Abstract
Maternal pre- and post-delivery stress levels might be different for vaginal or cesarean deliveries. This study aimed to investigate the effects of type of delivery (vaginal or cesarean) and time of delivery (pre- and post-delivery) on the stress axes of the body, namely the hypothalamic-pituitary adrenal axis (HPA) and autonomic nervous system (ANS).
Ninety-one pregnant women were volunteered to participate this prospective study. In these women, pre- and post-delivery HPA and ANS activities were measured noninvasively by salivary cortisol and heart rate variability (HRV), respectively. HRV was measured by 5-min electrocardiogram recording and time- and frequency-domain parameters were computed.
Salivary cortisol concentration and HRV parameters were higher in women having vaginal delivery than those having cesarean delivery (p < 0.05). Cortisol levels did not differ between pre- and post-delivery (p > 0.05) but the time-domain parameters of HRV decreased post-delivery (p < 0.05). No interactions were observed between the types and times of delivery (p > 0.05).
HPA and ANS axes had different activity patterns throughout the delivery process and they were higher during vaginal delivery, suggesting that they are integral parts of normal birth process and that cesarean delivery perturbs the activity of both axes.
1. Introduction
Vaginal delivery is a process causing numerous physiological changes in the woman and the newborn. Along with hormonal and anatomic changes, vaginal delivery requires activation of the hypothalamo-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS). End-product of the HPA axis, namely cortisol, increases threefold by third trimester (Duthie & Reynolds, Citation2013). Cortisol is considered to be a requisite for initiation of normal birth process and has numerous homeostatic functions (Wang et al., Citation2020). It regulates cardiovascular, metabolic, and immunologic functions (Wang et al., Citation2020) that are important for adaptations of the mother and the off-spring to the stress caused during pregnancy, delivery and post-delivery periods.
The other system which has a dynamic activity around delivery is the ANS. Sympathetic branch of the ANS increases the heart- and respiratory-rates before delivery (LoMauro & Aliverti, Citation2015; Söhnchen et al., Citation2011). This increase is observed toward the end of the last trimester, and reaches to the levels comparable to moderate to heavy exercise during labor (Söhnchen et al., Citation2011). A similar trend was also observed for heart rate variability (HRV), which is beat-to-beat variation in heart rate. It has been reported that HRV is a result of competing activities of two branches of the ANS, namely sympathetic and parasympathetic (vagal) branches (Shaffer et al., Citation2014). Therefore, HRV has generally been accepted as a noninvasive surrogate technic for determination of cardiac sympathovagal balance (Shaffer et al., Citation2014).
In the last decades, there has been a significant increase in the rate of cesarean delivery. According to data of the World Health Organization , rate of cesarean section is 32.8% in the USA (Betrán et al., Citation2016); whereas, this rate is as high as 47.5% in Turkey (Santas & Santas, Citation2018). The reason for this high rate is primarily reported to be due to the fear of vaginal delivery (Ozdemir et al., Citation2018). Although cesarean delivery is necessary when the health of the infant or the mother is at risk for vaginal delivery, the rate of cesarean delivery is two to three times more than it is medically indicated (Martinez et al., Citation2020). Moreover, cesarean delivery may have impact on the activities of the stress axes. For example, perception of comfort of painless surgery due to anesthesia might be compromised by the stress caused by anticipation of upcoming surgery (Prete et al., Citation2018). Moreover, delayed breastfeeding and mother-infant bond (Sandall et al., Citation2018) and post-surgery pain (Moore et al., Citation2016), injury and discomfort may increase activities of the stress axes following cesarean delivery.
Higher rates of cesarean delivery and associated risks dictate carrying out studies to find out the magnitude of deviations from the normal physiology. There is increasing evidence that cesarean delivery is associated with short-term and long-term health effects for women and infants (Sandall et al., Citation2018). These include altered immune development, increased likelihood of allergy, atopy, asthma, and obesity, etc. (Sandall et al., Citation2018). All of these immunologic or metabolic diseases have association with altered cortisol release (Carlsson et al., Citation2014; Herhaus et al., Citation2020; Hewagalamulage et al., Citation2016; Schleimer, Citation2000) and altered ANS function (Guarino et al., Citation2017; Voisin et al., Citation2017). Moreover, altered activity of stress axes of the mother is associated with disease development in the offspring (Chan et al., Citation2018). Despite these facts, the studies about the activities of stress axes before and after normal vaginal delivery and cesarean delivery are not well documented. Better understanding the deviations from normal physiology may help developing strategies for the management of cesarean delivery. Therefore, we aimed to compare pre- and post-delivery levels of cortisol and of autonomic nervous system activity in women undergoing cesarean or vaginal delivery.
