Abstract
Adolescent obesity augments and impedes the treatment of chronic pain. This is associated with increased systemic inflammation and is more prominent in females. In addition, pain and obesity each independently affect the hypothalamic-pituitary-adrenal (HPA) axis. However, the interaction of pain and obesity on the HPA axis and the potential for sexual dimorphism in this phenomenon is not established. We hypothesized that dysregulation of the HPA axis occurs in female human adolescents with chronic pain, obesity, or the combination of the two and is associated with gonadal steroids. We measured serum cortisol, estradiol, and testosterone in 13–17-year-old adolescent females (N = 79) from venous blood drawn during the daytime (0830–1730 h) and analyzed the data in toto and partitioned by morning vs. afternoon sampling time. Subjects were categorized as healthy weight/no pain (controls; BMI = 56th percentile [37–71]), healthy weight with chronic pain, obese without pain (BMI = 97th percentile [95–99]), or the combination of obesity and chronic pain. Serum cortisol was lower with chronic pain and/or obesity compared to healthy controls and was lower with chronic pain and obesity compared to chronic pain alone (healthy weight). The lower serum cortisol in the pain alone group was more prominent in the morning compared to the afternoon. There was no relationship between serum estradiol and testosterone and study group. The decrease in the anti-inflammatory and other pain-ameliorating effects of cortisol may contribute to chronic pain and its resistance to treatment with concurrent obesity in female adolescents.
Introduction
The prevalence of obesity in adolescents has increased dramatically in the past three decades (Gungor, Citation2014). We have previously demonstrated that adolescents with obesity have a higher incidence and sequelae of chronic pain (Hainsworth et al., Citation2009). Furthermore, the presence of obesity impedes the treatment of chronic pain, and the presence of chronic pain limits therapeutic approaches to obesity in adolescents (Stoner et al., Citation2017). Finally, we have demonstrated that the exacerbation of chronic pain in adolescents is sexually dimorphic and is associated with increased systemic inflammation assessed by the measurement of serum levels of inflammatory cytokines (Hainsworth et al., Citation2021; Raff et al., Citation2020).
Pain and obesity independently alter the dynamics of the hypothalamic-pituitary-adrenal (HPA) axis (Bose et al., Citation2009; Nees et al., Citation2019). There is a crosstalk between pain, inflammation (including inflammatory cytokines) and cortisol, the archetypal endogenous anti-inflammatory hormone – this is the so-called “immune-adrenal axis” (Chrousos, Citation1995). Both chronic pain and obesity affect women more than men (Hainsworth et al., Citation2021). In the US, the prevalence of severe obesity (9.2%) in adults is higher in women. Pediatric pain is greater in females for most pain types, and 70–85% of patients seen in pediatric pain clinics are female (Hainsworth et al., Citation2021). These studies and the work of others (Narouze & Souzdalnitski, Citation2015; Sorge & Totsch, Citation2017) suggest that females are also more affected by the co-occurrence of chronic pain and obesity than are males, and that gonadal steroids may be an important covariate (Torpy & Chrousos, Citation1996). Therefore, the current study focused on female adolescents differentially affected by obesity and pain. Finally, there is a circadian rhythm of the hypothalamic-pituitary-target gland axes (Raff & Trivedi, Citation2013; Rosner et al., Citation2007). Therefore, we measured serum estradiol and testosterone to determine if the time of day of blood sampling (morning [AM] vs. afternoon [PM]) revealed important differences.
The goal of the current study is to assess adrenal and gonadal steroids in female adolescents with chronic pain, obesity, or the combination of the two compared to a control group without obesity or chronic pain. We hypothesized that cortisol (reflecting HPA axis activity) is associated with the interaction of obesity and pain, and we examined whether gonadal steroids and/or time of day influenced this interaction.
Materials and methods
Subjects
Female (N = 79) adolescent (age 13–17) subjects were recruited from a larger study on pain control. This project was approved by the Children’s Wisconsin Institutional Review Board and written consent provided by participants and parents.
