Abstract
The autonomic nervous system (ANS) is activated by stress and is closely related to the female menstrual cycle. Women with premenstrual syndrome (PMS) have an imbalanced ANS response in their premenstrual period. However, no studies have explored the reasons for the differences in ANS response among women. In this study, we investigated how the female menstrual attitude and acute social stress influence the ANS response in women with PMS. First, 277 women [24.35 ± 2.1] were selected to measure the mediating role of women’s menstrual attitude between PMS severity and perceived ANS response. Second, participants’ (50 women [23.23 ± 1.25] with and 46 women [22.92 ± 2.00] without PMS) heart rate (HR) and HR variability (HRV; reflecting the functioning of ANS) under social stress were measured during various menstrual cycle phases. The results indicated that menstrual attitude (bothersome and predictable) had mediating effects between the degree of PMS and perceived ANS response; when undergoing a high cognitive load (e.g. mental-arithmetic) task, the ANS of the PMS group demonstrated hypo-arousal and delayed recovery in the late luteal phase; Therefore, menstrual attitude could influence female perceived ANS response, which may be a risk factor for PMS. When women with PMS experience high-strength cognitive pressure in the premenstrual period, their ANS showed hypo-arousal and delayed recovery, which may be another risk factor for PMS.
LAY SUMMARY
This study revealed that the attitude to menstruation (bothersome and predictable) could affect the women’s autonomic nervous system (ANS) response, and this may be a risk factor of premenstrual syndrome (PMS). Meanwhile, when women with PMS experience high-strength cognitive social pressure, their ANS showed hypo-arousal and delayed recovery. This imbalanced ANS reaction may result in their inability to cope with the stressful stimuli and emotional experiences, which may be another risk factor for PMS.
1. Introduction
In women, the menstrual cycle is a periodic physiological change lasting for an average of 28 days. Almost 80% of women report having some symptoms during the week prior to menstruation (Lustyk & Gerrish, Citation2010). These symptoms interfere with normal life and therefore qualify as premenstrual syndrome (PMS) in 20–30% of women. PMS refers to a series of cyclical physical, emotional, and cognitive symptoms that regularly recur during the late luteal phase of the menstrual cycle (Ryu & Kim, Citation2015). The sex hormones secreted by the ovaries can adversely affect the function of the hypothalamus, thereby causing changes in related stress hormones (Girdler et al., Citation1998; Lustyk & Gerrish, Citation2010). Therefore, numerous researches explored the mechanism of PMS from the perspective of stress. Among them, the autonomic nervous system (ANS, including sympathetic and parasympathetic systems), one of the stress physiological responses (Kahle et al., Citation2016), had received special attention.
Compared to the healthy group, women with PMS have an imbalanced ANS response (higher sympathetic and lower parasympathetic response) in the resting state of the luteal phase (Porges, Citation2003; Pu et al., Citation2010), which may be related to the underlying mechanism of PMS (Matsumoto et al., Citation2007). Although hormonal changes with the menstrual cycle could affect the ANS response (Baker et al., Citation2008; Yildirir et al., Citation2002), they are secreted normally in women with PMS (Güllner, Citation1983; Hedqvist, Citation1977). This suggests that other factors may affect ANS imbalance.
Firstly, the imbalanced ANS response in PMS could be influenced by their psychological cognition to menstruation. A survey of 1,809 Chinese female college students found that three kinds of attitudes (Debilitating, Predictable and Bothersome) toward menstruation were correlated with menstrual symptoms (Yongai et al., Citation2015). The more serious the PMS in women, the higher their score of menstrual attitude (Li, Citation2020; Lustyk et al., Citation2011). In addition, these attitudes are closely related to the physiological response to stress. It could affect the ANS response in different degrees (Brosschot et al., Citation2010; Mcewen & Gianaros, Citation2010). Thus, the first study detected the mediating role of menstrual attitudes (Debilitating, Predictable and Bothersome) between the degree of PMS and ANS response.
Secondly, the imbalanced ANS response in PMS could be the manifestation of physiological allostatic load (AL) to stress. The AL theory believed that when individuals are frequently subjected to chronic or acute stressors, their physiological reactions may be dysregulated (low reactivity and delayed recovery), leading to the loss of ability to cope with other stressors (Duchesne & Pruessner, Citation2013; Kahle et al., Citation2016; McEwen & Stellar, Citation1993). In other words, in addition to chronic menstrual stress, acute social pressure could cause an imbalanced ANS response in women with PMS, so that it is hard for them to cope with menstrual stress in the luteal phase. Thus, based on the AL theory, it is necessary to investigate the ANS activity in women with PMS. Actually, previous studies found their imbalanced response under acute stress (Girdler et al., Citation1998; Klatzkin et al., Citation2010; Citation2014), but they left certain limitations. First, only one study detected both sympathetic and parasympathetic aspects, but there were limited participants, which did not achieve statistical significance (Oda, Citation2007). Second, to our knowledge, no studies measured the recovery phase of women with PMS. Delayed recovery has been recognized as a key mechanism underlying the relationship between stress and health. It indicates disorders in the regulation of stress response and the associated negative emotions (Brosschot et al., Citation2005; Willmann et al., Citation2012). Therefore, the second study introduced more data to explore the ANS response in the PMS group during stress task and recovery period.
In summary, in the first study, we supposed that attitude toward menstruation (Debilitating, Predictable and Bothersome) plays mediating roles between PMS degree and ANS response. In the second study, we hypothesized that under acute stressors, the ANS of the PMS group exhibit hypo-arousal and delayed recovery compared to the control group.