2. Materials-methods
2.1. Study design
This prospective case-controlled study was started after approval of Adiyaman University Noninvasive Clinical Trials Ethics Committee (Approval number: 2019/3-11). The study was conducted between 1 June 2018 and 31 August 2018 at Gynecology and Obstetrics Clinic of Adiyaman University hospital. Ninety-one pregnant women (age >18 years) who did not have any disease registered to the regular pregnancy follow-up program. Pregnant women who were using anxiolytics and antidepressants, receiving psychological support in any period of pregnancy, and having high risk pregnancy (such as hypertension, diabetes, and fetal anomaly) were not included in the study. In the study, the pregnant women were divided into two groups as planning to have cesarean section or vaginal delivery. Pregnant women who would have vaginal delivery were examined by an experienced obstetrician in the delivery room. They were included to the study if they had a cervical opening of <2 cm, if they did not feel pain (visual analog score of <3) and if they did not have any obstacle for vaginal delivery. A total of 35 women were eligible to participate to the study and filled in the written consent form. They provided a 5-minute ECG record and a saliva sample (pre-delivery) for the determination of the activities of the autonomic nervous system (ANS) and HPA, respectively. Six hours after the delivery, when the pain had been relieved, procedures of saliva sampling and 5-min ECG recording were repeated (post-delivery). A total of 56 pregnant women who were decided to have a cesarean section and met the same inclusion and exclusion criteria were admitted to the service following examination by an experienced obstetrician. They signed in written consent form and provided saliva samples and 5-min ECG recordings before taking anesthesia for cesarean sectioning (pre-delivery). At 6th hour post-delivery, when the effect of anesthesia was over, procedures of saliva sampling and 5-min ECG recordings were repeated. Spinal anesthesia was administered to all pregnant women who had cesarean section. As the women did not feel well in the first 3–4 hours following cesarean section, we chose a six hours interval from the beginning of the first sampling for both groups of women. Moreover, the time of first sampling was also between 08:30 and 11:00 for both groups. The design of the study is summarized in and the information about the participants is presented in .
Figure 1. Experimental design. Women undergoing vaginal delivery (n = 35) or cesarean delivery (n = 56) accepted to participate to the current study. In the birth clinic, all parturitions took place between 08:30–11:00 h. Women provided saliva samples for cortisol measurement and had a 5-min electrocardiogram recording for HRV (heart rate variability) assessment before and after delivery (6 h after the first sampling).
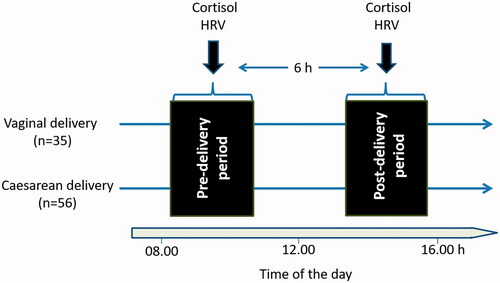
Table 1. Demographic and obstetrical variables of the participants (n = 91).
2.2. Saliva collection
Saliva samples were collected into 1.5 ml polypropylene tubes by passive droll method (Uçar et al. al., 2017 ; Ozgocer et al., Citation2021). The participants consumed no food or beverage until 30 minutes before saliva collection and did not brush their teeth. The saliva samples collected were kept at −45 °C until they were analyzed.
