Abstract
The aim of this study was to assess changes in mental health, gut microbiota composition, and stress marker serum cortisol due to COVID-19 lockdown in asymptomatic individuals. Healthy adults participated in anthropometric measurements, blood and stool sample collection pre-lockdown and post-lockdown (n = 38, 63.2% females), lifestyle and psychological questionnaires were included in pre-lockdown measurement and lockdown survey (n = 46, 67.4% females). Subjects reported significantly higher body dissatisfaction (p = 0.007) and anxiety (p = 0.002), and significantly lower positive affect (p = 0.001) during lockdown compared with pre-lockdown. According to perceived stress, 51.6% of females and 20% of males experienced moderate to high stress. This was reflected in serum cortisol levels that significantly increased only in females (p = 0.006) post-lockdown and correlated with perceived stress (p = 0.037) and anxiety (p = 0.031). In addition to psychological measures, changes in gut microbiota composition were observed. Gut microbial alpha diversity significantly decreased (p = 0.033), whereas relative abundance of Proteobacteria significantly increased (p = 0.043) post-lockdown. Depression during lockdown was moderately positively correlated with changes in Bacteroidetes abundance (p = 0.015) and negatively with changes in Firmicutes abundance (p = 0.008). Alistipes abundance post-lockdown was moderately positively correlated with anxiety (p = 0.004) and negative affect (p = 0.005) during lockdown. Despite a small sample size and not being able to perform objective measurements during lockdown, the results confirm the effect of lockdown on mental health and gut microbiota composition that could have a great impact on our health (ClinicalTrials identifier: NCT04347213).
1. Introduction
Due to the worldwide coronavirus disease (COVID-19) outbreak, the Slovenian government declared an epidemic status on 12 March 2020 (Vlada Republike Slovenije, Citation2020) and few days later strict measures were undertaken to prevent the spread of the virus. The measures included a ban on gathering in public places and crossing the regional borders, closure of schools, and all non-essential businesses, people were instructed to work from home with very few exceptions. The epidemic status ended on 31 May 2020, but many restrictions remained. The epidemic status was declared again between 9 October 2020 and 16 June 2021.
The ongoing pandemic and lockdown measures might affect people from many aspects. Fear of infection could lead to changes in daily lifestyle habits, self-isolation strategies, and changes in mental health, such as increased anxiety and depression levels (Xu et al., Citation2021). Lockdown measures lead to social isolation, less social interactions, and loneliness (Koyama et al., Citation2021). A disrupted daily routine, closed sport facilities, and restrictions of outdoor activities caused a decrease in physical activity and an increase in screen time, which was associated with increased insomnia, anxiety, and depression (Runacres et al., Citation2021). There are numerous studies showing that the epidemics and the related measures have taken on the mental health of different populations, such as depressive and anxiety symptoms, more intrusive thoughts, insomnia, acute stress, and worsened quality of life (Amerio et al., Citation2021). Several studies also show that females reported more intrusive thoughts, higher perceived stress, and lower quality of life than males (Kyprianidou et al., Citation2021; Siette et al., Citation2021).
Although stress can manifest in different physiological changes, there are only few studies investigating such effects arising during the COVID-19 lockdown. Of the known stress biomarkers, cardiovascular measures such as increased heart rate and systolic blood pressure mostly reflect acute stress and the elevated levels do not persist over a longer time period (Isowa et al., Citation2006). The same is true for the levels of adrenalin and noradrenalin (Nickel et al., Citation2009). The most known physiological markers typical of chronic stress are serum cortisol (Steckl & Ray, Citation2018) and the salivary α-amylase activity (Rohleder et al., Citation2004). One of the markers that has been associated with stress is also the composition of gut microbiota. The effect is bidirectional. The microorganisms in the gut contribute to the synthesis of the neurotransmitters serotonin, dopamine, and noradrenalin (Lyte et al., Citation2011) and to the metabolism of their precursors (O'Mahony et al., Citation2015) and affect mental health. In turn, microbiota diversity and the abundance of separate microbial strains can be altered by stress (Karl et al., Citation2018).
The aim of the present study was to investigate psychological measures before and during lockdown, including body dissatisfaction, anxiety, depression, positive and negative affect. We were particularly interested in whether the changes were also reflected in changes of stress-related biomarker cortisol and in the changes in gut microbiota composition post-lockdown. Due to the known differences in the perception of stay-at-home situation between males and females, we further focused on these differences.
2. Materials and methods
2.1. Study design
The present study is a sub-study of a larger study: The Link between Diets and Health Indicators (DIETE) started at the Faculty of Health Sciences, University of Primorska in Izola, Slovenia in December 2019 and was interrupted due to the COVID-19 pandemic. The study protocol was approved by the Slovenian National Medical Ethics Committee (No. 0120-557/2017/4) and was registered on ClinicalTrials.gov (Identifier: NCT04347213).
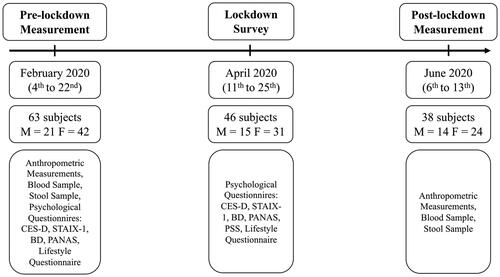
As shown in , measurements were performed prior, during, and after the COVID-19-related lockdown. The lockdown rules were the same for the whole Slovenian population, individual mobility was limited, everyone with few exceptions was obliged to work from home or refrain from work, schools were closed, there was no public transport, and all public events were canceled.
2.2. Study subjects
Healthy volunteers with BMI between 18.5 and 30.0 kg/m2, 20–60 years of age, and unchanged dietary pattern (diet) at least six months before the study were recruited through a web survey posted on social media in December 2019. Reported change in body mass and use of medication including antibiotics within three months before inclusion, pregnancy or lactation, and presence of any chronic disease served as exclusion criteria.
Pre-lockdown, a total of 63 subjects (21 males, 42 females) participated in the measurements, 46 subjects (15 males, 31 females) took part in the lockdown survey, and 38 subjects (14 males, 24 females) participated in the post-lockdown measurements (). Others dropped out of the study due to personal reasons, mainly for the fear of infection with SARS-CoV-2. Lockdown ended in May, but many restrictions remained, including working from home, closed restaurants, and the inability to perform research activities. The participants were invited immediately after it was allowed by the government.
We calculated a priori that a sample size of 34 subjects is required to achieve a statistical power of 0.8 (20% of a Type II error and 5% of a Type I error) when comparing the effect of lockdown on different variables, using G*Power 3.1.9.7 (Heinrich-Heine-Universität Düsseldorf, Germany). In addition, we calculated the statistical power post hoc for two main variables, for anxiety and serum cortisol levels, calculating the effect size from mean and standard deviation of difference for each variable. Statistical power for anxiety when all 46 subjects were included was 0.88; the same was for 38 completed cases. In addition, for serum cortisol levels, the statistical power was 0.82 for females and 0.83 for males.
