Abstract
The therapeutic activities of curcumin have long been investigated in some chronic and inflammatory diseases. This study was designed to investigate the protective effects of nanocurcumin on intestinal barrier function, apoptosis, and oxidative stress in rats exposed to traffic noise. Forty rats were divided into four groups: two traffic noise-exposed groups of animals that received either vehicle (NOISE) or nanocurcumin (NCUR + NOISE) and two control groups that either remained intact (CON) or received nanocurcumin (NCUR). Nanocurcumin injection (15 mg/Kg/ip) and traffic noise exposure were administered daily for two weeks. The relative protein expression of intestinal tight junctions, occludin, and ZO-1 and Bax/Bcl-2 ratio was measured to evaluate barrier integrity and apoptosis in intestinal samples, respectively. Plasma D-lactate concentration was examined as a criterion of intestinal permeability. Corticosterone, superoxide dismutase (SOD) activity, glutathione (GSH), total antioxidant capacity (TAC), and nitrite were measured in serum. The noise exposure increased Bax/Bcl-2 ratio, corticosterone, and oxidative stress in the NOISE animals. Nanocurcumin treatment improved the Bax/Bcl-2 ratio and reduced corticosterone and oxidative stress in the NCUR + NOISE animals. The expression of tight junction proteins was decreased while the concentration of D-lactate was increased in the NOISE animals. Nanocurcumin did not efficiently impact the expression of tight junction proteins and the D-lactate level in the NCUR + NOISE group. Nanocurcumin administration displayed antioxidant and anti-apoptotic roles in the noise-exposed rats, however, it did not affect the intestinal barrier integrity. We concluded that reduced apoptosis in the intestine might be related to the antioxidant activity of nanocurcumin and its modulatory effects on the HPA axis in the nanocurcumin-treated animals.
Introduction
Stress is a lifestyle factor associated with the deterioration of the intestinal barrier through gut-brain interactions and is a recognized risk factor for the onset and reactivation of chronic disorders (Rodiño-Janeiro et al., Citation2015). Today, noise stress is one of the most important environmental issues whose health effects are ignored (Jones, Citation1983; Bo et al., Citation2013). Noise is defined as an unpleasant and annoying sound of more than 90 decibels (Jones, Citation1983; Bo et al., Citation2013). Noise stress may reduce the function of the intestinal barrier (Bijlsma et al., Citation2001). Intestinal barrier defect is associated with a wide range of diseases and thus represents a new therapeutic target. Endothelial and, thus,epithelial barrier functions have fundamental roles in protecting the gut against potentially invasive pathogens (Yu et al., Citation2012). Intestinal barrier functions may be essential to prevent the development of multiple organ dysfunction (Odenwald & Turner, Citation2017). This barrier function is maintained by a set of proteins that form the tight junctions, transmembrane protein (occludin), and cytoplasmic membrane proteins (ZO-1). These proteins are involved in regulating paracellular permeability (Vancamelbeke & Vermeire, Citation2017). In the gastrointestinal tract, bacterial fermentation and decomposition produce D-lactate (Marcos et al., Citation1991). D-lactate, which is not metabolized by the liver, is effluxed into the bloodstream when intestinal permeability increases (Grootjans et al., Citation2010). Therefore, the plasma level of D-lactate is a valuable predictor for evaluating intestinal permeability (Xun et al., Citation2021; Ficek et al., Citation2017).
It is shown that environmental stress induces apoptosis (Xie et al., Citation2019). Apoptosis is the process of programmed cell death regulated by genes, such as the Bcl-2 family, caspase family, C-myc oncogenes and tumor suppressor gene P53, and so on (Zhao, Li, et al., Citation2021). The Bcl-2 family of proteins can be divided into two categories based on function. One kind inhibits apoptosis, such as Bcl-2, Bcl-XL, Bcl-W, and Mcl-1, while the other promotes apoptosis, such as Bax, Bcl-Xs, Bak, Bik/Nbk, and Bid (Lalier et al., Citation2022). In the process of apoptosis, Bax causes the release of cytochrome C and apoptosis-inducing factors and eventually leads to apoptosis (Lee et al., Citation2020). Meanwhile, Bcl-2 protein inhibits cytochrome C and apoptosis-inducing factors to prevent apoptosis (Li et al., Citation2013).
Environmental stress-induced apoptosis is associated with increased oxidative stress (Xie et al., Citation2019). Oxidative stress is caused by an imbalance between the production of reactive oxygen species (ROS) and the scavenger system (Song et al., Citation2020). We investigated oxidant/antioxidant balance by measuring serum nitric oxide (NO) glutathione (GSH), superoxide dismutase (SOD), and total antioxidant capacity (TAC).
Recent research has focused on the effects of natural antioxidant agents, such as curcumin, on bowel disease, mainly due to their safety profile and affordability (Lopresti, Citation2018). Curcumin, a naturally occurring polyphenolic compound, is known to have a wide range of therapeutic and pharmacological properties (Flora et al., Citation2013). In vivo and clinical studies have confirmed the antioxidant, anti-inflammatory, anti-tumor, analgesic, anti-arthritic, and immunoregulatory activities of curcuminoids relevant to the treatment of human diseases (Hewlings & Kalman, Citation2017). One of the effects of curcumin on the intestinal epithelium and immune system is to preserve the integrity of the intestinal barrier (Burge et al., Citation2019). However, the main obstacle to the clinical efficacy of curcumin is poor bioavailability due to its low aqueous solubility and rapid metabolism (Hassanzadeh et al., Citation2020). Therefore, efforts are devoted to developing curcumin formulations with greater bioavailability and systemic tissue distribution. In this study, curcumin was enhanced in bioavailability and stability by utilizing a novel nanomicelle formulation (Hatamipour et al., Citation2019). We designed this study to evaluate the protective effects of nanocurcumin on the intestinal barrier integrity, apoptosis, and oxidative stress status in the rats exposed to traffic noise.