Enrollment, inclusion and exclusion have been described in detail previously (Raff et al., Citation2020). Briefly, subjects were recruited into four groups based on presence/absence of chronic pain and presence/absence of obesity: Healthy Controls (normal weight and no pain; N = 20); Chronic Pain with Healthy Weight (N = 20); Obese (no chronic pain; N = 20); and Chronic Pain and Obesity (N = 19). Based on an expected difference and standard deviation in serum cortisol of 8.0 μg/dL, and a power of 0.85, a group sample size of 19 was determined to be required to generate an alpha of 0.05 (Sigmaplot 12.5, Systat Software Inc., San Jose, CA).
Psychotropic or long-acting analgesics were only allowed if their use was stable for at least one week prior to sampling. Subjects without obesity was defined as a sex-specific BMI of 5th to <85th percentile (Raff et al., Citation2020). Obesity was defined as a sex-specific BMI ≥95th percentile (Raff et al., Citation2020). The non-pain groups were defined as the absence of chronic illness, and lack of pain defined as “not at all or rarely” within the past three months. Self-reported days with pain (PFSD1) and worst pain intensity (PFSD4) over the past two weeks were assessed (Raff et al., Citation2020). The Pubertal Development Scale, a brief self-reported measure, was used to assess pubertal development (Hainsworth et al., Citation2021; Petersen et al., Citation1988).
Exclusion criteria included Type 1 or Type 2 diabetes mellitus and/or metabolic syndrome, hypertension, cancer-related pain, sickle cell disease, and inflammatory conditions (other than pain or obesity) or the current use of metformin, Accutane, medicinal marijuana/cannabidiol, immune modulating medications, or self-reported use of illicit “street drugs.” Patients using corticosteroids regularly (defined for this study as daily use within the past 2 weeks or ≥12 times in the past month) were excluded. Patients using NSAIDS or asthma inhalers were included in the study, but use of the former within 12 hours of blood draw or use of the latter within 48 hours, were excluded. Venous blood samples were obtained between 0830–1730 h and processed to serum for future hormone measurement in batches.
Hormone assays
Serum cortisol was measured by coated tube radioimmunoassay (MP Biomedicals, Solon, OH: 07–221102 R; RRID: AB_2801525). To convert cortisol to nmol/L, multiply by 27.6. Intraassay variation ranges from 8.9% at 4.7 μg/dl to 5.3% at 18.1 μg/dl. Inter-assay variation ranges from 9.3% at 5.0 μg/dl to 7.5% at 18.7 μg/dl. The only noteworthy cross-reactivities are 21-deoxycortisol (4.3%) and 17-hydroxy-progesterone (1.0%). The recovery ranges from 91–117%. No interference occurs with triglycerides, hemoglobin, or bilirubin. Serum 17β-estradiol was measured in females by coated tube radioimmunoassay (MP Biomedicals, Solon, OH: 07–238102; RRID: AB_2904013) as used by us previously (Tjoe et al., Citation2020). To convert estradiol to pmol/L, multiply by 3.67. Intraassay variation ranges from 15.7% at 25.4 pg/ml to 3.5% at 657.0 pg/ml. Inter-assay variation ranges from 15.4% at 49.2 pg/mL to 5.5% at 240.0 pg/ml. The only noteworthy cross-reactivities are estrone (6.2%) and estriol (1.5%). The recovery ranges from 96–110% at 300 pg/ml of estradiol. No interference occurs with triglycerides, hemoglobin, or bilirubin. Serum total testosterone was measured by liquid chromatography-tandem mass spectrometry as validated and described in detail previously (Raff et al., Citation2018). To convert testosterone to nmol/L, multiply by 0.347. The functional sensitivity is 0.7 ng/dl, and intra- and interassay coefficients of variation are 1.5–3.9% and 2.6−7.8%, respectively.
Statistical analyses
Continuous data are summarized with mean (SD) or median (25th-75th percentile) with parametric analysis of variance (type III Sum of squares) or exact nonparametric Kruskal–Wallis (suitably grouped so one way analysis of variance could be done) and Mann-Whitney tests with skew or ordinal data. Categorical data were summarized as number (%) with chi-square or Fisher-Halton exact tests. An unadjusted p < 0.05 is reported as significant. Software used was SPSS v26 (IBM Corporation, Somers, NY, and Cytel StatXact v8, Cambridge, MA).