The analysis of psychological factors was performed on 46 subjects (pre-lockdown measurement and lockdown survey), and gut microbiota and serum cortisol analysis included 38 subjects, subjects who participated in post-lockdown measurement. The subjects were Caucasians, homogenous by race, culture, and ethnicity. The average age of the subjects was 36.4 (SD 9.7) years with an average BMI 22.1 (SD 2.8) kg/m2, two-thirds (67.4%) were females. About 19.6% of the subjects were single and others were in a relationship or married; 19.6% of the subjects reported having a high school diploma, 67.4% a bachelor’s degree, and 13.0% a Master’s degree or a PhD; 78.2% of the subjects received regular payment and 21.7% received no regular payment. None of the subjects had a confirmed infection with SARS-CoV-2.
2.3. Psychological questionnaires
Well-being was assessed before and during lockdown through five scales: The State-Trait Anxiety Inventory (STAIX-1), The Center for Epidemiologic Studies Depression Scale (CES-D) and The Perceived Stress Scale (PSS) as measures of distress, and The Positive and Negative Affect Schedule (PANAS) and Body Dissatisfaction (BD) as indicators of the emotional component.
The STAIX-1 questionnaire was used as a reliable measure to evaluate the state of anxiety in adults (Spielberger et al., Citation1983). It measures the amount of anxiety experienced in a stressful situation and allows for the identification of the temporary emotional state of individuals. The STAIX-1 contains 20 items. A 4-point Likert scale is used for scoring; a higher score implies higher anxiety. Previous studies have used 45 points as a threshold for high anxiety levels (Barnes et al., Citation2002).
Symptoms associated with depression were assessed using CES-D. This self-report includes 20 items, through which factors such as sleep, appetite, and loneliness are evaluated on a 4-point scale. Higher score indicates more depressed mood (Radloff, Citation1977); depression is considered clinically significant when the score reaches or exceeds 16 (Okun et al., Citation1996).
The PSS was used for measuring the global perception of stress. It is a measure of the degree to which situations in one’s life are appraised as stressful based on 10 items (on a scale from 0–never to 4–very often) (Cohen et al., Citation1983). The PSS is widely used (Loft et al., Citation2007) and has high internal consistency with Cronbach’s alpha coefficient being reported at 0.85 (Cohen et al., Citation1983).
Body image evaluation was assessed with the Body Dissatisfaction subscale from the Eating Disorders Inventory-2 (EDI-2) (Garner, Citation1991). The scale consists of 10 items assessing how satisfied or dissatisfied an individual is with both overall body shape and the size/shape of specific body parts. Responses are rated on 5-point Likert scale from 0 (never) to 4 (always). Scores were summed to calculate an overall body dissatisfaction score with a possible range from 0 (lowest body dissatisfaction) to 40 (highest body dissatisfaction). The author of EDI-2 reported subscale internal consistency reliabilities (alphas) of 0.74–0.97, and test–retest reliabilities of 0.86–0.98.
The PANAS was used to determine subjective mood (Watson et al., Citation1988). It is a 20-item self-report measure of positive and negative affect and includes 20 adjectives, where participants indicate to which extent they feel a certain way. Positive affect (PA) refers to the extent to which a person feels enthusiastic, active or alert, whereas negative affect (NA) includes mood states such as anger, contempt, disgust, fear, and nervousness. Watson et al. (Citation1988) reported Cronbach’s alpha coefficient was 0.88 for positive affect and 0.87 for negative affect. Test–retest correlations for an eight-week period ranged from 0.47 to 0.68 for positive affect, 0.39 to 0.71 for negative affect (positive affect stability = 0.68, negative affect stability = 0.71).
2.4. Serum cortisol levels
Venous blood was withdrawn pre-lockdown and post-lockdown. The participants were instructed to restrain from eating and from physical exercise for 12 h preceding the withdrawal. The measurements were performed at the same time of the day both times for every subject, 2–3 h after waking up. Serum samples were prepared as described previously (Bogataj Jontez et al., Citation2021). Serum cortisol levels were determined in duplicate on a microplate reader (Tecan, Mannedorf, Switzerland) using human ELISA kits for cortisol (IBL international GMBH, Germany,Cat. No.. RE52611, lot no. ECO148).
2.5. Stool sample analysis
The stool samples were collected at home by the subjects, after an online explanation of the process. They were instructed to collect a pea-sized stool sample one day prior the measurements on several sheets of toilet paper, to prevent it from getting into contact with the toilet surface, to store it in the provided stool collection tube with attached spoon (Žikplast, Slovenia) and to freeze it immediately at −18 °C. Females were instructed not to take their stool sample during menstruation. At the day of the measurements they brought the sample wrapped in a frozen ice pack (provided). A stool sample from each subject was stored in stool collection tubes at −80 °C until analysis. For the assessment of the microbial community composition, DNA was extracted from the frozen fecal samples (1–2 g) using the commercial QIAamp DNA Stool Mini kit (Qiagen N. V., Venlo, The Netherlands) following the manufacturer’s instructions and stored at −80 °C for further analysis. Concentration of DNA was quantified with fluorometer Qubit® 3.0 and QubitTM dsDNA BR Assay kit (Thermo Fisher Scientific, Oregon, USA). A comparison of microbiota among subjects was performed with a metabarcoding approach using V4 hypervariable region of the 16S rRNA gene. Amplification of targeted region was performed with primers 515 F and 806 R, which were elongated on their 5′-end in order to make amplicons suitable for sequencing on Ion S5TM System. Primer 806 R (5′-GGACTACNVGGGTWTCTAAT-3′) was elongated with the sequence of P1 adapter and 515 F (5′-GTGYCAGCMGCCGCGGTAA-3′) primer was elongated with the sequence of linker, barcode, and A adapter. Each sample was amplified in triplicates. Negative control was included in amplicon library as well. PCR reaction mixture and temperature profile were set as described in Earth Microbiome Project (www.earthmicrobiome.org) (Thompson et al., Citation2017).
DNA concentration of PCR products (pool of three replicates) was measured using Ion Quantitation Library kit (Thermo Fisher Scientific, Vilnius, Lithuania). Equimolar amounts of each PCR product were pooled and cleaned with Agencourt AMPure XP beads (Beckman Coulter, USA) using bead-to-DNA ratio of 0.7:1. Concentration of final pooled amplicon library was determined with Agilent 2100 Bioanalyzer using High Sensitivity DNA Assay kit (Agilent Technologies, CA, USA) and Ion Spherical Particles with the template were prepared with Ion OneTouchTM 2 system using the kit Ion 520TM and Ion 530TM Kit-OT2. Samples were sequenced on two Ion 530TM chips using Ion S5TM System (Thermo Fisher Scientific, CA, USA).