Methods
Animals
Forty adult male Wistar rats weighing 200–250 g were used for the experiment. Experimental Animal Breeding Center of Tehran University of Medical Sciences provided rats. Rats were housed in a temperature-controlled room maintained at 22 ± 5 °C and relative humidity of 50% with a standard 12 h light/dark cycle and free access to food and water. The Ethical Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.865) approved this research.
Experimental groups
Rats were divided into four groups (n = 10/groups): two traffic noise-exposed groups that received either saline (NOISE) or nanocurcumin (NCUR + NOISE) and two control groups that either received saline (CON) or nanocurcumin (NCUR). The timeline of the research is shown in .
Noise exposure protocol
We recorded traffic noise using a standard recorder (Panasonic, RQ-L11, Japan) on a high-traffic square in Tehran, the capital city of Iran, and amplified by Sonar software (Cakewalk Inc., USA) to the level of 95 dB, which is comparable to the level of noise detected in some industrial workplaces. Rats were exposed to the noise in a metal-reflective chamber [60 cm (L) × 60 cm (w) × 90 cm (h)] equipped with two loudspeakers installed at the upper right and upper left corners. The sound intensity in the cage was consistently tracked by a precise sound level meter (Extech Instruments, USA). Rats were exposed to noise for 2 hours a day for two weeks (Alinaghipour, Salami, et al., Citation2022; Alinaghipour, Ashabi, et al., Citation2022).
Nanocurcumin administration
Nanocurcumin is a registered curcumin product (SinaCurcumin®, IRC: 1228225765; Exir Nano Sina, Tehran, Iran) in the form of capsules. Each capsule of nanocurcumin contained 40 mg curcumin in the form of nanomicelle dissolved in saline that was injected intraperitoneally (15 mg/kg) 30 min before the noise exposure. An equal volume of vehicles was injected in the CON and NOISE groups.
Western blotting
At the end of the study, the animals were deeply anesthetized using urethane (1.5 mg/Kg/ip) and duodenum samples were collected. Protein expression of Bax, Bcl2, occludin, and ZO-1 in the intestinal tissue was investigated by western blotting. Briefly, duodenum samples of different treatment groups homogenized in 1 mL of lysis buffer containing 50 mM NaCl, 10 mM Tris, 1 mM EDTA, 1 mM PMSF, 0.5 mM Na3VO4.12H2O, 50 mM NaF and 1 mM benzamidine. The samples were centrifuged at 12,500 g for 15 min at 4 °C and the supernatant was separated (Zhou et al., Citation2010). The protein concentration in the supernatants was measured using Bradford’s method (Bradford, Citation1976). Thirty µg of lysate protein were loaded on SDS-12.5% polyacrylamide electrophoresis gel and then transferred to PVDF membrane (Chemicon Millipore Co. Temecula, USA). Blots were blocked in a 3% Electrochemiluminescence (ECL) advanced blocking reagent kit (Amersham Bioscience Co. Piscataway, USA) and incubated with primary antibodies (1/1000, Cell Signaling Technology Co. New York, USA) for 18 h. Blots were then incubated with secondary antibody (1/3000, Cell Signaling Technology Co. New York, USA) for 1 h and then detected by chemiluminescence reagent kit (Amersham Bioscience Co. Piscataway, USA). Blots were stripped in stripping buffer (pH = 6.7) and then probed with anti-β-actin or GAPDH antibody (1/1000, Cell Signaling Technology Co. New York, USA). The picture of the whole membrane for western blot is provided in the supplementary material.
Biochemical analysis
Blood collection
At the end of the study, blood samples were collected from the left ventricle under anesthesia in two tubes to separate serum and plasma. D-lactate was measured in plasma and other factors in serum. For serum collection, the blood samples were centrifuged at 2000 rpm for 10 min and the supernatant was carefully removed and kept at −20 °C until biochemical analysis. For plasma separation, blood samples were poured into tubes containing anti-coagulation and centrifuged at 2500 rpm for 7 min.
D-lactate
The concentration of plasma D-lactate was measured using a commercially available assay kit (KA0869, Abnova, Taiwan), as a circulating marker of damage to the intestinal mucosa.
Corticosterone
The serum concentration of corticosterone was measured using a commercially available corticosterone enzyme-linked immunosorbent assay (ELISA) kit (Zellbio, Germany) according to the manufacturer’s instructions.
SOD enzyme activity assay
To assess SOD activity, we used Nasdox kit (Navand Salamat, Iran) that is based on the inhibition of Pyrogallol autoxidation by SOD activity. In the presence of SOD, pyrogallol autoxidation is inhibited so that the activity of the enzyme can be measured indirectly. The assay system contained pentenic acid, catalase, and Tris-Cacodylate buffer at pH=8.5.
GSH
The serum GSH content reacts with 5,5′-dithiobis 2-nitrobenzoic acid (DTNB) reagent to form a compound that is absorbed in 412 nm. A total of 100 µL of serum was added to 500 µL of sodium phosphate buffer with PH=8; next, 100 µL of DTNB was added. Optical densities were measured at 412 nm (blank) and the concentrations were calculated based on the standard samples provided by the manufacturer (Navand Salamat, Iran).
TAC
The TAC level of serum was determined by ferric ions reducing antioxidant power (FRAP) method according to TAC commercial kit (Navand Salamat, Iran). In this method, due to the presence of antioxidants, a reduction of ferric to ferrous ions at low pH occurs and a colored ferrous-tripyridyltriazine complex is formed which can be measured spectrophotometrically at 593 nm.
NO
The serum nitrite levels were measured as a proxy of NO using the Griess method (Navand Salamat, Iran). Using the protocol described by the manufacturer, all proteins were precipitated from the supernatants and aliquots of the remaining proteinless supernatant were reacted with the same volume of Griess reagent. After a 10 min incubation period, nitrite concentration was quantified spectrophotometrically at 570 nm (blank) concerning a standard curve plotted based on known-concentration standards provided by the manufacturer.
Statistical analysis
The normality of data was assessed using the Shapiro-Wilk test. Data were analyzed using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Statistical significance was set at p < .05.
