Abstract
Higher vagally mediated heart rate variability (vmHRV), reflecting vagal activity as indexed by heart function and lower stress vulnerability, is associated with higher perceived social support. Seeking social support is an adaptive stress response, and evolutionary theories suggest that females use this strategy more than males. The current study investigated the hypothesis that higher vmHRV is related to higher perceived social support under conditions of higher, relative to lower, stress, and that this association is most prominent in females. A healthy student sample (n = 143; 82 males, 61 females; mean age 19.9) completed the short version of the Medical outcomes study social support survey (MOS) and the Perceived stress scale (PSS). Activity in the high frequency band of heart rate variability (HF-HRV), deducted from five-minute resting electrocardiogram (ECG) recordings, indexed vmHRV. A moderation analysis was conducted, with PSS and sex as moderators of the association between vmHRV and MOS. Statistical effects were adjusted for age, education, physical activity, body mass index (BMI), alcohol and drug use, ECG-derived respiration (EDR), and mean heart rate. Higher PSS scores moderated the association between vmHRV and MOS in females but not males. Lower PSS scores did not moderate the relation between vmHRV and MOS. This suggests that higher vmHRV is associated with higher perceived social support under conditions of higher stress in females but not males, consistent with evolution of different stress management strategies in the sexes. The results may have implications for individualized intervention strategies for increasing vmHRV and perceived social support.
Introduction
Activity of the vagus nerve of the autonomic nervous system (ANS) as indexed by heart function is reflected in vagally mediated heart rate variability (vmHRV), deducted from resting electrocardiogram (ECG) recordings. Owing to its test–retest reliability (Bertsch et al., Citation2012; Tarkiainen et al., Citation2005), resting vmHRV is often used as a “trait” marker of a neurophysiological tendency to self-regulate and engage socially (Porges, Citation2007; Thayer & Lane, Citation2000). Supporting this, higher vmHRV has been associated with adaptive stress coping abilities (Kim et al., Citation2018), such as the increase of perceived social support (Fabes & Eisenberg, Citation1997; Gerteis & Schwerdtfeger, Citation2016; Holt-Lunstad et al., Citation2007; Kao et al., Citation2014; Schwerdtfeger & Schlagert, Citation2011) – defined as the perception of how one’s social network provides stress coping resources (Cohen, Citation2004). Furthermore, studies have found positive associations between perceived social support and flexible vmHRV changes during experimental stressors (Goodyke et al., Citation2021; Maunder et al., Citation2012; Schwerdtfeger & Schlagert, Citation2011).
Notably, there are sex differences in social support behavior during stress (Taylor et al., Citation2000), which might be reflected in vmHRV. The association between vmHRV and perceived social support might therefore relate differently to stress in males and females. For instance, social support has been found to be more consistently used during stress in females compared to males (Taylor, Citation2007). Moreover, females display higher vmHRV (Koenig & Thayer, Citation2016), and higher vmHRV flexibility during experimental stressors (Li et al., Citation2009; Sato & Miyake, Citation2004). Similar sex differences in vmHRV during stress have also been suggested in a neuroimaging study. In this study, there was found a positive correlation between blood flow in brain regions important for autonomic regulation and vmHRV during stress in females, whereas the association was negative in males (Nugent et al., Citation2011).
The aforementioned sex differences might originate from evolutionary processes. Compared to the “fight-or-flight” stress response, the use of social networks aiding in nurturant activities for oneself and the offspring – i.e. “tend-and-befriend” behavior (Taylor et al., Citation2000) – might have been more favorable for females. This was supported in a self-report study of stress and social support (Kneavel, Citation2021). The tend-and-befriend pattern might be mediated by oxytocin (Taylor et al., Citation2002), which increases vmHRV (Kemp et al., Citation2012). In line with this behavioral pattern, a study found that vmHRV increased in females and decreased in males during a stress task (Adjei et al., Citation2018). However, sex differences in the association between vmHRV and perceived social support in relation to self-reported stress have not been investigated previously.
In the current study, we investigated how self-reported stress and sex moderated the association between vmHRV and perceived social support – assessing whether vmHRV could reflect sex differences in tend-and-befriend behavior. As such, we hypothesized that higher vmHRV would associate with higher perceived social support specifically under conditions of higher, relative to lower, stress levels, and that this association would be most prominent in females.
Materials and methods
Design
Data for the present analyses were derived from the Ethnic Differences in the Experience of Noxious Stimuli (EDENS) study. The EDENS study is a cross-sectional study including behavioral pain testing while various psychophysiological measures were simultaneously recorded. In addition, participants completed several self-report questionnaires. Here, we only report on resting state ECG recordings in association with selected self-report measures, addressing the main hypotheses of the manuscript. Data on the Perceived Stress Scale (PSS) and the Medical outcomes study social support survey (MOS), as well as relevant HRV variables (i.e. HF-HRV and RMSSD; outlined below), were included. The authors state that they have reported all measures, conditions, data exclusions and how they determined their samples sizes in the current sub-study. In the EDENS study, several measures were assessed that are not reported in the current manuscript, as these were not included in the data analysis of the current study. The study protocol was approved by Institutional review board approval at the Ohio State University, Columbus, USA (Study number 2017B0179).