Results
The demographic and anthropomorphic data for the subject groups are shown in . There was a trend toward differences in race between the Pain and Obesity group and the groups without pain. There was no difference in overall pubertal status; most of the females (71 of 79) were post-pubertal. As the groups were selected for the presence or absence of pain and/or obesity, the differences in BMI and pain scores were different as predicted.
Table 1. Demographic and anthropomorphic data. Data are shown as mean [SD] or median [25th–75th %] where appropriate.
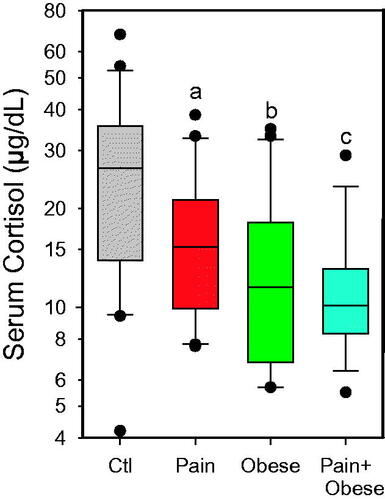
There was a significant group interaction on serum cortisol concentrations in females (p = 0.002) (). Compared to the Healthy Control group, serum cortisol was lower in the groups with pain and/or obesity. Those with chronic pain and obesity were lower than those with chronic pain (and healthy weight) (p = 0.042).
Figure 1. Serum cortisol in female adolescents. Healthy Controls (Ctl, normal weight and no pain; (N = 20); Chronic Pain with Healthy Weight (Pain, N = 20); Obese (no pain; N = 20); and Chronic Pain and Obesity (N = 19 females). a, different from Ctl (p = 0.03), b, different from Ctl (p = 0.002), c, different from Ctl (p < 0.001) and Pain (p = 0.042). To convert to nmol/L, multiply by 27.6.
There was no overall correlation of time of day and any of the hormonal data (serum cortisol shown in ). Despite this, we further parsed the data into subjects whose samples were drawn in the morning (between 8:25 and 11:50) and the afternoon (between 12:10–17:24) (). The overall differences in serum cortisol shown in were retained with the following interesting exceptions. It appears that the finding of lower serum cortisol in the group with chronic pain (and healthy weight) shown in was weighted toward samples drawn in the morning as shown in . It is therefore plausible that our finding of lower serum cortisol in the chronic pain with obesity group overall in (blue bar) compared to the chronic pain (and healthy weight) group (red bar), could mostly be accounted for by the higher PM samples in the chronic pain (healthy weight) group.
Figure 2. Linear regression analysis of sampling clock time vs. serum cortisol. There was no statistically significant relationship. (N = 79; r2 = 0.038; Adjusted r2 = 0.025; SEE = 11.6; p > 0.50).
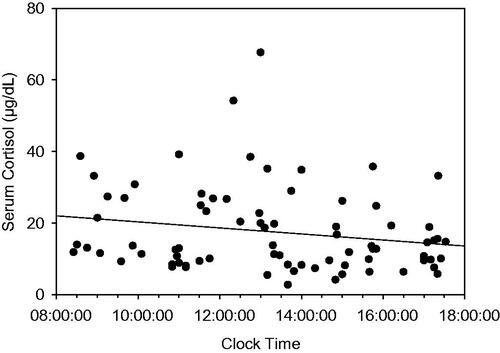
Table 2. Data parsed by time: AM vs PM (Median [IQR]).
Discussion
The purpose of this study was to determine the effects and interaction of chronic pain and obesity on serum cortisol in female adolescents, and then evaluate the potential influence of gonadal steroids and time of day on this interaction. The major findings were that (a) serum cortisol was lower with chronic pain and/or obesity compared to healthy controls, (b) serum cortisol was lower with chronic pain and obesity compared to chronic pain alone (healthy weight), (c) the lower serum cortisol in the pain alone group appeared to be more prominent in the morning compared to the afternoon and (d) neither testosterone nor estradiol showed a significant group or time effect.