Results
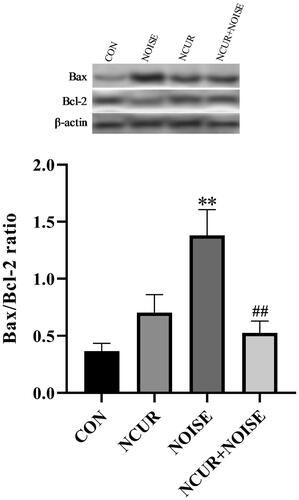
Bax/Bcl-2 protein expression
Two-way ANOVA showed a non-significant effect of the treatment (F(1,10) = 3.61, p > .05), a significant effect of noise (F(1,10) = 9.40, p < .05), and a significant effect of treatment and noise interaction (F(1,10) = 19.21, p < .01) on the Bax/Bcl-2 ratio in the duodenum samples among the different groups (). The post hoc analysis revealed that the Bax/Bcl-2 ratio was higher in the NOISE group than in the CON group (p < .01). Bax/Bcl-2 ratio was significantly decreased in the NCUR + NOISE animals compared to the NOISE group (p < .01). There was no significant difference between the NCUR and CON groups.
Figure 2. Bax/Bcl-2 ratio in duodenal cells of experimental groups. The densitometric quantification of Bax and Bcl-2 were normalized to β-actin. **p < .001 and ##p < .001 are compared to the CON and NOISE groups, respectively. Data were analyzed using two-way ANOVA followed by Tukey’s post hoc test. The values are represented as mean ± SEM (n = 4). CON: control; NCUR: nanocurcumin; NOISE: noise stress.
Tight junction protein expression
The relative protein expression of occludin and ZO-1 in the duodenum samples was determined using the band intensity ratio of these proteins to GAPDH band intensity in the same samples (). Two-way ANOVA revealed a significant effect of treatment (F(1,8) = 15.36, p < .01), noise (F(1,8) = 83.63, p < .001), and treatment and noise interaction (F(1,8) = 19.76, p < .001) on the occludin protein expression in the duodenum samples among groups (). The post-test analysis revealed that occludin protein was lower in the NOISE compared to CON animals (p < .001). There was no significant difference in occludin protein expression between the NCUR + NOISE and NOISE groups.
Figure 3. Occludin (A), ZO-1 (B) tight-junction protein expression in duodenal cells of experimental groups. The densitometric quantification of occludin and ZO-1 was normalized to GAPDH. ***p < .001 is compared to the CON group. Data were analyzed using two-way ANOVA followed by Tukey’s post hoc test. The values are represented as mean ± SEM (n = 3). ZO-1: Zonula occludens; CON: control; NCUR: nanocurcumin; NOISE: noise stress.
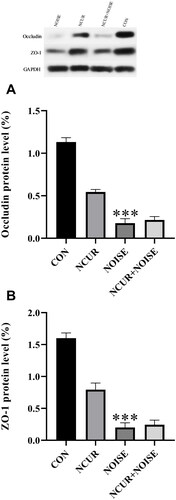
Two-way ANOVA also revealed a significant effect of treatment (F(1,8) = 20.79, p < .01), noise (F(1,8) = 135.80, p < .001), and treatment and noise interaction (F(1,8) = 25.79, p < .001) on the ZO-1 protein expression (). The post-test indicated that noise exposure declined the ZO-1 protein expression in the NOISE rats compared to the CON group (p < .001). No significant difference was observed in ZO-1 protein expression between the NCUR + NOISE and the NOISE groups.
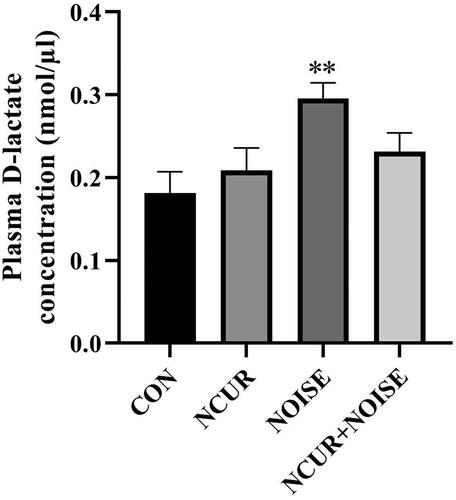
Plasma concentration of D-lactate
Plasma D-lactate concentration was assessed as an index of intestinal permeability. Two-way ANOVA showed a non-significant effect of treatment (F(1,40) = 0.59, p > .05), a significant effect of noise (F(1,40) = 8.10, p < .01), and a non-significant effect of treatment and noise interaction (F(1,40) =3.55, p > .05) on the plasma concentration of D-lactate among the different groups (). The post hoc analysis indicated that the noise exposure increased the D-lactate level in the NOISE compared to the CON group (p < .01). Nanocurcumin did not change the concentration of D-lactate in the NCUR + NOISE animals compared to their NOISE counterpart. The CON and NCUR groups resembled intestinal permeability characteristics.
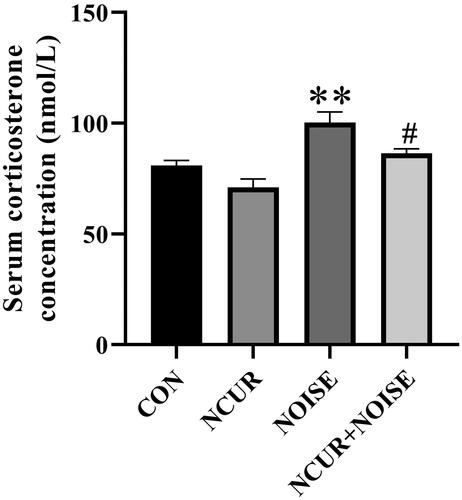
Serum level of corticosterone
Two-way ANOVA revealed a significant effect of treatment (F(1,16) = 2.26, p < .01), noise (F(1,16) = 26.35, p < .001), and a non-significant effect of treatment and noise interaction (F(1,16) = 0.35, p > .05) on the serum level of corticosterone among the different groups (). The post hoc analysis showed that serum corticosterone level in the NOISE animals was significantly higher than in their CON counterparts (p < .01). Nanocurcumin treatment decreased the stress hormone levels in the NCUR + NOISE animals compared to the NOISE group (p < .05).
