 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Worldwide, millions of people suffer from treatment-resistant depression. Ketamine, a glutamatergic receptor antagonist, can have a rapid antidepressant effect even in treatment-resistant patients. A proposed mechanism for the antidepressant effect of ketamine is the reduction of neuroinflammation. To further explore this hypothesis, we investigated whether a single dose of ketamine can modulate protracted neuroinflammation in a repeated social defeat (RSD) stress rat model, which resembles features of depression. To this end, male animals exposed to RSD were injected with ketamine (20 mg/kg) or vehicle. A combination of behavioral analyses and PET scans of the inflammatory marker TSPO in the brain were performed. Rats submitted to RSD showed anhedonia-like behavior in the sucrose preference test, decreased weight gain, and increased TSPO levels in the insular and entorhinal cortices, as observed by [11C]-PK11195 PET. Whole brain TSPO levels correlated with corticosterone levels in several brain regions of RSD exposed animals, but not in controls. Ketamine injection 1 day after RSD disrupted the correlation between TSPO levels and serum corticosterone levels, but had no effect on depressive-like symptoms, weight gain or the protracted RSD-induced increase in TSPO expression in male rats. These results suggest that ketamine does not exert its effect on the hypothalamic–pituitary–adrenal axis by modulation of neuroinflammation.
1. Introduction
About 350 million people worldwide suffer from major depressive disorder (MDD) and it is considered a major contributor to the global health economic burden due to high medication costs and disability (Mathers & Loncar, Citation2006). The symptomatology of MDD ranges from loss of enjoyment in social activities to increased anxiety on a daily basis (Mathers & Loncar, Citation2006). Treatment of MDD with classical antidepressants, such as selective serotonin-reuptake inhibitors and tricyclic antidepressants (TCA), is often not fully effective (Gartlehner et al., Citation2011), requires weeks to exert an effect and shows high rates of relapse (Zimmerman & Thongy, Citation2007). As a result, about 30% of the patients are treatment resistant (Hashimoto, Citation2015). One of the aspects that greatly contributes to treatment-resistant depression is our lack of understanding of the etiology of MDD (Friedman, Citation2014). Despite the extensive research pointing toward various pharmacological targets used for the treatment of MDD, increasing evidence suggests additional mechanisms could be involved in the pathogenesis of depression (Cai et al., Citation2019).
In recent years, more attention is given to the association between depression and the innate and adaptive immune system. It has been suggested that pro-inflammatory cytokine levels are increased in the plasma of depressed patients and these cytokine levels are correlated with the severity of depression (Miller et al., Citation2009; Raedler, Citation2011). However, this increase in inflammatory markers is not observed in all patients, but mainly in specific subpopulations of depressed patients (Beurel et al., Citation2020; Kiecolt-Glaser et al., Citation2015; Majd et al., Citation2020). Especially patients that are resistant to monoamine-targeting antidepressant drugs have shown an abnormal increase in various pro-inflammation markers (Leonard and Maes, Citation2012; Maes et al., Citation2012). In contrast, elevated inflammatory markers have been suggested to correlate with enhanced (rather than attenuated) treatment response to TCA, ketamine and electroconvulsive therapy (Carvalho et al., Citation2013; Yoshimura et al., Citation2009). Therefore, anti-inflammatory properties may play a role in the efficacy of some of the available treatments.
The use of imaging techniques such as positron emission tomography (PET) can be used for in vivo assessment of changes in neuroinflammatory markers. PET tracers targeting various inflammatory markers in the brain, including cyclooxygenase-2 (COX-2), cannabinoid receptor type 2 (CB2), and the mitochondrial 18 kDa translocator protein (TSPO), have been studied (Cumming et al., Citation2018). Among these, TSPO targeting tracers have been used most frequently. TSPO expression is increased in brain cells, such as microglia, astrocytes and endothelial cells, as a result of inflammatory processes in both neurodegenerative and psychiatric disorders in both humans and animal models (Barresi et al., Citation2021; Nutma et al., Citation2021; Werry et al., Citation2019; Zhang et al., Citation2021). Most studies have shown increased TSPO levels in MDD patients compared to healthy volunteers (Dahoun et al., Citation2019; Holmes et al., Citation2018; Li et al., Citation2018; Setiawan et al., Citation2018; Su et al., Citation2016), although differences in the location and intensity of the TSPO overexpression between studies were observed. It was suggested that these differences were related to factors like treatment, specific symptoms and the duration of the disorder (Holmes et al., Citation2018; Richards et al., Citation2018; Setiawan et al., Citation2018; Su et al., Citation2016). To date, only three PET studies assessing TSPO as a neuroinflammation marker in rodent models of chronic stress, mimicking depressive-like symptoms, have been performed. One study used the chronic mild stress mouse model and observed increased TSPO expression after 12 weeks of stress exposure (Wang et al., Citation2018). The other two studies investigated changes of TSPO expression in the repeated social defeat animal model. One study showed a regional increase in the inflammatory marker 1 week after the stress protocol (Kopschina Feltes et al., Citation2019), whereas the other did not find any changes 2 weeks after the stress procedure (Giacobbo et al., Citation2020). This discrepancy could reflect the reversible nature of the inflammatory response in this model. These results, however, also show that PET imaging of TSPO expression in animal models of chronic stress may be a useful tool to investigate the neuroinflammatory response to a stressor as potential feature of the neurobiology of MDD.
Among the different antidepressants available, ketamine has attracted a lot of interest as it shows fast antidepressant effects that are accompanied by a reduction in neuroinflammation, even in treatment-resistant depression (Abdallah et al., Citation2018). However, whether ketamine exerts its fast antidepressant effects through reducing neuroinflammation or, vice versa, the reduction in neuroinflammation is a consequence of successful treatment of the underlying cause of depression is still unclear. Therefore, the objective of this study was to evaluate if ketamine is able to modulate the protracted stress-induced neuroinflammation in the repeated social defeat (RSD) rat model, which induces responses that resemble features of depression. Compared to other chronic stress models, the RSD model mimics the exposure to social stress in humans better, resulting in behavior that resembles features of depressive disorders within a relatively short time frame (Beurel et al., Citation2020; Kiecolt-Glaser et al., Citation2015; Majd et al., Citation2020). In this model, neuroinflammation is still present one week after the last RSD session, as described previously (Kopschina Feltes et al., Citation2019). Therefore, we evaluated whether an acute injection of ketamine after the induction of depressive-like symptoms has an effect on neuroinflammation, as determined by PET with [11C]-PK11195 as a marker for TSPO overexpression.
2. Experimental section
2.1. Experimental animals
All animal experiments complied with the European Directive 2010/63/EU and the Law for Animal experiments of the Netherlands. The study was approved by both the Central Committee on Animal Experiments of the Netherlands (The Hague, license no. AVD1050020171706), and the Institutional Animal Care and Use Committee of the University of Groningen (IvD 171706-01-009). Thirty-six male outbred Wistar Unilever rats (HsdCpd:WU, age 8 weeks, weight 200–250 g) were purchased from Envigo (Horst, The Netherlands), individually housed with food and water available ad libitum and left for acclimatization for 7 days before the start of the experiments. The rats were kept in humidity-controlled, thermo-regulated (21 ± 2 °C) conditions and maintained on a 12/12-h light/dark cycle (lights on at 7.00 a.m.).