2. Study 1
2.1. Methods
2.1.1. Participants
This study was based on an online survey through questionnaires, namely Premenstrual Syndrome Scale (PSS) (Bancroft, Citation1993; Gengli et al., Citation1998), Menstrual Attitude Questionnaire (MAQ), and Menstrual Distress Questionnaire (MDQ), administered to 299 undergraduate or graduate female university students. The age of the subjects was between 18 and 30 years old. Exclusion criteria for all participants included a history of psychiatric disorder (n = 4), partly missing answers (n = 18). Finally, 277 individuals (24.35 ± 2.10) were included in the sample after screening.
2.1.2. Materials
The PSS was compiled by Bancroft, and the Chinese version was revised by Gengli (Bancroft, Citation1993; Gengli et al., Citation1998). It consists of 12 items and covers both psychological and physical symptoms, such as follows: “In the last 3 months, how much anxiety did you feel 5 days before menstruation?” The questionnaire is scored on a 4-point Likert-type scale, with 0 = no symptom, 1 = mild symptoms, 2 = symptoms had some effect on daily work and life, but were endurable, and 3 = symptoms had a severe effect on daily work and life and required treatment. The higher the score, the more serious is the symptom. Total scores of 6–10, 11–20, and >20 reflect mild, moderately severe, and severe PMS, respectively. The Cronbach’s alpha of the revised PSS is 0.80; here, it was 0.89.
The MAQ adopted the version of Kim (Kim Eun, Citation1993). The Chinese version consists of 30 items, divided into five categories (Yongai et al., Citation2011): Debilitating (10 items; e.g. “The physiological reaction during menstruation is normal”), Bothersome (5 items; e.g. “Men have a real advantage in not having the monthly interruption of a menstrual period”), Natural (4 items; e.g. “Menstruation allows women to be more aware of their bodies”), Predictable (5 items; e.g. “I have learned to anticipate my menstrual period by the mood changes which precede it”), and Denial of the negative effect (6 items; e.g. “Most women make too much of the minor physiological effects of menstruation”). It is scored on a 5-point Likert-type scale—ranging from 1 (strongly disagree) to 5 (strongly agree). The higher the score, the stronger is the participant’s menstrual attitude in that category. The Cronbach’s alpha for the Chinese version is 0.70; here, it was 0.74.
The MDQ was developed by Moos (Moos, Citation1968). The Chinese version includes 30 items (Yongai et al., Citation2015), divided into six categories: ANS reactions (9 items; e.g. “Cold sweats, cold hands and feet”), pain (5 items; e.g. “Cramps and headache”), Concentration (3 items; e.g. “Forgetful and distractible”), behavior change (5 items; e.g. “Decreased efficiency”), Negative affect (6 items; e.g. “anxiety and loneliness”), and Water retention (2 items; e.g. “swelling and painful breasts”). It is scored using a 5-point Likert-type scale ranging from 1 (no experience of the symptom) to 5 (severe experience of the symptom). The higher the score, the more severe is the participant’s symptom was in this category. The Cronbach’s alpha for the Chinese version is 0.93. Here, only the category MDQ-ANS was selected for analysis, the Cronbach’s alpha of which was 0.86.
2.2. Statistical analysis
It should be emphasized that the female ANS response data included here did not use objective observation variables, but uses their subjective evaluation data. The reason for this choice is because the severity of PMS and menstrual attitude are all subjective evaluation data, and the selection of ANS response data with the same measurement method is conducive to the fitting of the model.
Therefore, all data were analyzed using SPSS 22.0. First, the premise of the mediation analysis was that all variables need to be correlated in pairs. Therefore, correlational analyses were needed. Second, using the PROCESS macro in SPSS to examine the mediating role of menstrual attitude (Hayes, Citation2013). We used 1000 bootstrap samples, and biases were corrected at 95% confidence intervals (CIs) to calculate the indirect effect of each variable. If the CI of the indirect effect did not include zero, it indicated that the indirect effect was significant.
2.3. Results
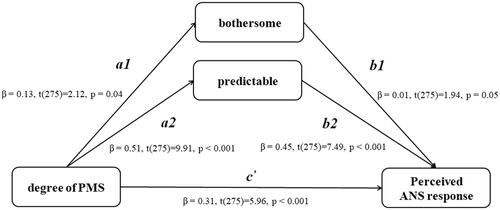
The correlation between the PSS and the MDQ-ANS was significant (p < 0.001). Debilitating, Bothersome, and Predictable categories in the MAQ were positively correlated with PSS and MDQ-ANS (all p < 0.001). Therefore, these three categories of MAQ could be used as mediating variables ().
Table 1. Means, standard deviations, and correlations between all study variables in study 1 (n = 277).
The mediation analysis revealed that the relationship between PMS severity and perceived ANS responses was partially mediated by two types of attitudes toward menstruation (bothersome and predictable; see ). The direct effect of PMS severity on perceived ANS responses was significant (β = 0.306, p < 0.001), and the indirect effects of PMS severity on perceived ANS responses through menstrual attitudes, namely bothersome and predictable, were both significant [indirect effect (95% CI) = 0.013 (0.005, 0.036) and 0.216 (0.139, 0.298), respectively]. Furthermore, the significant direct effect of PMS severity on perceived ANS responses indicates partial mediation.