Study site and population
The sample comprised 143 individuals between 18 and 38 years of age (mean age 19.94; SD 2.72; ). The majority of participants were male (n = 82, 57.8%). University students at the Ohio State University, USA, were recruited by study advertisement.
Table 1. Descriptive statistics for males and females.
Procedure
Minimum requirements for participation in the study involved two assessments (baseline and physiological assessment, and extended psychological assessment), which was completed at one appointment or within one week. Participants were given a detailed explanation of the procedure and the aim of the study, and then gave written consent to participation in accordance with the Declaration of Helsinki. The students received partial course credits for participating in the study, or if not applicable, 15$(13€). Upon completion of the consent form, the baseline assessment and initial physical examination were completed, or a future appointment was scheduled. The baseline assessment included the completion of several questionnaires, including a standard socio-demographic questionnaire on education (What is the highest level of education you have completed?). The item was scored on a scale from 1 (12th grade or less) through 5 (Masters or Doctorate degree). Participants also completed the International physical activity questionnaire (IPAQ), the Alcohol use disorders identification test (AUDIT), and the Drug abuse screening test (DAST; outlined below). The initial physical examination included assessment of height and weight, and a 5-minute ECG recording (outlined below). Subsequently, all participants underwent an extended psychological assessment where a set of questionnaires was administered, including the MOS and the PSS.
Measures
Physiological measures
A 5-minute ECG recording was obtained from each participant in a seated position. Participants were instructed to relax and try not to fall asleep while breathing spontaneously. The ECG signal (sampled at 1000 Hz) was conducted through three adhesive electrodes and assessed using a 16-channel bioamplifier (Nexus-16; Mind media B.V.; Roermond-Herten, The Netherlands), and the recording software Biotrace+ (Mind media B.V., Roermond-Herten: Mind Media BV, 2004). The inter-beat-interval time series was written and exported from BioTrace + in a single text file for each participant and further analyzed using the Kubios HRV analysis software, version 3.0.0 (Tarvainen et al., Citation2014). Automatic artifact corrections – which are commonly used, and supported under adequate recording conditions such as those in the current study (Laborde et al., Citation2017) – were performed on 32 of the recordings (22 males; 10 females; mean corrections: 4.04% of the recordings). An autoregressive model with smoothness priors detrending in Kubios HRV was used for spectral analysis, yielding a power spectrum displaying activity in the low frequency (LF; .04–.15 Hz) and high frequency (HF; .15–.4 Hz) domain. Although also influenced by other physiological systems, HF power (i.e. HF-HRV) mainly reflects vagal activity as indexed by heart function (Thayer et al., Citation2010). The square root of the mean squared differences of successive R-R intervals (RMSSD), also considered to mark vagal activity (Thayer et al., Citation2010) as indexed by heart function, was also calculated. HF-HRV was chosen as the primary measure of vmHRV (the term vmHRV will refer to HF-HRV in the following Methods and Results sections, unless otherwise specified), as it has been acknowledged as the most commonly reported index of vmHRV (Kemp et al., Citation2012). We used ECG-derived respiration (EDR) to control for respiratory frequency (Tarvainen et al., Citation2014). In addition, we assessed mean HR. The height (cm) and weight (kg) of each participant was recorded. These measures were used to derive their body mass index (BMI, by kg/m2), which has been related to vmHRV (Koenig et al., Citation2014). The BMI value was missing for one participant (male).
Questionnaires
The short version of the MOS (Hays et al., Citation1995) measured perceived social support. The short form has shown excellent psychometric properties similar to those of the original 19-item instrument (Hays et al., Citation1995). The questionnaire comprises 8 situations (If you needed it, how often is someone available…., e.g. who understands your problems?). Thus, it aims to measure the perceived availability, if needed, of social support. The situations are rated on a 5-point Likert scale from 1 (none of the time) to 5 (all of the time). A higher total score indicates higher perceived social support.
The PSS (Cohen et al., Citation1983) is a widely used self-report instrument to evaluate an individual’s level of perceived stress. In the current study, the 10-item short form was used. In general, the psychometric properties of the 10-item PSS have been found to be superior to those of the 14-item PSS (Lee, Citation2012). The PSS has shown adequate reliability and validity, and has often been assessed in students (Lee, Citation2012), as in the current sample. Asking for feelings and thoughts regarding the last month, it assesses the extent to which a person perceives life as stressful (e.g. How often have you found that you could not cope with all the things that you had to do?). The questionnaire uses a Likert-type response scale from 0 (never) to 4 (very often). Higher PSS scores indicate a higher level of perceived stress.
