Abstract
We and other research groups have previously described that levels of the anabolic hormone dehydroepiandrosterone sulfate (DHEA-S) are lowered in individuals who report prolonged stress. We have also shown that the DHEA-S production capacity during acute stress is attenuated in individuals reporting high prolonged stress. This study aimed to further investigate the DHEA and DHEA-S production capacity in relation to prolonged stress. Eighty-one healthy participants in the age 20–50 years old were included in the study and divided into a low stress (n = 45) and a high stress group (n = 36) according their response to a single question regarding perceived stress during the preceding month. They underwent the Trier Social Stress Test while blood samples were drawn before, during and after the stress test. The concentration of DHEA, DHEA-S, cortisol and ACTH was measured. The results showed that the high stress group exhibited a significantly lower response of DHEA-S (40% lower) than the low stress group, while DHEA, cortisol and ACTH responses did not differ between the groups. Reduced DHEA-S production may constitute one of the links between stress and poor health.
Introduction
Dehydroepiandrosterone (DHEA) and its sulfated metabolite DHEA-S are regenerative, anabolic, hormones with pleiotropic beneficial effects important for maintenance of health (Maninger et al., Citation2009; Theorell, Citation2009; Wolf & Kirschbaum, Citation1999). The levels of DHEA and DHEA-S peak in young adulthood and decrease thereafter with age (Hornsby, Citation1995). DHEA and DHEA-S are produced in the inner layer of the adrenal cortex (zona reticularis) in response to adrenocorticotropic hormone (ACTH). Thus, they are produced and released to the blood stream in parallel to the stress hormone cortisol, which is produced in the middle layer of the adrenal cortex (zona fasciculata). Several research groups, including ours, have found an association between prolonged stress in otherwise healthy individuals and reduced DHEA and DHEA-S levels (Brzoza et al., Citation2008; Heaney et al., Citation2014; Izawa et al., Citation2008; Jeckel et al., Citation2010; Lennartsson et al., Citation2013b; Mason et al., Citation1968). It is suggested that lowered DHEA-S constitutes one of the links between long-term stress and adverse health and seems to appear as an early sign of the negative physiological consequences of long-term stress, as marked effects are observed even in otherwise healthy individuals. Besides measuring baseline levels of DHEA and DHEA-S (thus taking a blood sample in a normal resting state), our research group has also investigated the production capacity of these hormones during acute stress, using the Trier Social Stress Test (TSST), and shown that the capacity to produce DHEA-S is attenuated in individuals who report high perceived stress (Lennartsson et al., Citation2013a). Lately, a few more publications have addressed this issue, using saliva samples, with contrary results (Lam et al., Citation2019; Prall et al., Citation2017). The aim of the present study was to further investigate, in a new larger sample of healthy individuals, the DHEA and DHEA-S production capacity during acute psychosocial stress in relation to perceived prolonged stress level. Since short-term changes in DHEA and DHEA-S levels are regulated by ACTH, we also took into account the changed levels of ACTH during the experiment, to further elucidate plausible mechanisms.
Method
The results from this study are based on baseline data from a randomized controlled trial (RCT), conducted at the Institute of Stress Medicine in Gothenburg, Sweden, from 2013 to 2016. Study procedures have previously been described in detail elsewhere (Arvidson et al., Citation2018, Citation2020). The intervention studied in the RCT was exercise training, explaining the focus on exercise in the inclusion and exclusion criteria. However, this present study only includes cross-sectional baseline data collected before the intervention and thus the RCT intervention is not included in this study.
Participants
Participants were recruited through advertisements in two major newspapers in the area of Gothenburg and through social media. Subjects included were subjects who were 20–50 years of age, essentially healthy (self-reported) regarding both mental and somatic health, working or studying at least 50% of full time and reporting themselves as sedentary according to the validated Saltin-Grimby Physical Activity Level Scale (Grimby et al., Citation2015). This scale is a single-item question with four response alternatives (Rodjer et al., Citation2012). The first level corresponds to a sedentary lifestyle, while levels 2–4 represent graded increases in activity level from light to strenuous exercise training. Exclusion criteria were high glucose level (≥ 7 mmol/L) or HbA1C (≥ 48 mmol/mol), abnormal electrocardiogram, high blood pressure ((> 140/90), anemia (Hb < 120 g/L for women and < 130 g/L for men) and high or low body mass index (< 18.5 or > 35 kg/m2). Also, medication with beta-blockers, psychopharmacological drugs or asthma medication and inability to exercise at a relatively high intensity led to exclusion. Using oral contraceptives was also set as an exclusion criterion. Locally working hormonal IUD (without estrogen) was allowed. Individuals who had an abnormal ECG were further examined by a cardiologist before inclusion/exclusion. The number of responders to the advertisement were 416, of which 170 individuals passed the inclusion criteria and were invited to a physical screening at the Institute of Stress Medicine. Twenty-two individuals declined participation in the study, and in addition 24 individuals were excluded after screening. Five individuals were excluded after the initial tests due to incomplete measures, resulting in 119 participants. Of these, 81 individuals had complete data on DHEA and DHEA-S and were included in the final analyses. A drop-out analysis revealed no differences in baseline characteristics in this group compared to the total sample of 119 individuals.