The most striking finding of this study was the lower serum cortisol with obesity and/or pain in female adolescents. It is well known that obesity can lead to a decrease in HPA axis activity and reduction of the cortisol-cortisone ratio in adults (Ceccato et al., Citation2020). This may reflect changes in negative feedback sensitivity as well as the activity of 11-beta-hydroxysteroid dehydrogenase (Nees et al., Citation2019). Furthermore, there are well known sexual dimorphisms in the activity of the HPA axis in humans in general (Goel et al., Citation2014). The key link in the specific interaction of the HPA axis and sexual dimorphism with chronic pain and obesity (Narouze & Souzdalnitski, Citation2015; Sorge & Totsch, Citation2017) is likely to be a heightened inflammatory state mediated by cytokines and lipid metabolites (Bose et al., Citation2009; Nees et al., Citation2019). In fact, this has been termed a “three-way interaction” between the HPA axis, gonadal steroids, and the immune system (Torpy & Chrousos, Citation1996). That said, we as yet do not know if the same relationship will hold in male adolescents.
At first, we were surprised that serum cortisol concentrations overall were not different between morning and afternoon samples. After carefully examining actual patterns of typical human serum cortisol circadian rhythms, it became clear that this was due to the fact that the AM samples were obtained well after the cortisol awakening response responsible for a large portion of the early morning peak in cortisol and that the PM samples were obtained before 18:00 hr after which cortisol starts to decline in earnest (Raff & Trivedi, Citation2013). This is consistent with studies using frequent blood sampling for plasma ACTH and cortisol in healthy humans that showed ultradian pulses but not a dramatic overall downward slope over the time period during which we sampled our subjects (Lightman et al., Citation2020; Lightman & Conway-Campbell, Citation2010; Spiga et al., Citation2014). It is interesting that the lower serum cortisol in the healthy weight subjects with pain was more prominent in the morning than the afternoon. This is consistent with the notion that pain intensity is higher toward that latter part of the active phase suggesting that pain is cumulative during the day (Bumgarner et al., Citation2021). This has important implications as to when pain is evaluated and perhaps how it is treated. It is also worth commenting about the potential mechanism of the effect of cortisol with regards to pain. Not only does cortisol work through the glucocorticoid receptor as an anti-inflammatory, but cortisol also may attenuate pain via its well-known binding to the mineralocorticoid receptor in the nervous system (Gomez-Sanchez & Gomez-Sanchez, Citation2014; Shaqura et al., Citation2020; Tafelski et al., Citation2019). Finally, considering the complexity of the HPA axis, there are many other factors in addition to time of day and the cumulative nature of pain that could have influenced the results (Herman et al., Citation2016; Keller-Wood, Citation2015; McEwen & Kalia, Citation2010; Raff & Findling, Citation2003).
The study has several limitations. First and foremost, a single blood sample for cortisol is not optimal for inferring overall activity of the HPA axis (Lightman et al., Citation2020; Lightman & Conway-Campbell, Citation2010; Spiga et al., Citation2014). Furthermore, the concentrations of serum cortisol we found were higher than the typical reference interval in female adolescents, even considering their higher corticosteroid-binding globulin concentrations compared to males (Dichtel et al., Citation2019; Mezzullo et al., Citation2021; Zrinski Topic & Lenicek Krleza, Citation2020). Therefore, we feel it is likely that the serum cortisol levels shown in and , and represent basal plus stress-induced HPA activity. The stress was likely due to the anticipation of venipuncture in our subjects. However, since the subjects were sampled under similar conditions, this limitation may suggest the novel idea that the effects we observed may represent an attenuation of the stress response with chronic pain and/or obesity which may be sexually dimorphic (Geva et al., Citation2022; Kidwell et al., Citation2022).
We would have liked to evaluate these phenomena over the entire range of pubertal status, but most of the females enrolled were in late puberty or were post pubertal. This is because we recruited by age (13–17 years old) rather than pubertal status, so we ended up with almost all post-pubertal female subjects (Mouritsen et al., Citation2010). This homogeneity of pubertal status can be considered a strength as it does allow us to better infer HPA axis activity from serum cortisol without the confounding factor of a wide range of pubertal stages (Reynolds et al., Citation2013). Another limitation is that we did not have access to EDTA plasma samples from these subjects so we could not measure plasma ACTH. As stated above, we feel the serum cortisol likely reflects overall glucocorticoid activity, and hence HPA axis dynamics, considering the consistency of pubertal status and serum gonadal steroid concentrations in our subjects.