The short form of the IPAQ (see https://sites.google.com/site/theipaq/, Craig et al., Citation2003) was used to assess the participants’ physical activity levels. The IPAQ’s measurement properties have been evaluated in 12 countries, where it has shown good reliability and acceptable validity (Craig et al., Citation2003). The 4-item short-form of the IPAQ collects information on the time (i.e. number of sessions and average time per session) spent on physical activities with moderate intensity, vigorous intensity, and sitting, reported from the last seven days. This is converted to metabolic equivalents (METs) expended at the various intensities per week. In the current study, the number of METs/week for vigorous intensity was chosen as the applied measure of physical activity levels. The reason for this was that vmHRV is related to maximum oxygen intake (VO2max; Goldsmith et al., Citation1997), and to elicit significant changes in VO2max it is necessary to perform physical activity at 75 percent or more of heart rate maximum (i.e. vigorous intensity; Burke & Franks, Citation1975).
The participants’ alcohol consumption, which has been associated with vmHRV (Cheng et al., Citation2019), was assessed by the AUDIT (Babor et al., Citation2001). The AUDIT is used to identify persons with hazardous and harmful patterns of alcohol consumption, and has been found to provide an accurate measure of risk across sex, age, and cultures (Babor et al., Citation2001). Items are rated on a five-point Likert-type scale ranging from “never” to “four times a week or more”. A higher total score corresponds to a higher alcohol consumption. The 10-item short form of the DAST (Skinner, Citation1982) was used to assess drug use – which also has been related to vmHRV (D’Souza et al., Citation2019) – within the sample. This is a well-validated instrument, which has been shown to have acceptable internal consistency (Skinner, Citation1982). Referring to the last 12 months, participants were asked to answer “yes” or “no” to a set of questions, which were then scored according to the scoring norm. A higher total score corresponds to a higher degree of problems related to drug use. We also assessed participants’ smoking habits in the current study, however few participants (n = 3, 2.0%) reported that they currently smoked daily, and smoking was consequently not included as a covariate in the current analyses.
Statistical analysis
Statistical analyses were performed in the Statistical package for the social sciences version 24.0 (SPSS; IBM Corp., Armonk, NY, 2016). Missing values were imputed with the sample mean. Due to missing data linking vmHRV to self-report scores and demographic information, four participants were excluded from the study. Outliers, defined as values > ±3 standard deviations (SD) from the mean of the total sample, would as a general rule be corrected with the value of ±3SD of the sample mean (for handling of missing data and outliers, see ). To approximate a normal distribution, vmHRV data were log-transformed (Thayer et al., Citation2010). If other variables were found to be severely skewed, they would be converted to percentile-based categorical variables: low (1; ≤16th percentile), intermediate (2; 16th–84th percentile) and high (3; ≥84th percentile). If the variable had been exactly normally distributed, the applied percentiles would translate to the mean –1SD, the mean, and the mean +1SD. However, the percentile-based approach avoids risking that one of the measures could be outside of the range of measurement, as with a SD-based approach (Hayes, Citation2017). The same rationale was applied for reporting PSS scores at these specific percentiles in the moderation analyses (outlined below).
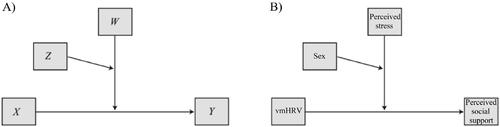
Figure 1. Model for multiple moderation analysis, as A) designed by Hayes, and B) applied in the current study. Note. vmHRV: Vagally mediated heart rate variability, indexed by high frequency power (HF-HRV).
We performed preliminary analyses of sex differences in age, vmHRV, MOS, PSS, BMI, AUDIT, DAST, EDR, and mean HR with the independent samples t-tests. Chi-square tests were used for analyses of sex differences in educational levels and IPAQ scores. Effect sizes were estimated with Cohen’s d in the t-tests, and with phi (φ) in the chi-square tests. Bivariate correlation analyses were conducted in the total sample, and for males and females, respectively, for correlations between vmHRV, MOS and PSS, age, educational levels, IPAQ, BMI, AUDIT, DAST, EDR and mean HR.
As our main analysis, we conducted a moderation (i.e. interaction) analysis in the PROCESS v.3.5 macro (Hayes, Citation2017) in SPSS. An a priori estimation of statistical power was performed in order to determine the sample size needed to estimate statistical effects in the moderation model. To our knowledge, no comparable prior studies had included two moderators in their model and the estimation was therefore based on two similar, prior studies using different statistical models (Fabes & Eisenberg, Citation1997; Schwerdtfeger & Schlagert, Citation2011). These studies revealed medium effect sizes (f 2 = 0.20 and 0.21, respectively). Considering that the current study applied two moderator variables, which tend to decrease effect sizes, and that there were few studies to provide a robust estimate of the expected effect size, we assumed a small to moderate effect size concerning the association between vmHRV and perceived social support: f2 = 0.15. Using g*power, we specified all eight predictors in the model: vmHRV, PSS scores, sex, the interaction of the aforementioned variables, and IPAQ scores (which were shown to covary significantly with the MOS scores, as described in the Results section). We specified the conventional level of sufficient power to be 1−β = 0.80 and a significance level of α = 0.05. The analysis revealed that a total number of n = 109 participants would be a required minimum to avoid a type II error in our study, which is below our sample size of n = 143.