All participants gave written informed consent before entering the study and were informed that they could withdraw their participation at any time. The study was conducted according to the 1964 Declaration of Helsinki and approved by the Regional Ethical Board, Gothenburg, Sweden, Dnr 917-12.
Perceived stress level groups
One week before the stress test, participants were asked to answer a questionnaire; the Perceived Stress Scale (PSS-14) (Cohen et al., Citation1983). This includes fourteen questions capturing perceived stress during the previous month in different ways. The 81 included participants were divided into two groups; “Low stress” (n = 45) and “High stress” (n = 36) based on their rating on a single item question about their perceived stress: “How often during the previous month did you feel nervous and stressed? The answering options were: “Never”, “Almost never”, “Sometimes”, “Quite often” and “Very often”. This item is one of the questions included in the PSS-14. None of the participants scored “Never”. The 14 participants (17%) who scored “Almost never” and the 31 (38%) who scored “Sometimes” constitute the “Low stress” group while the 28 participants (35%) who scored “Quite often” and the 8 (10%) who scored “Very often” constitute the “High stress” group. In addition, the total score of PSS-14 was also used in the analysis as a continuous variable.
Psychosocial stress test
To enable assessment of individual reactions to psychosocial stress, the Trier Social Stress Test (TSST) was used (Kirschbaum et al., Citation1993). It has previously been shown to elicit significant physiological stress responses in both women and men (Allen et al., Citation2014). The tests were performed between 1 p.m. and 3 p.m. To avoid any effects of food intake on the day of testing, all participants were served a standardized meal (around 500 kcal) containing controlled amounts of fat, protein and carbohydrates (20, 15 and 65 g, respectively) at least 2 h before the stress test. Participants entered the laboratory 10 minutes before the first sample (baseline) was drawn. The participant was introduced to a task in front of a committee consisting of three members. After a preparation period of 5 min in another room, the participant reentered the test room. In the first part of the test, a fictitious job interview, the participant presented him- or herself for 5 min. The second part of the test was an arithmetic task, not described beforehand. After the TSST the participant rested for 60 min.
Assessment of DHEA, DHEA-S levels and HPA-axis
DHEA and DHEA-S were assessed in plasma ten minutes prior to the stress test (pretest), directly after the stress test (+0 min) and 10, 20 and 60 min after the test. The peak value was identified as the highest one of the +0, 10- or 20-min values, while the last sample (60 min) was defined as the recovery value. For HPA-axis hormones, assessed as plasma ACTH and serum cortisol, samples were collected at −10, −0. +0, +10, +20, +40 and +60 min. Pretest values for cortisol and ACTH were the average level of the −10- and −0-min measure. Plasma samples were collected in EDTA -tubes. To separate the plasma, tubes were cold spun at 3500 revolutions per minute for 15 min and stored in micro tubes at −80 °C until analyzed. Serum samples were collected in Serum Sep Cloth Activator tubes, which were spun at 20 °C for 10 min at 3500 revolutions per minute and stored at 4 °C until analyzed the day after the test. Plasma concentrations of DHEA were determined using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (limit of quantitation 175 pmol/L), and concentrations of DHEA-S were assessed by radioimmunoassay techniques (RIA) (limit of detection 0.14 µmol/L, Diagnostic Products Corporation, Los Angeles, CA). Plasma concentrations of ACTH were assessed with electrochemiluminescence immunoassay (Elecsys, Cobas, Roche Diagnostics, Basel, Switzerland). Its assay measuring range is 0.220–440 pmol/L. At 3.4 pmol/L, the coefficient of variation (CV) is 1.8%; and, at 94.1 pmol/L, it is 2.3%. Serum concentrations of cortisol were assessed with immunochemiluminescence assay (Elecsys, Cobas, Roche Diagnostics). The samples from the participants enrolled before November 2015 (approximately 80% of the participants) were analyzed with the kit cortisol I. Its assay measuring range is 0.5-1750 nmol/L; CV is 7% at 100 nmol/L, 5% at 570 nmol/L and 5% at 990 nmol/L. Remaining samples were analyzed with cortisol II. Its assay measuring range is 3–1750 nmol/L; CV:s are 3.1% at 111 nmol/L, 2.1% at 242 nmol/L and 2.5% at 575 nmol/L.