3. Study 2
3.1. Methods
3.1.1. Participants
Participants were recruited from a local university and through an advertisement on a website. According to the criteria for PMS diagnosis of the American College of Obstetrics and Gynecology (ACOG), we required participants to report their daily symptoms during the next three months and fill out the PSS (the same as that in Study 1). Eventually, 66 women with PMS and 60 women without PMS were included. There are five affective symptoms (i.e. depression, nervousness, irritability, anxiety, and neurotic), three behavioral symptoms (i.e. restless, absent-minded, and insomnia or hypersomnia), and three somatic symptoms (i.e. abdominal bloating, headache, and swelling of extremities) in the criteria. The inclusion criteria for PMS were met: (1) the total score of PSS > 11; (2) symptoms must occur during the 5 days before menstrual bleeding for at least 3 consecutive menstrual cycles and at least one affective, one somatic symptom, and one behavioral symptom must be present; (3) symptoms are relieved within 4 days without recurrence until cycle day 13; (4) symptoms are present in the absence of medicine, hormone ingestion or alcohol use. Exclusionary criteria included pregnancy, breastfeeding, irregularity of menstrual cycle, history of psychiatric disorders, and current use of drugs. Thirteen participants were excluded – six had irregular menstrual cycles, five were taking antibiotics or hormonal drugs, and two had depression syndrome.
After that, 60 women with and 53 women without PMS were asked to participate in the experiments. No significant differences in age or variations in the menstrual cycle were observed between the groups. We assumed every participant’s late luteal and follicular phases according to their menstrual cycles in the previous 3 months and invited them to participate in the experiment twice: once in the late luteal phase and once in the follicular phase (the period after menstruation when premenstrual syndrome disappears). The phases during which the participants first came to our laboratory were assigned randomly. Finally, 11 participants did not come back for the second time, 6 participants’ resting heart rates were extreme to others (greater than 100/min or less than 50/min). Totally, these 17 participates were excluded. The reason why we set the lower limit of HR at 50 bpm was that in our initial data collection, we noticed that three girls of the participates exercised regularly every week and did not have any ANS-related diseases. It is well known that people who exercise a lot have a relatively low resting heart rate. Therefore, these three girls’ data were included. Ultimately, 50 PMS (23.23 ± 1.25) and 46 Non-PMS (22.92 ± 2.00) women were included in our analysis at last. According to G*Power instructions (Faul et al., Citation2007), 96 participants have sufficient analytical power (1 − β = 0.80, α = 0.05) for detecting medium-sized effects (r = 0.25). The statistical test we chose was the ANOVA: Repeated measures, within-between interaction.
The study was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki. All participants provided their written informed consent.
3.1.2. Materials
Social stress task was adopted to assess the ANS response (Oldehinkel et al., Citation2011; Sijtsema et al., Citation2015), which consists of public-speaking and mental-arithmetic tasks. Regarding the speech task, the participants had 3 minutes to prepare their speech content. After the preparation, a research assistant took the participants to a room, where two trained graduate students waited to examine their performance. Because everyone had to come twice, the test materials were divided into two versions (A and B). All participants were randomly assigned the order of these two versions on their initial arrival at the lab. Versions A and B of the speech task included self-introduction and job interview, respectively. The arithmetic task included mentally calculating every result starting from 1022 and repeatedly subtracting 13 and 16 until 0 was reached, respectively. Participants were asked to declare every result out loud until the end of the allotted time. In the event of an incorrect response, they were required to start again from 1022.
The Beck anxiety inventory (BAI), translated into Chinese (Zheng et al., Citation2002), was used to measure individual anxiety affect. The scale consists of 21 items, rated on a 4-point scale from 1 (no) to 4 (extremely heavy) to indicate the extent to which items were representative of respondents’ affect. The reliability of the questionnaire was acceptable (Cronbach’s alpha = 0.95; Zheng et al., Citation2002), and the scale had an acceptable Cronbach’s alpha of 0.90 in this study.
The Beck Depression Inventory (BDI), translated into Chinese (Zhang et al., Citation1990), was used to measure individual depression affect. The scale includes 21 items, rated on a 4-point scale from 0 (no) to 3 (extremely heavy) to indicate the extent to which items reflected respondents’ affect. The reliability of the questionnaire was acceptable (Cronbach’s alpha = 0.85), and the scale had an acceptable Cronbach’s alpha of 0.83 in this study.
3.1.3. Measures
ANS function can be measured using heart rate (HR) and HRV, an index of consecutive changes in HR. The frequency-domain index of HRV [high-frequency (HF), low-frequency (LF), and LF/HF] can reflect an individual’s ANS functioning, and it is a more sensitive indicator than skin electrical measurement or blood pressure (Bajkó et al., Citation2012; Baker et al., Citation2008). The HR is one of the indicators of the sympathetic nervous system (SNS) of the ANS. Power in the HF component reflects the influence of the parasympathetic nervous system (PNS) branch of the ANS, and power in the LF component is mediated by both SNS and PNS activities. The LF/HF ratio reflects sympathovagal balance. Increases in the ratio in response to stress are hypothesized to be an indicator of physiological response in the ANS such that higher values indicate the dominance of the SNS and lower values indicated the dominance of the PNS (García-Rubio et al., Citation2017; Luecken & Gallo, Citation2008). Although this interpretation has been disputed (Billman, Citation2013), it is still widely used in research and can explain our hypothesis to some extent, therefore, we kept them in the analysis.
3.1.4. Procedure
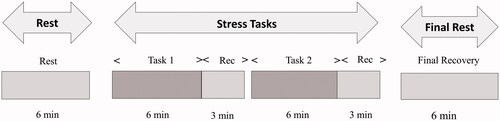
The experimental sessions were performed between 9:00am and 11:00am and 3:00pm and 5:00pm in soundproof rooms with blinded windows in the psychological laboratory of a school. After a 10-min rest, participants underwent baseline measurement, two stress tasks, and final-rest measurement (). The HR and HRV were monitored. The time for each task was 6 min and the recovery period for each task was 3 min. We measured the recovery period after each task. The recovery time was determined based on previous results of ANS showing that 3 min is sufficient to recover from a speech or math task (Sijtsema et al., Citation2015). To prevent interaction between tasks, half of each group first undertook the speech task, and the other half first underwent the mental-arithmetic task. After all tasks, participants finally underwent 6 min rest measurement.