Fastq files from each run were imported in QIIME2 v.2021.8 (Bolyen et al., Citation2019). Cutadapt (qiime cutadapt trim-single) was used to remove primers. A linked primer, with anchored 5′ primer, was defined and –p-discard-untrimmed parameter was used to discard untrimmed reads. DADA2 (Callahan et al., Citation2016) (qiime dada2 denois-pyro plugin) was used for denoising and determination of amplicon sequence variants (ASVs) using the following arguments: –p-trim-left 0 and –p-trunc-len 0 (which resulted in a final set of sequences with full length of V4 region). Feature tables and ASVs of samples from different runs were merged with feature-table merge and merge-seqs plugins. Taxonomy classification was made with feature-classifier plugin. Amplicon-region specific classifier was based on SILVA reference database, release 138.1, with representative sequences at 99% identity (Quast et al., Citation2013).
Bacterial phyla and genera present in more than 10% of the subjects, with relative abundance of at least 1% were presented. Microbiota diversity of stool samples was evaluated with the Shannon index (alpha index of diversity).
2.6. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26.0 (IBM, Armonk, NY, USA). Means and standard deviations were calculated. The normality of data distribution was evaluated using the Shapiro–Wilk test. Student’s paired samples t-test was used to compare the effect of lockdown between two normally distributed variables, while two non-normally distributed variables were compared with Wilcoxon’s signed-rank test. Changes of post-lockdown data from pre-lockdown (Δ) for serum cortisol levels, gut microbiota composition, and changes of lockdown data from pre-lockdown (Δ) for psychological measures were calculated by subtracting pre-lockdown values from post-lockdown/lockdown values. Student’s independent samples t-test was used to compare changes in variables between two groups for normally distributed variables, while changes in non-normally distributed variables were compared with Mann–Whitney’s U test. Pearson’s Correlation was used to investigate associations between gut microbiota composition, serum cortisol levels and psychological measures for normally distributed data, for non-normally distributed data Spearman’s Correlation was used. Partial correlation controlling for gender (male, female) and diet (regular meat consumption and no meat consumption) was used to investigate associations between gut microbial phyla and psychological measures. p-Values < 0.05 were considered statistically significant.
3. Results
3.1. Lifestyle changes during lockdown
Lifestyle and work changes during lockdown are presented in . Only one-third of the subjects experienced no changes at work during lockdown, and 37.0% of the subjects reported major or complete changes in their lifestyle. More than two-thirds of the subjects had no social interactions at the workplace. Only 17.0% of subjects had no fear of family’s infections with SARS-CoV-2. There were no significant differences in lifestyle changes during lockdown between males and females, except for work changes. In particular, 60.0% of males and only 19.4% of females experienced no changes in work arrangement.
Table 1. Lifestyle changes (n = 46).
3.2. Psychological measures and serum cortisol levels
Several changes in mental health were observed (). Subjects reported significantly higher body dissatisfaction during lockdown (t (45) = 2.807, p = 0.007), with an average of 13 (SD 7.3). They also reported more symptoms of anxiety during lockdown (t (45) = 3.211, p = 0.002), with an average of 37.8 (SD 7.3). Anxiety was scored from 20 to 80 and some previous studies have reported that a score of 45 or higher indicates clinically significant anxiety levels (Barnes et al., Citation2002). This high level of anxiety was experienced by 17.4% of subjects pre-lockdown and increased to 26.1% during lockdown. Subjects also reported a significantly lower positive affect (t (45) = −3.380, p = 0.001) during lockdown, with an average score of 31.3 (SD 6.0) ().
Table 2. Psychological measures pre-lockdown and during lockdown (n = 46) and serum cortisol levels (µg/dl) pre-lockdown and post-lockdown (n = 38).
We observed some differences between genders. Males reported significantly higher body dissatisfaction (t (14) = 2.902, p = 0.012) during lockdown, which was not seen in females. However, females already had significantly higher body dissatisfaction than males pre-lockdown (t (44) = −2.628, p = 0.012) and also during lockdown (t (44) = −2.208, p = 0.033). Males reported significantly more symptoms of anxiety (t (14) = 2.272, p = 0.039) and significantly lower positive affect (t (14) = −2.423, p = 0.030) during lockdown (). Like males, females reported significantly more symptoms of anxiety (t (30) = 2.419, p = 0.022), and significantly lower positive affect (t (30) = −2.871, p = 0.008) during lockdown (). Despite body dissatisfaction, there were no statistically significant differences in psychological measures and in the changes of psychological measures between genders. The same trend in psychological measures was observed also for the 38 subjects who completed the whole intervention.
We further analyzed whether experiencing more stress during lockdown manifested in increased serum cortisol levels. shows serum cortisol levels post-lockdown significantly decreased in males (Z = −2.605, p = 0.009) and increased in females (Z = 2.771, p = 0.006). We also observed a statistically significant difference for changes in serum cortisol levels between genders (t (36) = −4.286, p < 0.001). However, males already had significantly higher serum cortisol levels than females pre-lockdown (Z = 2.663, p = 0.008). We further divided the subjects into two groups based on experienced stress during lockdown. A statistically significant increase in serum cortisol levels was observed only in those who experienced moderate to high stress (Z = 2.229, p = 0.026), while no changes were observed in those who experienced low stress during lockdown. Increased levels of depression were observed in those who had no social interactions at the workplace compared with those who had social interactions (U = 104.0, p = 0.003).
We were interested in perceived stress during lockdown and the differences between genders. The results show no statistically significant differences in perceived stress during lockdown between genders (t (44) = −1.649, p = 0.107), the score increased from 9.7 (SD 5.4) pre-lockdown to 13.1 (SD 8.5) post-lockdown. However, when we divided subjects in groups according to perceived stress, where a score of 13 or less represented low stress and a score of 14 or more represented moderate to high stress (Cohen et al., Citation1983), we observed that 80% of males and 48.4% of females experienced low stress, while only 20% of males and 51.6% of females experienced moderate to high stress during lockdown.
3.3. Correlation analysis between psychological measures and serum cortisol
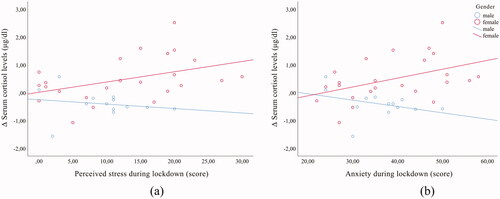
Changes in serum cortisol, a physiological marker of stress, were correlated with psychological measurements. The results show a weak positive correlation between changes in serum cortisol and perceived stress during lockdown for all subjects (r = 0.375, p = 0.022). However, by gender, a moderate positive correlation was observed only in females (r = 0.427, p = 0.037) (). Similarly, a moderate positive correlation between changes in serum cortisol and anxiety during lockdown was observed only in females (r = 0.441, p = 0.031) (). The results also show a weak positive correlation between the changes in serum cortisol and depression during lockdown for all subjects (r = 0.326, p = 0.049) and when the subjects were separated by gender, a weak positive correlation was observed only in females (r = 0.467, p = 0.021).
Figure 2. Correlation between changes in serum cortisol levels (µg/dl) and psychological measures in females (n = 24; darker line with a positive slope) and males (n = 14; lighter line with a negative slope) (a) Correlation between changes in serum cortisol levels (µg/dl) and perceived stress during lockdown; (b) Correlation between changes in serum cortisol levels (µg/dl) and anxiety during lockdown.