2.2. Study design
Thirty-six rats were randomly divided into three groups: (1) control + vehicle (CTL + VEH), (2) repeated social defeat + vehicle (RSD + VEH), and (3) repeated social defeat + ketamine (RSD + KET). Humane endpoints were applied to one RSD + VEH rat due to a severe wound obtained during the RSD protocol. One CTL + VEH rat was excluded due to abnormal behavior observed in the welfare diary evaluations along the experiment. Therefore, the CTL + VEH and RSD + VEH groups consisted of 11 rats and the RSD + KET group of 12 rats.
The overall study design is depicted in . In brief, groups 2 and 3 underwent social defeat on 5 consecutive days (days 0–4). In group 3, ketamine (20 mg/kg; Alfasan Nederland BV) dissolved in 1 ml of sterile saline was injected intraperitoneally on day 5. This dose was chosen because it induced antidepressant effects in a rat model of depression (Zhang et al., Citation2016). Vehicle-treated animals (groups 1 and 2) were injected with saline instead. All rats were weighed on day −1 (before the RSD), day 5 (after the RSD), day 6 (after the ketamine/vehicle injection), and day 11 (before the PET scan). Sucrose preference test (SPT) was used to determine changes in depressive-like behavior since it has been shown that this test was sensible enough to detect long-term changes after a single ketamine injection (Kato et al., Citation2019; Viana et al., Citation2021). Training was performed from day −6 to day −1 as specified below. SPT tests were performed overnight on days 4–5 (after RSD, before ketamine/vehicle administration), 5–6 (after ketamine/vehicle administration), and 10–11. Because of the high discomfort level of the RSD model, no other behavioral tests, such as tail suspension test or forced swim test, were used. An Open field evaluation was performed on days −1 and 6. On day 11, an [11C]-PK11195 PET scan was performed, followed by termination. This date was selected based on a previous study from our group showing changes in [11C]-PK11195 availability at one week, but not 1 day after the RSD protocol (Kopschina Feltes et al., Citation2019). Right before injection of the PET tracer, when animals were anesthetized, blood samples were taken for serum collection.
Figure 1. Study design. Repeated social defeat (RSD) was performed on 5 consecutive days, from day 0 to 4. Sucrose preference test (SPT) training was performed from day -6 to day -3, followed by an overnight training session with one bottle of sucrose solution and another bottle with tap water (day -2 to -1). SPT test sessions were performed overnight at different time points: after RSD (day 4-5), after ketamine (KET) or vehicle (VEH) administration (day 5-6) and before termination (day-10-11). The open field test (OFT) was conducted before RSD (day -1) and after ketamine administration (day 6). Either ketamine (20 mg/kg) or 0.9% saline (VEH) was administered intraperitoneally one day after the last session of RSD (day 5). [11C]-PK11195 PET scans were performed on the last day of the experiment (day 11). On the same day, right before the PET scan, blood samples were collected for serum corticosterone measurements.
![Figure 1. Study design. Repeated social defeat (RSD) was performed on 5 consecutive days, from day 0 to 4. Sucrose preference test (SPT) training was performed from day -6 to day -3, followed by an overnight training session with one bottle of sucrose solution and another bottle with tap water (day -2 to -1). SPT test sessions were performed overnight at different time points: after RSD (day 4-5), after ketamine (KET) or vehicle (VEH) administration (day 5-6) and before termination (day-10-11). The open field test (OFT) was conducted before RSD (day -1) and after ketamine administration (day 6). Either ketamine (20 mg/kg) or 0.9% saline (VEH) was administered intraperitoneally one day after the last session of RSD (day 5). [11C]-PK11195 PET scans were performed on the last day of the experiment (day 11). On the same day, right before the PET scan, blood samples were collected for serum corticosterone measurements.](/cms/asset/3ff33e72-896c-4b96-8551-d4ec5175db92/ists_a_2045269_f0001_b.jpg)
2.3. Repeated social defeat
The repeated social defeat (RSD) model is a commonly used animal model that induces symptoms that resemble features of depression with high ethological, face, construct, and predictive validity; it induces rapid depressive-like behavior in rodents (Czéh et al., Citation2016). Studies using this model have shown a relationship between anxiety-like and depressive-like behavior on the one hand and pro-inflammatory markers and microglia/macrophage activation on the other hand (Kopschina Feltes et al., Citation2019; Lisboa et al., Citation2018; Niraula et al., Citation2019).
Male Long Evans rats (HsdBlu:LE – Envigo, Indianapolis, IN) of 12-weeks old (450–500 g), were used as residents. To encourage territoriality, each male resident was housed in a large cage (80 × 50 × 40 cm) with an ovariectomized Long Evans female for at least one week prior to the start of the screening. Residents were screened for aggressive behavior at least five times prior to the experiment. In short, the screening was performed using RSD sessions on five consecutive days, measuring both attack latency (resident’s time to initiate the first attack) and submission time (time the intruder takes to show a submissive posture for at least 5 s). These parameters were used to select animals displaying the desired aggressive-behavior and exclude animals showing signs of pathological violence (attack latency ˂10 s, and/or attempts to kill the intruder by biting vital zones), or non-aggressive behavior (lack of attack or latency >60 s, lack of submission or latency >120, or resident being submitted). The animals selected for the experimental phase showed an attack latency ranging between 10 and 60 s and a submission time between 10 and 120 s.
The protocol for the training and experimental sessions were similar. The RSD sessions were conducted between 13:00 and 16:30 p.m. Each session lasted for 2 h and was divided into three stages. In the first stage, female rats were removed from resident cages 1 h before the exposure of the intruder. In the second stage, one experimental Wistar rat was placed in the cage of the resident and allowed to interact until it showed a submissive posture to the resident for at least 5 s, or until 10 min had passed if submission was not achieved. Intruders were never exposed more than once to the same resident. Finally, after either the submission took place or the maximum of 10 min had passed, the intruder was placed inside a wire mesh cage and put back in the cage of the resident to allow intense visual, auditory, and olfactory interactions. One hour after the first introduction to the resident’s cage, the intruder was placed back in its home cage. Control rats were not exposed to a resident but placed in a wire mesh cage inside a new clean cage for a period of 60 min instead.
2.4. Sucrose preference test
Anhedonia, a measure of depressive-like behavior, was assessed using the sucrose preference test (SPT). Before the SPT trial, experimental rats were trained by giving them access to a bottle with 1% sucrose in water for 1 h on 4 consecutive days (from day −6 to −3), followed by an overnight exposure (from day −2 to −1) to 2 bottles, one filled with 1% sucrose and the other with normal drinking water. This training ensured that animals get used to drinking sweet water, and to habituate them to the possibility to choose between the two bottles. Tests were performed on 3 time points: after RSD (days 4–5), after injection of ketamine/vehicle (days 5–6), and before termination (days 10–11). The test consisted of overnight exposure to 1 drinking bottle filled with drinking water and another with 1% sucrose solution, which were placed in the animal cages between 16:00 and 17:00 h and measured between 9:00 and 10:00 h the day after. The amount of fluid consumed from each bottle was measured. The percentage of preference for sucrose was used as the SPT outcome and was calculated as follows:
2.5. Open field test
Anxiety-like behavior and locomotion were measured using the open field test (OFT). Rats were placed in a circular arena (diameter: 100 cm) for 5 min, one day before RSD and one day after ketamine/vehicle injection. To measure anxiety-like behavior, the time spent in the center was measured. The border between the center and the periphery was set 20 cm from the walls of the arena. The total distance traveled and the cumulative duration of movement were estimated from offline video recordings using the Ethovision XT 8.5 software (Noldus Information Technology, Wageningen, The Netherlands). Eight videos had to be excluded because image quality or illumination was inadequate for the software program to perform proper analysis (baseline: CTL + VEH = 2; RSD + VEH = 1; RSD + KET = 1, after ketamine: CTL + VEH = 2; RSD + VEH = 1; RSD + KET = 1).