3.1.5. Electrocardiography recording
Electrocardiography (ECG) data were collected using the BIOPAC MP150 sampling at 500 Hz with 0.5-Hz high-pass and 35-Hz low-pass filters. The HR of each participant was obtained on the basis of the interbeat interval (R–R) that was immediately extracted from the ECG signal. The ground (GND) was connected to the right lower limb, the VIN + was connected to the left lower limb, and the VIN − was connected to the left upper limb. R waves were detected using an automated algorithm enabling the R–R interval to be calculated by the program. The detection of R waves was visually checked and edited where the automatic detection was incorrect.
3.2. Statistical analyses
HR and HRV data were calculated on AcqKnowledge 4.2. The original data of HRV were edited as follows: the power sums of the raw data are reported in units of s2/Hz. We transformed them into units of ms (Ryu & Kim, Citation2015) according to HF (0.15–0.4 Hz) and LF (0.04–0.15 Hz) power (Bajkó et al., Citation2012). It was obtained via Fast Fourier Transforms (FFTs) of the ECG signals. Afterward, the data were log-transformed to present the data clearly for reducing skewness and kurtosis. The measurement and data analysis followed the recommendations of the task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996).
We used the D-value, such as ΔHR, HRV-ΔHF, ΔLF, and ΔLF/HF, to indicate the degree of ANS response. Specifically, during the task period, the D-value used for comparison was the difference by subtracting baseline data from data of task period. During the recovery period, the D-value was the difference between data in the recovery period and data in the task period. The data in the first 3 min of the two tasks were used because stress responses were likely to be highest in the beginning (Sijtsema et al., Citation2015). In order to be consistent with the task and recovery time, we used the 3 min of data in the middle of baseline. 2 (time: task, recovery) × 2 (group: PMS, non-PMS) × 2 (phase: late luteal, follicular) repeated-measures analysis of variance (ANOVA) were performed to analyze every task (speech, arithmetic) respectively. Greenhouse–Geisser freedom correction was used. All data were analyzed using SPSS 22.0.
3.3. Results
3.3.1. Demographic variables
Results indicated no group differences in terms of age, BMI, menstrual flow duration, menstrual cycle length (all t [94] < 1.45, all ps > 0.15). However, scores on the BAI and BDI of women with PMS were higher than those of healthy women (all t [94] > 2.83, all ps < 0.01). Descriptive statistics for the demographic variables, questionnaires, and hormones of PMS and healthy groups are listed in .
Table 2. Descriptive statistics in study 2 (M ± SD).
3.3.2. ANS measurement
The HR and HRV values of the two groups during tasks are listed in . However, we used the D-value to indicate the degree of ANS arousal. As shown in , the speech task results revealed that the main effect of time in HR (F(1, 94) = 433.34, difference in means [95%CI, 17.06–21.29], p < 0.001, η2 = 0.82) and HRV-LF/HF (F(1, 94) = 6.57, difference in means [95%CI, 0.08–0.62], p = 0.01, η2 = 0.07) were significant. The values in the task period were higher than those in the recovery period. No significant main effects were observed under the group and phase conditions (all p > 0.15). The interaction among three factors concerning HR (F(1, 94) = 10.71, difference in means [95%CI, 2.36–9.22], p = 0.002, η2 = 0.12) was significant. In the late luteal phase, HR in the PMS group was higher than that in the non-PMS group during the recovery period (F(1, 94) = 11.21, difference in means [95%CI, 1.06–7.49], p = 0.02, η2 = 0.13). The interaction among groups and time concerning LF/HF was significant (F(1, 94) = 5.31, difference in means [95%CI, 0.03–1.04], p = 0.02, η2 = 0.05). LF/HF during the recovery period was lower than that in the task period in the non-PMS group (F(1, 94) = 11.37, difference in means [95%CI, 0.21–0.81], p = 0.001, η2 = 0.11). Furthermore, the group difference with regard to LF and HF did not reach statistical significance (all p > 0.35).
Figure 3. ΔHR and ΔLF/HF reactivity and recovery among two groups in the speaking task. During the task stage, ΔHR and Δ LF/HF were the difference between data in the task period and data in the baseline period; During the recovery stage, ΔHR and Δ LF/HF were the difference between data in the recovery period and data in the task period. Error bars depict standard error of the mean. *p < 0.05.
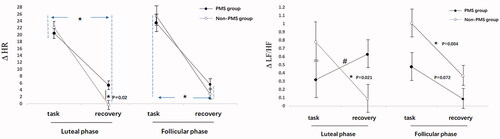
Table 3. HR and HRV (lg(ms2)) in the two tasks (M ± SD).