2.3. Measurement of cortisol in saliva
Following thawing, samples were centrifuged at 4000 g for 10 min and the supernatant was used for ELISA analyses as reported by Ozgocer et al. (Citation2017). Samples were diluted 5× with assay buffer and assayed in duplicate. Briefly, cortisol-BSA stock solution was diluted with carbonate buffer and added to a 96-well micro titer plate at 200 μL/well. Following incubation overnight at +4 °C, they were washed 5 times with wash buffer using eight-channel pipette. Binding sites not occupied by the coating antigen were blocked by the blocking buffer (200 μL/well) for 2 h at 37 °C. Following washing steps (5 times), standard solutions or samples (40 μL/well) and diluted primer antibody (antiserum, 40 μL/well) were added in duplicate and incubated at 37 °C for 45 min. Following washing 5 times, biotinylated anti-rabbit antibody was added (100 μL/well) and the plate was incubated at 37 °C for 30 min. The plate was washed 5 times and the streptavidin peroxidase solution (100 μL/well) was added and the plate was incubated for 15 min at +4 °C. Then, the plate was washed again for 5 times and the substrate solution (150 μL/well) was added and incubated in dark for 10 min. Following incubation, stop solution (50 μL/well) was added and the absorbance was measured at 450 nm using a microplate reader.
2.4. Heart rate variability
A 5-min continuous ECG was recorded to determine heart rate variability. ECG was recorded in supine positions with eyes open. Poly-Spectrum 8-E (Russia) was used for ECG record and a software program (Neurosoft, Ivanovo, Russia) provided with the same device was used to analyze heart rate variability (HRV) parameters.
Time- and frequency-domain parameters of the HRV are calculated by the software. The most widely used time-domain variables are SDNN (standard deviation of normal-to-normal intervals), RMSSD (root mean square of successive differences) and pNN50 (normal-to-normal R-R intervals that differ by more than 50 ms) parameters. The most used frequency-domain variables are TP (total power), LF (low frequency), HF (high frequency), LF n.u (low frequency normalized units), HF n.u (high frequency normalized units) and LF/HF ratio. All of these parameters were included in the study.
2.5. Statistical analyses
All statistical analyses were conducted using Minitab program (MINITAB 19, PA, USA). Data were analyzed by generalized linear model (GLM) as a 2 types of delivery (vaginal delivery vs. cesarean delivery) × 2 times of delivery (pre-delivery vs. post-delivery) factorial design including their interactions. Box-Cox transformation was applied for data which did not show normal distribution (optimal λ transformation was performed). The data were presented as mean ± standard deviation (SD). Partial eta-square values were reported to interpret the effect size. A p value ≤0.05 was considered to be statistically significant.
3. Results
3.1. Cortisol levels
Type of delivery affected salivary cortisol levels (p < 0.05) and it was higher in vaginal delivery group than the cesarean delivery group (p < 0.05, ). However, there were no differences between pre-delivery and post-delivery cortisol levels (p > 0.05). No interactions were observed in terms of cortisol levels (, p > 0.05).
Figure 2. Cortisol levels (mean ± SD) were higher in the women having vaginal delivery than that of the women having cesarean delivery (*p = 0.022). However, there were no differences between the cortisol levels measured before and after delivery (p > 0.05).
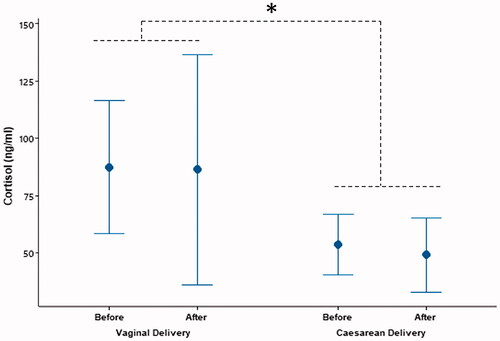
Table 2. Salivary cortisol and HRV parameters in vaginal and cesarean delivery before and after birth (mean ± SD).
3.2. HRV parameters
Heart rate (HR) was not affected by the type of delivery (p > 0.05) but it was higher pre-delivery than the post-delivery heart rates (p < 0.001). Even though HR was not influenced by the type of delivery, HRV parameters were significantly affected. Type of delivery affected both time-domain (SDNN, RMSSD, and pNN50) and frequency-domain parameters (TP, LF, and HF; p < 0.05, ). These parameters were higher in those of women who had vaginal delivery than those of women who had cesarean delivery (p < 0.05). For all these data, there were no interactions for type and time of delivery (p > 0.05).
HR, LF n.u., LF/HF, % VLF were higher before delivery (p < 0.05) but RR min, RR max, RRNN, RMSSD, p NN50, LF, HF, HF n.u., % LF and % HF were higher after delivery (p < 0.05).