Figure 5. The serum corticosterone level in the experimental groups. **p < .05 and #p < .05 are compared to the CON and NOISE groups, respectively. Data were analyzed using a two-way ANOVA followed by Tukey’s post hoc test. The values are represented as mean ± SEM (n = 5). CON: control; NCUR: nanocurcumin; NOISE: noise stress.
SOD enzyme activity assay
Two-way ANOVA revealed a non-significant effect of treatment (F(1,20) = 0.01, p > .05), a significant effect of noise (F(1,20) = 5.86, p < .05), and a significant effect of treatment and noise interaction (F(1,20) = 19.03, p < .001) on the serum level of SOD among the different groups (). The post hoc analysis showed that the serum level of SOD in the NOISE animals was significantly lower than the CON counterpart (p < .001). Nanocurcumin treatment increased the levels of SOD in the NCUR + NOISE animals compared to the NOISE group (p < .05).
Figure 6. Oxidative stress status markers in the experimental groups. The SOD (A), GSH (B), TAC (C), and NO (D) serum levels. *p < .05 and ***p < .001 are compared to the CON and #p < .05 is compared to the NOISE group. Data were analyzed using two-way ANOVA followed by Tukey’s post hoc test. The values are presented as the mean ± SEM (n = 6). SOD: Superoxide dismutase; GSH: Glutathione; TAC: Total antioxidant capacity; NO: Nitric oxide; CON: control; NCUR: nanocurcumin; NOISE: noise stress.
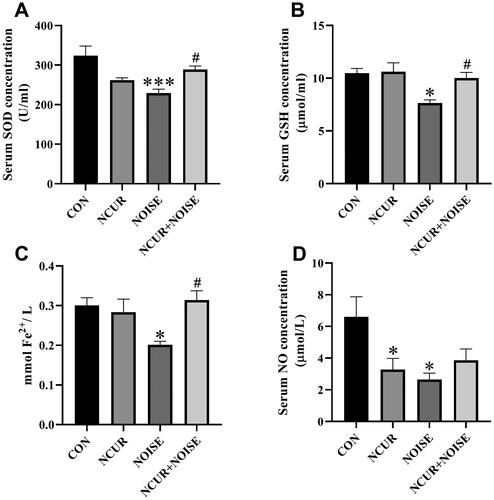
Serum level of GSH
Two-way ANOVA revealed a significant effect of treatment (F(1,20) =4.82, p < .05), noise (F(1,20) = 9.08, p < .01), and a non-significant effect of treatment and noise interaction (F(1,20) = 3.88, p > .05) on the serum level of GSH among the different groups (). The post hoc analysis showed that the GSH level was remarkably lower in the NOISE than in the CON group (p < .05). The GSH content was remarkably increased in the NCUR + NOISE compared to the NOISE animals (p < .05). There was no significant difference in the serum level of GSH between the NCUR + NOISE and CON groups (p > .05). The serum level of GSH was almost similar in the CON and NCUR rats.
Serum level of TAC
Two-way ANOVA revealed a non-significant effect of treatment (F(1,20) = 4.32, p > .05), noise (F(1,20) = 2.21, p > .05), and a significant effect of treatment and noise interaction (F(1,20) = 8.10, p < .01) on the serum level of TAC among the different groups (). The post-test analysis showed a lower concentration of TAC in the NOISE group than its CON counterpart (p < .05). Nanocurcumin treatment increased TAC in the NCUR + NOISE compared to the NOISE group (p < .05). No noticeable variation was evident between the measured TAC in the CON and NCUR + NOISE groups.
Serum level of NO
Two-way ANOVA revealed a non-significant effect of treatment (F(1,20) =1.62, p > .05), noise (F(1,20) = 4.14, p > .05), and a significant effect of treatment and noise interaction (F(1,20) = 7.50, p < .05) on the serum level of NO among the different groups (). The post hoc analysis showed that the NO level in the NOISE and NCUR animals were significantly lower than in the CON group (p < .05).
Discussion
In this study, we evaluated the effect of noise stress on intestinal barrier integrity and apoptosis and that how much these phenomena respond to nanocurcumin treatment. The relevancy of the oxidative/antioxidative factors with pathological alterations was also considered. Our findings showed that noise stress increased oxidative stress, D-lactate levels, and apoptosis and declined the expression of tight junction proteins in the duodenum. The nanocurcumin administration restored the detrimental effects of the noise stress on the oxidative stress status and apoptosis but displayed no significant effect on the intestinal barrier integrity.
Nanocurcumin treatment ameliorates pro-apoptotic effects of noise exposure
We found that Bax/Bcl-2 ratio was increased in the stressed animals, while the nanocurcumin treatment decreased the ratio. Whereas the Bax protein causes the release of cytochrome C and the apoptosis-inducing factor and ultimately leads to apoptosis, Bcl-2 protein inhibits cytochrome C and apoptosis-inducing factor to inhibit apoptosis (Li et al., Citation2013). Regulation of apoptosis depends not only on the expression level of Bcl-2 and Bax but also on their ratio. The increased ratio of Bax/Bcl-2 is an index of increased probability of apoptosis (Li et al., Citation2013). Consistent with our findings, Filho et al. showed that treatment with curcuminoids from Curcuma longa L. induces a slightly greater increase in the expression of Bcl-2 than in Bax in the epithelium of villi and crypts in mice intestine samples (Dos Santos Filho et al., Citation2016). Also, Qi et al. reported that curcumin pretreatment effectively protects trophoblast cells against oxidative stress-induced apoptosis by increasing the Bcl-2/Bax ratio (Qi et al., Citation2020). Accordingly, Xiang et al. reported that curcumin ameliorates copper-induced neurotoxicity by down-regulating Bax/Bcl-2 ratio (Xiang et al., Citation2021). Curcumin prevents apoptosis in intestinal epithelial cells and, thus, plays a role in relieving intestinal damage (Yucel et al., Citation2011). Our results demonstrated that the noise stress decreased the antioxidative factors SOD, GSH, and TAC. This, in turn, favors oxidative stress, which has been shown to induce apoptosis (Yang et al., Citation2019). On the other hand, the nanocurcumin treatment increased the level of SOD, GSH, and TAC in the noise-exposed animals. One of the other possible mechanisms may be the increased release of corticosterone in noise-exposed rats (Zhao et al., Citation2021). Therefore, a possible mechanism by which nanocurcumin reduces apoptosis may be due to its modulatory effects on the HPA axis and oxidative stress. We found that both the noise exposure and nanocurcumin administration reduced the NO level in the animals. NO, as a free radical, appears to be a potential antioxidant. It participates in the termination of lipid peroxidation reactions. It can also be an oxidant, especially in indirect reactions with oxygen molecules or superoxide anions (Kowalczyk et al., Citation2005). Therefore, the precise determination of the role of nitric oxide in apoptosis and the effects of stress and nanocurcumin on its level need more investigations.