Data handling
The peak levels of DHEA, DHEA-S, ACTH and cortisol were identified for each participant (at either the +0, or +20 time point for DHEA and DHEA-S, and at any of the time points from +0 to +40 for ACTH and cortisol). Delta values (acute stress-induced increase) of the concentrations of DHEA, DHEA-S, ACTH and cortisol were calculated for each participant as the difference between their peak level and their pretest level (log levels in cases of non-normal distribution). In addition, area under the curve (AUC) was calculated for ACTH, cortisol, DHEA and DHEA-S (using concentrations from all time points).
Statistical analyses
The Kolmogorov–Smirnov test was used on each study variable to test whether the data were normally distributed. Logarithmic transformation was used for the variables that showed a non-normal distribution. After logarithmic transformation (logln), the Kolmogorov–Smirnov test was used again to control whether the new variable showed normal distribution.
The distribution of men and women in the two groups was compared using the Chi-square test. Age, BMI, pretest ACTH and pretest cortisol in the groups were compared using the t-test. Log values of ACTH and cortisol were used. General linear model (one-way ANOVA) was used to compare pretest levels of DHEA, DHEA-S and the molar cortisol to DHEA-S ratio between the stress level groups, while controlling for age. Log values of DHEA, cortisol to DHEA-S ratio was used. Paired samples t-test were used to analyze the difference between pretest and after stress peak values for ACTH, cortisol, DHEA and DHEA-S. Log values were used for ACTH, cortisol and DHEA. T-tests and ANOVAs (or Mann–Whitney U test) were used to investigate the effect of prolonged perceived stress on acute hormone responses, with the stress group as the independent variable and hormonal stress response as dependent. Since ACTH stimulates the production of cortisol, DHEA and DHEA-S, we first analyzed whether the response of ACTH (delta ACTH and AUC ACTH, respectively) was different in the high stress group compared to the low stress group. Log values of delta ACTH and AUC ACTH was used. Second, we analyzed whether the cortisol response (delta cortisol and AUC cortisol, respectively) was different between the two groups. Thereafter, we investigated whether the response of DHEA (delta and AUC) and DHEA-S (delta and AUC DHEA-S) differed between the high stress group and the low stress group, respectively. T-tests were used in comparisons of delta values while univariate ANOVAs were used in analyses on AUC, controlling for pretest hormonal levels. The Mann–Whitney U test was used in group differences in AUC DHEA-S and AUC DHEA due to non-normal distributions which were not possible to logarithmically transform to normal distributions.
In addition to the analyses comparing the hormonal responses between the high stress group and low stress group, correlation analyses were performed between total scores on the PSS-14 scale and hormonal responses to the acute stress test. Spearman’s rank correlation analyses were conducted between PSS-14 total stress score on the one hand and all delta values and AUC values on the other hand, respectively. Further, we calculated a ratio between the delta ACTH and the delta DHEA-S (thus, the amount of produced DHEA-S in relation to the amount released of ACTH), reported separately for the stress groups in . To further investigate the effect of perceived prolonged stress on DHEA-S production capacity, ANCOVA was performed with delta DHEA-S as the dependent variable, the stress group as the independent variable and delta ACTH as covariate. Thus, in this analysis, we investigate the effect of the stress group on DHEA-S production capacity while controlling for ACTH production. Analyses were conducted with IBM Statistics 26 (SPSS Inc., Chicago, IL).
Results
As described in the method section, the participants were divided into the two groups “Low stress” and “High stress” based on their rating of the single item “previous month perceived stress”. Number of participants, age, BMI and pretest hormonal levels in the two stress level groups are reported in . The high stress group had significantly higher pretest cortisol to DHEA-S ratio than the low stress group. No other differences were seen between the groups.
Table 1. Number of men and women, age and basal hormonal levels in the two stress level groups.