No interactions were observed for time- and frequency-domain variables of HRV (p > 0.05).
4. Discussion
The current study, which investigated the functions of HPA and ANS axes pre- and post-delivery periods in women having vaginal or cesarean deliveries, revealed that both axes were activated during normal vaginal delivery but were quiescent during cesarean delivery. Moreover, activities of these axes (HPA and ANS) also differed from pre- to post-delivery stages, suggesting that they have different functions during transition from late pregnancy to early delivery stages. Thus, considering the role of HPA and ANS in the health of the mother and the baby, a special attention must be payed to the functioning of these axes during cesarean delivery.
4.1. Peri-partum cortisol levels were higher in women having vaginal delivery
Although cortisol is considered as a sign for stress, it also has vital beneficial functions in terms of coping with the demanding conditions like birth process. Increased cortisol secretion toward the end of pregnancy, appears to be a prerequisite for commencement of parturition process (Benfield et al., Citation2014). Elevated cortisol prepares both the mother and the fetus to parturition by causing maternal myometrial activation and contractility and fetal lung maturation (Kota et al., Citation2013). In the current study, cortisol levels before and after parturition were higher in women having vaginal delivery, suggesting that during normal vaginal delivery cortisol is high peri-partum. A study carried out by Miller et al. (Citation2019) showed that salivary cortisol levels increase gradually from the birth phases (latent phase, active phase, full dilation phase), reach to its maximum 2 min postpartum, and then gradually decreases at 2 h postpartum toward the basal levels at 24 h postpartum. The current study evaluated cortisol levels at the latent phase and at 6 h postpartum and the data show that both values are similar and high, suggesting that increased cortisol secretion is sustained until 6 h postpartum. The role of increased cortisol secretion early post-partum is not well known. But it is known that cortisol is necessary for lactogenesis. It has been reported that although the physiological trigger for lactogenesis is progesterone withdrawal postpartum, both prolactin and cortisol are needed for this triggering be efficient (Neville & Morton, Citation2001). Moreover, it has been shown that cortisol secreted into the milk, which is absorbed by the intestines afterwards, appears to protect neonates from developing obesity in humans (Hahn-Holbrook et al., Citation2016) and predicts infant growth patterns in rodents and non-human primates (Hinde, Citation2013; Hinde et al., Citation2015). Additionally, lower cortisol levels in neonates have been reported to limit their ability to handle stress and inhibit inflammation (Baud & Watterberg, Citation2019). Moreover, studies on the exact role of cortisol during peri-partum period are very scarce and the current study shows that it might be important to evaluate its effects on lactogenesis and on maternal and neonatal health.
4.2. Peripartum cortisol levels were lower in the women having cesarean delivery
In the current study, cortisol levels were lower in the women who had cesarean delivery. However, it is interesting to note that cortisol level was also lower in these women before commencement of cesarean section. Lower cortisol levels before parturition in the cesarean delivery might be due to psychological anticipation of painless delivery to be carried out by epidural anesthesia. On the other hand, vaginal delivery is associated with the increased cortisol secretion. Similar to the current study, Stjernholm et al. (Citation2016) and Kiriakopoulos et al. (Citation2019) observed higher cortisol levels in vaginal delivery group than that of cesarean group. During vaginal delivery, higher cortisol levels may be associated with physical stress, increased level of fear and anxiety, and increased pain. Although it was reported that the cortisol levels were not associated with pain during the labor (Benfield et al., Citation2014), it was observed that reducing the pain level with hydrotherapy decreased the cortisol levels 2-fold (Benfield et al., Citation2010). In terms of perceived stress between the women who were going to have vaginal or cesarean delivery, it was found women undergoing cesarean delivery were more anxious and had a lower sensitivity to physical pain (Tuschy et al., Citation2018). Available data, taken together with the current study, suggest that cortisol levels are lower during cesarean delivery. This finding is important and needs to be validated by further experiment taking into account pain, anxiety, and fear. Moreover, although it is difficult to determine whether cortisol is beneficial during labor, it seems that cesarean delivery compromises the activity of the HPA axis. Malfunctioning of the cortisol system during this time period may also have life-long effects on the offspring. It has been reported that cesarean section increases the risk for asthma up to 12 years and obesity up to 5 years (Sandall et al., Citation2018). Whether these risks have any correlation with decreased maternal cortisol secretion needs to be addressed. Moreover, in short-term, cesarean section is associated more with bleeding and infection due to invasive nature of this procedure (Nuruzzaman et al., Citation2020). Taking into consideration the prothrombotic activity (von Känel et al., Citation2008) and the anti-inflammatory (Nishiguchi & Taguchi, Citation2020) actions of cortisol, the current study also points out that lower cortisol during cesarean sectioning may also contribute to the risk for hemorrhage and infection.