2.6. Pet imaging
Small animal PET was used to assess changes in TSPO levels in brain regions, as a marker of neuroinflammation. In brief, rats were anesthetized with isoflurane mixed with oxygen (5% for induction, 2% for maintenance), followed by the injection of 16.5 ± 2.5 MBq of [11C]-PK11195 via the tail vein. Rats were maintained under anesthesia and placed in prone position inside the PET camera (MicroPET Focus 220, Siemens Medical Solutions, Malvern, PA) with the head in the field of view. Forty-five minutes after tracer injection, an emission scan of 30 min was acquired. A transmission scan using a 57Co point source was performed either before or after the emission scan and was used for correction of attenuation and scatter. Eye salve was used to prevent conjunctival dehydration. Thermal pads were used to maintain body temperature at 37 °C and both heart rate and blood oxygen saturation were recorded during the scan.
PET scans were iteratively reconstructed (OSEM2D, 4 iterations and 16 subsets) into a single frame. After reconstruction, images with a 128 × 128 × 95 matrix, a pixel width of 0.632 mm, and a slice thickness of 0.762 mm were obtained. PET images were automatically co-registered to a template [11C]-PK11195 PET scan of the rat brain (Vállez Garcia et al., Citation2015), which was spatially aligned with a stereotaxic T2-weighted MRI template in Paxinos space (Schwarz et al., Citation2006) using the PMOD software package (PMOD Technologies LLC, Zürich, Switzerland).
The co-registered images were divided into cubic voxels (0.2 mm) and converted into a standardized uptake value (SUV) image, using the following formula and assuming a tissue density of 1 g/ml:
Tracer uptake was calculated in several pre-defined volumes-of-interest (VOI), representing brain regions of sufficiently large size. Small brain regions were excluded to minimize the partial volume effects (Lehnert et al., Citation2012), due to the limited resolution of the PET scanner (1.4 mm) (Marx et al., Citation2012). Therefore, the selected brain regions were the amygdala, bed nucleus of the stria terminalis (BNST), cerebellum, corpus callosum, entorhinal cortex, frontal association cortex, insular cortex, medial prefrontal cortex, orbitofrontal cortex, striatum (which included the nucleus accumbens), temporal cortex, olfactory cortex, occipital cortex, parietal cortex, hippocampus, midbrain, brainstem and basal ganglia (which included olfactory tubercle, ventral pallidum, substantia nigra, subthalamic nucleus, and the ventral tegmental area (VTA)).
2.7. Serum corticosterone measurements
On day 11, just before injection of the PET tracer, blood samples were collected from the tail vein via a cannula and immediately centrifuged at 5000g for 3 min. Serum was collected, frozen in liquid nitrogen and stored at −80 °C to maintain the integrity of the sample. Assessment of serum corticosterone was performed via an enzyme-linked immunoassay (ELISA) using a commercially available kit (Arbor Assays, Ann Arbor, MI). Samples were diluted to 1:100 in appropriate assay buffers in order to determine a calibration curve as specified by the manufacturer. The ELISA plate with the samples was read at 450 nm and corticosterone concentrations were calculated using the calibration curve.
2.8. Statistical analysis
SPSS (IBM Corp. 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY) was used to perform statistical analyses of behavioral and physiological parameters. The generalized estimating equation (GEE) analysis was used for the statistical analyses of longitudinal data obtained from the SPT, OFT, and body weight measurements, to account for missing data and the irregular interval between the time points. For the GEE analysis, “RSD,” “ketamine,” and “time” were used as independent factors, and “RSD*ketamine*time” interactions were used to determine pairwise differences between and within groups. A generalized linear model (GLM) analysis was applied to analyze group differences in the PET data (SUV) and corticosterone serum concentrations, using “RSD” and “Ketamine” as independent factors, and “RSD*ketamine” interactions for pairwise comparison. Wald Chi-square (W) and degrees of freedom (df) are presented for GLM and GEE analyses. A possible relationship between corticosterone levels and neuroinflammation was explored using a Pearson’s test and expressed as coefficient of determination (R2) and probability value (p). p values <0.05 were considered as statistically significant. Data are presented as mean ± SD. The effect sizes were calculated for the percentage of change between two groups.
3. Results
3.1. Body weight
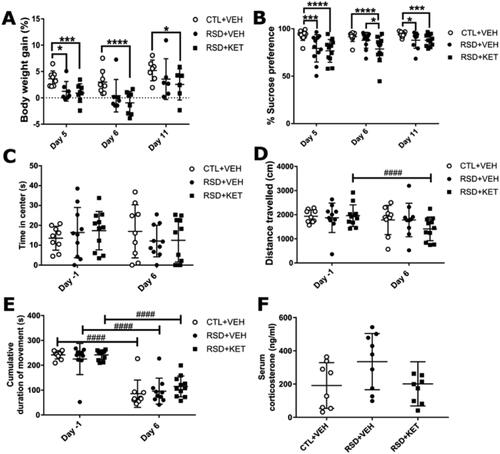
To determine changes in body weight gain due to RSD and/or ketamine injection, the body weight was measured on day 5 (after the RSD), day 6 (after the injection of ketamine), and day 11 (day of PET scan), and then compared to day −1 (baseline). The body weight gain, in percentage, is shown in . GEE analyses showed a main effect of RSD (W = 9.597; df = 1; p = 0.002) and of ketamine treatment (W = 6.587; df = 1; p = 0.010). Between-groups comparison (W = 200.594; df = 8; p < 0.0001) showed a statistically significant lower percentage body weight gain on day 5 for the RSD + VEH (n = 11, 1.9 ± 1.8, p < 0.05, −52%) and RSD + KET (n = 12, 1.3 ± 1.7, p < 0.001, −67%) group, as compared to the CTL + VEH group (n = 12, 3.9 ± 2.2). On days 6 and 11, however, a statistically significantly lower percentage body weight gain was still found in the RSD + KET group (day 6: −0.38 ± 1.9, p < 0.0001, −112%; day 11: 3.1 ± 3.1, p < 0.05, −47%) compared to controls (day 6: 3.2 ± 2.2; day 11: 5.9 ± 2.6), while the body weight gain of the RSD + VEH group was not statistically different any more (day 6: 1.2 ± 2.8, p = 0.051, −63%; day 11: 4.0 ± 4.2, p = 0.18, −32%).
Figure 2. Physiological and behavioral changes. (A) RSD induced a reduction in body weight gain which was not restored by ketamine injection. (B) Significant decrease in sucrose preference in the SPT is observed after RSD, which was not reversed by treatment with ketamine. (C) The open field test did not show any effect of RSD or ketamine on the time spent in the center of the arena, (D) on the distance traveled, or (E) on the total time they spent moving (cumulative duration of movement). (F) An increase in corticosterone serum concentrations due to RSD was observed, which was suppressed by ketamine treatment, but this difference was not statistically significant. Data are shown as mean ± SD. Statistically significant differences are indicated by asterisks: Between group comparison: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; within group comparison ####p < 0.0001.