Although increased intestinal apoptosis is associated with the pathogenesis of gastrointestinal injury, little is understood about its role in intestinal epithelial permeability (Williams et al., Citation2015). A link between apoptosis and changes in epithelial permeability has been highlighted by recent studies in which enterocyte apoptosis was induced by drugs, immune factors, or microbes (Buret & Bhargava, Citation2014; Bischoff et al., Citation2014; Kapczuk et al., Citation2020; Zeng et al., Citation2015; Ahmad et al., Citation2017).
Intestinal barrier integrity was not responsive to nanocurcumin treatment
Accumulating data emphasizes the role of permeability of the intestinal barrier in health and disease. Intestinal barrier dysfunction causes the passage of noxious molecules, resulting in the excessive activation of mucosal immune cells and inflammation (Vancamelbeke & Vermeire, Citation2017). The present study showed that noise exposure declined the tight junction protein expression in the duodenum, demonstrating a deficiency in intestinal barrier function. In this regard, Bijlsma et al. reported that exposure to a 95 dB noise induces a twofold increase in small intestinal permeability (Bijlsma et al., Citation2001). Also, Chi et al. confirmed that environmental noise stress diminishes tight junction protein expression in the intestine and hippocampus (Chi et al., Citation2021). The other types of stress are also reported to reduce tight junction protein expression. For instance, restraint stress induces intestinal mucosal injury by decreasing the expression of tight junction proteins ZO-1 and occludin (Lin et al., Citation2020). Loss of the occludin and ZO-1 proteins or reassembly of these proteins could lead to barrier dysfunction (Guo et al., Citation2019; Dodiya et al., Citation2020). There is ample evidence indicating that stress may damage intestinal barrier function, mainly through the systemic and peripheral release of corticotropin-releasing factors (Rodiño-Janeiro et al., Citation2015; Ait-Belgnaoui et al., Citation2012; Vanuytsel et al., Citation2014). Moreover, noise stress-induced oxidative stress may disrupt the intestinal barrier integrity. Our results show no significant effect of nanocurcumin on the expression level of tight junction proteins. Consistently, Yan et al. demonstrated that curcumin could not up-regulate tight junction ZO-1 protein in pigs following intestinal damage (Yan et al., Citation2019). However, some findings suggest that curcumin protects intestinal epithelial cells against disruption of tight junction and barrier dysfunction in hydrogen peroxide-induced epithelial barrier disruption (Wang et al., Citation2012), experimental colitis (Ohno et al., Citation2017), and intestinal ischemia-reperfusion injury (Tian et al., Citation2016).
Plasma D-lactate, as the end product of intestinal bacteria, has been proposed as a circulating marker to assess the extent of damage and repair of the intestinal mucosa (Fukudome et al., Citation2014). When the intestinal mucosa is damaged, almost all D-lactate is released into the blood due to D-lactate dehydrogenase deficiency in mammals. Therefore, D-lactate in peripheral blood can indicate damage to the intestinal barrier (Ruh et al., Citation2000). Our results showed that noise exposure increased the plasma D-lactate concentration. We found that nanocurcumin treatment did not reduce plasma D-lactate concentration in the stressed animals. Contrary to our finding, Xun et al. reported that curcumin reduces plasma D-lactate levels and protects the intestinal mucosal barrier function in weaned piglets challenged with enterotoxigenic Escherichia coli (Xun et al., Citation2015). We found that nanocurcumin reduced oxidative stress and corticosterone levels in noise-exposed rats while failing to improve intestinal integrity. Presumably, other mechanisms may be involved in altering intestinal permeability. One propose might be that nanocurcumin probably does not affect intestinal permeability in the animals exposed to noise. Although our findings are not consistent with some other research (Wang et al., Citation2012; Ohno et al., Citation2017; Tian et al., Citation2016), where the changes in relative protein expression of intestinal tight junctions were consistent with the plasma D-lactate level in our study. Moreover, discrepancies observed between our findings and others may be due to the different stress paradigms employed.
Conclusions
Altogether, the noise stress activates the HPA axis, increases oxidative stress, and induces intestinal barrier permeability and apoptosis in the duodenum. Nanocurcumin treatment ameliorates the pro-apoptotic effects of noise exposure with no significant effect on the intestinal barrier integrity. The effects of nanocurcumin might be mediated by its modulatory effects on the HPA axis and its antioxidant properties. However, further investigation is required to answer the questions raised about the probable mechanisms involved.
Supplemental Material
Download MS Word (862.2 KB)Acknowledgments
The present study was supported by a grant devoted to Fatemeh Nabavizadeh from Tehran University of Medical Sciences. We cordially thank the Physiology Research Center, Kashan University of Medical Sciences for collaborating in implementing this project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Azam Alinaghipour
Azam Alinaghipour, Ph.D, graduated from the Department of Physiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. She is interested in the gut-brain axis, focusing on the mechanisms involved in changing the permeability of the intestinal epithelial barrier and the blood brain barrier.