Cortisol secretion follows circadian rhythm, increasing immediately following awakening in the morning (cortisol awakening response), decreasing to basal levels within one hour and reaching to its nadir in the midnight (Kaushik et al., Citation2014). In the current study, pre-delivery samples were taken after cortisol awakening response. Moreover, it is known that there is not much difference in cortisol levels during the study period (i.e. from 08:00 to 16:00 h) (Adam et al., Citation2017). As all the births took place in the morning (pre-delivery samples) and salivary samples were taken before delivery and 6 h after delivery, a circadian effects might still be possible on cortisol secretion. However, sampling for both vaginal and cesarean delivery was carried out at the same time window and there was no difference in cortisol secretion during pre- and post-delivery stages. More importantly, higher cortisol secretion in vaginal delivery persisted both for pre- and post-delivery stages, suggesting that the difference in cortisol secretion was due to type of delivery rather than the circadian effect.
4.3. Time- and frequency-domain parameters of HRV was lower during cesarean delivery
In the current study, without a difference in the heart rate (HR), variability of the heart rate (HRV) differed significantly between delivery types. This provides an important example as to how HRV changes without changes in HR. Moreover, HR decreased following parturition in both groups of women, suggesting that HR and HRV changes dynamically around birth. Lower HRV parameters have generally been associated with negative health outcomes. For example, lower time- and frequency-domain parameters have been associated with gestational diabetes (Gasic et al., Citation2007), pre-eclampsia (Murphy et al., Citation2015) and metabolic problems (Stuckey et al., Citation2014).
LF/HF is used as an index for sympathovagal balance (Shaffer et al., Citation2014). Accordingly, it seems that sympathovagal balance has become lower postpartum in the current study. It might be suggested that, increased anxiety and stress before parturition increases sympathovagal balance but a relaxation occurs following birth. From that point of view, it appears that HRV, and more specifically LF/HF parameter, successfully reflects peripartum change from parturition stress to postpartum relaxation. In the literature search, we were not able to find any other study evaluating the effects of birth process on HRV in women. A study carried out in mares showed a similar trend and both HR and SDNN were higher immediately before the birth and decreased at 120 min after the birth (Nagel et al., Citation2020). Data from the current study suggests that assessment of HRV peripartum might be physiologically relevant as HRV parameters change dynamically in this time window. Likewise, revealing causes and consequences of these changes might provide valuable information that might be utilized for better understanding the neuronal control of birth process.
HRV parameters were, in general, lower in the women who had cesarean delivery but it is difficult to assess pathophysiological causes and consequences of these changes. As lower HRV has been associated with bad health outcomes, cesarean section seems unfavorable. This difference in heart rate and its variability in cesarean section might be due to epidural anesthesia but the samples were taken before and long after (6 h) anesthetic use. Moreover, trends of changes were quite similar with vaginal delivery, further suggesting that these effects are not likely to be due to the effects of anesthetics.
4.4. Correlations between cortisol and HRV data
In the current study we did not find any correlation between cortisol and the parameters of HRV. Cortisol levels were high peri-partum but HRV parameters were reduced post-delivery during vaginal delivery. It seems that both axes have different types of action and dynamically respond to the birth process.
4.5. Conclusions
The current study shows that vaginal delivery is associated with higher cortisol and higher HRV values, suggesting that cesarean section may compromise the activity of the HPA and ANS axes. Additionally, the activities of both axes had different patterns at pre- and post-delivery stages, suggesting that they respond differently to the parturition process. Moreover, salivary cortisol and HRV showed dynamical changes with type and time of the delivery and, therefore, they might be considered as useful parameters to study the physiology of birth process.
Acknowledgements
This study was not supported by any organization.