3.4. Gut microbiota composition
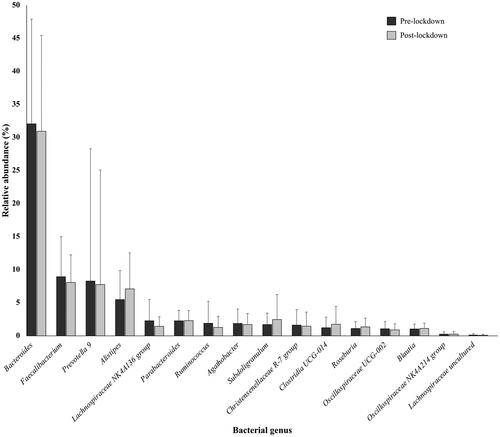
Psychological stress can also lead to changes in gut microbiota composition. To characterize the bacterial microbiota composition, 16S rRNA gene sequencing on collected stool samples was performed pre-lockdown and post-lockdown. The samples were not taken during lockdown due to lockdown restrictions. In the present study, the microbial profiles of 38 individuals were analyzed. The relative abundances of 7 bacterial phyla () and 16 genera () that were found in more than 10% of study subjects and with relative abundance of more than 1% are presented. We found that post-lockdown alpha diversity significantly decreased (t (37) = −2.320, p = 0.033) from 5.75 (SD 0.48) pre-lockdown to 5.42 (SD 0.50) post-lockdown. The phyla Bacteroidetes, Firmicutes, and Proteobacteria were detected in stool samples of all of the subjects. Pre-lockdown, Bacteroidetes was the predominant group at the phylum level, with an average abundance of 54.47% (SD 17.76%), followed by Firmicutes with an average abundance of 38.56% (SD 15.79%). Proteobacteria (M 3.69%, SD 3.30%), Tenericutes (M 1.57%, SD 3.09%), Cyanobacteria (M 1.10%, SD 2.72%), Lentisphaerae (M 0.99%, SD 1.0%), and Actinobacteria (M 0.58%, SD 1.37%) were also present. Post-lockdown, Proteobacteria abundance significantly increased (Z = 2.027, p = 0.043) from 3.69% (SD 3.30%) to 5.06% (SD 4.97%). No statistically significant differences were observed for the change of other phyla (). The most abundant genera, predominantly of the phyla Bacteroidetes and Firmicutes are presented in . No statistically significant differences were observed for the change of genera ().
Figure 3. Gut microbiota composition changes at the phylum level; the relative abundance of bacterial phyla pre-lockdown and post-lockdown (n = 38; 24 males, 14 females).
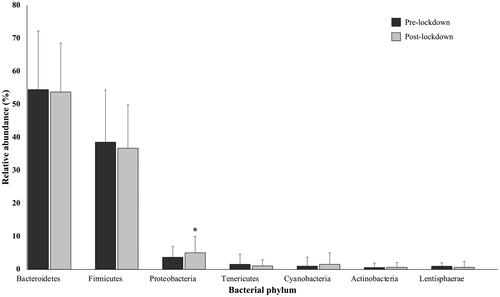
Figure 4. Gut microbiota composition changes at the genus level; the relative abundance of bacterial genera pre-lockdown and post-lockdown (n = 38; 24 males, 14 females).
3.5. Correlation analysis between psychological measures and gut microbiota composition
To investigate the relationship between psychological measures and gut microbiota, correlation analysis was performed. A moderate positive correlation between change in Bacteroidetes abundance and depression during lockdown (ρ = 0.404, p = 0.015) () and a moderate negative correlation between change in Firmicutes abundance and depression during lockdown (ρ = −0.433, p = 0.008) () was observed. In addition, Alistipes abundance post-lockdown moderately positively correlated with anxiety during lockdown (ρ = 0.460, p = 0.005) () and with negative affect during lockdown (ρ = 0.465, p = 0.004) (). A weak negative correlation between Bacteroides abundance post-lockdown and positive affect during lockdown (r = −0.399, p = 0.016) was also observed. No other significant correlations were observed between other bacteria and psychological measures. However, regarding lifestyle changes, increased relative abundance of Parabacteroides was observed in those who had no social interactions at the workplace during lockdown compared with those who had social interactions (U = 141.5, p = 0.032).
Figure 5. Correlation between gut microbiota composition and psychological measures (n = 38; 24 males, 14 females) (a) Correlation between changes in Bacteroidetes abundance and depression during lockdown; (b) Correlation between changes in Firmicutes abundance and depression during lockdown; (c) Correlation between Alistipes abundance post-lockdown and anxiety during lockdown; (d) Correlation between Alistipes abundance post-lockdown and negative affect during lockdown.
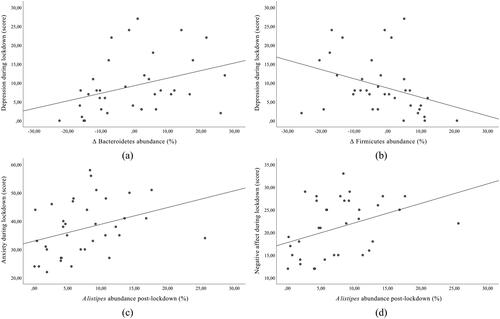
In addition to simple correlation, partial correlations between psychological measures and bacterial phyla abundance were calculated, controlling for gender and diet. Bacteroidetes abundance post-lockdown was moderately positively correlated with depression during lockdown (r = 0.551, p = 0.027), serum cortisol levels post-lockdown (r = 0.575, p = 0.020), and negatively with positive affect during lockdown (r = −0.558, p = 0.025). The results also show a strong negative correlation between Firmicutes relative abundance post-lockdown and serum cortisol levels post-lockdown (r = −0.656, p = 0.006). In addition, F/B ratio post-lockdown was moderately negatively correlated with depression during lockdown (r = −0.533, p = 0.033) and with serum cortisol levels post-lockdown (r = −0.571, p = 0.021). There were no statistically significant correlations between the relative abundance of other phyla and other psychological measures.
4. Discussion
The main aims of this study were to assess stress, anxiety, depression, body dissatisfaction, and positive and negative affect in healthy subjects before and during the COVID-19 lockdown, and to evaluate the relationship between psychological measures, cortisol levels, and gut microbiota. Subjects reported significantly higher body dissatisfaction and anxiety, and significantly lower positive affect during lockdown compared to pre-lockdown. Similarly, numerous studies reported higher levels of anxiety, stress, worry, and depression during lockdown (Gualano et al., Citation2020; Le & Nguyen, Citation2021; Pieh et al., Citation2020; Son et al., Citation2020). A longitudinal study reported increased symptoms of anxiety, depression, and loneliness that occurred shortly after the beginning of lockdown and remained high or slightly lower throughout lockdown (Mata et al., Citation2021), while another study reported increased anxiety levels for the duration of lockdown and a drop in anxiety symptoms shortly after the end of confinement measures (Quaglieri et al., Citation2021). As we did not measure the psychological measures post-lockdown, we cannot determine if the changes in our subjects persisted beyond lockdown. Although an increase in anxiety and depression symptoms was reported more frequently in females (Rodríguez-Domínguez et al., Citation2021; Rossi et al., Citation2020), and experiencing more stress during lockdown was associated with more anxiety and depression symptoms (Alsharawy et al., Citation2021; Jacques-Aviñó et al., Citation2020), in the present study, an increase in symptoms of anxiety during lockdown and lower positive affect were observed in both genders and no significant differences in the changes of these psychological measures were observed between genders.