In the moderation analysis, model 3 was chosen as our applied model (). This investigated the hypothesis that higher vmHRV would be associated with higher MOS scores only under conditions of higher PSS scores, and that this moderation would be found specifically in females. In the moderation model, vmHRV was included as the antecedent (i.e. independent, X) variable, MOS scores as the consequent (i.e. outcome, Y) variable, and PSS scores (W) and sex (Z) as moderators. A preliminary moderation analysis – performed to investigate which potential confounding factors would be included in the final model – included age, educational levels, IPAQ, BMI, AUDIT, DAST, EDR, and mean HR as covariates. Importantly, the effect of these covariates would only be controlled for in the final statistical model if they were shown to covary significantly with MOS scores in this preliminary model. In a conditional effects part of the final moderation analysis, effects of varying PSS scores on the relation between vmHRV and MOS were investigated. Here, PSS scores were reported at low (16th percentile), intermediate (50th percentile), and high (84th percentile) levels (while still being included as a continuous variable in the statistical model). To investigate whether these results were comparable when using another vmHRV index, we repeated the analyses with RMSSD as the antecedent variable. Furthermore, a linear regression analysis was included in the moderation analyses. Lastly, to test an alternative direction of our model, to account for the fact that perceived social support could predict vmHRV, we repeated the moderation analyses with MOS scores as the antecedent variable and vmHRV as the consequent variable.
Results
Preliminary analyses
There were detected three outliers for BMI values (one male, two females), and one for vmHRV (male). The BMI values were corrected with the value of ±3SD of the sample mean. The vmHRV outlier was a very low value, and we could not rule out that this had occurred due to underlying somatic issues. Therefore, we decided to deviate from our original rule of correcting outliers, and instead excluded this participant. No outliers were detected for educational levels, MOS, PSS, AUDIT or DAST scores, EDR or mean HR. The distribution of the IPAQ scores was severely skewed, and the scores were therefore re-categorized as low, intermediate, and high.
The preliminary analyses showed that there were no significant sex differences in age, educational levels, vmHRV, PSS, IPAQ, BMI, AUDIT, DAST, EDR, or mean HR (). Females reported significantly higher MOS scores than males. In the total sample, bivariate correlations analyses showed that higher vmHRV correlated with lower PSS scores, higher IPAQ scores and lower mean HR (). However, vmHRV was not correlated with MOS scores in the total sample. Higher MOS scores correlated with lower age and higher IPAQ scores. In males, higher vmHRV correlated with lower PSS scores and lower mean HR, but vmHRV was not correlated with MOS scores. Higher MOS scores correlated with higher IPAQ scores and lower educational levels. In females, higher vmHRV correlated with higher MOS scores, lower EDR and lower mean HR, but not with PSS scores. MOS and PSS scores were not correlated in any of the samples.
Table 2. Bivariate correlations among main study variables in the total sample and the two sexes, respectively.
Moderating effects
To test the hypothesis that higher vmHRV would be associated with higher MOS scores only under conditions of higher PSS scores specifically in females and not males, we performed a moderation analysis (). In the preliminary moderation model, IPAQ scores covaried significantly with MOS scores. Therefore, IPAQ scores were included in the final model. Age, educational level, BMI, AUDIT, DAST, EDR, and mean HR did not covary significantly with MOS scores and were not included in the final model.
Table 3. Moderation analyses of prediction of MOS.
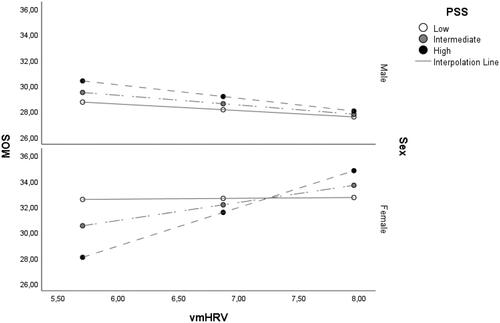
The conditional effects results of the final model showed that inclusion of both PSS scores and sex moderated the association between MOS scores and vmHRV, specifically for higher (i.e. intermediate and high) PSS scores in females and not males (). Low PSS scores alone did not moderate the relation in either females or males. The same results were found when using RMSSD as an index of vmHRV.
Figure 2. Regression lines for males and females on the association between vmHRV and MOS scores in relation to varying PSS scores. Note. vmHRV: Vagally mediated heart rate variability, indexed by high frequency power (HF-HRV). MOS: Medical Outcomes Study Social Support Survey; PSS: Perceived Stress Scale. Low, intermediate and high PSS scores correspond to the 16th, 50th and 84th percentiles, respectively. *p ≤ 0.05 **p ≤ 0.01.