Disclosure statement
No potential conflict of interest was reported by the author(s). The authors have notified that no competing interests conflict and published at the stage of preparation of this manuscript.
References
- Adam, E. K., Quinn, M. E., Tavernier, R., McQuillan, M. T., Dahlke, K. A., & Gilbert, K. E. (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. https://doi.org/10.1016/j.psyneuen.2017.05.018
- Baud, O., & Watterberg, K. L. (2019). Prophylactic postnatal corticosteroids: Early hydrocortisone. Seminars in Fetal & Neonatal Medicine, 24(3), 202–206. https://doi.org/10.1016/j.siny.2019.04.007
- Benfield, R. D., Hortobágyi, T., Tanner, C. J., Swanson, M., Heitkemper, M. M., & Newton, E. R. (2010). The effects of hydrotherapy on anxiety, pain, neuroendocrine responses, and contraction dynamics during labor. Biological Research for Nursing, 12(1), 28–36. https://doi.org/10.1177/1099800410361535
- Benfield, R. D., Newton, E. R., Tanner, C. J., & Heitkemper, M. M. (2014). Cortisol as a biomarker of stress in term human labor: physiological and methodological issues. Biological Research for Nursing, 16(1), 64–71. https://doi.org/10.1177/1099800412471580
- Betrán, A. P., Ye, J., Moller, A. B., Zhang, J., Gülmezoglu, A. M., & Torloni, M. R. (2016). The increasing trend in caesarean section rates: Global, regional and national estimates: 1990–2014. PLOS One, 11(2), e0148343 https://doi.org/10.1371/journal.pone.0148343
- Carlsson, E., Frostell, A., Ludvigsson, J., & Faresjö, M. (2014). Psychological stress in children may alter the immune response. The Journal of Immunology, 192(5), 2071–2081. https://doi.org/10.4049/jimmunol.1301713
- Chan, C., Law, B., Liu, Y. H., Ambrocio, A., Au, N., Jiang, M., & Chow, K. M. (2018). The association between maternal stress and childhood eczema: A systematic review. International Journal of Environmental Research and Public Health, 15(3), 395. https://doi.org/10.3390/ijerph15030395
- Duthie, L., & Reynolds, R. M. (2013). Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology, 98(2), 106–115. https://doi.org/10.1159/000354702
- Gasic, S., Winzer, C. h., Bayerle-Eder, M., Roden, A., Pacini, G., & Kautzky-Willer, A. (2007). Impaired cardiac autonomic function in women with prior gestational diabetes mellitus. European Journal of Clinical Investigation, 37(1), 42–47. https://doi.org/10.1111/j.1365-2362.2007.01752.x
- Guarino, D., Nannipieri, M., Iervasi, G., Taddei, S., & Bruno, R. M. (2017). The role of the autonomic nervous system in the pathophysiology of obesity. Frontiers in Physiology, 8, 665. https://doi.org/10.3389/fphys.2017.00665
- Hahn-Holbrook, J., Le, T. B., Chung, A., Davis, E. P., & Glynn, L. M. (2016). Cortisol in human milk predicts child BMI. Obesity, 24(12), 2471–2474. https://doi.org/10.1002/oby.21682
- Herhaus, B., Ullmann, E., Chrousos, G., & Petrowski, K. (2020). High/low cortisol reactivity and food intake in people with obesity and healthy weight. Translational Psychiatry, 10(1), 40. https://doi.org/10.1038/s41398-020-0729-6
- Hewagalamulage, S. D., Lee, T. K., Clarke, I. J., & Henry, B. A. (2016). Stress, cortisol, and obesity: a role for cortisol responsiveness in identifying individuals prone to obesity. Domestic Animal Endocrinology, 56, S112–S120. https://doi.org/10.1016/j.domaniend.2016.03.004
- Hinde, K. (2013). Lactational programming of infant behavioral phenotype. In K. Clancy, K. Hinde, & J. Rutherford (Eds.), Building babies. Developments in primatology: Progress and prospects (vol 37). Springer.