Interestingly, body dissatisfaction significantly increased during lockdown only in males. Nevertheless, it is important to note that females already had significantly higher body dissatisfaction than males pre-lockdown and also during lockdown, which could be one of the reasons why a further change was not observed. In contrast to our results, Robertson et al. (Citation2021) have recently shown that females reported more changes in thoughts and behaviors, and also more preoccupations with food and concerns about their appearance during lockdown. In addition, changes in eating patterns and body image were positively correlated with increased psychological distress. However, in the present study, we did not observe the same associations, and subjects’ BMI, body composition, and dietary patterns did not change significantly post-lockdown (Bogataj Jontez et al., Citation2021).
In addition, we also measured perceived stress during lockdown and the differences between genders. Unfortunately, we could not investigate changes in perceived stress due to the lack of pre-lockdown measurement. No statistically significant differences in perceived stress between genders during lockdown were observed. However, when we divided subjects in groups according to perceived stress, we observed that more than half of females and only 20% of males experienced moderate to high stress during lockdown. This was also prominent in serum markers, where serum cortisol post-lockdown significantly decreased in males and increased in females. To be noted, males had significantly higher serum cortisol levels than females pre-lockdown. A further analysis showed a statistically significant increase in serum cortisol levels only in those who experienced moderate to high stress, while no changes were observed in those who experienced low stress during lockdown. This is in line with Engert et al. (Citation2021) who reported the association between higher perceived stress and higher systemic cortisol and hair cortisone levels during the first wave of COVID-19 pandemic, regardless of gender. Moreover, Jopling et al. (Citation2021) found that loneliness due to COVID-19 lockdown was associated with higher levels of cortisol at waking in young subjects.
Lockdown induced many changes, including socio-economic and lifestyle changes. In the present study, 37.0% of the subjects reported major or complete changes in their lifestyle during lockdown and only 32.6% of them experienced no changes in their work arrangement. Interestingly, there were no significant differences between males and females, except for work changes. About 60.0% of males and only 19.4% of females experienced no changes in work arrangement. Those factors, in addition to fears related to the virus itself, could influence subjects’ subjective perceptions of stress during lockdown. Indeed, we have shown before that a significantly higher increase in serum AST levels was observed in subjects who were worried about infection with SARS-CoV-2 compared with those who were not worried and a trend of increased serum glucose, total cholesterol, and LDL levels was also observed (Bogataj Jontez et al., Citation2021). In addition, increased levels of depression were observed in those who had no social interactions at the workplace compared with those who had social interactions. No other significant differences in mental health were observed between subjects who experienced changes in lifestyle or socio-economic factors and those who did not.
In the present study, changes in serum cortisol were positively correlated with perceived stress and depression during lockdown. By gender, however, the correlations were observed only in females. The positive correlation between changes in serum cortisol and anxiety was also significant only in females. Similar to our results, studies observed significant gender differences, where females reported greater fear, more negative emotional experiences during the pandemic and more negative expectations about health consequences of COVID-19 than males (Alsharawy et al., Citation2021; Rodriguez-Besteiro et al., Citation2021). It has been shown that females were more likely to view the COVID-19 pandemic as a serious health problem and to agree and comply with preventative measures (Galasso et al., Citation2020). Perceived stress post-lockdown was not measured; however, an increase in serum cortisol levels at that time point might reflect higher levels of stress during lockdown in females and point to a prolonged physiological response. The notion is important, since a prolonged increase in cortisol levels results in a dynamic adaptation to achieve homeostasis under adverse conditions, termed allostasis (Almeida et al., Citation2021), which is associated with a higher risk of developing burnout, depression, and post-traumatic stress disorder (Sjörs Dahlman et al., Citation2021).
Although the mechanisms of anxiety and depression are not yet completely understood, neurotransmitters such as serotonin, dopamine, and norepinephrine are involved in the pathophysiology of anxiety and depression. These and other factors affecting mental health may change due to the changes in gut microbiota (O'Mahony et al., Citation2015). Considering the evidence for the role of microbiota in different mood and behavioral disorders (Valles-Colomer et al., Citation2019), we additionally investigated alterations in gut microbiota associated with COVID-19 lockdown. We identified that alpha diversity significantly decreased post-lockdown and by phylum, Proteobacteria relative abundance significantly increased. Even though to date, no studies have yet investigated the impact of lockdown due to COVID-19 on gut microbiota composition, studies have shown that the diversity of gut microbiota is closely related to human health, and that the decline in gut microbial alpha diversity is associated with the increased prevalence of common metabolic diseases (Wilmanski et al., Citation2019). The decrease in the diversity could be attributed to several indirect effects of the pandemic: increased hand hygiene, travel restrictions, altered dietary and exercise habits, and also to altered mental health (Burchill et al., Citation2021). Even a short exposure to stress can cause changes in gut microbiota composition and affect stress responsiveness, anxiety-like behavior, and HPA stress axis activation set point (Foster et al., Citation2017). Regarding the change of phyla, several studies demonstrated an increased abundance of Proteobacteria in several metabolic disorders, mostly with an inflammatory phenotype. The increase in Proteobacteria in the present study could therefore be a hallmark of a disrupted metabolic state (Rizzatti et al., Citation2017).
We were also interested if changes in psychological measures were associated with the changes in gut microbiota and serum cortisol levels. Simple correlation analysis revealed that changes in Bacteroidetes abundance were positively correlated, while changes in Firmicutes abundance were negatively correlated with symptoms of depression during lockdown. The observations are similar to those of Jiang et al. (Citation2015) who found significantly higher abundance of Bacteroidetes, Proteobacteria and Actinobacteria, and lower abundance of Firmicutes in patients with depression, compared with controls. Similarly, increased Bacteroidetes abundance and decreased Firmicutes abundance were also shown in an animal model of depression (Yu et al., Citation2017). Due to known effects of gender and diet on microbiota composition, we additionally performed partial correlation analysis and found the correlations of Bacteroidetes with psychological measures to be independent of the two factors. Partial correlation also revealed serum cortisol levels were positively correlated with Bacteroidetes abundance and negatively correlated with Firmicutes abundance post-lockdown. Regarding genera, our results show Alistipes abundance post-lockdown was positively correlated with anxiety and negative affect during lockdown. These findings are similar to Naseribafrouei et al. (Citation2014) that found higher abundance of Alistipes in patients with depression. Alistipes is potentially linked to depression through inflammatory pathways and has been associated with stress in mice. It has also been hypothesized that Alistipes could cause anxiety and depression by altering the serotonergic system (Dhaliwal, Citation2019). Furthermore, in an animal stress model, lower α and β diversity, and increased Bacteroidetes abundance, largely due to genus Alistipes, was observed. The transfer of this altered microbiota to germ-free animals impacted their memory, anxiety, and gene expression in the brain (Kraimi et al., Citation2022). We also observed increased relative abundance of Parabacteroides in those who had no social interactions at the workplace compared to those who had social interactions, which was also true for increased levels of depression. This is in line with Gomez-Nguyen et al. (Citation2021) that have shown the genus Parabacteroides was associated with depressive-like behavior and its administration to germ-free mice induced depressive-like behavior.
