468. https://doi.org/10.1016/b978-0-12-571148-7.50020-8
- Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions*. Endocrine Reviews, 21(1), 55–89. https://doi.org/10.1210/edrv.21.1.0389
- Schwerdtfeger, A. R., Schwarz, G., Pfurtscheller, K., Thayer, J. F., Jarczok, M. N., & Pfurtscheller, G. (2020). Heart rate variability (HRV): From brain death to resonance breathing at 6 breaths per minute. Clinical Neurophysiology, 131(3), 676–693. https://doi.org/10.1016/j.clinph.2019.11.013
- Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology, 5, 1040. https://doi.org/10.3389/fpsyg.2014.01040
- Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O., & Karjalainen, P. A. (2014). Kubios HRV-heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. https://doi.org/10.1016/j.cmpb.2013.07.024
- Tausczik, Y. R., & Pennebaker, J. W. (2010). The psychological meaning of words: LIWC and computerized text analysis methods. Journal of Language and Social Psychology, 29(1), 24–54. https://doi.org/10.1177/0261927x09351676
- Thorne, S. E., Bultz, B. D., & Baile, W. F. (2005). Is there a cost to poor communication in cancer care?: A critical review of the literature. Psycho-oncology, 14(10), 875–884. https://doi.org/10.1002/pon.947
- van Osch, M., Sep, M., van Vliet, L. M., van Dulmen, S., & Bensing, J. M. (2014). Reducing patients’ anxiety and uncertainty, and improving recall in bad news consultations. Health Psychology, 33(11), 1382–1390. https://doi.org/10.1037/hea0000097
- van Stegeren, A., Rohleder, N., Everaerd, W., & Wolf, O. T. (2006). Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology, 31(1), 137–141. https://doi.org/10.1016/j.psyneuen.2005.05.012
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. https://doi.org/10.1037/0022-3514.54.6.1063
- Weintraub, W. (1989). Verbal behavior in everyday life. Springer Publishing Co.
- Wolf, M., Horn, A. B., Mehl, M. R., Haug, S., Pennebaker, J. W., & Kordy, H. (2008). Computergestützte quantitative textanalyse: äquivalenz und robustheit der deutschen version des linguistic inquiry and word count. Diagnostica, 54(2), 85–98. https://doi.org/10.1026/0012-1924.54.2.85