ACTH and cortisol responses
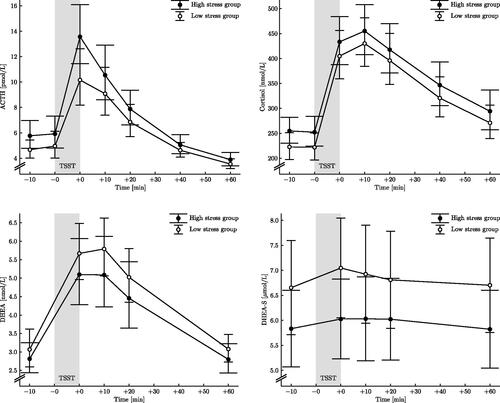
Paired samples t-test (with pretest and peak values) showed that levels of ACTH and cortisol significantly increased in response to the stress test (t = −14.68, d = 1.63, p < 0.001; t = −15.95, d = 1.78, p < 0.001, respectively), as expected from previous research using TSST. ACTH and cortisol levels in the two stress level groups are shown in . Since ACTH stimulates the production of DHEA and DHEA-S, possible differences in ACTH response between the two groups was first evaluated. The magnitude of increase of ACTH, measured as delta ACTH or AUC ACTH, did not differ between the low stress group and the high stress group (p = 0.408; p = 0.927, respectively). Similarly, no significant differences were seen for delta cortisol or AUC cortisol between the low stress group and the high stress group (p = 0.854; p = 0.635, respectively).
DHEA-S and DHEA response
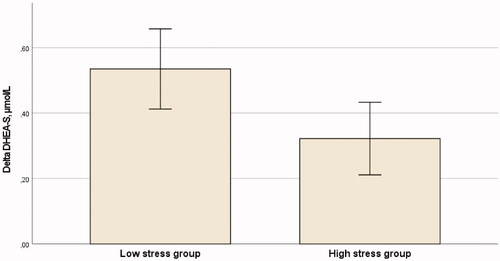
Paired samples t-test (with pretest and peak values) showed a significant increase in DHEA and DHEA-S responses to the acute psychosocial stressor (t = −15.86, d = 1.78, p < 0.001; t = −10.24, d = 1.14, p < 0.001, respectively). The DHEA and DHEA-S levels during the experiment in the two stress level groups are shown in . Delta DHEA-S (pretest levels subtracted from the peak levels) was significantly lower (on average 40%) in the high stress group compared to the low stress group (t = 2.093, d = 0.468, p = 0.040) (). Also, the AUC DHEA-S was significantly smaller in the high stress group compared to the low stress group (z = −2.276, r = 0.258, p = 0.023). Delta DHEA and AUC DHEA did not differ between the low stress group and the high stress group (p = 0.712; p = 0.586, respectively).
Correlation analysis between perceived stress score and hormonal responses
In addition to the above analyses, comparing the hormonal responses between the high stress group and the low stress group, correlation analysis was performed between scores on the PSS-14 and hormonal responses to the acute stress test. The PSS-14 score correlated negatively with Delta DHEA-S (r = −0.23, p = 0.037) and AUC DHEA-S (r = −0.27, p = 0.017), thus, the higher stress scores the lower DHEA-S production during the acute stress test. PSS-14 score did not correlate with responses of ACTH, cortisol or DHEA (data not shown).
The relation between DHEA-S increase and ACTH increase in the stress level groups
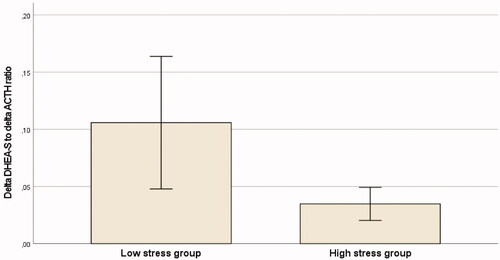
To further investigate the DHEA-S production capacity during the acute stress situation, we calculated the ratio between the acute stress-induced DHEA-S increase and increase in ACTH (thus, the amount of produced DHEA-S in relation to the amount released of its stimulus factor). shows the ratios in the high stress and low stress groups, respectively. ANCOVA was performed to investigate the effects of the stress group on DHEA-S production capacity while controlling for ACTH release. DHEA-S production was significantly lower in the high stress group, on an average, 38% lower (F (2, 78) = 5.729, p = 0.019), after controlling for the increase in ACTH. Thus, the low stress group exhibited larger DHEA-S production than the high stress group also in relation to their stress-induced increase of ACTH.
Figure 3. The ratio between delta DHEA-S and delta ACTH (mean, 95% CI) in the two stress level groups.