The limitation of the present study, apart from the small sample size, was not being able to perform measurements of serum cortisol levels and gut microbiota composition during lockdown at our faculty due to lockdown restrictions, so we performed the measurements immediately after the restrictions ended. Nevertheless, these findings confirm an effect of COVID-19 lockdown on mental health, with females being particularly at risk of increased mental health issues. Lockdown also had significant effects on changes in gut microbiota composition and decreased alpha diversity, which could have a great impact on our health.
The relationship between mental health, gut microbiota and physiological stress is complex and in this prospective study about the effect of COVID-19 lockdown, significant changes in gut microbiota composition and serum cortisol levels, and their relationship with mental health in healthy subjects were observed.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Slovenian National Medical Ethics Committee (No. 0120-557/2017/4) and was registered on ClinicalTrials.gov (Identifier: NCT04347213). Informed consent was obtained from all subjects involved in the study.
Author contributions
Conceptualization, Z.J.P. and N.M.; methodology, K.Š.N., N.B.J., M.Č.B., D.B., M.H., A.B.A., Z.J.P. and N.M.; software, M.H., N.B.J. and K.Š.N.; validation, M.H., K.Š.N., M.Č.B. and Z.J.P.; formal analysis, K.Š.N., Z.J.P. and M.H.; investigation, K.Š.N., N.B.J., N.M., A.P., S.K., Z.J.P.; resources, Z.J.P. and D.B.; data curation, M.H., K.Š.N. and Z.J.P.; writing—original draft preparation, K.Š.N.; writing—review and editing, S.K., A.P., N.M., M.Č.B., D.B., A.B.A. and Z.J.P.; visualization, K.Š.N., N.M., Z.J.P. and M.H.; supervision, N.M., Z.J.P. and D.B.; project administration, Z.J.P.; funding acquisition, Z.J.P. and D.B. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank all the subjects for volunteering in this study, and nurses of the University of Primorska, Faculty of Health Sciences for taking blood samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
Notes on contributors
Karin Šik Novak
Karin Šik Novak works as a Teaching/Research Assistant at the Department of Nutritional Counseling – Dietetics at the University of Primorska Faculty of Health Sciences. She is a PhD student, researching the relationship between different dietary patterns, gut microbiota composition, human behaviour and other health parameters.
Nives Bogataj Jontez
Nives Bogataj Jontez is a PhD student at the University of Primorska Faculty of Health Sciences. She is researching connections between different dietary patterns, health and longevity.
Saša Kenig
Saša Kenig, PhD, assistant professor at the University of Primorska, investigates the effects of different diets and the consumption of functional foods on health parameters, especially on the development of metabolic syndrome and obesity.
Matjaž Hladnik
Matjaž Hladnik works in the field of bioinformatics. In recent years, he has devoted most of his research work to the metabarcoding analysis of microbiota (DNA sequence analysis using Linux command line tools and R programming language for statistical analysis). He is a coordinator of Bioinformatics B.Sc. study programme at UP FAMNIT.
Alenka Baruca Arbeiter
Alenka Baruca Arbeiter is employed as assistant with PhD at the Faculty for Mathematics, Natural Sciences and Information Technologies (University of Primorska). Her fields of research are development and characterization of genomic and genic microsatellite markers for diverse genetic studies of Mediterranean agricultural species.
Dunja Bandelj
Dunja Bandelj works at Department of Applied Natural Sciences at University of Primorska. Her research work is focused on genetics of Mediterranean agricultural and herb plants and involve various research fields: diversity population studies, plant genetic resources management, high-throughput sequencing of genomes and transcriptomes, development of genomic and genic markers, plant breeding and metabolomics. She also works in the field of agroecology, focusing on microbial community analysis and metagenomics.
Maša Černelič Bizjak
Maša Černelič Bizjak works as an associate professor at the University of Primorska Faculty. Her research interests include various topics in health psychology, human behaviour, understanding stress responses, emotional responses, and the study of eating behaviours and understanding disordered eating behaviours.
Ana Petelin
Ana Petelin is an assistant professor at the University of Primorska Faculty of Health Sciences. Her research focus on identifying potential early biomarkers resulting from obesity and associated with obesity in asymptomatic healthy adults.
Nina Mohorko
Nina Mohorko studies the effects of lifestyle on metabolism and health. She is specially interested in connections between serum biomarkers and lifestyle parameters. She currently holds a position of Associate Professor at the University of Primorska Faculty of Health Sciences.
Zala Jenko Pražnikar
Zala Jenko Pražnikar, PhD, associate professor at the University of Primorska Faculty of Health Sciences, investigates the effects of different dietary patterns and functional foods on gut microbiota composition and health parameters. She is also interested in measuring biological and genetic variables in an attempt to relate them to psychological or behavioural variables.
References
- Almeida, F. B., Pinna, G., & Barros, H. M. T. (2021). The role of HPA axis and allopregnanolone on the neurobiology of major depressive disorders and PTSD. International Journal of Molecular Sciences, 22, 246. https://doi.org/10.3390/ijms22115495
- Alsharawy, A., Spoon, R., Smith, A., & Ball, S. (2021). Gender differences in fear and risk perception during the COVID-19 pandemic. Frontiers in Psychology, 12, 689467. https://doi.org/10.3389/fpsyg.2021.689467
- Amerio, A., Lugo, A., Stival, C., Fanucchi, T., Gorini, G., Pacifici, R., Odone, A., Serafini, G., & Gallus, S. (2021). COVID-19 lockdown impact on mental health in a large representative sample of Italian adults. Journal of Affective Disorders, 292, 398–404. https://doi.org/10.1016/j.jad.2021.05.117
- Barnes, L. L. B., Harp, D., & Jung, W. S. (2002). Reliability generalization of scores on the Spielberger State-Trait Anxiety Inventory. Educational and Psychological Measurement, 62(4), 603–618. https://doi.org/10.1177/0013164402062004005
- Bogataj Jontez, N., Novak, K., Kenig, S., Petelin, A., Jenko Pražnikar, Z., & Mohorko, N. (2021). The impact of COVID-19-related lockdown on diet and serum markers in healthy adults. Nutrients, 13(4), 1082. https://doi.org/10.3390/nu13041082
- Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., Alexander, H., Alm, E. J., Arumugam, M., Asnicar, F., Bai, Y., Bisanz, J. E., Bittinger, K., Brejnrod, A., Brislawn, C. J., Brown, C. T., Callahan, B. J., Caraballo-Rodríguez, A. M., Chase, J., … Caporaso, J. G. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology, 37(8), 852–857. https://doi.org/10.1038/s41587-019-0209-9
- Burchill, E., Lymberopoulos, E., Menozzi, E., Budhdeo, S., McIlroy, J. R., Macnaughtan, J., & Sharma, N. (2021). The unique impact of COVID-19 on human gut microbiome research. Frontiers in Medicine, 8, 652464. https://doi.org/10.3389/fmed.2021.652464
- Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., & Holmes, S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods, 13(7), 581–583. https://doi.org/10.1038/nmeth.3869
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- Dhaliwal, G. K. (2019). Alistipes: The influence of a commensal on anxiety and depression. Catalyst: Facets of Biochemistry and Biomedical Sciences, 3(1), 2–10.