Mahmoud Salami
Mahmoud Salami, Ph.D, Professor of Physiology and Head of the Physiology Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. He is pursuing a line of research on the potential use of probiotic supplementation to treat neurodegenerative disease, focusing on the gut-brain axis.
Esmail Riahi
Esmail Riahi, Ph.D, Associate Professor at Department of Physiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. He is pursuing a line of research on the potential use of deep brain stimulation (DBS) to treat intractable substance use disorders.
Ghorbangol Ashabi
Ghorbangol Ashabi, Ph.D, Associate Professor at Department of Physiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran. She has experience in cerebral injuries, focusing on molecular pathways.
Masoud Soheili
Masoud Soheili, Ph.D, Researcher of Physiology Research Center, Basic Sciences Research Institute, Kashan University of Medical Sciences, Kashan, Iran. His research focuses on neurodegenerative disease and the gut-brain axis, particularly identifying the relevant biochemical and histopathological mechanisms.
Fatemeh Nabavizadeh
Fatemeh Nabavizadeh, Ph.D, Professor of Physiology and Head of Electrophysiology Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran. Her research mainly focuses on the gastrointestinal tract, especially gastrointestinal cancers using animal models.
References
- Ahmad, R., Sorrell, M., Batra, S., Dhawan, P., & Singh, A. (2017). Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunology, 10(2), 307–317. https://doi.org/10.1038/mi.2016.128
- Ait-Belgnaoui, A., Durand, H., Cartier, C., Chaumaz, G., Eutamene, H., Ferrier, L., Houdeau, E., Fioramonti, J., Bueno, L., & Theodorou, V. (2012). Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology, 37(11), 1885–1895. https://doi.org/10.1016/j.psyneuen.2012.03.024
- Alinaghipour, A., Ashabi, G., Riahi, E., Soheili, M., Salami, M., & Nabavizadeh, F. (2022). Effects of nano-curcumin on noise stress-induced hippocampus-dependent memory impairment: Behavioral and electrophysiological aspects. Pharmacological Reports, 74(3), 461–469. https://doi.org/10.1007/s43440-022-00354-3
- Alinaghipour, A., Salami, M., & Nabavizadeh, F. (2022). Nanocurcumin substantially alleviates noise stress-induced anxiety-like behavior: The roles of tight junctions and NMDA receptors in the hippocampus. Behavioural Brain Research, 432, 113975. https://doi.org/10.1016/j.bbr.2022.113975
- Bijlsma, P. B., Van Raaij, M. T. M., Dobbe, C. J. G., Timmerman, A., Kiliaan, A. J., Taminiau, J. A. J. M., & Groot, J. A. (2001). Subchronic mild noise stress increases HRP permeability in rat small intestine in vitro. Physiology & Behavior, 73(1–2), 43–49. https://doi.org/10.1016/S0031-9384(01)00424-3
- Bischoff, S. C., Barbara, G., Buurman, W., Ockhuizen, T., Schulzke, J.-D., Serino, M., Tilg, H., Watson, A., & Wells, J. M. (2014). Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterology, 14(1), 1–25. https://doi.org/10.1186/s12876-014-0189-7
- Bo, C., Wu, M. Q., Zhu, L. X., She, X. J., Qiang, M., & Liu, H. T. (2013). Effect of chronic noise exposure on expression of N-methyl-D-aspartic acid receptor 2B and Tau phosphorylation in hippocampus of rats. Biomedical and Environmental Sciences, 26(3), 163–168.
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- Buret, A. G., & Bhargava, A. (2014). Modulatory mechanisms of enterocyte apoptosis by viral, bacterial and parasitic pathogens. Critical Reviews in Microbiology, 40(1), 1–17. https://doi.org/10.3109/1040841X.2012.746952
- Burge, K., Gunasekaran, A., Eckert, J., & Chaaban, H. (2019). Curcumin and intestinal inflammatory diseases: Molecular mechanisms of protection. International Journal of Molecular Sciences, 20(8), 1912. https://doi.org/10.3390/ijms20081912
- Chi, H., Cao, W., Zhang, M., Su, D., Yang, H., Li, Z., Li, C., She, X., Wang, K., Gao, X., Ma, K., Zheng, P., Li, X., & Cui, B. (2021). Environmental noise stress disturbs commensal microbiota homeostasis and induces oxi-inflammmation and AD-like neuropathology through epithelial barrier disruption in the EOAD mouse model. Journal of Neuroinflammation, 18(1), 9. https://doi.org/10.1186/s12974-020-02053-3
- Dodiya, H. B., Forsyth, C. B., Voigt, R. M., Engen, P. A., Patel, J., Shaikh, M., Green, S. J., Naqib, A., Roy, A., Kordower, J. H., Pahan, K., Shannon, K. M., & Keshavarzian, A. (2020). Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiology of Disease, 135, 104352. https://doi.org/10.1016/j.nbd.2018.12.012
- Dos Santos Filho, E. X., Ávila, P. H. M., Bastos, C. C. C., Batista, A. C., Naves, L. N., Marreto, R. N., Lima, E. M., Mendonca, E. F., & Valadares, M. C. (2016). Curcuminoids from Curcuma longaL. reduced intestinal mucositis induced by 5-fluorouracil in mice: Bioadhesive, proliferative, anti-inflammatory and antioxidant effects. Toxicology Reports, 3, 55–62. https://doi.org/10.1016/j.toxrep.2015.10.010
- Ficek, J., Wyskida, K., Ficek, R., Wajda, J., Klein, D., Witkowicz, J., Rotkegel, S., Spiechowicz-Zatoń, U., Kocemba-Dyczek, J., Ciepał, J., Więcek, A., Olszanecka-Glinianowicz, M., & Chudek, J. (2017). Relationship between plasma levels of zonulin, bacterial lipopolysaccharides, D-lactate and markers of inflammation in haemodialysis patients. International Urology and Nephrology, 49(4), 717–725. https://doi.org/10.1007/s11255-016-1495-5
- Flora, G., Gupta, D., & Tiwari, A. (2013). Nanocurcumin: A promising therapeutic advancement over native curcumin. Critical Reviews™ in Therapeutic Drug Carrier Systems, 30(4), 331–368. https://doi.org/10.1615/critrevtherdrugcarriersyst.2013007236
- Fukudome, I., Kobayashi, M., Dabanaka, K., Maeda, H., Okamoto, K., Okabayashi, T., Baba, R., Kumagai, N., Oba, K., Fujita, M., & Hanazaki, K. (2014). Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Medical Molecular Morphology, 47(2), 100–107. https://doi.org/10.1007/s00795-013-0055-7
- Grootjans, J., Thuijls, G., Verdam, F., Derikx, J. P., Lenaerts, K., & Buurman, W. A. (2010). Non-invasive assessment of barrier integrity and function of the human gut. World Journal of Gastrointestinal Surgery, 2(3), 61–69. https://doi.org/10.4240/wjgs.v2.i3.61
- Guo, Y., Li, H., Liu, Z., Li, C., Chen, Y., Jiang, C., Yu, Y., & Tian, Z. (2019). Impaired intestinal barrier function in a mouse model of hyperuricemia. Molecular Medicine Reports. 20, 3292–3300.