- Hinde, K., Skibiel, A. L., Foster, A. B., Del Rosso, L., Mendoza, S. P., & Capitanio, J. P. (2015). Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology, 26(1), 269–281. https://doi.org/10.1093/beheco/aru186
- Kaushik, A., Vasudev, A., Arya, S. K., Pasha, S. K., & Bhansali, S. (2014). Recent advances in cortisol sensing technologies for point-of-care application. Biosensors & Bioelectronics, 53, 499–512. https://doi.org/10.1016/j.bios.2013.09.060
- Kiriakopoulos, N., Grigoriadis, S., Maziotis, E., Philippou, A., Rapani, A., Giannelou, P., Tsioulou, P., Sfakianoudis, K., Kontogeorgi, A., Bakas, P., Mastorakos, G., Koutsilieris, M., & Simopoulou, M. (2019). Investigating stress response during vaginal delivery and elective cesarean section through assessment of levels of cortisol, interleukin 6 (IL-6), growth hormone (GH) and insulin-like growth factor 1 (IGF-1). Journal of Clinical Medicine, 8(8), 1112. https://doi.org/10.3390/jcm8081112
- Kota, S. K., Gayatri, K., Jammula, S., Kota, S. K., Krishna, S. V., Meher, L. K., & Modi, K. D. (2013). Endocrinology of parturition. Indian Journal of Endocrinology and Metabolism, 17(1), 50–59. https://doi.org/10.4103/2230-8210.107841
- LoMauro, A., & Aliverti, A. (2015). Respiratory physiology of pregnancy: Physiology masterclass. Breathe (Sheffield, England), 11(4), 297–301. https://doi.org/10.1183/20734735.008615
- Martinez, L. D., Glynn, L. M., Sandman, C. A., Wing, D. A., & Davis, E. P. (2020). Cesarean delivery and infant cortisol regulation. Psychoneuroendocrinology, 122, 104862. https://doi.org/10.1016/j.psyneuen.2020.104862
- Miller, N., Asali, A. A., Agassi-Zaitler, M., Neumark, E., Eisenberg, M. M., Hadi, E., Elbaz, M., Pasternak, Y., Fishman, A., & Biron-Shental, T. (2019). Physiological and psychological stress responses to labor and delivery as expressed by salivary cortisol: A prospective study. American Journal of Obstetrics and Gynecology, 221(4), 351.e1–e7. https://doi.org/10.1016/j.ajog.2019.06.045
- Moore, E. R., Bergman, N., Anderson, G. C., & Medley, N. (2016). Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Review, 11(11), CD003519. https://doi.org/10.1002/14651858.CD003519.pub4
- Murphy, M. S., Seaborn, G. E., Redfearn, D. P., & Smith, G. N. (2015). Reduced heart rate variability and altered cardiac conduction after pre-eclampsia. PLOS One, 10(9), e0138664. https://doi.org/10.1371/journal.pone.0138664
- Nagel, C., Melchert, M., Aurich, C., & Aurich, J. (2020). Differences in endocrine and cardiac changes in mares and her fetus before, during, and after parturition in horses of different size. Animals, 10(9), 1577. https://doi.org/10.3390/ani10091577
- Neville, M. C., & Morton, J. (2001). Physiology and endocrine changes underlying human lactogenesis II. Journal of Nutrition, 131(11), 3005S–3008S. https://doi.org/10.1093/jn/131.11.3005S
- Nishiguchi, A., & Taguchi, T. (2020). Designing an anti-inflammatory and tissue-adhesive colloidal dressing for wound treatment. Colloids and Surfaces B Biointerfaces, 188, 110737. https://doi.org/10.1016/j.colsurfb.2019.110737
- Nuruzzaman, K., Mostafizur, R., Aminur, R., Mahmudul, A., & Alam, K. (2020). Long-term effects of caesarean delivery on health and behavioural outcomes of the mother and child in Bangladesh. medRxiv, https://doi.org/10.1101/2020.03.12.20034975
- Ozdemir, M. E., Cilingir, I. U., Ilhan, G., Yildiz, E., & Ohanoglu, K. (2018). The effect of the systematic birth preparation program on fear of vaginal delivery and quality of life. Archives of Gynecology and Obstetrics, 298(3), 561–565. https://doi.org/10.1007/s00404-018-4835-0
- Ozgocer, T., Ucar, C., & Yildiz, S. (2021). Daily cortisol awakening response and menstrual symptoms in young females. Stress and Health, https://doi.org/10.1002/smi.3074