- Engert, V., Blasberg, J. U., Köhne, S., Strauss, B., & Rosendahl, J. (2021). Resilience and personality as predictors of the biological stress load during the first wave of the Covid-19 pandemic in Germany. Translational Psychiatry, 11(1), 443. https://doi.org/10.1038/s41398-021-01569-3
- Foster, J. A., Rinaman, L., & Cryan, J. F. (2017). Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136. https://doi.org/10.1016/j.ynstr.2017.03.001
- Galasso, V., Pons, V., Profeta, P., Becher, M., Brouard, S., & Foucault, M. (2020). Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. Proceedings of the National Academy of Sciences of the United States of America, 117(44), 27285–27291. https://doi.org/10.1073/pnas.2012520117
- Garner, D. M. (1991). Eating Disorder Inventory-2: Professional manual. Psychological assessment resources.
- Gomez-Nguyen, A., Basson, A. R., Dark-Fleury, L., Hsu, K., Osme, A., Menghini, P., Pizarro, T. T., & Cominelli, F. (2021). Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn's disease. Brain, Behavior, and Immunity, 98, 245–250. https://doi.org/10.1016/j.bbi.2021.08.218
- Gualano, M. R., Lo Moro, G., Voglino, G., Bert, F., & Siliquini, R. (2020). Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. International Journal of Environmental Research and Public Health, 17(13), 4779. https://doi.org/10.3390/ijerph17134779
- Isowa, T., Ohira, H., & Murashima, S. (2006). Immune, endocrine and cardiovascular responses to controllable and uncontrollable acute stress. Biological Psychology, 71(2), 202–213. https://doi.org/10.1016/j.biopsycho.2005.04.002
- Jacques-Aviñó, C., López-Jiménez, T., Medina-Perucha, L., de Bont, J., Gonçalves, A. Q., Duarte-Salles, T., & Berenguera, A. (2020). Gender-based approach on the social impact and mental health in Spain during COVID-19 lockdown: A cross-sectional study. BMJ Open, 10(11),. 44617. https://doi.org/10.1136/bmjopen-2020-044617
- Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., Wang, W., Tang, W., Tan, Z., Shi, J., Li, L., & Ruan, B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. https://doi.org/10.1016/j.bbi.2015.03.016
- Jopling, E., Rnic, K., Tracy, A., & LeMoult, J. (2021). Impact of loneliness on diurnal cortisol in youth. Psychoneuroendocrinology, 132, 105345. https://doi.org/10.1016/j.psyneuen.2021.105345
- Karl, J. P., Berryman, C. E., Young, A. J., Radcliffe, P. N., Branck, T. A., Pantoja-Feliciano, I. G., Rood, J. C., & Pasiakos, S. M. (2018). Associations between the gut microbiota and host responses to high altitude. American Journal of Physiology. Gastrointestinal and Liver Physiology, 315(6), G1003–G1015. https://doi.org/10.1152/ajpgi.00253.2018
- Koyama, Y., Nawa, N., Yamaoka, Y., Nishimura, H., Sonoda, S., Kuramochi, J., Miyazaki, Y., & Fujiwara, T. (2021). Interplay between social isolation and loneliness and chronic systemic inflammation during the COVID-19 pandemic in Japan: Results from U-CORONA study. Brain, Behavior, and Immunity, 94, 51–59. https://doi.org/10.1016/j.bbi.2021.03.007
- Kraimi, N., Lormant, F., Calandreau, L., Kempf, F., Zemb, O., Lemarchand, J., Constantin, P., Parias, C., Germain, K., Dupont, C., Rabot, S., Philippe, C., Foury, A., Moisan, M.-P., Carvalho, A. V., Coustham, V., Dardente, H., Velge, P., Cousin, P., … Leterrier, C. (2022). Microbiota and stress: A loop that impacts memory. Psychoneuroendocrinology, 136, 105594. https://doi.org/10.1016/j.psyneuen.2021.105594
- Kyprianidou, M., Christophi, C. A., & Giannakou, K. (2021). Perceived stress during the COVID-19-related confinement in Cyprus. Frontiers in Public Health, 9, 673411. https://doi.org/10.3389/fpubh.2021.673411
- Le, K., & Nguyen, M. (2021). The psychological burden of the COVID-19 pandemic severity. Economics and Human Biology, 41, 100979. https://doi.org/10.1016/j.ehb.2021.100979
- Loft, P., Thomas, M. G., Petrie, K. J., Booth, R. J., Miles, J., & Vedhara, K. (2007). Examination stress results in altered cardiovascular responses to acute challenge and lower cortisol. Psychoneuroendocrinology, 32(4), 367–375. https://doi.org/10.1016/j.psyneuen.2007.02.004
- Lyte, M., Vulchanova, L., & Brown, D. R. (2011). Stress at the intestinal surface: Catecholamines and mucosa-bacteria interactions. Cell and Tissue Research, 343(1), 23–32. https://doi.org/10.1007/s00441-010-1050-0
- Mata, J., Wenz, A., Rettig, T., Reifenscheid, M., Möhring, K., Krieger, U., Friedel, S., Fikel, M., Cornesse, C., Blom, A. G., & Naumann, E. (2021). Health behaviors and mental health during the COVID-19 pandemic: A longitudinal population-based survey in Germany. Social Science & Medicine, 287, 114333. https://doi.org/10.1016/j.socscimed.2021.114333
- Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linløkken, A., Wilson, R., & Rudi, K. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterology and Motility, 26(8), 1155–1162. https://doi.org/10.1111/nmo.12378
- Nickel, T., Deutschmann, A., Hanssen, H., Summo, C., & Wilbert-Lampen, U. (2009). Modification of endothelial biology by acute and chronic stress hormones. Microvascular Research, 78(3), 364–369. https://doi.org/10.1016/j.mvr.2009.07.008
- O'Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., & Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research, 277, 32–48. https://doi.org/10.1016/j.bbr.2014.07.027
- Okun, A., Stein, R. E., Bauman, L. J., & Silver, E. J. (1996). Content validity of the psychiatric symptom index, CES-depression scale, and state-trait anxiety inventory from the perspective of DSM-IV. Psychological Reports, 79(3 Pt 1), 1059–1069. https://doi.org/10.2466/pr0.1996.79.3.1059
- Pieh, C., Budimir, S., & Probst, T. (2020). The effect of age, gender, income, work, and physical activity on mental health during coronavirus disease (COVID-19) lockdown in Austria. Journal of Psychosomatic Research, 136, 110186. https://doi.org/10.1016/j.jpsychores.2020.110186