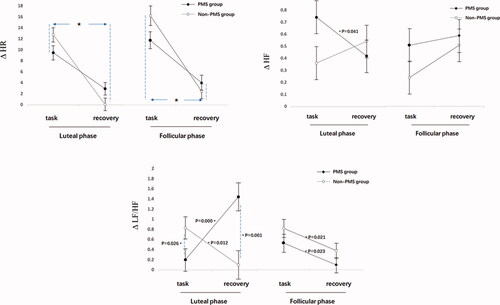
As shown in , the arithmetic task results revealed that the main effect of time in HR (F(1, 94)= 178.75, difference in means [95%CI, 8.66–11.69], p < 0.001, η2 = 0.66) was significant. The values in the task period were higher than those in the recovery period. No significant main effects were observed under the group and phase conditions (all p > 0.057). The interaction among groups and time concerning. HF during the recovery period was higher than that in the task period in the non-PMS group (F(1, 94) = 4.82, the difference in means [95%CI, 0.03–0.46], p = 0.04, η2 = 0.05). Furthermore, the interactions among three factors of HF and LF/HF were significant. During the late luteal phase, the HF of the PMS group during the recovery period was lower than that in the task period (F(1, 94) = 4.29, difference in means [CI 95%, 0.13–0.64], p = 0.04, η2 = 0.04); LF/HF in the PMS group was lower than that in the non-PMS group during task period in the late luteal phase (F(1, 94) = 5.20, the difference in means [95%CI, 0.08–1.19], p = 0.03, η2 = 0.05), but it became higher than that in the non-PMS group during the recovery period (F(1, 94) = 10.98, difference in means [95%CI, 0.73–1.96], p = 0.001, η2 = 0.11). LF was not significantly different throughout the process (all p > 0.07).
Figure 4. ΔHR, ΔHF and ΔLF/HF reactivity and recovery among two groups in the mental arithmetic task. During the task stage, ΔHR, ΔHF and Δ LF/HF were the difference between data in the task period and data in the baseline period; During the recovery stage, ΔHR, ΔHF and Δ LF/HF were the difference between data in the recovery period and data in the task period. Error bars depict standard error of the mean. *p < 0.05.
The data of the final rest was not significantly different from the previous baseline results (all p > 0.082).
4. Discussion
The first study analyzed the mediating role of menstrual attitudes in the relationship of PMS severity and ANS responses. Previous studies had reported that women who endorsed the attitude that menses are a bothersome and predicable event also reported more PMS (Lustyk et al., Citation2011). At the same time, in addition to the severity of PMS, these attitudes are closely related to the physiological response to stress. Nevertheless, they did not focus on the effect of attitudes on the relationship between the PMS degree and ANS response. Our results indicated that the attitudes concerning predictable and bothersome were retained as mediating variables, which affected the relationship between the degree of PMS and ANS response. Specifically, for one thing, it is the prediction toward events that can have an impact on the stress responses of individuals (Mcewen & Gianaros, Citation2010). For this reason, women with PMS regard the upcoming menstruation as a stressful event (Chayachinda et al., Citation2008), so that their prediction toward menstruation could affect the ANS response. For example, in one experiment, women who were led to believe that they were premenstrual reported experiencing a significantly higher degree of several physical symptoms than did women who were led to believe they were intermenstrual (Ruble, Citation1977). For another, Brosschot et al. (Citation2010) proved that conscious perseverative cognition, that is, worry and annoyance, has physiological effects (Brosschot et al., Citation2010). Meanwhile, negative attitudes toward menstruation can put women at odds with one another with regard to how to manage menstrual distress (Margaret et al., Citation2004), which may bring their different degrees of the stress response. Furthermore, the premise of mediation is that the self-report measures of PSS (PMS symptom) are closely related with MDQ-ANS (reflecting subjectively felt ANS response). Interestingly, we found that when subjects with PMS account for more than 40% of the total sample, the correlation of them is too high (r = 0.84), however, in the condition of PMS individuals account for 30% of the total sample (consistent with the distribution size of people with PMS in society), this correlation would become normal (r = 0.52). Thus, to accurately test the mediating effect, it is important to pay attention to the distribution of PMS samples. Overall, the attitude to menstruation (bothersome and predictable) could affect the women’s subjective ANS response, and this may be a risk factor of PMS.
The results of the second study indicated that the HR and HRV of women with PMS exhibited typical characteristics of AL (hypo-arousal and delayed recovery) under acute stress. First of all, compared to the non-PMS group, women with PMS showed slow recovery of sympathetic response under acute stress tasks, which was manifested by the high levels of HR and LF/HF. The HR is one of the indicators of the sympathetic nervous system (SNS). Besides, there are reports of higher LF/HF ratio values indicating the dominance of the SNS and lower values indicating the dominance of the PNS (Luecken & Gallo, Citation2008). All of this suggests that the SNS had a delayed recovery in the PMS group. However, rapid recovery from stress is a key indicator of health. In other words, prolonged activation can lead to a pathogenic state that may eventually lead to organic disease (Dragomir et al., Citation2014; Sijtsema et al., Citation2015). According to AL theory, delayed recovery can be defined as a post-stress rest period that provides information about the persistence of physiological arousal after the stressor is removed, and it has been recognized as a key mechanism underlying the relationship between stress and health (Brosschot et al., Citation2005; Willmann et al., Citation2012). Thus, the prolonged SNS response may be the reason why women with PMS experience negative emotions during the luteal phase. Next then, during the luteal phase, women with PMS had a hypo-arousal of parasympathetic responses to the arithmetic task. The HF (reflects the influence of the parasympathetic nervous system, PNS) of the control group during the recovery period