3.2. Sucrose preference test
The sucrose preference test was used to assess the reduction in preference for a sucrose solution over regular water, as a proxy of anhedonia-like behavior (). A main effect for both RSD (W = 29.913; df = 1; p < 0.0001) and ketamine treatment (W = 15.367; df = 1; p < 0.0001) on sucrose preference was found. Pairwise comparison analyses (W = 100.196; df = 8; p < 0.0001) revealed that rats submitted to the RSD protocol showed a reduction in sucrose preference compared to controls on day 5 (CTL + VEH: n = 12, 93.0 ± 5.3, RSD + VEH: n = 11, 79.3 ± 14.5 p < 0.01, −15%; RSD + KET: n = 12, 76.8 ± 12.02, p < 0.0001, −17%). On day 6, after ketamine injection, the RSD + KET group was significantly different from both the control and RSD + VEH group (CTL + VEH: 91.7 ± 5.0, RSD + VEH: 87.9 ± 8.3, p = 0.18, −4%; RSD + KET: 78.6 ± 13.4, p < 0.001, −14%). Both the RSD + VEH and RSD + KET groups presented a statistically significant decrease in sucrose preference relative to controls at the end of the experiment (day 11: CTL + VEH: 94.0 ± 0.8, RSD + VEH: 88.0 ± 2.7, p < 0.05, −7%; RSD + KET: 87.8 ± 1.8 p < 0.001, −7%).
Within-group comparison showed a consistent sucrose preference in the control group over time. There was an increase in sucrose preference between day 5 (completion of the RSD protocol) and day 6 (ketamine injection) in the RSD + VEH group (11%, p < 0.001), but not in the RSD + KET group (2%, p = 0.72). Both groups exposed to RSD showed a higher sucrose preference on day 11 than on day 5 (RSD + VEH: 11%, p < 0.05; RSD + KET: 14%, p < 0.05).
3.3. Open-field test
The OFT was used to assess the effect of RSD and ketamine on anxiety-like behavior and locomotion. The OFT was performed at baseline (day −1) and after ketamine injection (day 6). The time the animals spent in the center of the arena was used as a proxy for anxiety-like behavior (), while the distance traveled () and the cumulative duration of movement () were used to assess locomotion. GEE analysis showed a significant interaction of RSD*ketamine*time in the distance traveled (W = 14.884; df = 5; p = 0.011), the cumulative duration of movement (W = 224.935; df = 5; p < 0.0001), but not in the time spent in the center (W = 29.913; df = 1; p < 0.0001). There were no significant differences between groups on any time point for any of the analyzed parameters (p > 0.05 for all between group comparisons). Within-group analysis between days −1 and 6, showed that only the RSD + KET group had a statistically significant reduction in the distance traveled (p < 0.0001, −15%). Furthermore, a statistically significant decrease in the duration of movement between baseline and day 6 was found in all groups (CTL + VEH: −23%, RSD + VEH: −14%, RSD + KET: −13%, p < 0.0001 for all).
3.4. Serum corticosterone levels
Serum concentrations of corticosterone were measured at the end of the experiment (day 11, ). Although serum corticosterone levels in animals exposed to RSD alone (RSD + VEH: 335 ± 169 ng/mL) were 75% and 67% higher than those in the control group (CTL + VEH: 191 ± 138 ng/mL) and ketamine treated animals (RSD + KET: 201 ± 133 ng/mL) respectively, statistical analysis showed that the interaction RSD*ketamine did not reach significance (W = 5.843; df = 2; p = 0.54).
3.5 [11C]-PK11195 PET
To determine the effect of ketamine on RSD-induced neuroinflammation, we performed a [11C]-PK11195 PET scan on day 11. Tracer uptake in various brain regions, expressed as SUV, is presented in . Animals exposed to the RSD protocol showed a statistically significant increase in [11C]-PK11195 uptake in the insular cortex (RSD + VEH: p < 0.05, 18%) and entorhinal cortex (p < 0.05, 20%), when compared to the control group. This increase was not affected by ketamine injection, as RSD + KET animals also showed a similar statistically significant increase in [11C]-PK11195 uptake in the insula (p < 0.05, 18%) and entorhinal cortex (p < 0.01, 29%).
Table 1. [11C]-PK11195 uptake in the brain of control animals (CTL + VEH), and animals exposed to a 5-day repeated social defeat protocol followed by a single injection of 20 mg/kg ketamine (RSD + KET) or vehicle (RSD + VEH).
Interestingly, a significant positive association between [11C]-PK11195 uptake in the whole brain and serum corticosterone levels was observed in the RSD + VEH group (R2 0.79, p < 0.01), but not in the CTL + VEH group (R2 0.01, p = 0.99), or the RSD + KET group (R2 0.37, p = 0.24).
4. Discussion
In this study, we explored if a single ketamine injection is able to moderate protracted stress-induced neuroinflammation in the RSD rat model, which mimics certain features of depression. We found that RSD was able to induce anhedonia-like behavior in the SPT and decreased body weight gain. RSD also induced an increase in TSPO density in the insular and entorhinal cortex. TSPO levels in the whole brain of RSD exposed animals correlated with serum corticosterone levels. Contrary to our hypothesis, injection of ketamine one day after the RSD protocol abolished the correlation between TSPO levels and corticosterone levels, but did not reverse the effects of RSD on anhedonia-like behavior, body weight, and TSPO levels.
The 5-day RSD protocol used in this study was able to induce a reduction in bodyweight gain and sucrose preference, a feature that is used as a proxy for anhedonia . These results are in accordance with other studies in rodents describing similar effects (Kopschina Feltes et al., Citation2019; Pulliam et al., Citation2010; Xiong et al., Citation2019a). RSD also caused a 75% increase in serum corticosterone levels one week after RSD, which was not observed in RSD rats treated with ketamine. The interaction between RSD and ketamine, however, was not statistically significant yet, due to the large within-group variability and relatively small sample size. It is also plausible that the effect of RSD on corticosterone levels has largely disappeared during the relatively long interval between RSD and corticosterone assessment. Moreover, our study also failed to demonstrate effects of ketamine in RSD-exposed rats on locomotion and the time in the center of the OFT, which is a parameter that is often used a proxy for anxiety. Studies using the RSD protocol and the OFT to measure anxiety-like behavior have shown contradictory results, as either an increase in anxiety-like behavior (Jaisinghani and Rosenkranz, Citation2015; Lisboa et al., Citation2018; Liu et al., Citation2015) or no effect at all have been reported (Liu et al., Citation2017; Olivares et al., Citation2012). Our results are in accordance with the latter studies. Many factors may explain such differences, including differences in the RSD procedure, its duration, and the interval between the end of RSD and the OFT.
In addition to changes in behavior and weight gain, RSD also caused increased TSPO availability in the insular and entorhinal cortex, as observed with [11C]-PK11195 PET. These results are in line with earlier publications showing increases in brain inflammatory markers by social stress (Kopschina Feltes et al., Citation2019; Lehmann et al., Citation2016; Liu et al., Citation2019). [11C]-PK11195 PET for imaging of upregulation of TSPO has also been successfully used to detect immune activation in depressed patients (Setiawan et al., Citation2015) and in patients that had suicidal thoughts (Holmes et al., Citation2018). The immune activation in insular and entorhinal cortices observed in our study is interesting, since these areas have been suggested to participate in memory and in the emotional component of depression (Gerritsen et al., Citation2011; Sprengelmeyer et al., Citation2011). In rodent models of depression, increased activity (measured as glucose metabolism) in the insula and entorhinal cortices have been reported after chronic restraint stress (Wei et al., Citation2018), while in the chronic corticosterone model, a decrease in glucose metabolism was found in the insula (Van Laeken et al., Citation2018). Moreover, MRI studies suggest that patients suffering from major depression have decreased insular regional homogeneity (Guo et al., Citation2011; Yao et al., Citation2009), increased insular activity that correlates with depression severity (Henje Blom et al., Citation2015), decreased insular activation in depressed patients (Liu et al., Citation2013), reduced global fractional anisotropy in areas connected with the insula (Repple et al., Citation2019), and decreased or aberrant functional connectivity between insular sub-regions (Yang et al., Citation2019; Yin et al., Citation2018). Although some apparent contradictions are found in these observations, probably due to differences in the model and intensity of the symptoms and the fact that activation does not necessarily correlates with inflammation, it clearly suggests the insular and entorhinal cortices are involved in depression.