The linear regression results from the moderation analysis, including all key variables, found that the three-way-interaction of vmHRV, PSS scores, and sex significantly predicted MOS scores, where female sex, higher vmHRV, and higher PSS scores were associated with higher MOS scores. Furthermore, the interaction term of PSS scores and sex significantly predicted MOS scores. Specifically, there was found a more prominent relationship between lower stress levels and higher perceived social support in females compared to males. Neither vmHRV, PSS scores, nor the interaction of vmHRV and PSS scores associated with MOS scores. Furthermore, there were no significant main effects of sex, or interaction effect of sex and vmHRV on MOS scores. Lastly, IPAQ scores significantly predicted MOS scores, in that higher IPAQ scores were associated with higher MOS scores.
Using MOS scores as an antecedent variable and vmHRV as a consequent variable with PSS scores and sex as moderators did not yield significant results.
Discussion
The aim of the current study was to investigate if a positive relation between vmHRV and perceived social support was dependent on (i.e. moderated by) an interaction between stress levels and sex. The expectation was confirmed in that we found a significant three-way interaction of vmHRV, perceived stress and sex on perceived social support. Specifically, investigating how this association was related to various stress levels, we found that higher vmHRV was associated with higher perceived social support only in females and not males under conditions of higher stress levels. For lower stress levels, vmHRV was not associated with perceived social support in any of the sexes.
The results, indicating sex differences in the association between perceived social support and vagus nerve activity as reflected in vmHRV in relation to stress, are in line with evolutionary theories. As females have often been responsible for offspring, a tend-and-befriend response to stress – involving the creation and maintenance of social networks aiding in nurturant activities (Taylor et al., Citation2000) – might generally have been favored over the classic “fight-or-flight” response. Whereas the fight-or-flight response typically involves adrenaline and noradrenaline signaling, the tend-and-befriend pattern is partially mediated by oxytocin (Taylor, Citation2007; Taylor et al., Citation2000). Importantly, the female sex hormone estrogen potentiates the effects of oxytocin (Taylor et al., Citation2000). Furthermore, higher oxytocin levels are associated with increased vagal activity (Kemp et al., Citation2012), likely through oxytocin-type neurons from the paraventricular nucleus communicating with cardiovagal neurons in the nucleus tractus solitarius, the dorsal motor nucleus of the vagus, and the nucleus ambiguous (see Kanthak et al., Citation2016). This leads to an increase of vagal outflow especially during stress, as supported in a study where social support associated with higher vmHRV in the preparation for and during a stress task only in individuals with a gene variant increasing the effect of oxytocin (see Kanthak et al., Citation2016), Such an increase in vagus activity as indexed by heart function, reflected by higher vmHRV, is considered to represent activity of a neural network including the prefrontal cortex and amygdala (Thayer & Lane, Citation2000). This network is important for adaptability and self-regulatory functions (Thayer & Lane, Citation2000), which might include social support seeking as a stress regulation strategy.
Associations between key variables in our study were largely in agreement with previous literature. Firstly, females reported higher perceived social support than males, as expected. Secondly, also as expected, vmHRV was more strongly associated with perceived social support under conditions of higher, relative to lower, stress levels. This is in line with a self-report study investigating the relation between vmHRV and a mixed measure of constructive coping (including social support seeking) in both sexes (Fabes & Eisenberg, Citation1997). Lastly, the relationship between higher perceived social support and lower stress levels was more prominent in females compared to males, which could be due to perceived social support reducing stress more effectively in females.
Contrary to expectations, when including all key variables in the same moderation model, some interaction effects (e.g. sex and vmHRV, and vmHRV and stress) were non-significant predictors of perceived social support. This underlines the importance of investigating vmHRV, stress and sex in a total model in relation to perceived social support, as the variables moderate each other. Therefore, although we found non-significant associations between some of the key variables considered separately, this does not question the validity of the total model (Hayes, Citation2017). Further contrary to our expectations, we did not find higher vmHRV in females compared to males. As vmHRV shows age-dependent sex differences – with lower vmHRV in girls compared to boys, and higher vmHRV in females compared to males (Koenig et al., Citation2017; Koenig & Thayer, Citation2016) – this developmental shift in vmHRV could cause less apparent sex differences in a young adult sample such as ours, explaining the respective non-finding.
In contrast to the majority of previous studies, which have relied on experimental data, our results are derived from what is often considered “trait” assessments of vagal function and self-reports. Self-reports might more accurately represent how perceived social support and stress affect participants in their daily lives, compared to experimental studies that often are non-naturalistic and situation-based (see Uchino et al., Citation2011). Self-reported perceived stress further accounts for that females might experience higher stress and thus greater ANS activation compared to males despite exposure to the same stressor (Tamres et al., Citation2002) – possibly due to effects of menstrual cycles (Simon et al., Citation2021). In addition, self-reports might contribute to health-related insights, as perceived support has consistently shown a beneficial influence on psychological and physiological outcomes such as cardiovascular disease, in contrast to received support (Uchino et al., Citation2011). Furthermore, we assessed vmHRV derived from resting ECGs, which is considered to reflect “trait” vagal activity as indexed by heart function to a large degree. This notion is supported by studies showing that resting vmHRV has a high test–retest reliability (Bertsch et al., Citation2012; Tarkiainen et al., Citation2005), and by widely supported theoretical frameworks that consider resting vmHRV as a neurophysiological marker of a tendency to self-regulate and engage socially (Porges, Citation2007; Thayer & Lane, Citation2000). Moreover, our results point in similar directions as experimental data assessing “state” vagal responses to stress protocols, in that higher vmHRV and flexible vagal reactivity under stressful conditions are associated with higher perceived social support (Goodyke et al., Citation2021). As such, our results are likely valid. Taken together, our study might provide important complementing insights to experimental studies.