- Quaglieri, A., Lausi, G., Fraschetti, A., Burrai, J., Barchielli, B., Pizzo, A., Cordellieri, P., De Gennaro, L., Gorgoni, M., Ferlazzo, F., Sdoia, S., Zivi, P., Giannini, A. M., & Mari, E. (2021). Stay at Home’ in Italy during the COVID-19 outbreak: A longitudinal study on individual well-being among different age groups. Brain Sciences, 11(8), 993. https://doi.org/10.3390/brainsci11080993
- Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., & Glöckner, F. O. (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research, 41(Database issue), D590–D596. https://doi.org/10.1093/nar/gks1219
- Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. https://doi.org/10.1177/014662167700100306
- Rizzatti, G., Lopetuso, L. R., Gibiino, G., Binda, C., & Gasbarrini, A. (2017). Proteobacteria: A common factor in human diseases. BioMed Research International, 2017, 9351507. https://doi.org/10.1155/2017/9351507
- Robertson, M., Duffy, F., Newman, E., Prieto Bravo, C., Ates, H. H., & Sharpe, H. (2021). Exploring changes in body image, eating and exercise during the COVID-19 lockdown: A UK survey. Appetite, 159, 105062. https://doi.org/10.1016/j.appet.2020.105062
- Rodriguez-Besteiro, S., Tornero-Aguilera, J. F., Fernández-Lucas, J., & Clemente-Suárez, V. J. (2021). Gender differences in the COVID-19 pandemic risk perception, psychology, and behaviors of Spanish university students. International Journal of Environmental Research and Public Health, 18(8), 3908. https://doi.org/10.3390/ijerph18083908
- Rodríguez-Domínguez, C., Carrascal-Caputto, B., & Durán, M. (2021). Anxiety and intimate relationships in times of lockdown due to COVID-19. Psychological Trauma: Theory, Research, Practice and Policy, 14(2), 237–246. http://dx.doi.org/10.1037/tra0001094
- Rohleder, N., Nater, U. M., Wolf, J. M., Ehlert, U., & Kirschbaum, C. (2004). Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Annals of the New York Academy of Sciences, 1032, 258–263. https://doi.org/10.1196/annals.1314.033
- Rossi, R., Socci, V., Talevi, D., Mensi, S., Niolu, C., Pacitti, F., Di Marco, A., Rossi, A., Siracusano, A., & Di Lorenzo, G. (2020). COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatry, 11, 790. https://doi.org/10.3389/fpsyt.2020.00790
- Runacres, A., Mackintosh, K. A., Knight, R. L., Sheeran, L., Thatcher, R., Shelley, J., & McNarry, M. A. (2021). Impact of the COVID-19 pandemic on sedentary time and behaviour in children and adults: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health, 18(21), 11286. https://doi.org/10.3390/ijerph182111286
- Siette, J., Seaman, K., Dodds, L., Ludlow, K., Johnco, C., Wuthrich, V., Earl, J. K., Dawes, P., Strutt, P., & Westbrook, J. I. (2021). A national survey on COVID-19 second-wave lockdowns on older adults’ mental wellbeing, health-seeking behaviours and social outcomes across Australia. BMC Geriatrics, 21(1), 400. https://doi.org/10.1186/s12877-021-02352-1
- Sjörs Dahlman, A., Jonsdottir, I. H., & Hansson, C. (2021). The hypothalamo-pituitary-adrenal axis and the autonomic nervous system in burnout. Handbook of Clinical Neurology, 182, 83–94. https://doi.org/10.1016/B978-0-12-819973-2.00006-X
- Son, C., Hegde, S., Smith, A., Wang, X., & Sasangohar, F. (2020). Effects of COVID-19 on college students' mental health in the United States: Interview survey study. Journal of Medical Internet Research, 22(9), e21279. https://doi.org/10.2196/21279
- Spielberger, C., Gorsuch, R., Lushene, R., Vagg, P., & Jacobs, G. (1983). Manual for the State-Trait Anxiety Inventory (Form Y1 – Y2) (Vol. IV). Consulting Psychologists Press.
- Steckl, A. J., & Ray, P. (2018). Stress biomarkers in biological fluids and their point-of-use detection. ACS Sensors, 3(10), 2025–2044. https://doi.org/10.1021/acssensors.8b00726
- Thompson, L. R., Sanders, J. G., McDonald, D., Amir, A., Ladau, J., Locey, K. J., Prill, R. J., Tripathi, A., Gibbons, S. M., Ackermann, G., Navas-Molina, J. A., Janssen, S., Kopylova, E., Vázquez-Baeza, Y., González, A., Morton, J. T., Mirarab, S., Zech Xu, Z., Jiang, L., … Knight, R, Earth Microbiome Project Consortium. (2017). A communal catalogue reveals Earth's multiscale microbial diversity. Nature, 551(7681), 457–463. https://doi.org/10.1038/nature24621
- Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., Schiweck, C., Kurilshikov, A., Joossens, M., Wijmenga, C., Claes, S., Van Oudenhove, L., Zhernakova, A., Vieira-Silva, S., & Raes, J. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 4(4), 623–632. https://doi.org/10.1038/s41564-018-0337-x
- Slovenije, V. R. (2020). Slovenija razglasila epidemijo novega koronavirusa | GOV.SI. Portal GOV.SI. https://www.gov.si/novice/2020-03-12-slovenija-razglasila-epidemijo-novega-koronavirusa
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. https://doi.org/10.1037/0022-3514.54.6.1063
- Wilmanski, T., Rappaport, N., Earls, J. C., Magis, A. T., Manor, O., Lovejoy, J., Omenn, G. S., Hood, L., Gibbons, S. M., & Price, N. D. (2019). Blood metabolome predicts gut microbiome α-diversity in humans. Nature Biotechnology, 37(10), 1217–1228. https://doi.org/10.1038/s41587-019-0233-9
- Xu, Z., Zhang, D., Xu, D., Li, X., Xie, Y. J., Sun, W., Lee, E. K.-P., Yip, B. H.-K., Xiao, S., & Wong, S. Y.-S. (2021). Loneliness, depression, anxiety, and post-traumatic stress disorder among Chinese adults during COVID-19: A cross-sectional online survey. PLOS One, 16(10), e0259012. https://doi.org/10.1371/journal.pone.0259012
- Yu, M., Jia, H., Zhou, C., Yang, Y., Zhao, Y., Yang, M., & Zou, Z. (2017). Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. Journal of Pharmaceutical and Biomedical Analysis, 138, 231–239. https://doi.org/10.1016/j.jpba.2017.02.008