Discussion
The main results of this study show that higher perceived stress during the previous month was related to an attenuated DHEA-S response during acute psychosocial stress in healthy individuals. These results confirm our previous findings (Lennartsson et al., Citation2013a) that perceived long-term stress negatively affects the capacity to produce DHEA-S during acute stress. In our previous study on DHEA-S production capacity, including stress at work during the previous week as exposure measure, it was found that the magnitude of response of DHEA-S was around 50% lower in participants reporting medium–high stress compared to those reporting low stress. These results are comparable to the results in the present study; the individuals who reported higher perceived prolonged stress exhibited 40% smaller DHEA-S production during the stress test. As in the previous study, DHEA response was not shown to the related to perceived stress. However, since the levels of DHEA are about 300 times lower than levels of DHEA-S, and since DHEA-S can rapidly convert to DHEA and vice versa (Rosenfeld et al., Citation1975) the non-finding in DHEA levels could be considered to be of minor importance. It should be noted that although the relative change (percentage increase) of DHEA during acute stress is greater than the relative change of DHEA-S, the increased concentration of DHEA-S during acute stress is much larger than for DHEA. Thus, the increase of DHEA-S accounts for the majority of the total increase of DHEA and DHEA-S together. Recent publications addressing DHEA and DHEA-S responses during acute stress, show contrary results. Lam et al. (Citation2019) studied responses of salivary cortisol and DHEA (but not DHEA-S) to TSST in 61 young adults and related the responses to their scores on measures of exposure to acute life events and “chronic difficulty.” They found a positive correlation between chronic life stress and DHEA response and a negative correlation between chronic life stress and cortisol response during the stress test. Thus, greater lifetime stress exposure was associated with blunted cortisol and heightened DHEA responses. This is in contrast to the results on DHEA in our two studies were no difference in DHEA were seen between the groups. Prall et al. (Citation2017) studied responses of salivary DHEA, DHEA-S and cortisol in 27 young adult males exposed to a modified version of TSST. Their main aim was to investigate the relation between endocrine responses and markers of innate immunity. However, they also performed correlation analyses between total score of the PSS and DHEA and DHEA-S responses. They did not find significant relations between stress score and DHEA response (although the correlation coefficient was −0.34) or DHEA-S response. It is not clear whether there was enough variance of scores in PSS in the study group to be able to find a linear correlation, which the authors also pointed out. In the present study, we also performed a correlation analysis, between PSS total score and DHEA- and DHEA-S production capacity, respectively. While no correlation was seen between total stress score and DHEA response, a significant negative correlation was seen between stress score and DHEA-S response. Taken together, there are few studies investigating the relationship between prolonged stress and DHEA and DHEA-S production capacity. The published studies vary somewhat with regard to size of the study populations and which hormones are measured. To our knowledge, our studies are the only ones published including both DHEA and DHEA-S in blood samples (contrary to saliva samples) and using liquid chromatography-tandem mass spectrometry (LC-MS/MS) for DHEA measurements. This should be taken into account when interpreting results.
DHEA-S production capacity during acute stress has also been studied in patients with stress-related exhaustion disorder (Lennartsson et al., Citation2015) and in patients diagnosed with depression (Jiang et al., Citation2017). Patients with stress-related exhaustion disorder exhibited 43% lower DHEA-S production during acute stress test (TSST) compared to healthy control subjects. The patients with depression, studied by Jiang et al. (Citation2017) also exhibited attenuated DHEA-S production capacity measured in salivary samples (an average increase of 34% in the depression group compared to 52% in the control group).
Mechanisms behind the attenuated DHEA-S production
Regulation of short-term changes of DHEA and DHEA-S is governed by ACTH (Parker et al., Citation1996). ACTH is in turn stimulated by corticotropic releasing hormone (CRH) from the pituitary which is released according to a diurnal rhythm (low during sleep and higher at awakening) and when higher brain areas perceive stress exposure. The magnitude of ACTH increase was not different between the two stress level groups and thus cannot explain the differences in DHEA-S response. However, we also analyzed the DHEA-S production capacity controlling for the amount of ACTH increase by calculating the ratio between the increases in ACTH and the increase in DHEA-S for each individual. The results showed significantly lower delta DHEA-S in the high stress group, even after controlling for delta ACTH, which means that for a given amount of ACTH, a lower amount of DHEA-S is produced in this group compared to those reporting low stress levels. To our knowledge, this finding has not been previously reported. Mechanisms linking prolonged stress to reduced DHEA-S production capacity during acute stress are thus not likely to include reduced ACTH levels. A more likely interpretation is that prolonged stress affects the factors that regulate long-term changes in DHEA-S production. Long-term changes in DHEA-S levels are modulated by the number of zona reticularis cells and levels of the enzymes (17-hydroxylase, dehydroepiandrosterone sulfotransferase and 3b-hydroxysteroid dehydrogenase) within the cells. Aging is associated with a reduced number of zona reticularis cells that produce DHEA (Parker et al., Citation1997) as well as shifted enzymatic activity within the zona reticularis leading to reduced capacity to produce DHEA (Liu et al., Citation1990). Plausibly, changes in the zona reticularis after long-term stress are comparable to changes that occur during aging. Lower DHEA-S production during acute stress shown in the individuals that reported higher perceived stress the previous month compared to the individuals that reported low stress indicate that, during prolonged stress, steroid biosynthesis may be shifted from biosynthesis of adrenal androgens to corticosteroid pathways ensuring maintained production of cortisol, which is essential during exposure to stressors.