- Ozgocer, T., Yildiz, S., & Uçar, C. (2017). Development and validation of an enzyme-linked immunosorbent assay for detection of cortisol in human saliva. Journal of Immunoassay & Immunochemistry, 38(2), 147–164. https://doi.org/10.1080/15321819.2016.1230130
- Prete, A., Yan, Q., Al-Tarrah, K., Akturk, H. K., Prokop, L. J., Alahdab, F., Foster, M. A., Lord, J. M., Karavitaki, N., Wass, J. A., Murad, M. H., Arlt, W., & Bancos, I. (2018). The cortisol stress response induced by surgery: A systematic review and meta-analysis. Clinical Endocrinology, 89(5), 554–567. https://doi.org/10.1111/cen.13820
- Sandall, J., Tribe, R. M., Avery, L., Mola, G., Visser, G. H., Homer, C. S., Gibbons, D., Kelly, N. M., Kennedy, H. P., Kidanto, H., Taylor, P., & Temmerman, M. (2018). Short-term and long-term effects of caesarean section on the health of women and children. Lancet, 392(10155), 1349–1357. https://doi.org/10.1016/S0140-6736(18)31930-5
- Santas, G., & Santas, F. (2018). Trends of caesarean section rates in Turkey. Journal of Obstetrics and Gynaecology, 38(5), 658–662. https://doi.org/10.1080/01443615.2017.1400525
- Schleimer, R. P. (2000). Interactions between the hypothalamic-pituitary-adrenal axis and allergic inflammation. The Journal of Allergy and Clinical Immunology, 106(5 Suppl), S270–S274. https://doi.org/10.1067/mai.2000.110162
- Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Frontiers in Psychology, 5, 1040. doi:https://doi.org/10.3389/fpsyg.2014.01040.
- Söhnchen, N., Melzer, K., Tejada, B. M., Jastrow-Meyer, N., Othenin-Girard, V., Irion, O., Boulvain, M., & Kayser, B. (2011). Maternal heart rate changes during labour. European Journal of Obstetrics & Gynecology and Reproductive Biology, 158(2), 173–178. https://doi.org/10.1016/j.ejogrb.2011.04.038
- Stjernholm, Y. V., Nyberg, A., Cardell, M., & Höybye, C. (2016). Circulating maternal cortisol levels during vaginal delivery and elective cesarean section. Archives of Gynecology and Obstetrics, 294(2), 267–271. https://doi.org/10.1007/s00404-015-3981-x
- Stuckey, M. I., Tulppo, M. P., Kiviniemi, A. M., & Petrella, R. J. (2014). Heart rate variability and the metabolic syndrome: A systematic review of the literature. Diabetes Metabolism Research and Review, 30(8), 784–793. https://doi.org/10.1002/dmrr.2555
- Tuschy, B., Berlit, S., Stützer, P., Lis, S., Schmahl, C., Baumgärtner, U., & Sütterlin, M. (2018). Evaluation of psychosocial and biological parameters in women seeking for a caesarean section and women who are aiming for vaginal delivery: A cross-sectional study. Archives of Gynecology and Obstetrics, 297(4), 897–905. https://doi.org/10.1007/s00404-018-4654-3
- Uçar, C., Özgöçer, T., & Yildiz, S. (2017). Late-night exercise affects the autonomic nervous system activity but not the hypothalamo-pituitary-adrenal axis in the next morning. The Journal of Sports Medicine and Physical Fitness, 58(1–2), 57–65. https://doi.org/10.23736/S0022-4707.16.06766-9
- Voisin, T., Bouvier, A., & Chiu, I. M. (2017). Neuro-immune interactions in allergic diseases: Novel targets for therapeutics. International Immunology, 29(6), 247–261. https://doi.org/10.1093/intimm/dxx040
- von Känel, R., Mausbach, B. T., Kudielka, B. M., & Orth-Gomér, K. (2008). Relation of morning serum cortisol to prothrombotic activity in women with stable coronary artery disease. Journal of Thrombosis and Thrombolysis, 25(2), 165–172. https://doi.org/10.1007/s11239-007-0035-7
- Wang, W. S., Guo, C. M., & Sun, K. (2020). Cortisol regeneration in the fetal membranes, a coincidental or requisite event in human parturition? Frontiers in Physiology, 11, 462 https://doi.org/10.3389/fphys.2020.00462