In this study, RSD exposed animals were treated with a single dose of 20 mg/kg of ketamine but showed no improvement in sucrose preference or weight loss. Instead, it delayed weight recovery compared to animals only exposed to RSD. The dose of 20 mg/kg of ketamine has previously been reported to reduce behavioral deficits in chronic stress models (Zhang et al., Citation2016). Several studies have even used a lower dose of 10 mg/kg of ketamine and found a reduction in behavioral parameters that resemble features of depression (Tornese et al., Citation2019; Wan et al., Citation2018; Wang et al., Citation2018). However, all these studies tested the effects of ketamine either on the day of administration or 24 h afterwards. Yet, one study using maternal deprivation in Wistar rats reported a reduction in depressive-like behavior in both the forced swim test and splash test 14 days post injection of a dose of 15 mg/kg of ketamine (Réus et al., Citation2015). We evaluated the effects of a higher dose of ketamine 1 and 6 days after injection, so it is less likely that the dose or time of assessment are responsible for the lack of efficacy.
The studies that have shown a reduction in behavioral deficits associated with depression after acute ketamine injection have used different animal models of chronic stress (Ghosal et al., Citation2020; Rincón-Cortés and Grace, Citation2020; Tornese et al., Citation2019; Wan et al., Citation2018; Xiong et al., Citation2019a). Studies in rats have used either chronic mild stress or chronic unpredictable mild stress to evaluate the antidepressant effects of ketamine. Studies that reported antidepressant effects of ketamine after RSD used different species. For example, Xiong and colleagues showed antidepressant effects in C57BL/6 mice 4 h after administration of 10 mg/kg ketamine (Xiong et al., Citation2019a). It is possible that the difference in the stress model or species could account for the lack of efficacy of ketamine in the SPT in our RSD model.
Since several studies reported that ketamine seemed to reduce circulating pro-inflammatory markers in animal models of inflammation, we expected that ketamine would decrease the levels of the neuroinflammatory marker TSPO (Réus et al., Citation2017; Wang et al., Citation2017; Xiong et al., Citation2019b; Zhang et al., Citation2016, Citation2019). Contrary to our hypothesis, we observed that the RSD-induced increase in TSPO levels in the insular and entorhinal cortices were unaffected by the acute injection of ketamine. Moreover, the association between neuroinflammation and serum corticosterone levels in the brain of animals exposed to RSD disappeared after the administration of ketamine, which is likely due to the normalization of corticosterone levels by ketamine (67% reduction), while neuroinflammation was unaffected. These observations seem to suggest that the effect of ketamine on the hypothalamic–pituitary–adrenal axis is not directly related to modulation of neuroinflammation. One issue that must be emphasized is related to the timing of inflammatory processes and the frequency of ketamine administration. Differences in the timing of evaluations can have profound effects on the results of different studies. In studies evaluating the effects of ketamine on neuroinflammation in the RSD model, a single administration of ketamine induced a reduction in neuroinflammatory markers measured after 3 h (Zhang et al., Citation2019), 1 h and 24 h (Zhang et al., Citation2016), and 2 days (Xiong et al., Citation2019a). Studies in other models found effects on neuroinflammation after 7 days of chronic ketamine administration (Réus et al., Citation2017; Wang et al., Citation2017). Thus, it should be taken into consideration that the effects of ketamine may last longer after chronic than after a single administration. Here we measured TSPO levels as a marker of inflammation with [11C]-PK11195 PET 6 days after ketamine injection. At this time point the effects of the single ketamine injection on neuroinflammation may already have worn out. This suggests that more PET studies on the effect of ketamine on earlier time points may be needed in order to confirm that a single ketamine injection, for example, 1 day after RSD has also has not any effect on inflammation at earlier time points. Additional experiments using chronic administration and different doses in the RSD model could also be useful to further determine the role of ketamine on brain changes in TSPO.
Limitations of this study include the inability of [11C]-PK11195 to discriminate between a pro- and anti-inflammatory immune response. Consequently, it is not possible to determine if the increase in TSPO expression in the insular and entorhinal cortex are caused by a more anti-inflammatory or pro-inflammatory immune response. Assessment of circulating inflammatory cytokines and postmortem immunohistochemical analyses of the brains could provide this complementary information. Additionally, more studies using other dosing regimens and/or different evaluation time points after the ketamine injection could help in the exploration of the relationship between ketamine’s antidepressant effects and neuroinflammation. Moreover, in our study, only the SPT test for parameters associated with depressive-like behavior was included. The use of additional behavioral tests to assess different types of depressive-like behavior, such as learned helplessness, could improve translation of these results to the clinical situation.
5. Conclusions
In conclusion, our results show that a single dose of ketamine one day after of RSD does not reverse the RSD-induced reduction in sucrose preference, suggesting anhedonic-like behavior was not restored by the drug. Moreover, the protracted RSD-induced immune response in the insular and entorhinal cortices was also not moderated by a single dose of ketamine, suggesting that the drug did not act as an anti-inflammatory drug. Yet, the association between TSPO levels in the brain and serum corticosterone levels disappear after injection of ketamine, which is likely due to normalization of corticosterone levels 6 days after administration of the drug. This suggests that the positive effect of ketamine on stress hormone levels is not mediated by a modulating effect on neuroinflammation.
Author contributions
Author R. M. and C. G. designed the study, wrote the protocol, performed the experiments and statistical analyses, and wrote the first draft of the manuscript. L. R. and B. L. participated in the performance of experiments, data analysis and interpretation, and contributed to the correction of the first draft of the manuscript. R. D., J. S., J. D., and E. d. V. participated in the planning and the design of the study and the interpretation of the data, and corrected the draft manuscript. All authors have approved the final manuscript.
Acknowledgments
The authors would like to thank David Vallez-García, Jurgen Sijbesma, Anne-Sophie Enthoven, Daniel Vazquez-Matias, and Laura Kracht for experimental and analytical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available upon request to the corresponding author.