was higher than it during the task, whereas it did not differ in the PMS group. Under normal circumstances, the PNS should be evoked in the recovery period, which indicated more positive coping responses and the inhibitory effect of the PNS on the SNS by shifting resources to restorative, homeostatic functions (Pu et al., Citation2010). Therefore, the unraised HF of the PMS group may indicate their poor regulation after stress. However, we did not find a significant change with regard to the HF in the speech task. Similarly, some studies have not observed significant changes in HF under pressure (García-Rubio et al., Citation2017; Jönsson et al., Citation2010; Rodrigues et al., Citation2018). This may be due to the domination of the SNS over the PNS under certain tasks (García-Rubio et al., Citation2017; Kaur et al., Citation2013). Lastly, in the arithmetic task, in addition to finding a hypo-arousal of HF in women with PMS, it was also found that there were phase differences in their LF/HF index. During the late luteal phase, LF/HF in the PMS group was lower than the control group in the task period. Instead, it was higher in the recovery period. Thus, the results of the PMS group showed that their SNS was not dominant (hypo-arousal) in the task period, while their PNS were also not dominant in the recovery period. This indicated both SNS and PNS in the PMS group exhibited hypo-arousal and a delayed recovery existed in ANS. The delayed recovery has been recognized as a manifestation of an imbalanced stress response, which leads to negative emotions (Brosschot et al., Citation2005; Willmann et al., Citation2012). Meanwhile, the hypo-arousal to stress in individuals increases their risk for disease and predicts heightened premenstrual symptom severity (Klatzkin et al., Citation2010; Citation2014). In short, this hypo-arousal and delayed recovery are typical characteristics of AL. The AL can be thought of as the biological “wear and tear” on the body as a result of its inability to cope with the stressful stimuli and emotional experiences (Booth et al., Citation2015; Kahle et al., Citation2016; Oldehinkel et al., Citation2011)
However, we analyzed the speech and arithmetic stressor separately because of the inconsistent results from previous studies. For example, Klatzkin et al. (Citation2010) verified that the HR from the speech task in the PMS group was lower than that of the healthy group, but they did not find differences between the two groups during the math task (Klatzkin et al., Citation2010). However, Oda (Citation2007) proved that the LF/HF in women with PMS during the math task was lower than that of the healthy individuals (Oda, Citation2007). Certainly, our results show that the ANS of women with PMS exhibited typical characteristics of AL (hypo-arousal and delayed recovery) under arithmetic stressors. Compared with the speech task, the math task could cause an increased cognitive load to people, which implies that participants need to be allocated sufficient cognitive resources to perform it (Galy & Mélan, Citation2015). In brief, we believed that when women with PMS experience high-strength cognitive pressure in the late luteal phase, their ANS showed typical characteristics of AL (hypo-arousal and delayed recovery) so that it is hard for them to cope with menstrual pressure at that time. Therefore, a higher cognitive load social stress may be another risk factor for PMS.
Some limitations must be noted. First, for the fitting of the mediating model, the first study used female subjective evaluation data of ANS response. More researches will be needed to explore to what extent the subjective ANS data represents objective physiological data. Second, owing to the particularity and limitation of the participants, we included the data of three girls whose resting HR was close to 60 bmp. Future studies should have sufficient subjects to replicate the results. Second, there is no abnormal threshold for HRV indicators and the LF/HF index has been argued more recently 38. Thus, the interpretation of this result should be cautious. Future research can use more ways to measure ANS response. Third, the menstrual cycle was determined by retrospective information, which may decrease the reliability of the results. Future studies should choose more objective measurements to estimate cycle time (Mccarthy & Rockette, Citation1983).
In summary, our study revealed that the attitude to menstruation (bothersome and predictable) could affect the women’s perceived ANS response, and this may be a risk factor of PMS. Meanwhile, when women with PMS experience high-strength cognitive social pressure, their ANS showed typical characteristics of AL (hypo-arousal and delayed recovery). This imbalanced ANS reaction may result in their inability to cope with stressful stimuli and emotional experiences, which may be another risk factor for PMS.
Acknowledgments
The authors thank the volunteers of this study and the funders who supported the project. The authors would also like to thank Dr. Lirong Chen, Weiyi Zhou, and Yaling Deng for their assistance in giving comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yao Meng
Yao Meng, PhD, Assistant Professor of the School of Nursing, Nanjing Medical University, China. She conducts research in the areas of cognitive and health psychology.
Lei Chang
Lei Chang, PhD, Chair Professor of Psychology and Head of the Department of Psychology, University of Macau. He has published over 250 journal papers in the areas of evolutionary and developmental psychology, with over 20,000 citations and h-Index of 63 (by Google Scholar).
Lulu Hou
Lulu Hou, PhD, Assistant Professor of the Department of Psychology, Shanghai Normal University, China. She conducts research in the areas of cognitive and health psychology.
Renlai Zhou
Renlai Zhou, PhD, Professor and Head of the Department of Psychology, Nanjing University, China. He has published over 240 journal papers in the areas of cognitive neuroscience and cognitive psychology, especially emotion and working memory training.