Regarding limitations of the study, we did not control for effects of varying estrogen levels in relation to menstrual cycles, which has been shown to influence the relation between vmHRV and stress (Simon et al., Citation2021). However, estrogen levels are generally higher in females than males. Thus, if all females in the current study had been in the same menstrual (i.e. follicular/luteal) phase, we expect that we still would have found a more prominent association between higher vmHRV and higher perceived social support in females compared to males. Furthermore, our cross-sectional design leaves us unable to draw on causal inferences. However, our findings are supported by theoretical frameworks such as Porges’ influential polyvagal theory, which suggest that higher vmHRV allows for adaptive social engagement (Porges, Citation2007). In addition, there is evidence that vmHRV prospectively predicts feelings of social connectedness (Kok & Fredrickson, Citation2010). While an opposite relation is possible, where perceived social support influences vmHRV, using perceived social support as a predictor of vmHRV in our moderation analyses did not yield significant results. Thus, the question of causality is not yet clearly elucidated. In addition, our study cannot fully explicate the complexity of the investigated constructs. Further insight might be provided by assessing multiple markers of ANS responses, possible genetic contributing factors such as oxytocin gene receptor polymorphism (see Kanthak et al., Citation2016), sub-categories of perceived social support (see Taylor, Citation2007), which sex social support is perceived to be provided from (see Taylor, Citation2007), and accounting for the fact that social support might not universally have a positive effect (see Kneavel, Citation2021; Taylor, Citation2007).
Our results might have important theoretical and clinical implications. Firstly, they add to the literature underlining the importance of assessing sex differences in the relations between vmHRV and psychological functioning (see e.g. Simon et al., Citation2021). This could also apply to studies of psychiatric illnesses, where differences in ANS regulation in relation to stress could explain sex-based variations in symptomatology (see Klein & Corwin, Citation2002). Secondly, we found that physical activity levels predicted perceived social support, in line with previous literature (e.g. Mendonça et al., Citation2014). This highlights the importance of controlling for the possible confounding effects of physical activity in the current research field. Thirdly, our results give reason to suspect that increasing vmHRV, for example by vagus nerve stimulation or biofeedback, could be an intervention strategy for individuals with high perceived stress and low perceived social support (see Porges, Citation2007). Interventions for increasing vmHRV could be a cost-effective prevention for the wide range of morbidities and increased mortality associated with lower vmHRV (Thayer & Lane, Citation2007), higher stress (Cohen et al., Citation2007) and lack of social support (Holt-Lunstad et al., Citation2010). Our findings suggest that such interventions may be especially relevant in females, which could contribute to targeted therapy. Possibly, such interventions could also be relevant for psychiatric illnesses, which often represent the activation of evolutionary defense strategies, especially in contexts with few indications of social support (see Petrocchi & Cheli, Citation2019). Yet, the generalizability of the findings in the current study awaits future validation in other and larger populations.
Supplemental Material
Download MS Word (188.1 KB)Acknowledgements
The authors thank Anthony Bernardi and Ravi R. Bhatt. We would also like to thank the individuals who participated in the study.
Disclosure statement
JH has received lecture honoraria as part of continuing medical education programs sponsored by Shire, Takeda and Medice. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interest.
Data availability statement
The data that support the findings of this study are available on request from JK.
Additional information
Funding
References
- Adjei, T., Xue, J., & Mandic, D. P. (2018). The female heart: Sex differences in the dynamics of ECG in response to stress. Frontiers in Physiology, 9, 1616–1618. https://doi.org/10.3389/fphys.2018.01616
- Babor, T. F., Higgins-Biddle, J., Saunders, J., & Monteiro, M. (2001). The alcohol use disorders identification test: Guidelines for use in primary care. World Health Organization.
- Bertsch, K., Hagemann, D., Naumann, E., Schächinger, H., & Schulz, A. (2012). Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology, 49(5), 672–682. https://doi.org/10.1111/j.1469-8986.2011.01341.x
- Burke, E. J., & Franks, B. D. (1975). Changes in V02max resulting from bicycle training at different intensities holding total mechanical work constant. Research Quarterly, 46(1), 31–37.