References
- Abdallah, C. G., Sanacora, G., Duman, R. S., & Krystal, J. H. (2018). The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacology & Therapeutics, 190, 148–158. https://doi.org/10.1016/j.pharmthera.2018.05.010
- Barresi, E., Robello, M., Costa, B., Da Pozzo, E., Baglini, E., Salerno, S., Da Settimo, F., Martini, C., & Taliani, S. (2021). An update into the medicinal chemistry of translocator protein (TSPO) ligands. European Journal of Medicinal Chemistry, 209, 112924. https://doi.org/10.1016/j.ejmech.2020.112924
- Beurel, E., Toups, M., & Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: Double trouble. Neuron, 107(2), 234–256. https://doi.org/10.1016/j.neuron.2020.06.002
- Cai, M., Wang, H., & Zhang, X. (2019). Potential anti-depressive treatment maneuvers from bench to bedside. In Y. Fang (Ed.), Depressive disorders: Mechanisms, measurement and management (pp. 277–295). Springer Singapore. https://doi.org/10.1007/978-981-32-9271-0_15
- Carvalho, L. A., Torre, J. P., Papadopoulos, A. S., Poon, L., Juruena, M. F., Markopoulou, K., Cleare, A. J., & Pariante, C. M. (2013). Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. Journal of Affective Disorders, 148(1), 136–140. https://doi.org/10.1016/j.jad.2012.10.036
- Cumming, P., Burgher, B., Patkar, O., Breakspear, M., Vasdev, N., Thomas, P., Liu, G.-J., & Banati, R. (2018). Sifting through the surfeit of neuroinflammation tracers. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 38(2), 204–224. https://doi.org/10.1177/0271678X17748786
- Czéh, B., Fuchs, E., Wiborg, O., & Simon, M. (2016). Animal models of major depression and their clinical implications. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 64, 293–310. https://doi.org/10.1016/j.pnpbp.2015.04.004
- Dahoun, T., Calcia, M. A., Veronese, M., Bloomfield, P., Reis Marques, T., Turkheimer, F., & Howes, O. D. (2019). The association of psychosocial risk factors for mental health with a brain marker altered by inflammation: A translocator protein (TSPO) PET imaging study. Brain, Behavior, and Immunity, 80, 742–750. https://doi.org/10.1016/j.bbi.2019.05.023
- Friedman, A. (2014). Neuroscience. Jump-starting natural resilience reverses stress susceptibility . Science (New York, N.Y.), 346(6209), 555–555. https://doi.org/10.1126/science.1260781
- Gartlehner, G., Hansen, R. A., Morgan, L. C., Thaler, K., Lux, L., Van Noord, M., Mager, U., Thieda, P., Gaynes, B. N., Wilkins, T., Strobelberger, M., Lloyd, S., Reichenpfader, U., & Lohr, K. N. (2011). Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: An updated meta-analysis. Annals of Internal Medicine, 155(11), 772–785. https://doi.org/10.7326/0003-4819-155-11-201112060-00009
- Gerritsen, L., Comijs, H. C., van der Graaf, Y., Knoops, A. J. G., Penninx, B. W. J. H., & Geerlings, M. I. (2011). Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—The SMART Medea study. Biological Psychiatry, 70(4), 373–380. https://doi.org/10.1016/j.biopsych.2011.01.029
- Ghosal, S., Duman, C. H., Liu, R.-J., Wu, M., Terwilliger, R., Girgenti, M. J., Wohleb, E., Fogaca, M. V., Teichman, E. M., Hare, B., & Duman, R. S. (2020). Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiology of Disease, 134, 104669. https://doi.org/10.1016/j.nbd.2019.104669
- Giacobbo, B. L., Doorduin, J., Moraga-Amaro, R., Nazario, L. R., Schildt, A., Bromberg, E., Dierckx, R. A. J. O., & de Vries, E. F. J. (2020). Chronic harmine treatment has a delayed effect on mobility in control and socially defeated rats. Psychopharmacology, 237(6), 1595–1606. https://doi.org/10.1007/s00213-020-05483-2
- Guo, W., Sun, X., Liu, L., Xu, Q., Wu, R., Liu, Z., Tan, C., Chen, H., & Zhao, J.-P. (2011). Disrupted regional homogeneity in treatment-resistant depression: A resting-state fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry , 35(5), 1297–1302. https://doi.org/10.1016/j.pnpbp.2011.02.006
- Hashimoto, K. (2015). Inflammatory biomarkers as differential predictors of antidepressant response. International Journal of Molecular Sciences, 16(4), 7796–7801. https://doi.org/10.3390/ijms16047796
- Henje Blom, E., Connolly, C. G., Ho, T. C., LeWinn, K. Z., Mobayed, N., Han, L., Paulus, M. P., Wu, J., Simmons, A. N., & Yang, T. T. (2015). Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. Journal of Affective Disorders, 178, 215–223. https://doi.org/10.1016/j.jad.2015.03.012
- Holmes, S. E., Hinz, R., Conen, S., Gregory, C. J., Matthews, J. C., Anton-Rodriguez, J. M., Gerhard, A., & Talbot, P. S. (2018). Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: A Positron Emission Tomography Study. Biological Psychiatry, 83(1), 61–69. https://doi.org/10.1016/j.biopsych.2017.08.005
- Jaisinghani, S., & Rosenkranz, J. A. (2015). Repeated social defeat stress enhances the anxiogenic effect of bright light on operant reward-seeking behavior in rats. Behavioural Brain Research, 290, 172–179. https://doi.org/10.1016/j.bbr.2015.04.048
- Kato, T., Pothula, S., Liu, R. J., Duman, C. H., Terwilliger, R., Vlasuk, G. P., Saiah, E., Hahm, S., & Duman, R. S. (2019). Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. The Journal of Clinical Investigation, 129(6), 2542–2554. https://doi.org/10.1172/JCI126859
- Kiecolt-Glaser, J. K., Derry, H. M., Fagundes, C. P., & Health, B. (2015). Inflammation: Depression fans the flames and feasts on the heat. The American Journal of Psychiatry, 172(11), 1075–1091. https://doi.org/10.1176/appi.ajp.2015.15020152
- Kopschina Feltes, P., de Vries, E. F., Juarez-Orozco, L. E., Kurtys, E., Dierckx, R. A., Moriguchi-Jeckel, C. M., & Doorduin, J. (2019). Repeated social defeat induces transient glial activation and brain hypometabolism: A positron emission tomography imaging study. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 39(3), 439–453. https://doi.org/10.1177/0271678X17747189
- Lehmann, M. L., Cooper, H. A., Maric, D., & Herkenham, M. (2016). Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. Journal of Neuroinflammation, 13(1), 224. https://doi.org/10.1186/s12974-016-0672-x
- Lehnert, W., Gregoire, M.-C., Reilhac, A., & Meikle, S. R. (2012). Characterisation of partial volume effect and region-based correction in small animal positron emission tomography (PET) of the rat brain. Neuroimage, 60(4), 2144–2157. https://doi.org/10.1016/j.neuroimage.2012.02.032
- Leonard, B., & Maes, M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience and Biobehavioral Reviews, 36(2), 764–785. https://doi.org/10.1016/j.neubiorev.2011.12.005
- Li, H., Sagar, A. P., & Kéri, S. (2018). Microglial markers in the frontal cortex are related to cognitive dysfunctions in major depressive disorder. Journal of Affective Disorders, 241, 305–310. https://doi.org/10.1016/j.jad.2018.08.021