References
- Bajkó, Z., Szekeres, C.-C., Kovács, K. R., Csapó, K., Molnár, S., Soltész, P., Nyitrai, E., Magyar, M. T., Oláh, L., Bereczki, D., & Csiba, L. (2012). Anxiety, depression and autonomic nervous system dysfunction in hypertension. Journal of the Neurological Sciences, 317(1–2), 112–116. https://doi.org/10.1016/j.jns.2012.02.014
- Baker, F. C., Colrain, I. M., & Trinder, J. (2008). Reduced parasympathetic activity during sleep in the symptomatic phase of severe premenstrual syndrome. Journal of Psychosomatic Research, 65(1), 13–22. doi:https://doi.org/10.1016/j.jpsychores.2008.04.008
- Bancroft, J. (1993). The premenstrual syndrome-a reappraisal of the concept and the evidence. Psychological Medicine, 24(24), 1–47. https://doi.org/10.1017/s0264180100001272
- Billman, G. E. (2013). The lf/hf ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in Physiology, 4, 26. https://doi.org/10.3389/fphys.2013.00026
- Booth, T., Royle, N. A., Corley, J., Gow, A. J., Valdés Hernández, M. d-C., Muñoz Maniega, S., Ritchie, S. J., Bastin, M. E., Starr, J. M., Wardlaw, J. M., & Deary, I. J. (2015). Association of allostatic load with brain structure and cognitive ability in later life. Neurobiology of Aging, 36(3), 1390–1399. https://doi.org/10.1016/j.neurobiolaging.2014.12.020
- Brosschot, J. F., Pieper, S., & Thayer, J. F. (2005). Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology, 30(10), 1043–1049. https://doi.org/10.1016/j.psyneuen.2005.04.008
- Brosschot, J. F., Verkuil, B., & Thayer, J. F. (2010). Conscious and unconscious perseverative cognition: Is a large part of prolonged physiological activity due to unconscious stress? Journal of Psychosomatic Research, 69(4), 407–416. doi:https://doi.org/10.1016/j.jpsychores.2010.02.002
- Chayachinda, C., Rattanachaiyanont, M., Phattharayuttawat, S., & Kooptiwoot, S. (2008). Premenstrual syndrome in Thai nurses. Journal of Psychosomatic Obstetrics and Gynaecology, 29(3), 199–205. https://doi.org/10.1080/01674820801970306
- Dragomir, A. I., Gentile, C., Nolan, R. P., & D’Antono, B. (2014). Three-year stability of cardiovascular and autonomic nervous system responses to psychological stress. Psychophysiology, 51(9), 921–931. doi:https://doi.org/10.1111/psyp.12231
- Duchesne, A., & Pruessner, J. C. (2013). Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology, 38(12), 3155–3159. doi:https://doi.org/10.1016/j.psyneuen.2013.08.009
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. G. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/bf03193146
- Galy, E., & Mélan, C. (2015). Effects of cognitive appraisal and mental workload factors on performance in an arithmetic task. Applied Psychophysiology and Biofeedback, 40(4), 313–325. https://doi.org/10.1007/s10484-015-9302-0
- García-Rubio, M. J., Espín, L., Hidalgo, V., Salvador, A., & Gómez-Amor, J. (2017). Autonomic markers associated with generalized social phobia symptoms: Heart rate variability and salivary alpha-amylase. Stress, 20(1), 61–68. https://doi.org/10.1080/10253890.2016.1265939
- Gengli, Z., Linhong, W., & Q, C. (1998). Occurrence and influencing factors of premenstrual syndrome in women of childbearing age. Chinese Journal of Obstetrics and Gynecology, 4, 222–224.
- Girdler, S. S., Pedersen, C. A., Straneva, P. A., Leserman, J., Stanwyck, C. L., Benjamin, S., & Light, K. C. (1998). Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Research, 81(2), 163–178. doi:https://doi.org/10.1016/S0165-1781(98)00074-2
- Güllner, H. G. (1983). The interactions of prostaglandins with the sympathetic nervous system-a review. Journal of the Autonomic Nervous System, 8(1), 1–12. https://doi.org/10.1016/0165-1838(83)90017-6
- Hayes, A. (2013). Introduction to mediation, moderation, and conditional process analysis. Journal of Educational Measurement, 51(3), 335–337. doi:https://doi.org/10.1111/jedm.12050
- Hedqvist, P. (1977). Basic mechanisms of prostaglandin action on autonomic neurotransmission. Annual Review of Pharmacology and Toxicology, 17(1), 259–279. https://doi.org/10.1146/annurev.pa.17.040177.001355
- Jönsson, P., Wallergård, M., Osterberg, K., Hansen, A. M., Johansson, G., & Karlson, B. (2010). Cardiovascular and cortisol reactivity and habituation to a virtual reality version of the trier social stress test: A pilot study. Psychoneuroendocrinology, 35(9), 1397–1403. https://doi.org/10.1016/j.psyneuen.2010.04.003
- Kahle, S., Miller, J. G., Lopez, M., & Hastings, P. D. (2016). Sympathetic recovery from anger is associated with emotion regulation. Journal of Experimental Child Psychology, 142, 359–371.doi:https://doi.org/10.1016/j.jecp.2015.10.004
- Kaur, S., Bhalla, P., Bajaj, S., Sanya, S., & Babbar, R. (2013). Effect of physical and mental stress on heart rate variability in type-a and type-b personalities. Indian Journal of Applied Basic Medical Sciences, 15a:20, 59–70. Print ISSN:0975–8917
- Kim Eun, J. (1993). A model of the theoretical structure of factors influencing college womens’ attitudes toward menstruation Journal of korean Academy of Nursing, 23, 224–243. doi:https://doi.org/10.4040/jnas.1993.23.2.224
- Klatzkin, R. R., Bunevicius, A., Forneris, C. A., & Girdler, S. (2014). Menstrual mood disorders are associated with blunted sympathetic reactivity to stress. Journal of Psychosomatic Research, 76(1), 46–55. doi:https://doi.org/10.1016/j.jpsychores.2013.11.002
- Klatzkin, R. R., Lindgren, M. E., Forneris, C. A., & Girdler, S. S. (2010). Histories of major depression and premenstrual dysphoric disorder: Evidence for phenotypic differences. Biological Psychology, 84(2), 235–247. https://doi.org/10.1016/j.biopsycho.2010.01.018