One would initially assume that chronic pain with or without obesity would increase cortisol (the ‘stress’ hormone) but that was clearly not the case in our study. This may be due to HPA axis habituation to chronic stressors like obesity-mediated increases in inflammatory cytokines and to increased pain afferent input (Herman et al., Citation2016). It remains to be seen if interventions to normalize basal and stress induced HPA axis activity in female adolescents with chronic pain and/or obesity could be a valid approach to mitigate the negative effects we have previously observed (Hainsworth et al., Citation2009).
Ethics approval
This project was approved by the Children’s Wisconsin Institutional Review Board.
Informed consent to participate
Written informed consent was obtained from participants and their parents.
Author contributions
All authors contributed to the study conception and design. Patient recruitment, sample obtainment, and patient demographic obtainment was performed by Keri Hainsworth. Assays were performed by Hershel Raff and Jonathan Phillips. Statistical analyses and figure preparation were performed by Pippa Simpson. The first draft of the manuscript was written by Hershel Raff, and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work could not have been done without the support of the Children’s Wisconsin Pediatric Translational Research Unit and the Children’s Research Institute.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Hershel Raff
Hershel Raff received his PhD at Johns Hopkins, did a post-doctoral fellowship at UCSF, and is currently a Professor of Medicine, Surgery, and Physiology at the Medical College of Wisconsin. His research focuses on the HPA axis (clinical and basic research), as well as neonatal and adult stress models.
Jonathan Phillips
Jonathan Phillips received his B.S. in chemistry from the University of Wisconsin-Stevens Point. He is a Research Technologist Senior in the Endocrine Research Laboratory and specializes in using LC-MS/MS instrumentation.
Pippa Simpson
Pippa Simpson received an MSc with distinction in computing from the University of London and a PhD from the University of Kentucky. She is Professor Emeritus at the Medical College of WI after leading a biostatistical consulting team in Pediatrics and at other universities. Her publications on pediatric studies exceed 400.
Steven J. Weisman
Steven Weisman received his MD from Albert Einstein College of Medicine. He is currently a Professor of Anesthesiology and Pediatrics at the Medical College of Wisconsin. He also serves as the Jane B. Pettit Chair in Pain Management, at Children’s Wisconsin. His research focuses on all aspects of pediatric pain.
Keri R. Hainsworth
Keri Hainsworth received her PhD from the University of Wisconsin–Milwaukee, did a post-doctoral fellowship at Children’s WI. She is an Associate Professor of Anesthesiology at the Medical College of WI and Research Director for the pain program at Children’s Wisconsin. Her research focuses on pediatric acute and chronic pain.
References
- Bose, M., Olivan, B., & Laferrere, B. (2009). Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Current Opinion in Endocrinology, Diabetes, and Obesity, 16(5), 340–346. https://doi.org/10.1097/MED.0b013e32832fa137
- Bumgarner, J. R., Walker, W. H., 2nd, & Nelson, R. J. (2021). Circadian rhythms and pain. Neuroscience and Biobehavioral Reviews, 129, 296–306. https://doi.org/10.1016/j.neubiorev.2021.08.004
- Ceccato, F., Lizzul, L., Barbot, M., & Scaroni, C. (2020). Pituitary-adrenal axis and peripheral cortisol metabolism in obese patients. Endocrine, 69(2), 386–392. https://doi.org/10.1007/s12020–020–02392–4
- Chrousos, G. P. (1995). The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. The New England Journal of Medicine, 332(20), 1351–1362. https://doi.org/10.1056/NEJM199505183322008
- Dichtel, L. E., Schorr, M., Loures de Assis, C., Rao, E. M., Sims, J. K., Corey, K. E., Kohli, P., Sluss, P. M., McPhaul, M. J., & Miller, K. K. (2019). Plasma Free Cortisol in States of Normal and Altered Binding Globulins: Implications for Adrenal Insufficiency Diagnosis. The Journal of Clinical Endocrinology and Metabolism, 104(10), 4827–4836. https://doi.org/10.1210/jc.2019–00022
- Geva, N., Golan, S., Pinchas, L., & Defrin, R. (2022). Sex effects in the interaction of acute stress and pain perception. Pain. Advance online publication. https://doi.org/10.1097/j.pain.0000000000002743
- Goel, N., Workman, J. L., Lee, T. T., Innala, L., & Viau, V. (2014). Sex differences in the HPA axis. Comprehensive Physiology, 4(3), 1121–1155. https://doi.org/10.1002/cphy.c130054
- Gomez-Sanchez, E., & Gomez-Sanchez, C. E. (2014). The multifaceted mineralocorticoid receptor. Comprehensive Physiology, 4(3), 965–994.