- Hassanzadeh, K., Buccarello, L., Dragotto, J., Mohammadi, A., Corbo, M., & Feligioni, M. (2020). Obstacles against the marketing of curcumin as a drug. International Journal of Molecular Sciences, 21(18), 6619. https://doi.org/10.3390/ijms21186619
- Hatamipour, M., Sahebkar, A., Alavizadeh, S. H., Dorri, M., & Jaafari, M. R. (2019). Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iranian Journal of Basic Medical Sciences, 22(3), 282–289.
- Hewlings, S. J., & Kalman, D. S. (2017). Curcumin: A review of its effects on human health. Foods, 6(10), 92. https://doi.org/10.3390/foods6100092
- Jones, M. (1983). JM Ramsay, basic pathophysiology—Modern stress and the disease process, Addison-Wesley, London (1982), p. 555, illus,£13.25. Churchill Livingstone.
- Kapczuk, P., Kosik-Bogacka, D., Kupnicka, P., Metryka, E., Simińska, D., Rogulska, K., Skórka-Majewicz, M., Gutowska, I., Chlubek, D., & Baranowska-Bosiacka, I. (2020). The influence of selected gastrointestinal parasites on apoptosis in intestinal epithelial cells. Biomolecules, 10(5), 674. https://doi.org/10.3390/biom10050674
- Kowalczyk, E., Kopff, A., Kopff, M., Fijałkowski, P., & Błaszczyk, J. (2005). Nitric oxide–oxidant or antioxidant? Wiadomosci Lekarskie, 58, 540–542.
- Lalier, L., Vallette, F., & Manon, S. (2022). Bcl-2 family members and the mitochondrial import machineries: The roads to death. Biomolecules, 12(2), 162. https://doi.org/10.3390/biom12020162
- Lee, Y.-S., Kalimuthu, K., Park, Y. S., Luo, X., Choudry, M. H. A., Bartlett, D. L., & Lee, Y. J. (2020). BAX-dependent mitochondrial pathway mediates the crosstalk between ferroptosis and apoptosis. Apoptosis, 25(9–10), 625–631. https://doi.org/10.1007/s10495-020-01627-z
- Li, Y., Han, F., & Shi, Y. (2013). Increased neuronal apoptosis in medial prefrontal cortex is accompanied with changes of Bcl-2 and Bax in a rat model of post-traumatic stress disorder. Journal of Molecular Neuroscience, 51(1), 127–137. https://doi.org/10.1007/s12031-013-9965-z
- Lin, R., Wang, Z., Cao, J., Gao, T., Dong, Y., & Chen, Y. (2020). Role of melatonin in intestinal mucosal injury induced by restraint stress in mice. Pharmaceutical Biology, 58(1), 342–351. https://doi.org/10.1080/13880209.2020.1750659
- Lopresti, A. L. (2018). The problem of curcumin and its bioavailability: Could its gastrointestinal influence contribute to its overall health-enhancing effects? Advances in Nutrition, 9(1), 41–50. https://doi.org/10.1093/advances/nmx011
- Marcos, M., Vila, J., Gratacos, J., Brancos, M., & Jimenez De Anta, M. (1991). Determination of D-lactate concentration for rapid diagnosis of bacterial infections of body fluids. European Journal of Clinical Microbiology & Infectious Diseases, 10(11), 966–969. https://doi.org/10.1007/BF02005455
- Odenwald, M. A., & Turner, J. R. (2017). The intestinal epithelial barrier: A therapeutic target? Nature Reviews. Gastroenterology & Hepatology, 14(1), 9–21. https://doi.org/10.1038/nrgastro.2016.169
- Ohno, M., Nishida, A., Sugitani, Y., Nishino, K., Inatomi, O., Sugimoto, M., Kawahara, M., & Andoh, A. (2017). Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One, 12(10), e0185999. https://doi.org/10.1371/journal.pone.0185999
- Qi, L., Jiang, J., Zhang, J., Zhang, L., & Wang, T. (2020). Curcumin protects human trophoblast HTR8/SVneo cells from H2O2-induced oxidative stress by activating Nrf2 signaling pathway. Antioxidants, 9(2), 121. https://doi.org/10.3390/antiox9020121
- Rodiño-Janeiro, B. K., Alonso-Cotoner, C., Pigrau, M., Lobo, B., Vicario, M., & Santos, J. (2015). Role of Corticotropin-releasing factor in gastrointestinal permeability. Journal of Neurogastroenterology and Motility, 21(1), 33–50. https://doi.org/10.5056/jnm14084
- Ruh, J., Vogel, F., Schmidt, E., Werner, M., Klar, E., Secchi, A., Gebhard, M. M., Glaser, F., & Herfarth, C. (2000). Effects of hydrogen peroxide scavenger Catalase on villous microcirculation in the rat small intestine in a model of inflammatory bowel disease. Microvascular Research, 59(3), 329–337. https://doi.org/10.1006/mvre.1999.2201
- Song, K., Li, Y., Zhang, H., An, N., Wei, Y., Wang, L., Tian, C., Yuan, M., Sun, Y., Xing, Y., & Gao, Y. (2020). Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Medicine and Cellular Longevity, 2020, 1–27. https://doi.org/10.1155/2020/4356386
- Tian, S., Guo, R., Wei, S., Kong, Y., Wei, X., Wang, W., Shi, X., & Jiang, H. (2016). Curcumin protects against the intestinal ischemia-reperfusion injury: Involvement of the tight junction protein ZO-1 and TNF-α related mechanism. The Korean Journal of Physiology & Pharmacology, 20(2), 147–152. https://doi.org/10.4196/kjpp.2016.20.2.147