- Cheng, Y. C., Huang, Y. C., & Huang, W. L. (2019). Heart rate variability as a potential biomarker for alcohol use disorders: A systematic review and meta-analysis. Drug and Alcohol Dependence., 204, 1–10. https://doi.org/10.1016/j.drugalcdep.2019.05.030
- Cohen, S. (2004). Social relationships and health. The American Psychologist, 59(8), 676–684. https://doi.org/10.1037/0003-066X.59.8.676
- Cohen, S., Janicki-Deverts, D., & Miller, G. E. (2007). Psychological stress and disease. JAMA, 298(14), 1685–1687. https://doi.org/10.1001/jama.298.14.1685
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Craig, C. L., Marshall, A. L., Sjöström, M., Bauman, A. E., Booth, M. L., Ainsworth, B. E., Pratt, M., Ekelund, U., Yngve, A., Sallis, J. F., & Oja, P. (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35(8), 1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB
- D'Souza, J. M., Wardle, M., Green, C. E., Lane, S. D., Schmitz, J. M., & Vujanovic, A. A. (2019). Resting heart rate variability: Exploring associations with symptom severity in adults with substance use disorders and posttraumatic stress. Journal of Dual Diagnosis, 15(1), 2–7. https://doi.org/10.1080/15504263.2018.1526431
- Fabes, R. A., & Eisenberg, N. (1997). Regulatory control and adults' stress-related responses to daily life events. Journal of Personality and Social Psychology, 73(5), 1107–1117. https://doi.org/10.1037/0022-3514.73.5.1107
- Gerteis, A. K., & Schwerdtfeger, A. R. (2016). When rumination counts: Perceived social support and heart rate variability in daily life. Psychophysiology, 53(7), 1034–1043. https://doi.org/10.1111/psyp.12652
- Goldsmith, R. L., Bigger, J. T., Jr., Bloomfield, D. M., & Steinman, R. C. (1997). Physical fitness as a determinant of vagal modulation. Medicine and Science in Sports and Exercise, 29(6), 812–817. https://doi.org/10.1097/00005768-199706000-00012
- Goodyke, M. P., Hershberger, P. E., Bronas, U. G., & Dunn, S. L. (2021). Perceived social support and heart rate variability: An integrative review. Western Journal of Nursing Research, 1–11. https://doi.org/10.1177/01939459211028908
- Hayes, A. F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Publications.
- Hays, R. D., Sherbourne, C. D., & Mazel, R. M. (1995). User's manual for the Medical outcomes study (MOS) core measures of health-related quality of life. Rand Corporation Santa Monica.
- Holt-Lunstad, J., Smith, T. B., & Layton, J. B. (2010). Social relationships and mortality risk: A meta-analytic review. PLoS Medicine, 7(7), e1000316–20. https://doi.org/10.1371/journal.pmed.1000316
- Holt-Lunstad, J., Uchino, B. N., Smith, T. W., & Hicks, A. (2007). On the importance of relationship quality: The impact of ambivalence in friendships on cardiovascular functioning. Annals of Behavioral Medicine: a Publication of the Society of Behavioral Medicine, 33(3), 278–290. https://doi.org/10.1007/BF02879910
- Kanthak, M. K., Chen, F. S., Kumsta, R., Hill, L. K., Thayer, J. F., & Heinrichs, M. (2016). Oxytocin receptor gene polymorphism modulates the effects of social support on heart rate variability. Biological Psychology, 117, 43–49. https://doi.org/10.1016/j.biopsycho.2016.02.007
- Kao, C. W., Tseng, L. F., Lin, W. S., & Cheng, S. M. (2014). Association of psychosocial factors and heart rate variability in heart failure patients. Western Journal of Nursing Research, 36(6), 769–787. https://doi.org/10.1177/0193945913505922
- Kemp, A. H., Quintana, D. S., Kuhnert, R.-L., Griffiths, K., Hickie, I. B., & Guastella, A. J. (2012). Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One, 7(8), 1–6.
- Kim, H. G., Cheon, E. J., Bai, D. S., Lee, Y. H., & Koo, B. H. (2018). Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investigation, 15(3), 235–245. https://doi.org/10.30773/pi.2017.08.17
- Klein, L. C., & Corwin, E. J. (2002). Seeing the unexpected: How sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Current Psychiatry Reports, 4(6), 441–448. https://doi.org/10.1007/s11920-002-0072-z
- Kneavel, M. (2021). Relationship between gender, stress, and quality of social support. Psychological Reports, 124(4), 1421–1481. https://doi.org/10.1177/0033294120939844
- Koenig, J., & Thayer, J. F. (2016). Sex differences in healthy human heart rate variability: A meta-analysis. Neuroscience and Biobehavioral Reviews, 64, 288–310. https://doi.org/10.1016/j.neubiorev.2016.03.007
- Koenig, J., Jarczok, M. N., Warth, M., Ellis, R. J., Bach, C., Hillecke, T. K., & Thayer, J. F. (2014). Body mass index is related to autonomic nervous system activity as measured by heart rate variability—A replication using short term measurements. The Journal of Nutrition, Health & Aging, 18(3), 300–302. https://doi.org/10.1007/s12603-014-0022-6
- Koenig, J., Rash, J. A., Campbell, T. S., Thayer, J. F., & Kaess, M. (2017). A meta-analysis on sex differences in resting-state vagal activity in children and adolescents. Frontiers in Physiology, 8, 1–11. https://doi.org/10.3389/fphys.2017.00582
- Kok, B. E., & Fredrickson, B. L. (2010). Upward spirals of the heart: Autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biological Psychology, 85(3), 432–436. https://doi.org/10.1016/j.biopsycho.2010.09.005
- Laborde, S., Mosley, E., & Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213.