- Gungor, N. K. (2014). Overweight and obesity in children and adolescents. Journal of Clinical Research in Pediatric Endocrinology,.6(3), 129–143. https://doi.org/10.4274/Jcrpe.1471
- Hainsworth, K. R., Davies, W. H., Khan, K. A., & Weisman, S. J. (2009). Co-occurring chronic pain and obesity in children and adolescents: the impact on health-related quality of life. The Clinical Journal of Pain, 25(8), 715–721. https://doi.org/10.1097/AJP.0b013e3181a3b689
- Hainsworth, K. R., Simpson, P. M., Raff, H., Grayson, M. H., Zhang, L., & Weisman, S. J. (2021). Circulating inflammatory biomarkers in adolescents: evidence of interactions between chronic pain and obesity. Pain Reports, 6(1), e916. https://doi.org/10.1097/PR9.0000000000000916
- Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. https://doi.org/10.1002/cphy.c150015
- Keller-Wood, M. (2015). Hypothalamic-pituitary–adrenal axis-feedback control. Comprehensive Physiology, 5(3), 1161–1182.
- Kidwell, K. M., Reiter-Purtill, J., Decker, K., Howarth, T., Doland, F., & Zeller, M. H. (2022). Stress and eating responses in adolescent females predisposed to obesity: A pilot and feasibility study. Appetite, 179, 106308. https://doi.org/10.1016/j.appet.2022.106308
- Lightman, S. L., & Conway-Campbell, B. L. (2010). The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nature Reviews. Neuroscience, 11(10), 710–718. https://doi.org/10.1038/nrn2914
- Lightman, S. L., Birnie, M. T., & Conway-Campbell, B. L. (2020). Dynamics of ACTH and cortisol secretion and implications for disease. Endocrine Reviews, 41(3), bnaa002. https://doi.org/10.1210/endrev/bnaa002
- McEwen, B. S., & Kalia, M. (2010). The role of corticosteroids and stress in chronic pain conditions. Metabolism: clinical and Experimental, 59(Suppl 1), S9–S15. https://doi.org/10.1016/j.metabol.2010.07.012
- Mezzullo, M., Gambineri, A., Di Dalmazi, G., Fazzini, A., Magagnoli, M., Baccini, M., Vicennati, V., Pelusi, C., Pagotto, U., & Fanelli, F. (2021). Steroid reference intervals in women: influence of menopause, age and metabolism. European Journal of Endocrinology, 184(3), 395–407. https://doi.org/10.1530/EJE-20–1147
- Mouritsen, A., Aksglaede, L., Sorensen, K., Mogensen, S. S., Leffers, H., Main, K. M., Frederiksen, H., Andersson, A. M., Skakkebaek, N. E., & Juul, A. (2010). Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. International Journal of Andrology, 33(2), 346–359. https://doi.org/10.1111/j.1365–2605.2010.01051.x
- Narouze, S., & Souzdalnitski, D. (2015). Obesity and chronic pain: systematic review of prevalence and implications for pain practice. Regional Anesthesia and Pain Medicine, 40(2), 91–111. https://doi.org/10.1097/AAP.0000000000000218
- Nees, F., Loffler, M., Usai, K., & Flor, H. (2019). Hypothalamic-pituitary-adrenal axis feedback sensitivity in different states of back pain. Psychoneuroendocrinology, 101, 60–66. https://doi.org/10.1016/j.psyneuen.2018.10.026
- Petersen, A. C., Crockett, L., Richards, M., & Boxer, A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. https://doi.org/10.1007/BF01537962
- Raff, H., & Findling, J. W. (2003). A physiologic approach to diagnosis of the Cushing syndrome. Annals of Internal Medicine, 138(12), 980–991. https://doi.org/10.7326/0003–4819–138–12–200306170–00010
- Raff, H., & Trivedi, H. (2013). Circadian rhythm of salivary cortisol, plasma cortisol, and plasma ACTH in end-stage renal disease. Endocrine Connections, 2(1), 23–31. https://doi.org/10.1530/EC-12–0058