Physiological relevance of attenuated DHEA-S production capacity
While cortisol is a catabolic hormone, and thus has an energy mobilizing effect in the body, DHEA and DHEA-S are anabolic hormones that play a protective and regenerative role (Maninger et al., Citation2009; Theorell, Citation2009). DHEA and DHEA-S have been shown to have neuroprotective, anti-oxidative, anti-inflammatory, and anti-glucocorticoid effects (Kalimi et al., Citation1994; Maninger et al., Citation2009). Attenuated production of DHEA-S, seen during acute psychosocial stress in individuals reporting higher perceived long-term stress could thus be seen as unfavorable, expressing a lower anabolic activity in the body. DHEA and DHEA-S production during acute stress have been suggested to play a protective role during the stress reaction, as an antagonist to the effects of cortisol (Hechter et al., Citation1997; Morgan et al., Citation2004). The stress-induced increase of DHEA and DHEA-S likely has, besides the favorable anabolic effects in the body, also beneficial behavioral and emotional effects. Studies on mice have shown antidepressant, anxiolytic, anti-aggression, and memory-enhancing effects of DHEA-S (Melchior & Ritzmann, Citation1994). While DHEA supplementation does not seem to be able to affect well-being or cognition in humans (Grimley Evans et al., Citation2006; Kritz-Silverstein et al., Citation2008), endogenous DHEA-S levels have been shown to be related to cognitive function (Davis et al., Citation2008; Sorwell & Urbanski, Citation2010) and some aspects of well-being (Bell et al., Citation2006; Berr et al., Citation1996). It has also been shown that lower DHEA levels during TSST were associated with an increased negative mood during the recovery period (Izawa et al., Citation2008) and increased DHEA concentration during stressful situations was suggested to contribute to reduction of negative mood. Furthermore, higher DHEA-S/cortisol ratio during acute psychosocial stress has been associated with better cognitive and decision-making abilities during acute stress (Morgan et al., Citation2004). Our study suggests that individuals exposed to prolonged stress might attain less of the physiological and psychological benefits described above, since the DHEA-S production during acute stress is lower in these individuals. This could plausibly be seen as an early sign of the negative consequences of long-term psychosocial stress.
Limitations of the study
There are some issues that need to be considered when interpreting the results. First, the aim of the present study, thus; investigating DHEA-S production capacity in relation to different levels of prolonged stress, was not initially defined as one of the primary aims of the randomized controlled trial which this study is based on. The RCT study aimed to investigate the effects of a six month exercise training intervention on acute stress responses. Thus, inclusion of participants in the RCT were not focused on recruiting individuals with different stress levels. However, the stress scale which was included in the RCT could be used to differentiate participants regarding prolonged stress level and the participants could be divided to a low stress group and a high stress group. There were several individuals that could not be included in the analysis which is considered as a limitation, but the drop-out analysis revealed no difference between those included in the analysis and those who were not. It is relevant to raise the issue that hormonal differences in low versus high stress groups could be caused by differences in mental health status, such as depression. However, no study participants included in this study report psychiatric disorders, as they were initially recruited as healthy participants, including being healthy with regard to mental health. Post doc analysis was performed and showed that there was no difference between the groups regarding depression score, measured with Hospital and Anxiety depression scale (HAD) (mean score 8.73 and 9.03 in low stress and high stress groups, respectively, p = 0.345).
Conclusions
This study indicates that prolonged psychosocial stress, measured as perceived stress during the previous month using a single item question, negatively affects the production of DHEA-S during acute psychosocial stress. Given the protective functions of DHEA-S, attenuated DHEA-S production during acute stress may lead to higher risk for adverse effects on psychological and physiological health, particularly if perceived stress levels remain high during a longer period of time. Future studies are needed to study the clinical relevance of the changes seen in this study as well as on possible plausible methods that may have beneficial effects on stress level and the DHEA-S production capacity.