- Vancamelbeke, M., & Vermeire, S. (2017). The intestinal barrier: A fundamental role in health and disease. Expert Review of Gastroenterology & Hepatology, 11(9), 821–834. https://doi.org/10.1080/17474124.2017.1343143
- Vanuytsel, T., Van Wanrooy, S., Vanheel, H., Vanormelingen, C., Verschueren, S., Houben, E., Salim Rasoel, S., Tόth, J., Holvoet, L., Farré, R., Van Oudenhove, L., Boeckxstaens, G., Verbeke, K., & Tack, J. (2014). Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 63(8), 1293–1299. https://doi.org/10.1136/gutjnl-2013-305690
- Wang, N., Wang, G., Hao, J., Ma, J., Wang, Y., Jiang, X., & Jiang, H. (2012). Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Digestive Diseases and Sciences, 57(7), 1792–1801. https://doi.org/10.1007/s10620-012-2094-7
- Williams, J., Duckworth, C., Burkitt, M., Watson, A., Campbell, B., & Pritchard, D. (2015). Epithelial cell shedding and barrier function: A matter of life and death at the small intestinal villus tip. Veterinary Pathology, 52(3), 445–455. https://doi.org/10.1177/0300985814559404
- Xiang, B., Li, D., Chen, Y., Li, M., Zhang, Y., Sun, T., & Tang, S. (2021). Curcumin ameliorates copper-induced neurotoxicity through inhibiting oxidative stress and mitochondrial apoptosis in SH-SY5Y cells. Neurochemical Research, 46(2), 367–378. https://doi.org/10.1007/s11064-020-03173-1
- Xie, X., He, Z., Chen, N., Tang, Z., Wang, Q., & Cai, Y. (2019). The roles of environmental factors in regulation of oxidative stress in plant. BioMed Research International, 2019, 1–11. https://doi.org/10.1155/2019/9732325
- Xun, W., Fu, Q., Shi, L., Cao, T., Jiang, H., & Ma, Z. (2021). Resveratrol protects intestinal integrity, alleviates intestinal inflammation and oxidative stress by modulating AhR/Nrf2 pathways in weaned piglets challenged with diquat. International Immunopharmacology, 99, 107989. https://doi.org/10.1016/j.intimp.2021.107989
- Xun, W., Shi, L., Zhou, H., Hou, G., Cao, T., & Zhao, C. (2015). Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. International Immunopharmacology, 27(1), 46–52. https://doi.org/10.1016/j.intimp.2015.04.038
- Yan, E., Zhang, J., Han, H., Wu, J., Gan, Z., Wei, C., Zhang, L., Wang, C., & Wang, T. (2019). Curcumin alleviates IUGR jejunum damage by increasing antioxidant capacity through Nrf2/Keap1 pathway in growing pigs. Animals, 10(1), 41. https://doi.org/10.3390/ani10010041
- Yang, F., Pei, R., Zhang, Z., Liao, J., Yu, W., Qiao, N., Han, Q., Li, Y., Hu, L., Guo, J., Pan, J., & Tang, Z. (2019). Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicology In Vitro, 54, 310–316. https://doi.org/10.1016/j.tiv.2018.10.017
- Yu, L. C.-H., Wang, J.-T., Wei, S.-C., & Ni, Y.-H. (2012). Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World Journal of Gastrointestinal Pathophysiology, 3(1), 27–43. https://doi.org/10.4291/wjgp.v3.i1.27
- Yucel, A. F., Kanter, M., Pergel, A., Erboga, M., & Guzel, A. (2011). The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. Journal of Molecular Histology, 42(6), 579–587. https://doi.org/10.1007/s10735-011-9364-0
- Zeng, N., Mignet, N., Dumortier, G., Olivier, E., Seguin, J., Maury, M., Scherman, D., Rat, P., & Boudy, V. (2015). Poloxamer bioadhesive hydrogel for buccal drug delivery: Cytotoxicity and trans-epithelial permeability evaluations using TR146 human buccal epithelial cell line. International Journal of Pharmaceutics, 495(2), 1028–1037. https://doi.org/10.1016/j.ijpharm.2015.09.045
- Zhao, L., Li, H., Huang, X., Liu, T., Xin, Y., Xiao, Z., Zhao, W., Miao, S., Chen, J., Li, Z., & Mi, Y. (2021). The endocytic pathway of Pt nanoclusters and their induced apoptosis of A549 and A549/Cis cells through c-Myc/p53 and Bcl-2/caspase-3 signaling pathways. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 144, 112360. https://doi.org/10.1016/j.biopha.2021.112360
- Zhao, Y. Q., Chen, R. R., Kong, Q. Q., An, J. S., Zhao, X. Y., Gong, S., Yuan, H. J., & Tan, J. H. (2021). Corticosterone induced apoptosis of mouse oviduct epithelial cells independent of the TNF-α system. The Journal of Reproduction and Development, 67(1), 43–51. https://doi.org/10.1262/jrd.2020-122
- Zhou, J., Wang, Y. X., Xiong, Y. F., Wang, H. X., Feng, Y. M., & Chen, J. A. (2010). Delivery of Tfpi-2 Using Ultrasound with a Microbubble Agent (Sonovue) Inhibits Intimal Hyperplasia after Balloon Injury in a Rabbit Carotid Artery Model. Ultrasound in Medicine & Biology, 36(11), 1876–1883. https://doi.org/10.1016/j.ultrasmedbio.2010.08.013