- Lisboa, S. F., Niraula, A., Resstel, L. B., Guimaraes, F. S., Godbout, J. P., & Sheridan, J. F. (2018). Repeated social defeat-induced neuroinflammation, anxiety-like behavior and resistance to fear extinction were attenuated by the cannabinoid receptor agonist WIN55,212-2. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(9), 1924–1933. https://doi.org/10.1038/s41386-018-0064-2
- Liu, C.-H., Ma, X., Wu, X., Fan, T.-T., Zhang, Y., Zhou, F.-C., Li, L.-J., Li, F., Tie, C.-L., Li, S.-F., Zhang, D., Zhou, Z., Dong, J., Wang, Y.-J., Yao, L., & Wang, C.-Y. (2013). Resting-state brain activity in major depressive disorder patients and their siblings. Journal of Affective Disorders, 149(1–3), 299–306. https://doi.org/10.1016/j.jad.2013.02.002
- Liu, L., Zhao, Z., Lu, L., Liu, J., Sun, J., & Dong, J. (2019). Icariin and icaritin ameliorated hippocampus neuroinflammation via mediating HMGB1 expression in social defeat model in mice. International Immunopharmacology, 75, 105799. https://doi.org/10.1016/j.intimp.2019.105799
- Liu, Y.-Y., Zhou, X.-Y., Yang, L.-N., Wang, H.-Y., Zhang, Y.-Q., Pu, J.-C., Liu, L.-X., Gui, S.-W., Zeng, L., Chen, J.-J., Zhou, C.-J., & Xie, P. (2017). Social defeat stress causes depression-like behavior with metabolite changes in the prefrontal cortex of rats. PLoS One, 12(4), e0176725. https://doi.org/10.1371/journal.pone.0176725
- Liu, Y., Yang, L., Yu, J., & Zhang, Y. Q. (2015). Persistent, comorbid pain and anxiety can be uncoupled in a mouse model. Physiology & Behavior, 151, 55–63. https://doi.org/10.1016/j.physbeh.2015.07.004
- Maes, M., Song, C., & Yirmiya, R. (2012). Targeting IL-1 in depression. Expert Opinion on Therapeutic Targets, 16(11), 1097–1112. https://doi.org/10.1517/14728222.2012.718331
- Majd, M., Saunders, E. F. H., & Engeland, C. G. (2020). Inflammation and the dimensions of depression: A review. Frontiers in Neuroendocrinology, 56, 100800. https://doi.org/10.1016/j.yfrne.2019.100800
- Marx, C., Lex, B., Calaminus, C., Hauber, W., Backes, H., Neumaier, B., Mies, G., Graf, R., & Endepols, H. (2012). Conflict processing in the rat brain: Behavioral analysis and functional μPET imaging using [F]fluorodeoxyglucose. Frontiers in Behavioral Neuroscience, 6, 4–12. https://doi.org/10.3389/fnbeh.2012.00004
- Mathers, C. D., & Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine, 3(11), e442–e2030. https://doi.org/10.1371/journal.pmed.0030442
- Miller, A. H., Maletic, V., & Raison, C. L. (2009). Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65(9), 732–741. https://doi.org/10.1016/j.biopsych.2008.11.029
- Niraula, A., Witcher, K. G., Sheridan, J. F., & Godbout, J. P. (2019). Interleukin-6 induced by social stress promotes a unique transcriptional signature in the monocytes that facilitate anxiety. Biological Psychiatry, 85(8), 679–689. https://doi.org/10.1016/j.biopsych.2018.09.030
- Nutma, E., Ceyzériat, K., Amor, S., Tsartsalis, S., Millet, P., Owen, D. R., Papadopoulos, V., & Tournier, B. B. (2021). Cellular sources of TSPO expression in healthy and diseased brain. European Journal of Nuclear Medicine and Molecular Imaging, 49(1):146–163. https://doi.org/10.1007/s00259-020-05166-2
- Olivares, E. L., Silva-Almeida, C., Pestana, F. M., Sonoda-Côrtes, R., Araujo, I. G., Rodrigues, N. C., Mecawi, A. S., Côrtes, W. S., Marassi, M. P., Reis, L. C., & Rocha, F. F. (2012). Social stress-induced hypothyroidism is attenuated by antidepressant treatment in rats. Neuropharmacology, 62(1), 446–456. https://doi.org/10.1016/j.neuropharm.2011.08.035
- Pulliam, J. V. K., Dawaghreh, A. M., Alema-Mensah, E., & Plotsky, P. M. (2010). Social defeat stress produces prolonged alterations in acoustic startle and body weight gain in male Long Evans rats. Journal of Psychiatric Research, 44(2), 106–111. https://doi.org/10.1016/j.jpsychires.2009.05.005
- Raedler, T. J. (2011). Inflammatory mechanisms in major depressive disorder. Current Opinion in Psychiatry, 24(6), 519–525. https://doi.org/10.1097/YCO.0b013e32834b9db6
- Repple, J., Mauritz, M., Meinert, S., de Lange, S. C., Grotegerd, D., Opel, N., Redlich, R., Hahn, T., Förster, K., Leehr, E. J., Winter, N., Goltermann, J., Enneking, V., Fingas, S. M., Lemke, H., Waltemate, L., Nenadic, I., Krug, A., Brosch, K., … van den Heuvel, M. P. (2019). Severity of current depression and remission status are associated with structural connectome alterations in major depressive disorder. Molecular Psychiatry, 25(7):1550–1558. https://doi.org/10.1038/s41380-019-0603-1
- Réus, G. Z., Carlessi, A. S., Titus, S. E., Abelaira, H. M., Ignácio, Z. M., da Luz, J. R., Matias, B. I., Bruchchen, L., Florentino, D., Vieira, A., Petronilho, F., & Quevedo, J. (2015). A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Developmental Neurobiology, 75(11), 1268–1281. https://doi.org/10.1002/dneu.22283
- Réus, G. Z., Simões, L. R., Colpo, G. D., Scaini, G., Oses, J. P., Generoso, J. S., Prossin, A. R., Kaddurah-Daouk, R., Quevedo, J., & Barichello, T. (2017). Ketamine potentiates oxidative stress and influences behavior and inflammation in response to lipolysaccharide (LPS) exposure in early life. Neuroscience, 353, 17–25. https://doi.org/10.1016/j.neuroscience.2017.04.016
- Richards, E. M., Zanotti-Fregonara, P., Fujita, M., Newman, L., Farmer, C., Ballard, E. D., Machado-Vieira, R., Yuan, P., Niciu, M. J., Lyoo, C. H., Henter, I. D., Salvadore, G., Drevets, W. C., Kolb, H., Innis, R. B., & Zarate C. A. Jr. (2018). PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Research, 8(1), 57. https://doi.org/10.1186/s13550-018-0401-9
- Rincón-Cortés, M., & Grace, A. A. (2020). Antidepressant effects of ketamine on depression-related phenotypes and dopamine dysfunction in rodent models of stress. Behavioural Brain Research, 379, 112367. https://doi.org/10.1016/j.bbr.2019.112367
- Schwarz, A. J., Danckaert, A., Reese, T., Gozzi, A., Paxinos, G., Watson, C., Merlo-Pich, E. V., & Bifone, A. (2006). A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: Application to pharmacological MRI. Neuroimage, 32(2), 538–550. https://doi.org/10.1016/j.neuroimage.2006.04.214
- Setiawan, E., Attwells, S., Wilson, A. A., Mizrahi, R., Rusjan, P. M., Miler, L., Xu, C., Sharma, S., Kish, S., Houle, S., & Meyer, J. H. (2018). Association of translocator protein total distribution volume with duration of untreated major depressive disorder: A cross-sectional study. The Lancet Psychiatry, 5(4), 339–347. https://doi.org/10.1016/S2215-0366(18)30048-8
- Setiawan, E., Wilson, A. A., Mizrahi, R., Rusjan, P. M., Miler, L., Rajkowska, G., Suridjan, I., Kennedy, J. L., Rekkas, V., Houle, S., & Meyer, J. H. (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry, 72(3), 268–275. https://doi.org/10.1001/jamapsychiatry.2014.2427