Acknowledgments
The authors thank Karin Nygren and Anna Björntorp for their professional work with the participants, the samplings, and the completion of the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allen, A. P., Kennedy, P. J., Cryan, J. F., Dinan, T. G., & Clarke, G. (2014). Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews, 38, 94–124. https://doi.org/10.1016/j.neubiorev.2013.11.005
- Arvidson, E., Dahlman, A. S., Borjesson, M., Gullstrand, L., & Jonsdottir, I. H. (2018). Exercise training and physiological responses to acute stress: Study protocol and methodological considerations of a randomised controlled trial. BMJ Open Sport & Exercise Medicine, 4(1), e000393. https://doi.org/10.1136/bmjsem-2018-000393
- Arvidson, E., Dahlman, A. S., Borjesson, M., Gullstrand, L., & Jonsdottir, I. H. (2020). The effects of exercise training on hypothalamic-pituitary-adrenal axis reactivity and autonomic response to acute stress-a randomized controlled study. Trials, 21(1), 888. https://doi.org/10.1186/s13063-020-04803-3
- Bell, R. J., Donath, S., Davison, S. L., & Davis, S. R. (2006). Endogenous androgen levels and well-being: Differences between premenopausal and postmenopausal women. Menopause (New York, N.Y.), 13(1), 65–71. https://doi.org/10.1097/01.gme.0000191212.58856.96
- Berr, C., Lafont, S., Debuire, B., Dartigues, J. F., & Baulieu, E. E. (1996). Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: A French community-based study. Proceedings of the National Academy of Sciences of the United States of America, 93(23), 13410–13415. https://doi.org/10.1073/pnas.93.23.13410
- Brzoza, Z., Kasperska-Zajac, A., Badura-Brzoza, K., Matysiakiewicz, J., Hese, R. T., & Rogala, B. (2008). Decline in dehydroepiandrosterone sulfate observed in chronic urticaria is associated with psychological distress. Psychosomatic Medicine, 70(6), 723–728. https://doi.org/10.1097/PSY.0b013e31817bcc8d
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Davis, S. R., Shah, S. M., McKenzie, D. P., Kulkarni, J., Davison, S. L., & Bell, R. J. (2008). Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. The Journal of Clinical Endocrinology and Metabolism, 93(3), 801–808. https://doi.org/10.1210/jc.2007-2128
- Grimby, G., Borjesson, M., Jonsdottir, I. H., Schnohr, P., Thelle, D. S., & Saltin, B. (2015). The "Saltin-Grimby Physical Activity Level Scale" and its application to health research. Scandinavian Journal of Medicine & Science in Sports, 25 (Suppl 4), 119–125. https://doi.org/10.1111/sms.12611
- Grimley Evans, J., Malouf, R., Huppert, F., & van Niekerk, J. K. (2006). Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database of Systamatic Reviews, CD006221.
- Heaney, J. L., Carroll, D., & Phillips, A. C. (2014). Physical activity, life events stress, cortisol, and DHEA: Preliminary findings that physical activity may buffer against the negative effects of stress. Journal of Aging and Physical Activity, 22(4), 465–473. https://doi.org/10.1123/japa.2012-0082
- Hechter, O., Grossman, A., & Chatterton, R. T. Jr. (1997). Relationship of dehydroepiandrosterone and cortisol in disease. Medical Hypotheses, 49(1), 85–91. https://doi.org/10.1016/S0306-9877(97)90258-9
- Hornsby, P. J. (1995). Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Annals of the New York Academy of Sciences, 774, 29–46. https://doi.org/10.1111/j.1749-6632.1995.tb17370.x
- Izawa, S., Sugaya, N., Shirotsuki, K., Yamada, K. C., Ogawa, N., Ouchi, Y., Nagano, Y., Suzuki, K., & Nomura, S. (2008). Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biological Psychology, 79(3), 294–298. https://doi.org/10.1016/j.biopsycho.2008.07.003
- Jeckel, C. M., Lopes, R. P., Berleze, M. C., Luz, C., Feix, L., Argimon, I. I., Stein, L. M., & Bauer, M. E. (2010). Neuroendocrine and immunological correlates of chronic stress in 'strictly healthy' populations. Neuroimmunomodulation, 17(1), 9–18. https://doi.org/10.1159/000243080
- Jiang, X., Zhong, W., An, H., Fu, M., Chen, Y., Zhang, Z., & Xiao, Z. (2017). Attenuated DHEA and DHEA-S response to acute psychosocial stress in individuals with depressive disorders. Journal of Affective Disorders, 215, 118–124. https://doi.org/10.1016/j.jad.2017.03.013
- Kalimi, M., Shafagoj, Y., Loria, R., Padgett, D., & Regelson, W. (1994). Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry, 131(2), 99–104. https://doi.org/10.1007/BF00925945
- Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The 'Trier Social Stress Test'–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/10.1159/000119004
- Kritz-Silverstein, D., von Muhlen, D., Laughlin, G. A., & Bettencourt, R. (2008). Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. Journal of the American Geriatrics Society, 56(7), 1292–1298. https://doi.org/10.1111/j.1532-5415.2008.01768.x
- Lam, J. C. W., Shields, G. S., Trainor, B. C., Slavich, G. M., & Yonelinas, A. P. (2019). Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress and Health : Journal of the International Society for the Investigation of Stress, 35(1), 15–26. https://doi.org/10.1002/smi.2835
- Lennartsson, A. K., Sjors, A., & Jonsdottir, I. H. (2015). Indication of attenuated DHEA-s response during acute psychosocial stress in patients with clinical burnout. Journal of Psychosomatic Research, 79(2), 107–111. https://doi.org/10.1016/j.jpsychores.2015.05.011
- Lennartsson, A. K., Theorell, T., Kushnir, M. M., Bergquist, J., & Jonsdottir, I. H. (2013a). Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology, 38(9), 1650–1657. https://doi.org/10.1016/j.psyneuen.2013.01.010
- Lennartsson, A. K., Theorell, T., Rockwood, A. L., Kushnir, M. M., & Jonsdottir, I. H. (2013b). Perceived stress at work is associated with lower levels of DHEA-S. PLoS One, 8(8), e72460. https://doi.org/10.1371/journal.pone.0072460
- Liu, C. H., Laughlin, G. A., Fischer, U. G., & Yen, S. S. (1990). Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women: Evidence for a reduced 17,20-desmolase enzymatic activity. The Journal of Clinical Endocrinology and Metabolism, 71(4), 900–906. https://doi.org/10.1210/jcem-71-4-900
- Maninger, N., Wolkowitz, O. M., Reus, V. I., Epel, E. S., & Mellon, S. H. (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology, 30(1), 65–91. https://doi.org/10.1016/j.yfrne.2008.11.002
- Mason, J. W., Tolson, W. W., Robinson, J. A., Brady, J. V., Tolliver, G. A., & Johnson, T. A. (1968). Urinary androsterone, etiocholanolone, and dehydroepiandrosterone responses to 72-hr. avoidance sessions in the monkey. Psychosomatic Medicine, 30(Suppl 5), 710–720.
- Melchior, C. L., & Ritzmann, R. F. (1994). Dehydroepiandrosterone is an anxiolytic in mice on the plus maze. Pharmacology, Biochemistry, and Behavior, 47(3), 437–441. https://doi.org/10.1016/0091-3057(94)90140-6
- Morgan, C. A., Southwick, S., Hazlett, G., Rasmusson, A., Hoyt, G., Zimolo, Z., & Charney, D. (2004). Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Archives of General Psychiatry, 61(8), 819–825. https://doi.org/10.1001/archpsyc.61.8.819
- Parker, C. R., Jr., Azziz, R., Potter, H. D., & Boots, L. R. (1996). Adrenal androgen production in response to adrenocorticotropin infusions in men. Endocrine Research, 22(4), 717–722. https://doi.org/10.1080/07435809609043767
- Parker, C. R., Jr., Mixon, R. L., Brissie, R. M., & Grizzle, W. E. (1997). Aging alters zonation in the adrenal cortex of men. The Journal of Clinical Endocrinology and Metabolism, 82(11), 3898–3901. https://doi.org/10.1210/jcem.82.11.4507
- Prall, S. P., Larson, E. E., & Muehlenbein, M. P. (2017). The role of dehydroepiandrosterone on functional innate immune responses to acute stress. Stress and Health : Journal of the International Society for the Investigation of Stress, 33(5), 656–664. https://doi.org/10.1002/smi.2752
- Rodjer, L., Jonsdottir, I. H., Rosengren, A., Bjorck, L., Grimby, G., Thelle, D. S., Lappas, G., & Borjesson, M. (2012). Self-reported leisure time physical activity: A useful assessment tool in everyday health care. BMC Public Health, 12, 693. https://doi.org/10.1186/1471-2458-12-693
- Rosenfeld, R. S., Rosenberg, B. J., & Hellman, L. (1975). Direct analysis of dehydroisoandrosterone in plasma. Steroids, 25(6), 799–805. https://doi.org/10.1016/0039-128X(75)90044-6
- Sorwell, K. G., & Urbanski, H. F. (2010). Dehydroepiandrosterone and age-related cognitive decline. Age (Dordrecht, Netherlands), 32(1), 61–67. https://doi.org/10.1007/s11357-009-9113-4
- Theorell, T. (2009). Anabolism and catabolism. In S. Sunnentag, P. L. Perrewé, & D. C. Ganster (Eds.), Research in occupational stress and well-being. Current perspectives on job-stress recovery. 7, 249–276.
- Wolf, O. T., & Kirschbaum, C. (1999). Actions of dehydroepiandrosterone and its sulfate in the central nervous system: Effects on cognition and emotion in animals and humans. Brain Research. Brain Research Reviews, 30(3), 264–288. https://doi.org/10.1016/S0165-0173(99)00021-1