- Raff, H., Hoeynck, B., Jablonski, M., Leonovicz, C., Phillips, J. M., & Gehrand, A. L. (2018). Insulin sensitivity, leptin, adiponectin, resistin, and testosterone in adult male and female rats after maternal-neonatal separation and environmental stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 314(1), R12–R21. https://doi.org/10.1152/ajpregu.00271.2017
- Raff, H., Phillips, J. M., Simpson, P. M., Weisman, S. J., & Hainsworth, K. R. (2020). Serum soluble urokinase plasminogen activator receptor in adolescents: interaction of chronic pain and obesity. Pain Reports, 5(4), e836. https://doi.org/10.1097/PR9.0000000000000836
- Reynolds, R. M., Hii, H. L., Pennell, C. E., McKeague, I. W., de Kloet, E. R., Lye, S., Stanley, F. J., Mattes, E., & Foster, J. K. (2013). Analysis of baseline hypothalamic-pituitary-adrenal activity in late adolescence reveals gender specific sensitivity of the stress axis. Psychoneuroendocrinology, 38(8), 1271–1280. https://doi.org/10.1016/j.psyneuen.2012.11.010
- Rosner, W., Auchus, R. J., Azziz, R., Sluss, P. M., & Raff, H. (2007). Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. The Journal of Clinical Endocrinology and Metabolism, 92(2), 405–413. https://doi.org/10.1210/jc.2006–1864
- Shaqura, M., Li, L., Mohamed, D. M., Li, X., Treskatsch, S., Buhrmann, C., Shakibaei, M., Beyer, A., Mousa, S. A., & Schafer, M. (2020). Neuronal aldosterone elicits a distinct genomic response in pain signaling molecules contributing to inflammatory pain. Journal of Neuroinflammation, 17(1), 183. https://doi.org/10.1186/s12974–020–01864–8
- Sorge, R. E., & Totsch, S. K. (2017). Sex Differences in Pain. Journal of Neuroscience Research, 95(6), 1271–1281. https://doi.org/10.1002/jnr.23841
- Spiga, F., Walker, J. J., Terry, J. R., & Lightman, S. L. (2014). HPA axis-rhythms. Comprehensive Physiology, 4(3), 1273–1298.
- Stoner, A. M., Jastrowski Mano, K. E., Weisman, S. J., & Hainsworth, K. R. (2017). Obesity impedes functional improvement in youth with chronic pain: An initial investigation. European Journal of Pain (London, England), 21(9), 1495–1504. https://doi.org/10.1002/ejp.1051
- Tafelski, S., Mohamed, D., Shaqura, M., Assaf, C., Beyer, A., Treskatsch, S., Schafer, M., & Mousa, S. A. (2019). Identification of mineralocorticoid and glucocorticoid receptors on peripheral nociceptors: Translation of experimental findings from animal to human biology. Brain Research, 1712, 180–187. https://doi.org/10.1016/j.brainres.2019.02.015
- Tjoe, J. A., Piacentine, L. B., Papanek, P. E., Raff, H., Richards, J., Harkins, A. L., Yin, J., & Ng, A. V. (2020). Team triathlon effects on physiological, psychological, and immunological measures in women breast cancer survivors. Supportive Care in Cancer : official Journal of the Multinational Association of Supportive Care in Cancer, 28(12), 6095–6104. https://doi.org/10.1007/s00520–020–05457–2
- Torpy, D. J., & Chrousos, G. P. (1996). The three-way interactions between the hypothalamic-pituitary-adrenal and gonadal axes and the immune system. Bailliere’s Clinical Rheumatology, 10(2), 181–198. https://doi.org/10.1016/S0950–3579(96)80014–8
- Zrinski Topic, R., & Lenicek Krleza, J. (2020). Verification of the Canadian Laboratory Initiative on Paediatric Reference Intervals (CALIPER) reference values in Croatian children and adolescents. Biochemia Medica, 30(2), 020710. https://doi.org/10.11613/BM.2020.020710