- Sprengelmeyer, R., Steele, J. D., Mwangi, B., Kumar, P., Christmas, D., Milders, M., & Matthews, K. (2011). The insular cortex and the neuroanatomy of major depression. Journal of Affective Disorders, 133(1–2), 120–127. https://doi.org/10.1016/j.jad.2011.04.004
- Su, L., Faluyi, Y. O., Hong, Y. T., Fryer, T. D., Mak, E., Gabel, S., Hayes, L., Soteriades, S., Williams, G. B., Arnold, R., Passamonti, L., Rodríguez, P. V., Surendranathan, A., Bevan-Jones, R. W., Coles, J., Aigbirhio, F., Rowe, J. B., & O’Brien, J. T. (2016). Neuroinflammatory and morphological changes in late-life depression: The NIMROD study. The British Journal of Psychiatry: The Journal of Mental Science, 209(6), 525–526. https://doi.org/10.1192/bjp.bp.116.190165
- Tornese, P., Sala, N., Bonini, D., Bonifacino, T., La Via, L., Milanese, M., Treccani, G., Seguini, M., Ieraci, A., Mingardi, J., Nyengaard, J. R., Calza, S., Bonanno, G., Wegener, G., Barbon, A., Popoli, M., & Musazzi, L. (2019). Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine. Neurobiology of Stress, 10, 100160. https://doi.org/10.1016/j.ynstr.2019.100160
- Vállez Garcia, D., Casteels, C., Schwarz, A. J., Dierckx, R. A. J. O., Koole, M., & Doorduin, J. (2015). A standardized method for the construction of tracer specific PET and SPECT rat brain templates: Validation and implementation of a toolbox. PLoS One, 10(3), e0122363. https://doi.org/10.1371/journal.pone.0122363
- Van Laeken, N., Pauwelyn, G., Dockx, R., Descamps, B., Brans, B., Peremans, K., Baeken, C., Goethals, I., Vanhove, C., & De Vos, F. (2018). Regional alterations of cerebral [18F]FDG metabolism in the chronic unpredictable mild stress- and the repeated corticosterone depression model in rats. Journal of Neural Transmission (Vienna), 125(9), 1381–1393. https://doi.org/10.1007/s00702-018-1899-8
- Viana, G. S. B., Do Vale, E. M., de Araujo, A. R. A., Coelho, N. C., Andrade, S. M., da Costa, R. O., de Aquino, P. E. A., de Sousa, C. N. S., de Medeiros, I. S., de Vasconcelos, S. M. M., & Neves, K. R. T. (2021). Rapid and long-lasting antidepressant-like effects of ketamine and their relationship with the expression of brain enzymes, bdnf, and astrocytes. Brazilian Journal of Medical and Biological Research, 54, 1–9. https://doi.org/10.1590/1414-431x202010107
- Wan, Y.-Q., Feng, J.-G., Li, M., Wang, M.-Z., Liu, L., Liu, X., Duan, X.-X., Zhang, C.-X., & Wang, X.-B. (2018). Prefrontal cortex miR-29b-3p plays a key role in the antidepressant-like effect of ketamine in rats. Experimental & Molecular Medicine, 50(10), 1–14. https://doi.org/10.1038/s12276-018-0164-4
- Wang, C.-Q., Ye, Y., Chen, F., Han, W.-C., Sun, J.-M., Lu, X., Guo, R., Cao, K., Zheng, M.-J., & Liao, L.-C. (2017). Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience, 343, 30–38. https://doi.org/10.1016/j.neuroscience.2016.11.029
- Wang, Y.-L., Han, Q.-Q., Gong, W.-Q., Pan, D.-H., Wang, L.-Z., Hu, W., Yang, M., Li, B., Yu, J., & Liu, Q. (2018). Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. Journal of Neuroinflammation, 15(1), 21. https://doi.org/10.1186/s12974-018-1054-3
- Wei, K., Bao, W., Zhao, Z., Zhou, W., Liu, J., Wei, Y., Li, M., Wu, X., Liu, B., Du, Y., Gong, W., & Dong, J. (2018). Changes of the brain activities after chronic restraint stress in rats: A study based on 18F-FDG PET. Neuroscience Letters, 665, 104–109. https://doi.org/10.1016/j.neulet.2017.11.047
- Werry, E. L., Bright, F. M., Piguet, O., Ittner, L. M., Halliday, G. M., Hodges, J. R., Kiernan, M. C., Loy, C. T., Kril, J. J., & Kassiou, M. (2019). Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. International Journal of Molecular Sciences, 20(13), 3161. https://doi.org/10.3390/ijms20133161
- Xiong, Z., Fujita, Y., Zhang, K., Pu, Y., Chang, L., Ma, M., Chen, J., & Hashimoto, K. (2019a). Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behavioural Brain Research, 368, 111904. https://doi.org/10.1016/j.bbr.2019.111904
- Xiong, Z., Zhang, K., Ishima, T., Ren, Q., Ma, M., Pu, Y., Chang, L., Chen, J., & Hashimoto, K. (2019b). Lack of rapid antidepressant effects of Kir4.1 channel inhibitors in a chronic social defeat stress model: Comparison with (R)-ketamine. Pharmacology, Biochemistry and Behavior, 176, 57–62. https://doi.org/10.1016/j.pbb.2018.11.010
- Yang, H., Wang, C., Ji, G., Feng, Z., Duan, J., Chen, F., Zhou, X. J., Shi, Y., & Xie, H. (2019). Aberrant interhemispheric functional connectivity in first-episode, drug-naïve major depressive disorder. Brain Imaging and Behavior, 13(5), 1302–1310. https://doi.org/10.1007/s11682-018-9917-x
- Yao, Z., Wang, L., Lu, Q., Liu, H., & Teng, G. (2009). Regional homogeneity in depression and its relationship with separate depressive symptom clusters: A resting-state fMRI study. Journal of Affective Disorders, 115(3), 430–438. https://doi.org/10.1016/j.jad.2008.10.013
- Yin, Z., Chang, M., Wei, S., Jiang, X., Zhou, Y., Cui, L., Lv, J., Wang, F., & Tang, Y. (2018). Decreased functional connectivity in insular subregions in depressive episodes of bipolar disorder and major depressive disorder. Frontiers in Neuroscience, 12, 842–849. https://doi.org/10.3389/fnins.2018.00842
- Yoshimura, R., Hori, H., Ikenouchi-Sugita, A., Umene-Nakano, W., Ueda, N., & Nakamura, J. (2009). Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(4), 722–726. https://doi.org/10.1016/j.pnpbp.2009.03.020
- Zhang, G. F., Wang, J., Han, J. F., Guo, J., Xie, Z. M., Pan, W., Yang, J. J., & Sun, K. J. (2016). Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model. Neuroscience Letters, 631, 7–12. https://doi.org/10.1016/j.neulet.2016.08.006
- Zhang, J., Qu, Y., Chang, L., Pu, Y., & Hashimoto, K. (2019). (R)-Ketamine rapidly ameliorates the decreased spine density in the medial prefrontal cortex and hippocampus of susceptible mice after chronic social defeat stress. International Journal of Neuropsychopharmacology, 22(10), 675–679. https://doi.org/10.1093/ijnp/pyz048
- Zhang, L., Hu, K., Shao, T., Hou, L., Zhang, S., Ye, W., Josephson, L., Meyer, J. H., Zhang, M.-R., Vasdev, N., Wang, J., Xu, H., Wang, L., & Liang, S. H. (2021). Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharmaceutica Sinica. B, 11(2), 373–393. https://doi.org/10.1016/j.apsb.2020.08.006
- Zimmerman, M., & Thongy, T. (2007). How often do SSRIs and other new-generation antidepressants lose their effect during continuation treatment? Evidence suggesting the rate of true tachyphylaxis during continuation treatment is low. Journal of Clinical Psychiatry, 68(8), 1271–1276. https://doi.org/10.4088/jcp.v68n0814