- Li, J. (2020). Survey on the influence of personality traits, menstrual attitude and sex hormones levels on premenstrual syndrome in female college students. Maternal & Child Health Care of China, 35(5), 924–927. doi:https://doi.org/10.19829/j.zgfybj.issn.1001-4411.2020.05.046
- Luecken, L., & Gallo, L. (2008). Handbook of physiological research methods in health psychology. Sage. doi:https://doi.org/10.4135/9781412976244
- Lustyk, M. K. B., & Gerrish, W. G. (2010). Premenstrual syndrome and premenstrual dysphoric disorder: Issues of quality of life, stress and exercise. In V. R. Preedy, & R. R. Watson (Eds.), Handbook of disease burdens and quality of life measures (pp. 1951–1975). Springer. doi:https://doi.org/10.1007/978-0-387-78665-0_115
- Lustyk, M. K. B., Gerrish, W. G., Douglas, H., Bowen, S., & Marlatt, G. A. (2011). Relationships among premenstrual symptom reports, menstrual attitudes, and mindfulness. Mindfulness, 2(1), 37–48. https://doi.org/10.1007/s12671-011-0041-x
- Margaret, L., Stubbs , & Costos, D. (2004). Negative attitudes toward menstruation. Women & Therapy, 27(3–4), 37–54. doi:https://doi.org/10.1300/J015v27n03_04
- Matsumoto, T., Ushiroyama, T., Kimura, T., Hayashi, T., & Moritani, T. (2007). Altered autonomic nervous system activity as a potential etiological factor of premenstrual syndrome and premenstrual dysphoric disorder. BioPsychoSocial Medicine, 1(1), 24. doi:https://doi.org/10.1186/1751-0759-1-24
- Mccarthy, J. J., & Rockette, H. E. (1983). A comparison of methods to interpret the basal body temperature graph. Fertility and Sterility, 39(5), 640–646. doi:https://doi.org/10.1016/S0015-0282(16)47059-7
- Mcewen, B. S., & Gianaros, P. J. (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186(1), 190–222. doi:https://doi.org/10.1111/j.1749-6632.2009.05331.x
- McEwen, B. S., & Stellar, E. (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. doi:https://doi.org/10.1001/archinte.1993.00410180039004
- Moos, R. H. (1968). The development of a menstrual distress questionnaire. Psychosomatic Medicine, 30(6), 853–867. doi:https://doi.org/10.1097/00006842-196811000-00006
- Oda, Y. (2007). Influences of premenstrual syndrome on psychophysiological responses to acute stress. Psychologia, 50(3), 192–202. https://doi.org/10.2117/psysoc.2007.192
- Oldehinkel, A. J., Johan, O., Bosch, N. M., Bouma, E. M. C., Roon, A. M., Van Rosmalen, J. G. M., & Harriette, R. (2011). Stressed out? Associations between perceived and physiological stress responses in adolescents: The TRAILS study. Psychophysiology, 48(4), 441–452. doi:https://doi.org/10.1111/j.1469-8986.2010.01118.x
- Porges, S. W. (2003). The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503–513. https://doi.org/10.1016/S0031-9384(03)00156-2
- Pu, J., Schmeichel, B. J., & Demaree, H. A. (2010). Cardiac vagal control predicts spontaneous regulation of negative emotional expression and subsequent cognitive performance. Biological Psychology, 84(3), 531–540. https://doi.org/10.1016/j.biopsycho.2009.07.006
- Rodrigues, S., Paiva, J., Dias, D., Aleixo, M., Filipe, R., & Cunha, J. (2018). Cognitive impact and psychophysiological effects of stress using a biomonitoring platform. International Journal of Environmental Research and Public Health, 15(6), 1080. https://doi.org/10.3390/ijerph15061080
- Ruble, D. N. (1977). Premenstrual symptoms: A reinterpretation. Science, 197(4300), 291–292. doi:https://doi.org/10.1126/science.560058
- Ryu, A., & Kim, T. H. (2015). Premenstrual syndrome: A mini review. Maturitas, 82(4), 436–440. doi:https://doi.org/10.1016/j.maturitas.2015.08.010
- Sijtsema, J. J., Roon, A. M. V., Groot, P. F. C., & Riese, H. (2015). Early life adversities and adolescent antisocial behavior: The role of cardiac autonomic nervous system reactivity in the TRAILS study. Biological Psychology, 110, 24–33. https://doi.org/10.1016/j.biopsycho.2015.06.012
- Willmann, M., Langlet, C., Hainaut, J.-P., & Bolmont, B. (2012). The time course of autonomic parameters and muscle tension during recovery following a moderate cognitive stressor: Dependency on trait anxiety level. International Journal of Psychophysiology, 84(1), 51–58. doi:https://doi.org/10.1016/j.ijpsycho.2012.01.009
- Yildirir, A., Kabakci, G., Akgul, E., Tokgozoglu, L., & Oto, A. (2002). The effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Journal of the American College of Cardiology, 39(1), 208. https://doi.org/10.1016/S0735-1097(02)80922-6
- Yongai, Z., Liqin, W., Xiaolan, Z., Yaru, X., & L, J. (2011). Analysis of influence of dysmenorrhea on menstrual attitudes and measures approach of female college. Nursing Research, 25(17), 1526–1528. doi:https://doi.org/10.3969/j.issn.1009-6493.2011.17.009
- Yongai, Z., Xiaomei, L., Haimiao, Z., Zhengyan, T., & L, J. (2015). Reliability and validity evaluation of the Chinese version of the menstrual symptom scale. Nursing Research, 50(3), 374–377. https://doi.org/10.3761/j.issn.0254-1769.2015.03.028
- Zhang, Y. X., Wang, Y., & Qian, M. Y. (1990). Reliability and validity of Beck Depression Inventory. Chinese Mental Health Journal, 4, 164–168.
- Zheng, J., Huang, Z., Huang, J., Zhuang, X., Wang, D., Zheng, S., & Wu, J. (2002). A study of psychometric properties, normative scores and factor structure of Beck Anxiety Inventory Chinese version. Chinese Journal of Clinical Psychology, 10(1), 4–6. doi:https://doi.org/10.3969/j.issn.1005-3611.2002.01.002