- Lee, E.-H. (2012). Review of the psychometric evidence of the perceived stress scale. Asian Nursing Research, 6(4), 121–127. https://doi.org/10.1016/j.anr.2012.08.004
- Li, Z., Snieder, H., Su, S., Ding, X., Thayer, J. F., Treiber, F. A., & Wang, X. (2009). A longitudinal study in youth of heart rate variability at rest and in response to stress. International Journal of Psychophysiology, 73(3), 212–217. https://doi.org/10.1016/j.ijpsycho.2009.03.002
- Maunder, R. G., Nolan, R. P., Hunter, J. J., Lancee, W. J., Steinhart, A. H., & Greenberg, G. R. (2012). Relationship between social support and autonomic function during a stress protocol in ulcerative colitis patients in remission. Inflammatory Bowel Disease, 18(4), 737–742. https://doi.org/10.1002/ibd.21794
- Mendonça, G., Cheng, L. A., Mélo, E. N., & de Farias Júnior, J. C. (2014). Physical activity and social support in adolescents: a systematic review. Health Education Research, 29(5), 822–839. https://doi.org/10.1093/her/cyu017
- Nugent, A. C., Bain, E. E., Thayer, J. F., Sollers, J. J., & Drevets, W. C. (2011). Sex differences in the neural correlates of autonomic arousal: A pilot PET study. International Journal of Psychophysiology, 80(3), 182–191. https://doi.org/10.1016/j.ijpsycho.2011.03.001
- Petrocchi, N., & Cheli, S. (2019). The social brain and heart rate variability: Implications for psychotherapy. Psychology and Psychotherapy, 92(2), 208–223. https://doi.org/10.1111/papt.12224
- Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009
- Sato, N., & Miyake, S. (2004). Cardiovascular reactivity to mental stress: Relationship with menstrual cycle and gender. Journal of Physiological Anthropology and Applied Human Science, 23(6), 215–223. https://doi.org/10.2114/jpa.23.215
- Schwerdtfeger, A. R., & Schlagert, H. (2011). The conjoined effect of naturalistic perceived available support and enacted support on cardiovascular reactivity during a laboratory stressor. Annals of Behavioral Medicine : a Publication of the Society of Behavioral Medicine, 42(1), 64–78. https://doi.org/10.1007/s12160-011-9272-2
- Simon, S. G., Sloan, R. P., Thayer, J. F., & Jamner, L. D. (2021). Taking context to heart: Momentary emotions, menstrual cycle phase, and cardiac autonomic regulation. Psychophysiology, 58(4), e13765. https://doi.org/10.1111/psyp.13765
- Skinner, H. A. (1982). The drug abuse screening test. Addictive Behaviors, 7(4), 363–371. https://doi.org/10.1016/0306-4603(82)90005-3
- Tamres, L. K., Janicki, D., & Helgeson, V. S. (2002). Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personality and Social Psychology Review, 6(1), 2–30. https://doi.org/10.1207/S15327957PSPR0601_1
- Tarkiainen, T. H., Timonen, K. L., Tiittanen, P., Hartikainen, J. E. K., Pekkanen, J., Hoek, G., Ibald-Mulli, A., & Vanninen, E. J. (2005). Stability over time of short-term heart rate variability. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, 15(6), 394–399. https://doi.org/10.1007/s10286-005-0302-7
- Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O., & Karjalainen, P. A. (2014). Kubios HRV-heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. doi: https://doi.org/10.1016/j.cmpb.2013.07.024
- Taylor, S. E. (2007). Social support. In H. S. Friedman (Ed.), The Oxford handbook of health psychology. Oxford Library of Psychology: Oxford University Press.
- Taylor, S. E., Dickerson, S. S., & Klein, L. C. (2002). Toward a biology of social support. In C. R. Snyder, Shane J. Lopez (Ed.), Handbook of Positive Psychology (pp. 556–569). New York: Oxford University Press.
- Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A., & Updegraff, J. A. (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. https://doi.org/10.1037/0033-295x.107.3.411
- Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. doi: https://doi.org/10.1016/S0165-0327(00)00338-4
- Thayer, J. F., & Lane, R. D. (2007). The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology, 74(2), 224–242. doi: https://doi.org/10.1016/j.biopsycho.2005.11.013
- Thayer, J. F., Hansen, A. L., & Johnsen, B. H. (2010). The non-invasive assessment of autonomic influences on the heart using impedance cardiography and heart rate variability. Handbook of Behavioral Medicine (pp. 723–740). Springer. doi: 10.1007/978-0-387-09488-5_47
- Uchino, B. N., Carlisle, M., Birmingham, W., & Vaughn, A. A. (2011). Social support and the reactivity hypothesis: conceptual issues in examining the efficacy of received support during acute psychological stress. Biological Psychology, 86(2), 137–142. https://doi.org/10.1016/j.biopsycho.2010.04.003
